Abstract
Previous experiments have demonstrated that the vestibular system contributes to regulating sympathetic nervous system activity, particularly the discharges of vasoconstrictor fibres. In the present study, we examined the physiological significance of vestibulosympathetic responses by comparing blood flow and vascular resistance in the forelimb and hindlimb during head-up tilt from the prone position before and after the removal of vestibular inputs through a bilateral vestibular neurectomy. Experiments were performed on conscious cats that were trained to remain sedentary on a tilt table during rotations up to 60 deg in amplitude. Blood flow through the femoral and brachial arteries was recorded during whole-body tilt using perivascular probes; blood pressure was recorded using a telemetry system and vascular resistance was calculated from blood pressure and blood flow measurements. In vestibular-intact animals, 60 deg head-up tilt produced ∼20% decrease in femoral blood flow and ∼37% increase in femoral vascular resistance relative to baseline levels before tilt; similar effects were also observed for the brachial artery (∼25% decrease in blood flow and ∼38% increase in resistance). Following the removal of vestibular inputs, brachial blood flow and vascular resistance during head-up tilt were almost unchanged. In contrast, femoral vascular resistance increased only ∼6% from baseline during 60 deg head-up rotation delivered in the first week after elimination of vestibular signals and ∼16% in the subsequent 3-week period (as opposed to the ∼37% increase in resistance that occurred before lesion). These data demonstrate that vestibular inputs associated with postural alterations elicit regionally specific increases in vascular resistance that direct blood flow away from the region of the body where blood pooling may occur. Thus, the data support the hypothesis that vestibular influences on the cardiovascular system serve to protect against the occurrence of orthostatic hypotension.
Numerous animal studies have demonstrated that electrical or natural stimulation of vestibular receptors elicits changes in the activity of sympathetic efferents (Yates et al. 1993; Yates & Miller, 1994), particularly vasoconstrictor fibres (Kerman & Yates, 1998; Kerman et al. 2000a, b). In human subjects, modulation of vestibular nerve activity through head-down neck flexion (Essandoh et al. 1988; Shortt & Ray, 1997; Hume & Ray, 1999), caloric stimulation of the ear (Cui et al. 1997) or off-vertical axis rotation (Kaufmann et al. 2002) also elicits alterations in the discharges of vasoconstrictor efferents. It is thus not surprising that removal of vestibular inputs in animal models affects the regulation of blood pressure during gravitational challenges. Conscious rats lacking labyrinthine inputs were less able to appropriately adjust blood pressure during gravitational stress than vestibular-intact animals (Gotoh et al. 2004; Tanaka et al. 2006). In both anaesthetized and conscious cats, bilateral transection of the VIIIth cranial nerve (Doba & Reis, 1974; Jian et al. 1999; Holmes et al. 2002) or bilateral lesions of the caudal vestibular nuclei (Mori et al. 2005) resulted in labile blood pressure during head-up tilt of the body.
Although the vestibular system elicits net increases in vascular resistance to prevent orthostatic hypotension during postural alterations, vasoconstriction does not need to be equivalent in every vascular bed. Because blood pooling occurs in the lower extremities during head-up rotation, it would be of benefit if the sympathetic nervous system induced powerful vasoconstriction in the lower body during such movements to limit this pooling. As blood pooling is unlikely in the upper body during head-up postural alterations, vasoconstriction does not need to be as powerful in the forelimbs as in the hindlimbs. Thus, it is beneficial for vestibulosympathetic reflexes to be patterned, and to vary in magnitude in different regions of the body. Accordingly, simultaneous recordings from sympathetic efferents innervating blood vessels in muscle of the hindlimb, forelimb and face of cats revealed that rostrally and caudally located vasoconstrictor fibres have different responses to electrical stimulation of the vestibular nerve (Kerman et al. 2000b). Another study showed that electrical stimulation of VIIIth cranial nerve afferents produces opposite changes in blood flow to the forelimb and hindlimb in cats (Kerman et al. 2000a).
These latter findings are not in agreement with a general notion that the central nervous system lacks the capacity to evoke anatomically patterned changes in blood flow. Instead, many have concluded that patterning of blood flow mediated by the sympathetic nervous system is in accordance with tissue type, but not the location of the tissue within the body. For example, mild unloading of cardiopulmonary afferents results in an increase in the discharges of muscle vasoconstrictor fibres in both the arms and legs (Rea & Wallin, 1989), but no change in the activity of cutaneous vasoconstrictor fibres (Vissing et al. 1994). Mental stress produces parallel increases in arm and leg muscle sympathetic nerve activity (Carter et al. 2005), but variable changes in cutaneous blood flow depending on the ambient temperature (Wallin, 1990). When microinjection of sodium glutamate was used to activate neurons in the principal vasomotor region of the brainstem, the rostral ventrolateral medulla (RVLM), no differences could be found between sites that caused vasoconstriction in forelimb and hindlimb muscles (McAllen & Dampney, 1990), despite the fact that injection sites that specifically altered blood flow to particular tissues were readily identifiable (Dampney & McAllen, 1988; Dean et al. 1992; McAllen & May, 1994). As the RVLM mediates vestibular influences on sympathetic nervous system activity (Yates et al. 1995), these experiments suggest that the brainstem does not have the capacity to produce differing vestibulosympathetic responses in the forelimb and hindlimb. Furthermore, a study in humans (Monahan & Ray, 2002) failed to show that vestibular signals evoked by head-down neck flexion produce the anatomically related patterning in arm and leg muscle vasoconstrictor activity and vascular resistance elicited by vestibular nerve stimulation in the cat (Kerman et al. 2000a, b).
A number of explanations could potentially account for the disagreement in the literature regarding whether or not the vestibular system can elicit anatomically patterned changes in blood flow. Studies in animals examining the patterning of vestibulosympathetic responses have utilized anaesthetics that could affect regulation of sympathetic outflow, as well as stimuli that were not physiological (Kerman et al. 2000a, b). The one pertinent study in humans employed head-down neck flexion to modulate vestibular inputs (Monahan & Ray, 2002). This is a complex stimulus, which can activate many different sensory receptors, including neck proprioceptors, baroreceptors, and receptors in the airway. It is feasible that patterning of sympathetic efferent activity related to vestibular system activation during head-down neck flexion was masked by cardiovascular responses to other sensory inputs. To determine more definitively whether the vestibular system has the capacity to evoke anatomically patterned alterations in blood flow, we compared blood flow responses in the forelimb and hindlimb of conscious cats during graded head-up tilt up to 60 deg in amplitude, both before and after the removal of vestibular inputs through a bilateral vestibular neurectomy. We tested the hypothesis that bilateral transection of the VIIIth cranial nerve affects the patterning of limb blood flow that ordinarily occurs during postural alterations.
