Abstract
Recurrent inhibition of the bladder C fibre reflex was studied in adult female cats anaesthetized with α-chloralose. Test reflexes were evoked by electrical stimulation of bladder Aδ and C afferents in the right pelvic nerve and were recorded from the proximal end of a small ipsilateral pelvic nerve branch, transected close to the bladder. Such test reflexes were consistently depressed by repetitive electrical stimulation of the contralateral bladder pelvic nerve (20 Hz, 20 s) at intensities sufficient to recruit axons of bladder preganglionic neurones. The inhibition could be evoked after transection of the left dorsal roots S1–S4 and the sympathetic supply to the bladder but was abolished by transection of the pelvic nerve central to the site of stimulation. Hence, it most likely involved central recurrent collaterals of antidromically activated bladder preganglionic neurones. The reflex suppression was quite considerable – maximal C fibre reflexes were reduced to a group mean of 25% (± 9% confidence interval) of their control size. The effect had a slow onset, requiring a few seconds of conditioning stimulation to be revealed, and was very long lasting (minutes). Naloxone (0.01–0.5 mg kg−1i.v.) abolished the recurrent inhibition of both the C fibre and Aδ bladder reflexes, while inhibition from afferents in the dorsal clitoris nerve remained unchanged. It is concluded that the segmental bladder C fibre reflex and the spino-ponto-spinal Aδ micturition reflex are both targets of recurrent inhibition from bladder parasympathetic preganglionic neurones and that the effect involves an enkephalinergic mechanism.
Recurrent inhibition, first described by Renshaw (1941) for spinal motoneurones, is a common phenomenon in the central nervous system. Typically, the inhibition involves a short disynaptic pathway, with axon collaterals driving an inhibitory interneuron that feeds back to the cells of origin. An entirely different type of recurrent inhibition was identified by De Groat & Ryall (1968b) in relation to sacral preganglionic parasympathetic neurones. Repetitive antidromic activation of these neurones in the cat resulted in a prolonged central depression of the Aδ micturition reflex. Yet, there was no obvious inhibition of the bladder preganglionic neurones themselves, indicating that the inhibition took place at an interneuronal level before the bladder preganglionic neurones (De Groat, 1976). The inhibitory effect was seen bilaterally, in keeping with later findings that sacral preganglionic neurones issue axon collaterals that terminate on both sides of the spinal cord (Morgan et al. 1991; Morgan, 2001).
We have now explored if a similar recurrent mechanism affects also the bladder C fibre reflex. This reflex originates from cold receptors (and presumably nociceptors) in the bladder and urethral walls, with their unmyelinated afferents in the pelvic and pudendal nerves (Fall et al. 1990; Jiang et al. 2002). In contrast to the spino-ponto-spinal Aδ micturition reflex, the bladder C fibre reflex is primarily a segmental polysynaptic reflex (Mazières et al. 1998). It has been proposed to facilitate bladder evacuation during cold stress and bladder inflammation (Fall et al. 1990; Häbler et al. 1990). It is also believed to play a crucial role in bladder function after spinal cord injury (De Groat et al. 1990; De Groat & Yoshimura, 2006) and in triggering involuntary bladder contractions in other pathological conditions (Yoshimura et al. 2002; Cruz, 2004).
Remarkably little is known of the detailed organization of the Aδ and C fibre reflex pathways at the spinal level. Findings so far point to bladder preganglionic neurones as their only common link (De Groat et al. 1981; Mazières et al. 1998). As the recurrent inhibition affects the Aδ reflex before the preganglionic neurones, it may be asked if it also influences the segmental C fibre reflex. The answer to this question might provide a tool to identify the spinal organization of both reflexes and ultimately help to understand the role of the C fibre reflex in bladder pathophysiology.
To test for recurrent inhibition, electrically elicited bladder C fibre reflexes were conditioned by repetitive antidromic activation of contralateral bladder preganglionic neurones. Since these neurones contain leu-enkephalin (Glazer & Basbaum, 1980; Kawatani et al. 1983; De Groat & Kawatani, 1989), we also tested the effect of the general opioid receptor antagonist naloxone. Some early results have been reported in an abstract (Mazières et al. 1998).
Methods
Twelve adult female cats were used for the experiments. Anaesthesia was induced by a ketamine–xylazine mixture (Ketalar, Parke-Davis and Rompun vet., Bayer; 15 + 1 mg kg−1, i.m.) followed by α-chloralose (55 mg kg−1i.v., supplemented as needed). An adequate depth of anaesthesia was ascertained by the lack of blood pressure and heart rate changes upon strong paw-pinches or electrical stimulation of C afferents. Heart rate was monitored by the electrocardiogram and blood pressure by a catheter in the right femoral artery. Mean arterial blood pressure was maintained above 110 mmHg by substituting fluid losses during the experiment by a slow i.v. infusion of bicarbonate buffered Ringer–glucose. Central body temperature was recorded by a thermode in the lower oesophagus and maintained between 37.5 and 38.5°C by a feedback controlled heating device. Urethra and bladder neck were exposed extraperitoneally by a ventral midline approach. A thin catheter was inserted into the bladder through a slit in the proximal urethra and used for bladder filling and draining, in some situations also for intravesical pressure recordings. Another catheter was inserted in the urethra in the distal direction for urethral perfusion. A laminectomy was made of the L6–L7 vertebrae to allow for transection of the S1–S4 dorsal roots (DRs) before or during the course of experiments. Animals were killed at the end of experiments by an overdose of anaesthetics, followed by severance of the heart. Experimental procedures were approved by the Animal Research Ethical Committee of Linköping in accordance with Swedish law.