Methods
All experimental procedures conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh. Data were collected from seven purpose-bred adult female cats obtained from Liberty Research (Waverly, NY, USA). Animals were spayed prior to being included in this study to eliminate cyclic changes in hormonal levels.
Surgical procedures
Two recovery surgeries were required for each animal. Both surgeries were performed in a dedicated operating suite with the use of sterile procedures. Animals were initially anaesthetized with an intramuscular injection of ketamine (20 mg kg−1) and acepromazine (0.2 mg kg−1). Subsequently, an endotracheal tube and intravenous catheter were inserted. Anaesthesia was supplemented as necessary by using 1%–1.5% isoflurane vaporized in O2 so that limb withdrawal reflexes were absent and heart rate was stable. Lactate-containing Ringer solution was infused intravenously to replace fluid loss during the surgery. A heating pad and heat lamp were used to maintain core temperature near 38°C.
The first surgery was performed to mount a fixation plate to the skull and to implant a blood pressure recording device and perivascular probes to monitor blood flow. The blood pressure recording device consisted of a transducer and attached transmitter (Data Sciences International, St Paul, MN, USA), which permitted data collection using telemetry. To implant the blood pressure transducer, an incision was made in the right hindlimb to expose the femoral artery. A small opening was then made in the arterial wall so that the tip of the transducer could be introduced and advanced ∼4 cm rostrally into the abdominal aorta. The incision in the arterial wall was closed using Vetbond tissue adhesive (3M, St Paul, MN, USA) and a ligature was used to secure the transducer to the artery. An incision was made through the abdominal wall, and the transmitter unit was passed subcutaneously, deep to the inguinal ligament, into the peritoneal cavity and secured to the abdominal muscles using sutures. In addition, the left brachial and femoral arteries were accessed through a ventral approach; in two animals, the right brachial artery was also isolated. Care was taken not to disturb surrounding tissue while a perivascular probe (Transonic Systems, Ithaca, NY, USA) was placed around each artery and secured in place using sutures. The cable from each probe was routed subcutaneously and the connector was attached using dental cement to the skull behind the fixation bolt. Animals recovered for at least 3 weeks after this surgery before data collection was initiated. For 72 h following the surgery, analgesia was provided through the transdermal delivery of 25 μg h−1 fentanyl (Janssen Pharmaceutical Products, Titusville, NJ, USA). In addition, 50 mg antibiotic (amoxicillin) was administered orally twice daily for 10 days following surgery to prevent infection.
A second surgery was performed after initial data collection was complete to eliminate vestibular inputs. For this purpose, the tympanic bulla on each side of the skull was opened using a ventrolateral approach to expose the cochlea. A drill was used to remove temporal bone near the base of the cochlea, thereby producing a labyrinthectomy that rendered the vestibular apparatus dysfunctional. This procedure also provided access to the internal auditory canal. The VIIIth cranial nerve was transected under microscopic observation within the internal auditory canal. Thus, two independent lesions affecting the vestibular system were made on both sides to ensure that vestibular inputs were eliminated. In no case did nystagmus or a tonic deviation in eye position occur after the surgery, suggesting that the peripheral lesions were complete. Furthermore, post-mortem histological examinations performed as part of our previous studies utilizing this surgical method revealed that it is always completely effective in eliminating vestibular inputs (Jian et al. 1999; Cotter et al. 2001, 2004). To ensure that animals received proper hydration and nutrition during the post-surgical period, ∼100 ml Ringer solution was administered subcutaneously each day and animals were fed by hand until the spontaneous consumption of food and water returned to pre-lesion levels (which only required 2–3 days). In addition, 3 mg kg−1 ketoprofen, a non-steroidal anti-inflammatory drug (NSAID) with analgesic effects, was administered intramuscularly every 12 h for 3 days following surgery. After all data recording was completed, animals were deeply anaesthetized with an intraperitoneal injection of 40 mg kg−1 pentobarbital sodium and killed by transcardial perfusion with saline.
Data collection procedures
The animals were trained over a period of 1–2 months to remain sedentary, with hindlimbs fully extended, on a tilt table during head-up whole body rotation of 20, 40 or 60 deg. A jacket with attached Velcro straps was placed around the torso; the straps were secured to the sides of the tilt table to prevent the position of the animal from shifting during tilting. The head was immobilized by inserting a screw into the bolt mounted on the skull.
Prior experiments revealed that the consequences of vestibular lesions on cardiovascular regulation could differ depending on whether the laboratory was dark or illuminated (Jian et al. 1999; Wilson et al. 2006). Accordingly, blood pressure and limb blood flow were monitored during body tilt under two conditions in these experiments. During some trials, normal lighting was present in the laboratory, such that an animal could potentially employ visual cues to determine its body position in space. During other trials, the laboratory was darkened and black cardboard was mounted to the front and sides of the tilt table so that the animal's visual field rotated with its body. Under this condition, an animal was unable to use visual information to determine the orientation of its body.
Data were collected during recording sessions lasting 30 min. Head-up tilt at 20, 40 and 60 deg was randomly used throughout the recording sessions so that animals could not anticipate the amplitude of the next rotation. Tilts persisted for 45 s and were separated by at least 60 s. The tilt table was rotated manually and was secured in the tilted position using a locking device that permitted movement to one of the three predetermined tilt positions. Rotations from the Earth-horizontal to the head-up position were performed rapidly, at a velocity of ∼30 deg s−1 at all three amplitudes, to produce a sudden orthostatic challenge. Data collected during tilts in which animals vocalized or failed to remain stationary were discarded; as data collection was not initiated until animals were well-acclimated to the experimental protocol, the vast majority of trials yielded usable data. After baseline recordings were performed, vestibular inputs were surgically eliminated; data recording sessions resumed the day after the surgery, and continued for 30 days (although data collection was discontinued in one cat at 7 days after the lesion, due to an injury sustained by the animal). Experimental sessions were typically conducted 5 days per week, but were performed daily for the first week after vestibular lesions.
Data recording and analysis
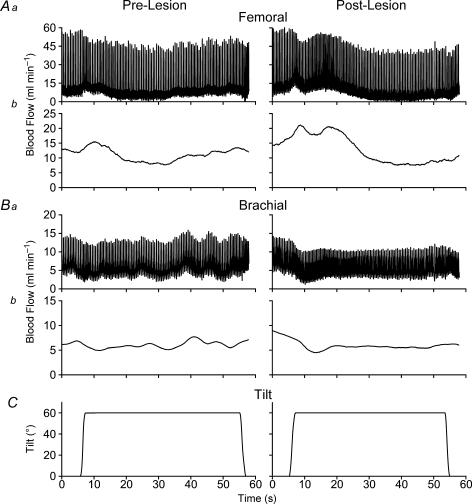
During recording sessions, each perivascular probe was connected to a Transonics Systems TS420 perivascular flow module, which provided instantaneous volume flow measurements. Both the pulsatile volume flow and mean volume flow outputs from these units were recorded digitally using a Cambridge Electronic Design (Cambridge, UK) 1401-plus data collection system interfaced with a Macintosh (Apple Computer, Cupertino, CA, USA) G4 computer. A voltage proportional to blood pressure produced by the Data Sciences International telemetric blood pressure system was also digitized. In addition, the voltage supplied by a potentiometer mounted on the tilt table provided a recording of table position. All signals were sampled at 100 Hz. Examples of the blood flow data recorded are shown in Fig. 1.
Figure 1. Examples of blood flow responses to head-up tilt recorded from Animal 2.
Recording of femoral artery (A) and brachial artery (B) blood flow. For each of these panels, trace a shows the pulsatile volume flow output and trace b illustrates the mean volume flow output from a Transonics Systems TS420 perivascular flow module. C, recording of table position provided by a potentiometer. The left-hand column shows responses recorded prior to removal of vestibular inputs, whereas the right-hand column provides data recorded during the first week after removal of vestibular inputs.
The Spike-2 software package (Cambridge Electronic Design) was used for data analysis. Each data file considered a 60-s interval, consisting of the 15-s period before the onset of each tilt and the 45-s period during which the animal was rotated head-up. For each 1-s bin in this interval, mean systolic blood pressure, mean diastolic blood pressure and mean blood flow through each of the arteries were determined. Mean arterial pressure was calculated using the formula: (⅔× diastolic blood pressure) + (⅓× systolic blood pressure).
Resistance of the limb vasculature was calculated by dividing mean arterial pressure by forelimb or hindlimb blood flow. When animals were tilted, blood pressure values employed for resistance calculations were corrected for the height of the hydrostatic column separating the heart and each perivascular probe. Typically, 32 successful trials were conducted per day per animal, with 16 performed in the dark and 16 executed when the laboratory was illuminated. Approximately one-third of the tilts were performed at each of the three amplitudes. The mean values obtained for identical trials performed on a particular day (i.e. at a particular rotation amplitude and laboratory lighting condition) were pooled to generate an average curve of responses to that testing condition. Thus, results from 32 trials were condensed into six average curves that provided the data used for subsequent analyses (i.e. the number of daily average curves was equivalent to the number of data points employed for statistical tests). Table 1 summarizes the number of days for which average curves were generated for each animal.
Table 1.
Approximately 32 trials were performed per animal per day, which included tilts at three amplitudes (20, 40 and 60 deg) conducted when the laboratory was either dark or illuminated
| Animal | Pre-lesion | Post-W1 | Post-W2–4 |
|---|---|---|---|
| 1 | 44 | 6 | — |
| 2 | 35 | 7 | 14 |
| 3 | 30 | 7 | 14 |
| 4 | 37 | 7 | 15 |
| 5 | 32 | 7 | 15 |
| 6 | 25 | 7 | 14 |
| 7 | 27 | 7 | 15 |
Data from trials performed using a particular tilt amplitude and laboratory lighting condition on each day were pooled to generate an average daily curve of responses to each testing situation. The number of days for which average curves were generated for each animal prior to removal of vestibular inputs (Pre-lesion), in the first week after removal of vestibular inputs (Post-W1), and in subsequent weeks (Post-W2–4) are shown.
Statistical analyses considered the effects of rotation amplitude, the availability of visual information reflecting body position in space, and vestibular lesions on limb blood flow and vascular resistance. In particular, we considered data obtained 5 s before each tilt (baseline condition) and at 2, 4, 6, 8, 10, 15 and 40 s after the onset of tilt. In a previous study we reported that compensation for the effects of vestibular lesions on regulation of blood pressure during postural alterations occurred after 1 week (Jian et al. 1999). For this reason, data recorded in the first 7 days after the removal of labyrinthine inputs and in subsequent weeks were considered as separate groups during analyses.
Statistical analyses were performed using SPSS Version 11 software (SPSS Inc., Chicago, IL, USA). Statistical significance was set at P < 0.05, and pooled data are presented as means ± s.e.m. To determine the main effect of tilt amplitude on limb blood flow and vascular resistance in labyrinth-intact animals, multivariate analyses of variance (MANOVA) were performed. These analyses in conjunction with the least significant difference (LSD) test were used to identify significant differences at each time point. Specifically, the analyses were conducted to identify differences in limb blood flow and vascular resistance values determined during 40 or 60 deg tilts and values determined during 20 deg tilt. To determine the effects of vestibular lesions on baseline limb blood flow and vascular resistance, univariate analyses of variance (ANOVA) were performed, with the presence of vestibular lesions (pre-lesion, first week post lesion and subsequent post-lesion period) serving as the dependent variable. Separate analyses were executed for data recorded when the laboratory was illuminated, and those recorded when the laboratory was dark. Planned comparisons using the LSD test were performed after obtaining significant F-values from the ANOVA to determine whether post-lesion baseline limb blood flow and vascular resistance differed from values prior to lesion. To ascertain whether vestibular lesions altered the patterning of blood distribution to the limbs during postural alterations, the percentage change in brachial and femoral blood flow or vascular resistance from baseline resulting from head-up tilt at 60 deg was considered. Specifically, manova combined with LSD tests compared the percentage change in limb blood flow and vascular resistance from baseline at each time point, for trials conducted prior to removal of vestibular inputs, trials performed in the first week after the vestibular neurectomy, and trials carried out subsequently. To ascertain directly whether removal of vestibular inputs had different effects on forelimb and hindlimb blood flow and vascular resistance, the mean data from each animal were included in a two-way ANOVA that compared percentage changes in brachial and femoral blood flow or vascular resistance from baseline at different time points during head-up tilt at 60 deg. Bonferroni post hoc test then established whether the changes in blood flow or vascular resistance from baseline at each time point were different for the forelimb and hindlimb. In this analysis, data recorded when the laboratory was dark or illuminated, as well as in the first week and subsequent weeks following the bilateral vestibular neurectomy, were considered separately.
Results
Both femoral and brachial blood flow were recorded successfully throughout the course of the experiment in four of the seven animals, as evidenced by the continuous presence of a stable pulsatile signal obtained from the Transonics Systems TS420 perivascular flow module. However, in one case (Animal 3), brachial blood flow could be recorded but the femoral perivascular probe became dislodged from the artery soon after the initiation of data collection. Similarly, in two other cats (Animals 1 and 5), the brachial probe became dislodged soon after implantation, although the femoral probe provided data. Post-mortem analyses indicated that in cases where a probe was detached from an artery, no apparent injury to the artery and adjacent tissues had occurred and that the artery had not been obstructed. Additionally, one of the animals (Animal 1) sustained an injury 1 week after the vestibular neurectomy; this animal was immediately killed, so that only 6 days of post-lesion data are available in this case.