Bladder pelvic nerves on both sides were dissected for recording or stimulation, carefully excluding branches to the urethra or other pelvic organs. Unless specified, the nerves were left in continuity with the bladder. Test reflexes were monitored from the proximal stump of a small pelvic nerve branch on the right side, transected close to the bladder (Fig. 1). The filament was distal to some parasympathetic ganglia and contained a mixture of pre- and postganglionic axons. The remaining ipsilateral pelvic nerve to the bladder was lifted onto a pair of silver hook electrodes for stimulation, as was the contralateral (left) bladder pelvic nerve. To isolate the stimulation sites from surrounding structures the exposed nerves were covered by paraffin oil in a pool formed by sown-up skin flaps. The temperature of the pool was maintained at 36–38°C by a heating lamp.
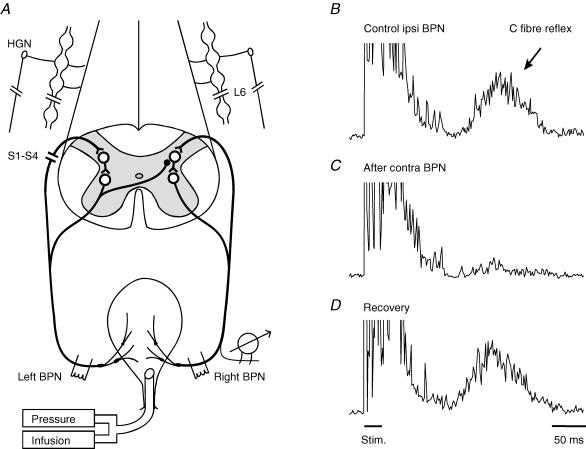
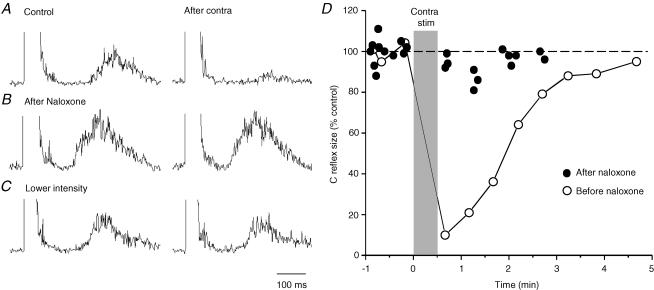
Figure 1. Inhibitory effect of contralateral pelvic nerve stimulation on the bladder C fibre reflex.
A, schematic diagram of the experimental arrangement. B–D, C fibre reflex discharges evoked in a small ipsilateral bladder branch by stimulation of the remaining right bladder pelvic nerve (BPN) at an intensity maximal for C fibres (3 × 0.5 ms at 10 ms intervals, 50 × Aδ threshold, repetition rate 1 Hz). Each trace is an average of 20 rectified responses. B, the reflex response in the control situation; C, depression of the reflex immediately after conditioning stimulation of the contralateral left BPN at optimal parameters (5 × efferent threshold, 20 Hz, 20 s); D, recovery of the reflex 5 min after the end of conditioning stimulation. The left S1–S4 dorsal roots and the sympathetic supply to the bladder were transected, as indicated in A. Time calibration in D refers to all traces.
The left pelvic nerve was used to elicit recurrent inhibition by antidromic activation of bladder preganglionic fibres. To eliminate afferent inputs, the left dorsal roots S1–S4 were transected in all experiments but three. In some animals, the left pelvic nerve was cut centrally and/or distally to the stimulation site at the end of the experiment to confirm a central course of the presumed recurrent pathway. The hypogastric nerves and the sympathetic chains below the L6 ganglia were transected bilaterally in three cats prior to recordings to eliminate a sympathetic link. To allow comparison with another type of inhibition, stimulation electrodes were also placed bilaterally on the dorsal clitoris branch of the pudendal nerve in three cats.
Naloxone (0.01–0.5 mg kg−1, i.v.) was used in nine experiments to further characterize the recurrent inhibition. These rather high doses were used to obtain sufficient testing times. In experiments with multiple injections (2–3 h apart), increasing doses were used to counteract any effect of naloxone tolerance (Roppolo et al. 1983; Hisamitsu & De Groat, 1983). In three experiments menthol solution (0.06 mm in saline; Mazières et al. 1998) was perfused through the distal urethra to facilitate the C fibre reflex.
C fibre test reflexes were evoked by low frequency stimulation (0.5–1 Hz) with trains of three shocks (0.5 ms, 10 ms intervals) at appropriate intensity. For comparison between experiments, strength of afferent stimulation was expressed in multiples of threshold for an Aδ reflex, as determined with the bladder filled (Mazières et al. 1998). On the deafferented (left) side, threshold intensity for direct activation of parasympathetic preganglionic axons was assessed by monitoring evoked bladder contractions (cathode distal). For antidromic activation the polarity of the stimulation electrode was reversed (cathode central). Latencies were measured from the first pulse in the stimulus train to onset of a particular reflex discharge. In most trials with C fibre reflexes, the bladder was left empty with open outlet to avoid eliciting Aδ micturition reflexes (Mazières et al. 1998).
Nerve signals were fed to differential amplifiers equipped with appropriate filters and displayed on a multichannel chart recorder (Hioki 8830; Hioki EE Corporation, Japan) together with recordings of heart rate, blood pressure and intravesical pressure. Reflex responses were also full-wave rectified, digitized and stored on a PC for averaging (20–40 responses). For quantification, the area under the curve of averaged responses was measured. Since naloxone greatly enhances bladder reflexes, afferent stimulation intensity was sometimes adjusted to obtain matching test reflexes before and after naloxone. Data are expressed as means ± 95% confidence interval (c.i.). ANOVA was used for comparisons, taking P < 0.05 as significant.
Results
Recurrent inhibition of the bladder C fibre reflex
General observations
A segmental bladder C fibre reflex was readily evoked in all animals by ipsilateral (right) bladder pelvic nerve stimulation (Fig. 1). The C fibre reflex was distinguished from the ordinary Aδ micturition reflex by its high threshold (> 20 × Aδ reflex threshold) and long latency (> 150 ms; Mazières et al. 1998). To obtain the C fibre reflex uncontaminated by a low threshold Aδ reflex, recordings were taken with the bladder empty and the catheter outlet open. In this situation, the Aδ reflex is subthreshold since it typically requires background facilitation by ongoing activity from bladder mechanoreceptors to emerge (Mazières et al. 1998). Evoked C fibre reflexes were consistently depressed following repetitive stimulation of the contralateral (left) bladder pelvic nerve (20 Hz, 20 s) at an intensity sufficient to recruit bladder preganglionic neurones (Fig. 1B, middle trace). The inhibitory effect remained for more than a minute after the period of conditioning stimulation. Full recovery was in the illustrated case obtained 5 min later (Fig. 1B, lower trace).
A similar longlasting inhibition, induced by contralateral bladder pelvic nerve stimulation, was found in all eight cats with the contralateral S1–S4 DRs transected. Three of these animals had also the sympathetic supply to the bladder and pelvic ganglia transected bilaterally. A total of 219 test–conditioning trials were performed (as in Fig. 1). The reflex suppression was quite considerable – with optimal conditioning parameters (see below), maximal C fibre test reflexes were reduced to a group mean of 25% (± 9%c.i.; range 7–35% in different cats) of their control size. There was no significant difference between the inhibitory effects in experiments with intact or damaged sympathetic innervation (mean 25% compared to 26%). The inhibition also remained after severance of the contralateral bladder pelvic nerve between the stimulation site and the bladder but was abolished by a central transection. These observations are consistent with a recurrent inhibitory mechanism involving antidromically activated preganglionic parasympathetic fibres to the bladder (De Groat & Ryall, 1968b; De Groat, 1976).
A prolonged inhibition was also observed after conditioning stimulation of the contralateral pelvic nerve in animals with intact S1–S4 DRs (47 trials in 3 cats) or with the conditioning stimulation applied to the ipsilateral pelvic nerve (34 trials in 3 cats). In fact there was no discernable difference in strength of inhibition in recordings obtained before and after transection of the contralateral DRs in the same animal (Fig. 2A and B).
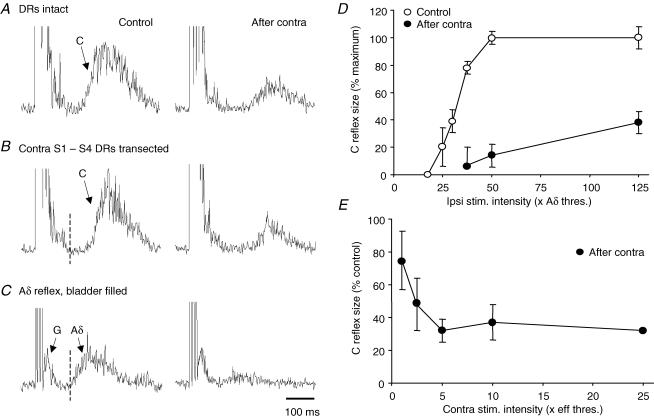
Figure 2. Recurrent inhibition of bladder Aδ and C fibre reflexes after transection of contralateral S1–S4 dorsal roots.