In all animals, stable blood pressure recordings were obtained from the transducer throughout the course of the experiment, permitting the calculation of vascular resistance. Removal of vestibular inputs resulted in blood pressure lability during head-up tilt, as has been previously described (Jian et al. 1999; Holmes et al. 2002). Thus, it was necessary to calculate vascular resistance in order to determine whether changes in limb blood flow were due to alterations in vasoconstriction or variations in perfusion pressure.
In two animals, we compared the consequences of tilt amplitude, laboratory lighting conditions and vestibular lesions on left and right brachial blood flow and vascular resistance. In both cases, manipulations produced similar effects on the left and right forelimb vasculature. Thus, data recorded from only one brachial artery of these two animals (the left side) were considered for subsequent analysis. An initial goal of these experiments was to determine whether the effects of removal of labyrinthine inputs on limb blood flow and vascular resistance varied depending on the availability of visual cues reflecting body position in space. However, the consequences of vestibular lesions on these parameters were found to be indistinguishable when animals were tested in the dark and when the lights were illuminated. For this reason, only data recorded in the dark are presented below.
Effects of postural alterations on limb blood in animals with an intact vestibular apparatus
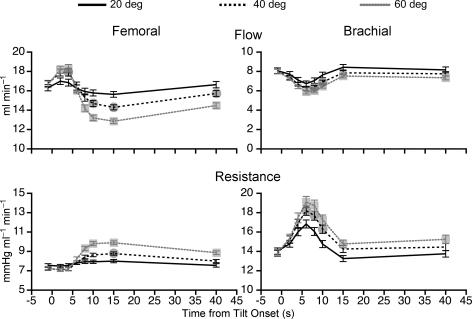
Figure 2 shows the effects of head-up tilts of different amplitudes on femoral (left-hand panel) and brachial (right-hand panel) blood flow and vascular resistance of all animals prior to removal of vestibular inputs. Head-up rotation produced alterations in femoral blood flow and vascular resistance that were graded depending on the tilt amplitude. In every animal, 60 deg head-up tilt elicited increases in femoral resistance and decreases in flow that were significantly larger than responses elicited by 20 deg tilt. These effects of tilt amplitude on femoral blood flow and vascular resistance were maximal 8–15 s after the onset of rotation. At 10 s after the onset of rotation with head-up tilt at 60 deg, average femoral blood flow was 21% lower than the baseline and average vascular resistance was 35% higher than baseline.
Figure 2. Average changes in femoral and brachial blood flow and resistance during 20, 40 and 60 deg head-up tilt prior to removal of vestibular inputs.
Data were recorded in a darkened laboratory. Changes in blood flow or vascular resistance elicited by 40 ( ) and 60 deg (
) and 60 deg ( ) tilt were significantly different from those resulting from 20 deg tilt. For points where no symbols are present, no significant differences were present. Error bars indicate one s.e.m.
) tilt were significantly different from those resulting from 20 deg tilt. For points where no symbols are present, no significant differences were present. Error bars indicate one s.e.m.
Head-up tilt produced similar changes in brachial blood flow and resistance as were observed for the femoral artery (see right-hand panel of Fig. 2). The only tangible differences in the consequences of rotation at 60 deg on the forelimb and hindlimb vasculature related to timing: the maximal changes in brachial blood flow and vascular resistance occurred at 6 s after the onset of tilt, whereas maximal effects on the femoral artery occurred at 8–10 s after the onset of rotation. On average, 60 deg head-up tilt produced a maximum decrease of 27% in brachial blood flow from baseline that was accompanied by 38% increase in brachial vascular resistance.
Effects of removal of vestibular inputs on baseline limb blood flow and vascular resistance
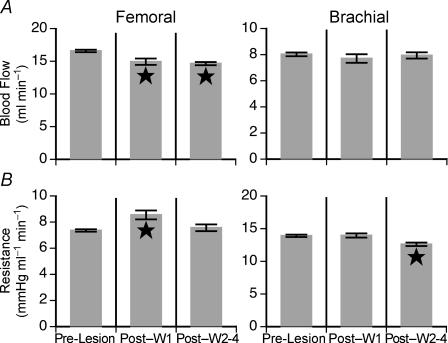
Baseline limb blood flow and vascular resistance were ascertained from recordings made 5 s before each tilt, while animals were in the prone position. Prior to vestibular lesion, baseline femoral blood flow was fairly consistent between animals, ranging from 13.5 ± 0.2 to 20.5 ± 0.4 ml min−1. A bilateral vestibular neurectomy had varying effects on baseline femoral blood flow. In three of the six animals the lesions produced a slight decrease in baseline femoral blood flow, in one cat there was a slight increase in blood flow, and in the other two cases no clear statistical change in blood flow was evident. The left panel of Fig. 3 shows the consequences of removal of vestibular inputs on mean baseline femoral blood flow (Fig. 3A) and vascular resistance (Fig. 3B) for all animals. These group averages reveal that for the population of animals studied, removal of vestibular inputs elicited a small decrease in baseline femoral blood flow and a small increase in baseline femoral resistance. However, vascular resistance was not significantly different from pre-lesion values after the first week following vestibular lesion.
Figure 3. Effects of removal of vestibular inputs on baseline femoral and brachial blood flow (A) and vascular resistance (B) measured when the animals were in the prone position in a darkened laboratory.
Baseline blood flow determined in the first week after the removal of vestibular inputs (Post-W1) and in subsequent weeks (Post-W2–4) are indicated separately. Significant differences in blood flow measured after the removal of vestibular inputs from that recorded prior to lesion are indicated by a star on the relevant bar. Error bars indicate one s.e.m.
When the labyrinths were intact, baseline brachial blood flow was similar between animals, ranging from 6.9 ± 0.1 to 10.2 ± 0.6 ml min−1. A bilateral vestibular neurectomy had little effect on either baseline brachial blood flow or resistance, as reflected in the mean values for all animals shown in the right-hand panel of Fig. 3. Although the vestibular lesions resulted in ∼10% decrease in baseline brachial vascular resistance, this effect was not evident until 1 week after surgery.