A and B, inhibition of bladder C fibre reflex by stimulation of contralateral BPN before (A) and after (B) transection of the contralateral dorsal roots (DRs) S1–S4 (left, control responses; right, conditioned responses; other details as in Fig. 1). The strength of inhibition was unchanged by this transection. C, similar recurrent inhibition of a bladder Aδ reflex, same experiment after DR transection. The test reflex was evoked at maximal intensity for Aδ afferents (5 × Aδ threshold) with the bladder partly filled. Note shorter latency of the Aδ reflex compared to that of the C fibre reflex (vertical dotted lines in B and C). Arrow G indicates a small ganglionic component, similar responses of much larger size merge with the stimulus artefacts in all records with C fibre reflexes, as in A and B. Time calibration in C refers to all records. D, bladder C fibre reflex size plotted against test stimulation intensity; control (○) and corresponding response with maximal recurrent inhibition (•). E, increase in strength of recurrent inhibition with conditioning stimulation intensity; the inhibitory effect appeared at threshold intensity for bladder preganglionic fibres and was maximal at 5 × eff. threshold. Reflex size in D and E was measured as the area under the curve of the averaged reflex response (time window 150–450 ms after onset of test stimulation). Points show the mean (± 95%c.i.) of data pooled from several experiments (4 in D, n = 141 reflex responses; 2 in E, n = 25).
Contralateral conditioning stimulation was also tried on Aδ micturition reflexes (16 trials in 5 animals). Aδ reflexes were readily evoked by ipsilateral stimulation at low intensity, when the bladder was partly filled (Fig. 2C). Note that this reflex had shorter latency than the C fibre reflex in the same animal (Fig. 2B and C; dotted line). Confirming earlier findings by De Groat & Ryall (1968b), such Aδ micturition reflexes were effectively inhibited by contralateral pelvic nerve stimulation (Fig. 2C). Both components of combined Aδ–C fibre reflex discharges, evoked by high intensity stimulation in the same situation (bladder partly filled; Mazières et al. 1998), were decreased in parallel (not illustrated). At the same time any ongoing efferent activity associated with bladder contraction was effectively curtailed.
Stimulation intensity
C fibre reflexes of different sizes were systematically tested in four experiments. Typically, the C fibre reflex appeared and increased in size within the intensity range 20–40 × Aδ reflex threshold (Fig. 2D). As expected, the effectiveness of the recurrent inhibition was somewhat larger with small test C reflexes than at the highest test stimulation intensity. Graded stimulation of the contralateral bladder pelvic nerve was tried in two experiments. The inhibitory effect appeared at threshold intensity for direct efferent activation of the detrusor (Methods) and reached maximum at about 5 times this intensity (Fig. 2E). This intensity range corresponded to that for the emergence and growth of a ganglionic component in nerve recordings on ipsilateral stimulation (De Groat & Ryall, 1969; Mazières et al. 1998). A similar effective range was found in trials with intact dorsal roots or when conditioning Aδ test reflexes. To increase the sensitivity of the testing procedure most trials were performed with a submaximal C fibre test reflex, combined with conditioning stimulation at supramaximal intensities.
Time course
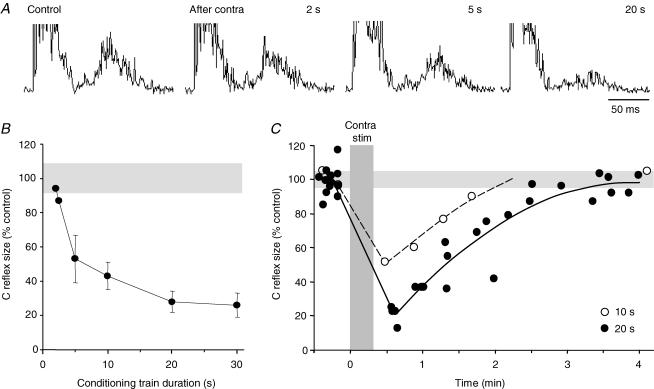
The inhibitory effect was also highly dependent on the frequency and duration of the conditioning stimulation. For a significant effect, stimulation frequencies above 5 Hz were required with optimal effects attained at about 20 Hz. The recurrent inhibition became evident first after a few seconds of conditioning stimulation at 20 Hz and then gradually increased in potency over the next 5–10 s. Optimal inhibition was not seen until the conditioning train was prolonged to 20–30 s (Fig. 3A and B). The duration of the recurrent inhibition changed in parallel; it could extend for several minutes with optimal conditioning parameters (Fig. 3C). In the illustrated experiment, a 10 s conditioning train at maximal intensity decreased the test response to about 50% with recovery after about 2 min while a 20 s train reduced the test response to about 20% and prolonged the effect with another minute. Thus, the inhibition was optimal at a stimulation frequency corresponding to the maximal physiological discharge rate of bladder preganglionic neurones in the cat (De Groat & Ryall, 1968a) and at train durations roughly similar to normal voiding times.
Figure 3. Recurrent inhibition by conditioning trains of different duration.