Effects of removal of vestibular inputs on limb blood flow and vascular resistance during head-up rotation
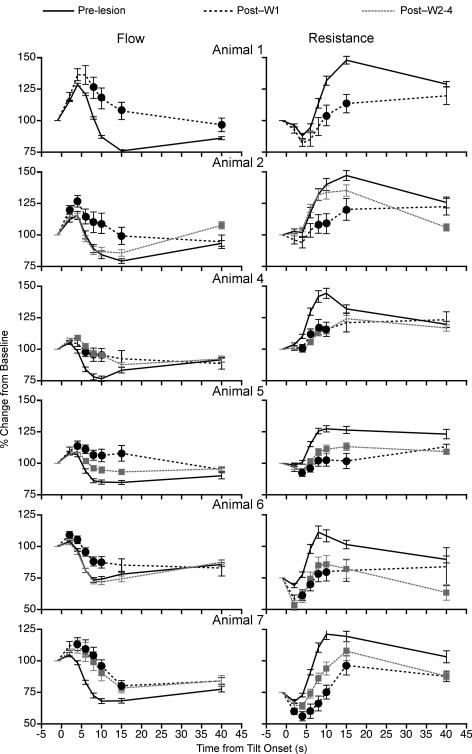
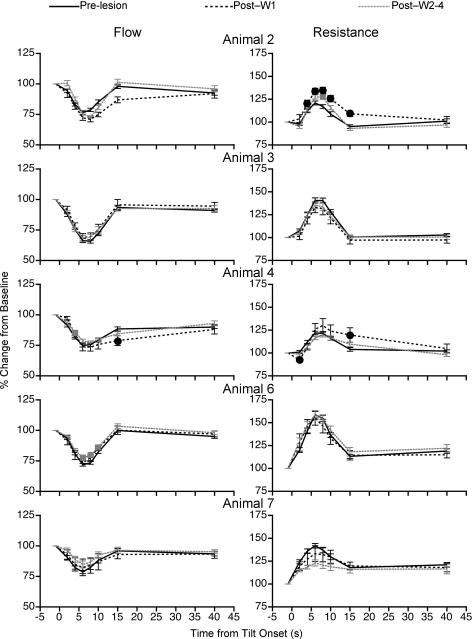
To determine whether the vestibular system contributes to regulating the blood distribution in the limbs during postural alterations, the changes in femoral and brachial blood flow and vascular resistance during 60 deg head-up tilt were compared before and after bilateral transection of the VIIIth cranial nerve. Figure 4 shows the effects of removal of vestibular inputs on the percentage changes in femoral blood flow and vascular resistance in individual animals during 60 deg head-up rotation. The data were normalized to the baseline values ascertained before each tilt while the animals were in the prone position. In every case, removal of labyrinthine inputs decreased the reduction in femoral blood flow and the increase in vascular resistance that accompanied head-up rotation when vestibular signals were present. The consequences of the vestibular neurectomy on regulation of femoral blood flow and vascular resistance were most prominent during the first week after lesion. However, in four of the six animals (Animals 4, 5, 6 and 7), the effects of the lesions on tilt-related alterations in femoral vascular resistance were still evident more than 7 days after the transaction of VIIIth cranial nerve (see Fig. 4). Only Animal 2 fully recovered the ability to regulate femoral vascular resistance at the onset of head-up rotation by 1 week after the vestibular neurectomy. Animal 1 was killed 7 days after transection of the VIIIth nerve, so the long-term consequences of the lesion cannot be ascertained for this cat.
Figure 4. Effects of removal of vestibular inputs on changes in femoral blood flow and vascular resistance from pre-tilt levels that occurred at different times during 60 deg head-up rotation.
Data were recorded in a darkened laboratory. Responses recorded from each animal are shown in separate rows. Distinct lines illustrate data recorded prior to vestibular lesion (Pre-lesion), values ascertained during the first week after vestibular neurectomy (Post-W1), and responses recorded subsequently (Post-W2–4). Symbols (Post-W1, •; Post-W2–4,  ) indicate post-lesion changes in blood flow or vascular resistance during tilts that were significantly different from those recorded when vestibular inputs were present. For points where no symbols are present, no significant differences were found. Error bars indicate one s.e.m.
) indicate post-lesion changes in blood flow or vascular resistance during tilts that were significantly different from those recorded when vestibular inputs were present. For points where no symbols are present, no significant differences were found. Error bars indicate one s.e.m.
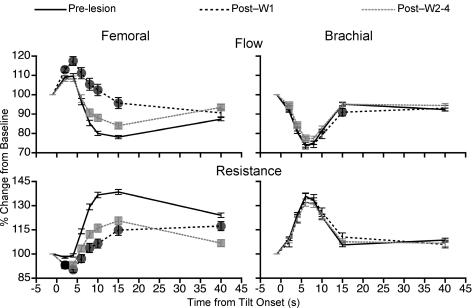
The mean effects of removal of vestibular inputs on femoral blood flow and vascular resistance for all the animals combined are shown in the left-hand panel of Fig. 5. Across the group, it was evident that removal of labyrinthine inputs profoundly diminished the hindlimb vascular responses that occurred in vestibular-intact animals during rotation with head-up tilt, particularly at 8–15 s after the onset of the rotation. For example, at 10 s after the tilt was initiated, femoral blood flow dropped an average of 20 ± 1% from baseline in labyrinth-intact animals, but increased 2 ± 3% from baseline during the first week after vestibular lesion and decreased an average of 12 ± 2% from baseline during the subsequent 3 weeks of data recording. At the same time point, femoral vascular resistance increased an average of 37 ± 2% from baseline values when the vestibular system was intact, but increased only 6 ± 3% from baseline during the first week after elimination of vestibular signals and 16 ± 3% during the subsequent 3-week period.
Figure 5. The average effects of removal of vestibular inputs on femoral and brachial blood flow and vascular resistance relative to pre-tilt baseline values.
Data were recorded in a darkened laboratory. Lines illustrate mean data recorded prior to vestibular lesion (Pre-lesion), mean values recorded during the first week after the vestibular neurectomy (Post-W1), and mean responses recorded subsequently (Post-W2–4). Symbols (Post-W1, •; Post-W2–4,  ) indicate post-lesion changes in blood flow and vascular resistance during tilt that were significantly different from those recorded when vestibular inputs were present. For points where no symbols are present, no significant differences were found. Error bars indicate one s.e.m.
) indicate post-lesion changes in blood flow and vascular resistance during tilt that were significantly different from those recorded when vestibular inputs were present. For points where no symbols are present, no significant differences were found. Error bars indicate one s.e.m.
The effects of removal of vestibular inputs on brachial blood flow and vascular resistance were much smaller than those observed for the femoral artery. Figure 6 shows the percentage changes in brachial blood flow (left-hand column) and vascular resistance (right-hand column) from pre-tilt baseline values for each animal during the course of 60 deg head-up rotation. During the first week after the bilateral vestibular neurectomy, for most time points the changes in brachial blood flow and resistance during the rotation were virtually identical to those prior to lesioning. When any disparities existed between the tilt-related changes in brachial resistance before and during the first week after the removal of labyrinthine inputs, there was a tendency for resistance to increase slightly more in the lesioned cats (see the right-hand panel of Fig. 6). In contrast, vestibular lesioning diminished the tilt-related increase in femoral vascular resistance that occurred when the labyrinths were intact (see Figs 4 and 5).
Figure 6. Effects of removal of vestibular inputs on changes in brachial blood flow and vascular resistance from pre-tilt levels that occurred at different times during 60 deg head-up rotation.
The symbols and abbreviations, and recording of data are the same as for Fig. 4. Data were recorded in a darkened laboratory.