Sample records in A show C fibre test reflexes before and after conditioning stimulation of the contralateral BPN by trains of 2, 5 and 20 s duration (20 Hz, 5 × efferent threshold). The pooled results of similar trials in 3 experiments are plotted in B with the size of the conditioned C fibre reflex expressed as a percentage of the control reflex (± c.i.; other details as in Fig. 2); the recurrent inhibition became apparent with 2 s long trains and was optimal with trains of 20 s. C, change in time course of recurrent inhibition induced by conditioning trains, 10 s (○) and 20 s (•) long. Each point is the average of 20 consecutive reflex responses (1 Hz) and is plotted at half the collecting period; data points for 20 s trains are from 4 conditioning trials. Gray bands in B and C mark 95%c.i. range of control reflexes. The time of conditioning stimulations (20 s long) of the contralateral BPN is indicated by the vertical bar.
Preparations with intact or transected DRs only differed with respect to the development phase of the recurrent inhibition. While the inhibition became apparent during the conditioning train in experiments with transected DRs, it was delayed until the period after the train with intact DRs. In the latter situation, the reflex response was in fact facilitated during the conditioning stimulation, due to the high frequency concomitant stimulation of Aδ afferents (with thresholds below that of preganglionic fibres; Jiang & Lindström, 1999). In the postconditioning period the inhibition was quite comparable in strength with or without the dorsal roots transected (Fig. 2A and B). Notably, no facilitation that could be ascribed to recurrent activation of bladder preganglionic neurones was observed.
Effects of naloxone
C fibre reflex
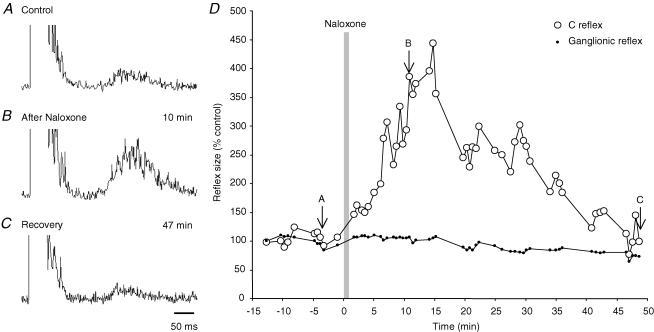
Naloxone, in the dose range 0.01–0.5 mg kg−1i.v., greatly enhanced the C fibre reflex (15 trials in 9 cats; Fig. 4A and B upper and middle records). The mean increase after a first dose was 4.3 times (± 1.3 c.i.; range 2.4–6.2 in different experiments), measured at peak enhancement 10–20 min after the injection (Fig. 4D). In the illustrated case, full recovery was observed after 45 min while several hours seemed to be required with larger doses. In two experiments, two additional doses were given at 2–3 h interval, at which time the C fibre reflex had not completely returned to prenaloxone levels. With cumulative doses there was a dose dependent increase in reflex size from about 3 times at 0.01 mg kg−1 to 11 times at 0.5 mg kg−1 (R2 = 0.73), with an extrapolated threshold at about a quarter of the lowest dose. The naloxone enhancement of C fibre reflexes occurred without concomitant changes in the peripheral ganglionic component of the evoked discharge (Fig. 4D, small dots; Mazières et al. 1998) pointing to facilitation at the central level of the reflex pathway.
Figure 4. Effect of naloxone on the size of the bladder C fibre reflex.
A–C, bladder C fibre reflexes obtained before (A) and at indicated times after systemic naloxone (0.025 mg kg−1i.v.; B and C). Time scale in C is for all records. D, time course of naloxone induced enhancement of the bladder C fibre reflex (○) and lack of effect on peripheral ganglionic discharge (dots). Reflex sizes are expressed as a percentage of the mean value before naloxone. Vertical bar marks the time of naloxone injection, arrows the sample records A–C.
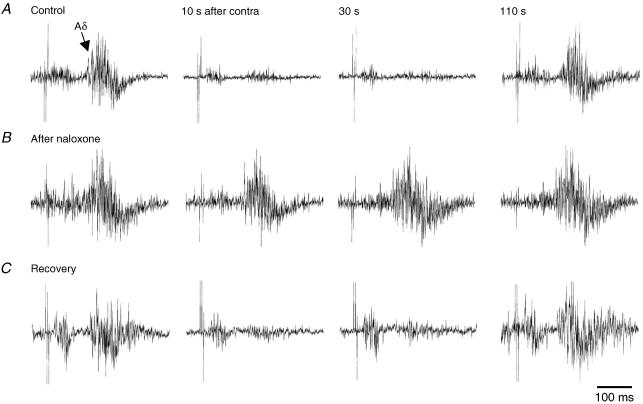
Naloxone also decreased the recurrent inhibition of the C fibre reflex in all but 1/14 sessions (8 cats). Effective doses ranged from 0.01 to 0.5 mg kg−1i.v. without obvious dose–response relationship. Using standard parameters and optimal intervals, the recurrent conditioning stimulation reduced the C fibre reflex to 24% of the test response before and to 90% after naloxone, a highly significant difference (P < 0.001). A representative naloxone trial is illustrated in Fig. 5. In the control situation, the conditioned C fibre reflex was reduced to 20% of the test response (Fig. 5A, upper pair of records). This recurrent inhibition was completely abolished 3 min after the naloxone injection (conditioned response 101%; Fig. 5B, middle pair of records). Since the enhanced reflex could conceal a recurrent inhibitory effect, the intensity of the C fibre stimulation was reduced to obtain a test reflex of comparable size to that obtained before naloxone (Fig. 5C, lower pair of records). Even with this precaution the recurrent inhibition was virtually abolished (conditioned response 88%). The diagram compares the time course of recurrent inhibition before and after naloxone in the same experiment, the latter data points obtained in four conditioning trials during the first 30 min after the drug injection.