The relative ineffectiveness of vestibular lesions in affecting posturally related alterations in brachial blood flow and vascular resistance is particularly evident from a pooling of the data from all of the animals shown in the right-hand panel of Fig. 5. Removal of vestibular inputs maximally affected brachial blood flow and vascular resistance relative to baseline values at 15 s after the onset of tilt. At this time point, average blood flow was 5 ± 1% lower than baseline in labyrinth-intact cats, but 9 ± 2% lower than baseline during the first week after the removal of vestibular inputs (only a 4% difference). However, more than 1 week following lesion, mean brachial blood flow relative to baseline at 15 s after tilt onset was identical to that measured when vestibular inputs were present. The largest disparities in mean brachial vascular resistance relative to baseline noted in labyrinth-intact cats and those lacking vestibular inputs also occurred at 15 s after tilt onset. At this time point average brachial resistance was 6 ± 1% higher than the baseline value in vestibular-intact cats, but 11 ± 2% higher during the first week after the vestibular neurectomy (only a 5% difference). Brachial vascular resistance at this time point was virtually identical to pre-lesion values 1 week after transection of the VIIIth cranial nerve. In contrast, during the first week after removal of vestibular inputs, average femoral blood flow relative to baseline maximally differed by 22% from pre-lesion values; mean femoral resistance relative to baseline maximally differed by 31% when the labyrinths were intact and in the first week after lesions (see Fig. 5).
Differences in the effects of removal of vestibular inputs on forelimb and hindlimb blood flow and vascular resistance during head-up rotation
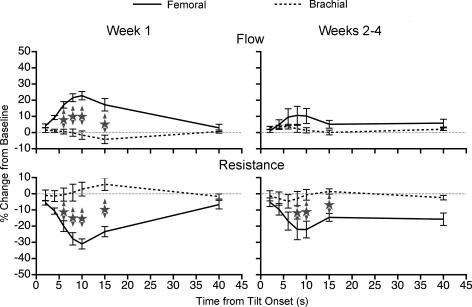
To directly ascertain whether removal of vestibular inputs had different effects on forelimb and hindlimb blood flow or vascular resistance, the mean data from each animal were included in a two-way ANOVA to compare percentage changes in these parameters from baseline at different time points during 60 deg head-up tilt. The results of this analysis are shown in Fig. 7. The percentage changes in femoral and brachial blood flow or vascular resistance from the baseline are illustrated on the same graph; data recorded after the removal of vestibular inputs are plotted relative to those recorded before vestibular lesion (i.e. the differences between pre- and post-lesion responses are shown). The left-hand panel of Fig. 7 shows the differences between responses recorded during the first week after vestibular lesion and those determined beforehand; the right-hand panel illustrates data recorded after the first week following removal of vestibular inputs relative to pre-lesion responses. Stars show significant differences in mean femoral and brachial blood flow or resistance values at the same time point. Figure 7 shows that during the first week after lesion the effects of removal of vestibular inputs on femoral and brachial blood flow and vascular resistance differed significantly at 6–15 s from the onset of tilt. After animals had recovered for more than 1 week following lesion, significant differences in femoral and brachial resistance values were still evident at 8–15 s from the onset of tilt. It is noteworthy that significant differences could be found despite the limited power of the analysis (because mean values from each animal were considered, the n = 6 for the femoral artery and n = 5 for the brachial artery).
Figure 7. Comparison of the effects of removal of vestibular inputs on forelimb and hindlimb blood flow and vascular resistance during 60 deg head-up rotation performed in a darkened laboratory.
The percentage changes in femoral and brachial blood flow or vascular resistance from the pre-tilt baseline are illustrated on the same graph; data collected after the removal of vestibular inputs are plotted relative to those before vestibular lesion. The left-hand panel shows differences between responses recorded during the first week after vestibular lesion and those determined prior to lesion; the right-hand panel illustrates data recorded subsequent to the first week after removal of vestibular inputs relative to pre-lesion responses. Stars show significant differences in mean femoral and brachial blood flow or resistance values at the same time point. Error bars indicate one s.e.m.
Discussion
This study showed that head-up tilt in conscious cats typically results in a decrease in blood flow and an increase in vascular resistance in both the forelimb and hindlimb. However, immediately following the removal of vestibular inputs through a bilateral vestibular neurectomy, the hindlimb responses were appreciably attenuated, whereas the forelimb responses were slightly augmented. Furthermore, baseline femoral blood flow was diminished and baseline femoral resistance increased acutely after the lesions, although little change occurred in baseline brachial blood flow and vascular resistance. These findings demonstrate that during postural alterations, the vestibular system elicits regionally specific increases in vascular resistance that serve to direct blood flow away from the region of the body where blood pooling may occur. Thus, these data support the hypothesis that vestibular influences on the cardiovascular system act to protect against the occurrence of orthostatic hypotension. In addition, the present results demonstrate that the central nervous system has the capacity to evoke anatomically patterned changes in blood flow, in contradiction to studies suggesting that patterning of sympathetic outflow to blood vessels occurs mainly in accordance with tissue type but not the location of the tissue within the body.
Previous studies have demonstrated that vestibular inputs modulate the activity of sympathetic vasoconstrictor fibres (Essandoh et al. 1988; Cui et al. 1997; Shortt & Ray, 1997; Kerman & Yates, 1998; Hume & Ray, 1999; Kerman et al. 2000b; Kaufmann et al. 2002), and it thus seems likely that the consequences of vestibular lesion on regulation of limb blood flow observed in this study were due to a loss of vestibulosympathetic responses. Nonetheless, one possibility that must be considered is that alterations in the tone of limb skeletal muscles after the removal of labyrinthine inputs indirectly resulted in the alterations in blood flow observed in the present experiments. However, previous studies have demonstrated that vestibular reflexes have similar effects on both forelimb and hindlimb muscles of cats (for review see Wilson & Peterson, 1981), so that it is improbable that the loss of vestibulospinal reflexes would selectively alter hindlimb blood flow. Moreover, removal of vestibular inputs does not appreciably alter the responses of hindlimb muscles to postural perturbations, but only the dynamics of the responses, presumably because somatosensory inputs also have a major role in adjusting limb muscle activity during postural disturbances (Thomson et al. 1991; Macpherson & Inglis, 1993; Inglis & Macpherson, 1995). These observations suggest that the effects of vestibular lesion on limb blood flow observed in this study were the consequence of a selective loss of vestibulosympathetic reflexes, and not indirect factors such as changes in the tone of limb muscles.
The effects of a bilateral vestibular neurectomy on the regulation of limb blood flow during postural alterations dissipated over time. This observation is in agreement with previous studies, which also demonstrated that the loss of vestibular inputs has only a transient effect on cardiovascular regulation (Jian et al. 1999; Jauregui-Renaud et al. 2003). Regions of the vestibular nuclei that influence autonomic control receive both vestibular inputs and non-labyrinthine signals that could be used to determine body location is space (Jian et al. 2002, 2005). Furthermore, the firing of some vestibular nucleus neurons is modulated in accordance with body position following a bilateral vestibular neurectomy (Yates et al. 2000). It has been suggested that plasticity occurs in the vestibular nuclei after bilateral transection of the VIIIth cranial nerve, which permits the ‘substitution’ of non-labyrinthine signals for the missing inputs from the inner ear and the reappearance of responses that are typically elicited by vestibular inputs (Yates et al. 2000; Jian et al. 2002, 2005; Mori et al. 2005). The occurrence of such sensory substitution in vestibulosympathetic reflex pathways would account for the findings in the present study.
In summary, we have shown in the present study that removal of vestibular inputs attenuates the increase in vascular resistance and the decrease in blood flow in the hindlimbs that usually occurs during head-up rotation. These data support the hypothesis that vestibulosympathetic reflexes serve to redirect blood from areas where pooling might occur during gravitational challenges. However, further experiments will be needed to support definitively this premise. In particular, it will be necessary to demonstrate directly that removal of labyrinthine inputs results in a reduction of venous return to the heart during head-up tilt in order to establish that the vestibular system serves to elicit patterned cardiovascular responses that protect against orthostatic intolerance. Furthermore, additional studies will be needed to determine the neural pathways that mediate the anatomically patterned alterations in blood flow that are elicited by vestibular inputs. One possibility is that the vestibular system provides selective inputs to neurons in the RVLM that regulate sympathetic outflow to blood vessels in the upper and lower body. It is also feasible that different regions of the brainstem, such as the medullary raphe nuclei (Blessing & Nalivaiko, 2000) and the A5 region (Stanek et al. 1984), are responsible for mediating the selective vestibular influences on vasculature in each body region. Experiments involving the use of multiple transneuronal tracers in the same animal to label the circuitry involved in regulating sympathetic outflow to several body areas may be helpful to elucidate the neural pathways responsible for producing the anatomically patterned vestibulosympathetic responses.
Acknowledgments
The authors thank Katie Wilkinson and Brian Sadacca for valuable technical assistance with studies and Drs Ilan Kerman and Alan Sved for their helpful suggestions regarding a previous version of this manuscript. This work was supported by grant R01 DC00693 from the National Institutes of Health (NIH). Core support was provided by NIH grant P30-DC05205.
References
- Blessing WW, Nalivaiko E. Regional blood flow and nociceptive stimuli in rabbits: patterning by medullary raphe, not ventrolateral medulla. J Physiol. 2000;524:279–292. doi: 10.1111/j.1469-7793.2000.t01-2-00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JR, Kupiers NT, Ray CA. Neurovascular responses to mental stress. J Physiol. 2005;564:321–327. doi: 10.1113/jphysiol.2004.079665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter LA, Arendt HE, Cass SP, Jian BJ, Mays DF, II, Olsheski CJ, Wilkinson KA, Yates BJ. Effects of postural changes and vestibular lesions on genioglossal muscle activity in conscious cats. J Appl Physiol. 2004;96:923–930. doi: 10.1152/japplphysiol.01013.2003. [DOI] [PubMed] [Google Scholar]
- Cotter LA, Arendt HE, Jasko JG, Sprando C, Cass SP, Yates BJ. Effects of postural changes and vestibular lesions on diaphragm and rectus abdominis activity in awake cats. J Appl Physiol. 2001;91:137–144. doi: 10.1152/jappl.2001.91.1.137. [DOI] [PubMed] [Google Scholar]
- Cui J, Mukai C, Iwase S, Sawasaki N, Kitazawa H, Mano T, Sugiyama Y, Wada Y. Response to vestibular stimulation of sympathetic outflow to muscle in humans. J Auton Nerv Syst. 1997;66:154–162. doi: 10.1016/s0165-1838(97)00077-5. [DOI] [PubMed] [Google Scholar]
- Dampney RAL, McAllen RM. Differential control of sympathetic fibres supplying hindlimb skin and muscle by subretrofacial neurones in the cat. J Physiol. 1988;395:41–56. doi: 10.1113/jphysiol.1988.sp016907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C, Seagard JL, Hopp FA, Kampine JP. Differential control of sympathetic activity to kidney and skeletal muscle by ventral medullary neurons. J Auton Nerv Syst. 1992;37:1–10. doi: 10.1016/0165-1838(92)90139-8. [DOI] [PubMed] [Google Scholar]
- Doba N, Reis DJ. Role of the cerebellum and vestibular apparatus in regulation of orthostatic reflexes in the cat. Circ Res. 1974;34:9–18. doi: 10.1161/01.res.40.4.9. [DOI] [PubMed] [Google Scholar]
- Essandoh LK, Duprez DA, Shepherd JT. Reflex constriction of human resistance vessels to head-down neck flexion. Am J Physiol. 1988;64:767–770. doi: 10.1152/jappl.1988.64.2.767. [DOI] [PubMed] [Google Scholar]
- Gotoh TM, Fujiki N, Matsuda T, Gao S, Morita H. Roles of baroreflex and vestibulosympathetic reflex in controlling arterial blood pressure during gravitational stress in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R25–R30. doi: 10.1152/ajpregu.00458.2003. [DOI] [PubMed] [Google Scholar]
- Holmes MJ, Cotter LA, Arendt HE, Cass SP, Yates BJ. Effects of lesions of the caudal cerebellar vermis on cardiovascular regulation in awake cats. Brain Res. 2002;938:62–72. doi: 10.1016/s0006-8993(02)02495-2. [DOI] [PubMed] [Google Scholar]
- Hume KM, Ray CA. Sympathetic responses to head-down rotations in humans. J Appl Physiol. 1999;86:1971–1976. doi: 10.1152/jappl.1999.86.6.1971. [DOI] [PubMed] [Google Scholar]
- Inglis JT, Macpherson JM. Bilateral labyrinthectomy in the cat: effects on the postural response to translation. J Neurophysiol. 1995;73:1181–1191. doi: 10.1152/jn.1995.73.3.1181. [DOI] [PubMed] [Google Scholar]