Figure 5. Suppression of recurrent inhibition after systemic naloxone.
A–C, recurrent inhibition of bladder C fibre reflexes as observed before (A) and after naloxone (0.05 mg kg−1i.v.; B and C); left control, right conditioned response. Test stimulation intensity was the same for records in A and B (50 × threshold for the Aδ reflex) but decreased in C (20 × threshold) to match the control response in A; other stimulation parameters remained the same throughout. Note that the recurrent inhibition was abolished by naloxone, even after the decrease in test stimulation intensity. D, graphic illustration of the same effect showing conditioned reflex sizes (as a percentage of control) before (○) and after naloxone (•). The latter points were obtained during 4 conditioning trials in the 30 min period following naloxone administration. The time of conditioning stimulations of the contralateral BPN is indicated by the grey bar.
To further exclude that the reflex enhancement per se counteracted the recurrent inhibition, the C fibre reflex was in three experiments facilitated by urethral exposure to menthol (Methods). This procedure increased the test response considerably (Fig. 6A and B, upper and middle pair of records), yet the conditioned C fibre reflex was reduced to the same extent as in the control situation (menthol 23% of test, control 29%). A subsequent dose of naloxone gave a C fibre reflex of about the same size as with menthol but now the recurrent inhibition was completely eliminated (conditioned reflex 118% of corresponding test response; Fig. 6C, lower pair of records). The diagram shows a time series of recurrent inhibition following three doses of naloxone in the same experiment (Fig. 6D). For clarity, the conditioned reflex responses have in this plot been normalized with respect to the size of each preceding control response. It is obvious that the recurrent inhibition was abolished after each naloxone injection and that the effect of the first two doses was fully reversible within 1–2 h. Full recovery of the recurrent inhibition was obtained in three more experiments with sufficient recovery time after naloxone administration.
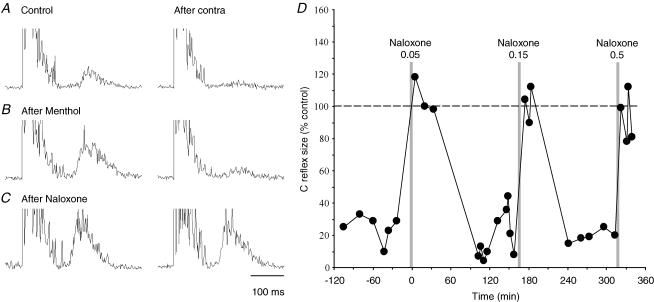
Figure 6. Time course of naloxone suppression of recurrent inhibition.
A–C, test bladder C fibre reflexes conditioned by contralateral BPN stimulation. In B, test reflexes were enhanced to match the response expected after naloxone (C), using a preceding slow urethral perfusion with menthol (0.06 mm in saline) to sensitize urethral cold receptors. This enhanced bladder C fibre reflex was virtually abolished by the recurrent inhibition while the similar sized response after naloxone was unaffected by the conditioning stimulation. D, plot showing consistent suppression by naloxone of the recurrent inhibition of bladder C fibre reflexes (three consecutive doses 0.05, 0.15 and 0.5 mg kg−1i.v.). The size of conditioned reflexes, as a percentage of their controls, is plotted against time from the first naloxone injection. The conditioned reflexes attained the size of their controls after each naloxone injection, implying abolished recurrent inhibition. The illustrated points represent a subset of reflexes evoked at identical test stimulation intensity, taken from a larger series of trials illustrated in Fig. 7.
There was no complete concordance between the naloxone modulation of the recurrent inhibition and the enhancement of the C fibre reflex. The recurrent inhibition was usually abolished within 2–5 min after naloxone, before the enhancement of the C reflex reached its maximum. At the latter time the recurrent inhibition had already started to recover as tested with weak C fibre reflexes and it was back to the control efficacy well before the C fibre reflex had recovered. After the first two naloxone injections in Fig. 6D, the size of the C reflex remained at 3 and 4 times the control size at times when the recurrent inhibition had returned to the prenaloxone efficacy.
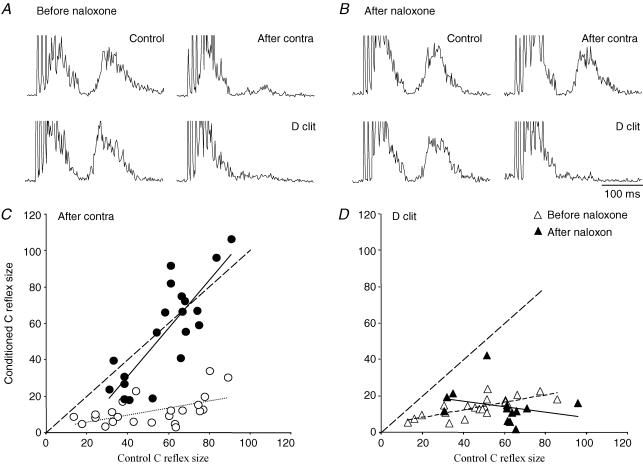
The effect of naloxone on another inhibitory mechanism – namely the inhibition of the bladder C fibre reflex from dorsal clitoris afferents – was also assessed to evaluate the specificity of the naloxone action on recurrent inhibition (Fig. 7). In contrast to the recurrent inhibition this inhibitory effect is short-lasting and the effect was estimated by averaging individually conditioned responses (D clit, 50 Hz for 200 ms, starting 50 ms before the test train) rather than responses in the postconditioning period. As seen from the sample records both types of inhibition decreased the test response to less than 20% of its control value before naloxone (Fig. 7A). After naloxone the recurrent inhibition was dramatically reduced, with the conditioned response amounting to 95% of its control value, while inhibition from dorsal clitoris afferents was unchanged (Fig. 7B). The diagrams below (Fig. 7C and D) summarize data from many trials with recurrent or dorsal clitoris inhibition before and after naloxone in the same experiment. Each point in the diagrams represents the size of a conditioned response plotted against the size of its control response, both as a percentage of the largest test response. Results from three naloxone exposures in the same animal are pooled. Only trials during the first 20 min after a naloxone injection were included. It can be seen that the dorsal clitoris inhibition was unchanged by naloxone (Fig. 7D) while the recurrent inhibition (Fig. 7C) was virtually abolished at all reflex sizes (data points close to the dotted line representing conditioned reflexes equal in size to the control response). Only with the smallest test reflexes could a weak residual recurrent inhibition be demonstrated. Preserved dorsal clitoris inhibition after naloxone was observed in two more experiments.
Figure 7. Selective naloxone effect on recurrent inhibition.
A, sample records of bladder C fibre reflexes inhibited by antidromic activation of contralateral preganglionic neurones (After contra; upper pair) or orthodromic activation of ipsilateral dorsal clitoris afferents (D clit, lower pair). The latter induced a shortlasting inhibition, so each test reflex was conditioned by a brief stimulus train to the dorsal clitoris nerve (50 Hz, 200 ms, starting 50 ms before test stimulation). The recurrent inhibition was assed as usual in the period after contralateral BPN stimulation (20 Hz, 20 s). The two conditioning stimuli were adjusted to give roughly similar inhibition of the bladder C fibre reflex. B, similar responses after naloxone (0.5 mg kg−1i.v.). The recurrent inhibition was abolished by the drug while the afferent inhibition remained unchanged. C and D, effects of the two conditioning stimuli on bladder C fibre reflexes of different sizes evoked by varying the test stimulation intensity. Each point represents the size of the conditioned reflex plotted against the size of its control reflex, before (○) and after naloxone (•). Hatched lines indicate conditioned reflexes equal in size to their controls, thin dotted and continuous lines are best fits for data points from before and after naloxone. The diagrams show pooled data from three naloxone sessions in the same experiment (same as in Fig. 6). For the naloxone effect, only reflexes sampled during the first 20 min after injections are included. Naloxone clearly suppressed the recurrent inhibition at all test reflex sizes while leaving the inhibition from dorsal clitoris afferents unchanged.
Ongoing efferent activity and Aδ reflex
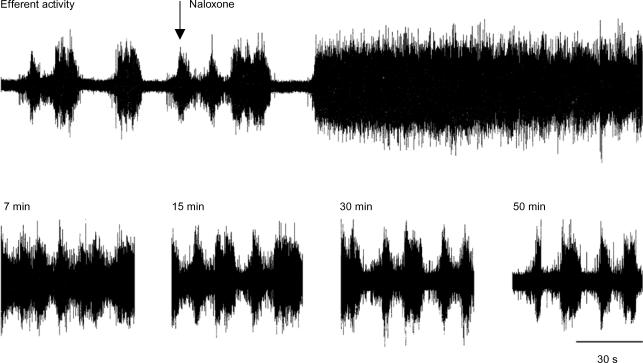
With fluid in the bladder, naloxone transformed ongoing phasic efferent activity into a continuous discharge which lasted for several minutes before periodic activity slowly resumed (Fig. 8; Roppolo et al. 1983). Full recovery was observed after 40–90 min, depending on naloxone dose. The drug also enhanced the Aδ micturition reflex and antagonized its recurrent inhibition as illustrated by single sample records obtained in the same experiment (Fig. 9).