- Jauregui-Renaud K, Hermosillo AG, Gomez A, Marquez MF, Cardenas M, Bronstein AM. Vestibular function interferes in cardiovascular reflexes. Arch Med Res. 2003;34:200–204. doi: 10.1016/s0188-4409(03)00023-7. [DOI] [PubMed] [Google Scholar]
- Jian BJ, Acernese AW, Lorenzo J, Card JP, Yates BJ. Afferent pathways to the region of the vestibular nuclei that participates in cardiovascular and respiratory control. Brain Res. 2005;1044:241–250. doi: 10.1016/j.brainres.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Jian BJ, Cotter LA, Emanuel BA, Cass SP, Yates BJ. Effects of bilateral vestibular lesions on orthostatic tolerance in awake cats. J Appl Physiol. 1999;86:1552–1560. doi: 10.1152/jappl.1999.86.5.1552. [DOI] [PubMed] [Google Scholar]
- Jian BJ, Shintani T, Emanuel BA, Yates BJ. Convergence of limb, visceral, and vertical semicircular canal or otolith inputs onto vestibular nucleus neurons. Exp Brain Res. 2002;144:247–257. doi: 10.1007/s00221-002-1042-8. [DOI] [PubMed] [Google Scholar]
- Kaufmann H, Biaggioni I, Voustianiouk A, Diedrich A, Costa F, Clarke R, Gizzi M, Raphan T, Cohen B. Vestibular control of sympathetic activity. An otolith-sympathetic reflex in humans. Exp Brain Res. 2002;143:463–469. doi: 10.1007/s00221-002-1002-3. [DOI] [PubMed] [Google Scholar]
- Kerman IA, Emanuel BA, Yates BJ. Vestibular stimulation leads to distinct hemodynamic patterning. Am J Physiol Regul Integr Comp Physiol. 2000a;279:R118–R125. doi: 10.1152/ajpregu.2000.279.1.R118. [DOI] [PubMed] [Google Scholar]
- Kerman IA, Yates BJ. Regional and functional differences in the distribution of vestibulosympathetic reflexes. Am J Physiol Regul Integr Comp Physiol. 1998;275:R824–R835. doi: 10.1152/ajpregu.1998.275.3.R824. [DOI] [PubMed] [Google Scholar]
- Kerman IA, Yates BJ, McAllen RM. Anatomic patterning in the expression of vestibulosympathetic reflexes. Am J Physiol Regul Integr Comp Physiol. 2000b;279:R109–R117. doi: 10.1152/ajpregu.2000.279.1.R109. [DOI] [PubMed] [Google Scholar]
- McAllen RM, Dampney RAL. Vasomotor neurons in the rostral ventrolateral medulla are organized topographically with respect to type of vascular bed but not body region. Neurosci Lett. 1990;110:91–96. doi: 10.1016/0304-3940(90)90793-9. [DOI] [PubMed] [Google Scholar]
- McAllen RM, May CN. Differential drives from rostral ventrolateral medullary neurons to three identified sympathetic outflows. Am J Physiol Regul Integr Comp Physiol. 1994;267:R935–R944. doi: 10.1152/ajpregu.1994.267.4.R935. [DOI] [PubMed] [Google Scholar]
- Macpherson JM, Inglis JT. Stance and balance following bilateral labyrinthectomy. Prog Brain Res. 1993;97:219–228. doi: 10.1016/s0079-6123(08)62281-5. [DOI] [PubMed] [Google Scholar]
- Monahan KD, Ray CA. Limb neurovascular control during altered otolithic input in humans. J Physiol. 2002;538:303–308. doi: 10.1113/jphysiol.2001.013131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori RL, Cotter LA, Arendt HE, Olsheski CJ, Yates BJ. Effects of bilateral vestibular nucleus lesions on cardiovascular regulation in conscious cats. J Appl Physiol. 2005;98:526–533. doi: 10.1152/japplphysiol.00970.2004. [DOI] [PubMed] [Google Scholar]
- Rea RF, Wallin BG. Sympathetic nerve activity in arm and leg muscles during lower body negative pressure in humans. J Appl Physiol. 1989;66:2778–2781. doi: 10.1152/jappl.1989.66.6.2778. [DOI] [PubMed] [Google Scholar]
- Shortt TL, Ray CA. Sympathetic and vascular responses to head-down neck flexion in humans. Am J Physiol Heart Circ Physiol. 1997;272:H1780–H1784. doi: 10.1152/ajpheart.1997.272.4.H1780. [DOI] [PubMed] [Google Scholar]
- Stanek KA, Neil JJ, Sawyer WB, Loewy AD. Changes in regional blood flow and cardiac output after L-glutamate stimulation of A5 cell group. Am J Physiol Heart Circ Physiol. 1984;246:H44–H51. doi: 10.1152/ajpheart.1984.246.1.H44. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Gotoh TM, Awazu C, Morita H. Roles of the vestibular system in controlling arterial pressure in conscious rats during a short period of microgravity. Neurosci Lett. 2006;397:40–43. doi: 10.1016/j.neulet.2005.11.052. [DOI] [PubMed] [Google Scholar]
- Thomson DB, Inglis JT, Schor RH, Macpherson JM. Bilateral labyrinthectomy in the cat: motor behaviour and quiet stance parameters. Exp Brain Res. 1991;85:364–372. doi: 10.1007/BF00229414. [DOI] [PubMed] [Google Scholar]
- Vissing SF, Scherrer U, Victor RG. Increase of sympathetic discharge to skeletal muscle but not to skin during mild lower body negative pressure in humans. J Physiol. 1994;481:233–241. doi: 10.1113/jphysiol.1994.sp020434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin BG. Neural control of human skin blood flow. J Auton Nerv Syst. 1990;30(suppl):S185–S190. doi: 10.1016/0165-1838(90)90128-6. [DOI] [PubMed] [Google Scholar]
- Wilson TD, Cotter LA, Draper JA, Misra SP, Rice CD, Cass SP, Yates BJ. Effects of postural changes and removal of vestibular inputs on blood flow to the head of conscious felines. J Appl Physiol. 2006;100:1475–1482. doi: 10.1152/japplphysiol.01585.2005. [DOI] [PubMed] [Google Scholar]
- Wilson VJ, Peterson BW. Vestibulospinal and reticulospinal systems. In: Brookhart JM, Mountcastle VB, editors. Handbook of Physiology, section 1, The Nervous System, Motor Control. vol. II. Bethesda: American Physiological Society; 1981. pp. 667–702. [Google Scholar]
- Yates BJ, Jakus J, Miller AD. Vestibular effects on respiratory outflow in the decerebrate cat. Brain Res. 1993;629:209–217. doi: 10.1016/0006-8993(93)91322-j. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Jian BJ, Cotter LA, Cass SP. Responses of vestibular nucleus neurons to tilt following chronic bilateral removal of vestibular inputs. Exp Brain Res. 2000;130:151–158. doi: 10.1007/s002219900238. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Miller AD. Properties of sympathetic reflexes elicited by natural vestibular stimulation: implications for cardiovascular control. J Neurophysiol. 1994;71:2087–2092. doi: 10.1152/jn.1994.71.6.2087. [DOI] [PubMed] [Google Scholar]
- Yates BJ, Siniaia MS, Miller AD. Descending pathways necessary for vestibular influences on sympathetic and inspiratory outflow. Am J Physiol Regul Integr Comp Physiol. 1995;268:R1381–R1385. doi: 10.1152/ajpregu.1995.268.6.R1381. [DOI] [PubMed] [Google Scholar]