Figure 8. Effect of naloxone on phasic BPN efferent discharges.
Upper trace is a continuous recording of typical phasic efferent activity in a small BPN branch, obtained with the bladder partly filled. Systemic naloxone (0.1 mg kg−1i.v.; arrow) rapidly (< 2 min) converted this phasic activity into a sustained tonic discharge, an activity that gradually returned to the control pattern during the following 50 min (lower traces).
Figure 9. Effect of naloxone on recurrent inhibition of bladder Aδ reflexes.
Bladder Aδ reflexes recorded with the bladder filled (same experiment as Fig. 8). Each horizontal row of single traces illustrates a conditioning trial with reflex responses taken before and at indicated times after conditioning stimulation of the contralateral BPN (20 s, 20 Hz). Conditioning trials were performed before (A), 17 min (B) and 65 min (C) after naloxone (0.1 mg kg−1i.v.) The test stimulation intensity was reduced in B (from 4 to 2 × threshold for the Aδ reflex) to obtain an Aδ reflex of about the same size as in A, yet the recurrent inhibition was virtually abolished.
As the C fibre reflex, the Aδ reflex increased in size, > 1.5 times as estimated from the area of the reflex response (not shown). Therefore, the test stimulation intensity was reduced in order to match the recurrent inhibition against an Aδ reflex of about the prenaloxone size (Fig. 9B, middle row of traces). Even so, the recurrent inhibition was hardly detectable. The naloxone effect on the Aδ reflex and its recurrent inhibition was fully reversible as shown by the lowermost records obtained 65 min after the naloxone injection. Similar results were obtained in two more experiments with Aδ test reflexes. Thus, naloxone enhanced the reflex size and reduced the recurrent inhibition in parallel for the bladder Aδ and C fibre reflexes.
Discussion
Two main findings emerge from the present experiments: (1) the segmental bladder C fibre reflex is controlled by recurrent inhibition from bladder parasympathetic preganglionic neurones just like the Aδ spino-ponto-spinal micturition reflex, and (2) the recurrent inhibition is antagonized by the opioid receptor antagonist naloxone.
The observed inhibition of the bladder C fibre reflex was obtained by electrical stimulation of the ipsi- and contralateral bladder pelvic nerves at an intensity sufficient to activate axons of preganglionic neurones. The inhibition remained unchanged after transection of the S1–S4 dorsal roots and the sympathetic supply of the bladder or of the pelvic nerve distal to the stimulation site. The effect was abolished, however, by a complete transection of the pelvic nerve central to its stimulation site. The inhibition required rather long trains of stimulation (> 2 s) at frequencies above 5 Hz and had, under optimal conditions, a duration that outlasted the conditioning stimulation by several minutes. All these characteristics conform to the original findings by De Groat & Ryall (1968b) concerning recurrent inhibition of the Aδ micturition reflex in the cat. A possible involvement of ventral root afferents (Ryall & Piercey, 1970) has previously been ruled out for the latter reflex (De Groat, 1976), leaving recurrent inhibition by antidromically activated preganglionic neurones as the remaining alternative. Our experimental design allows the conclusion that bladder preganglionic neurones – rather than those to other pelvic visceral organs – are the source of this recurrent inhibition (not conclusively shown before, see Discussion in Kruse et al. 1992).
Sacral preganglionic neurones have extensive intraspinal collateral arborizations, terminating on both ipsi- and contralateral sides of the spinal cord (Morgan et al. 1991; Morgan, 2001). It is unlikely that these collaterals form inhibitory terminals directly onto preganglionic neurones, since the excitability of these cells is unaffected by recurrent stimuli, as tested by iontophoretic application of an excitatory amino acid (De Groat, 1976). Based on this finding, De Groat proposed that the recurrent inhibition takes place at an earlier, interneuronal site in the Aδ micturition reflex pathway. This notion was reinforced by the observation that, following pontine stimulation, only the reflex-enhanced component of the bladder response is controlled by recurrent inhibition, while the direct descending effect is not (Kruse et al. 1992).
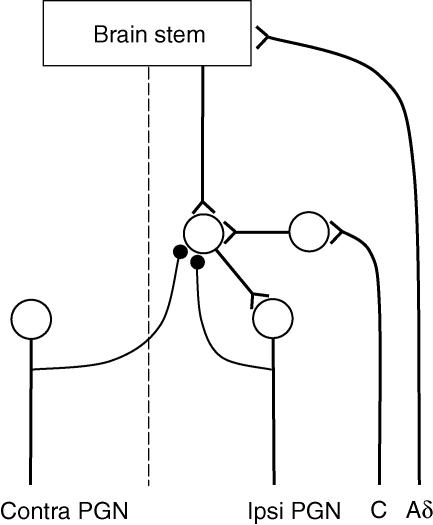
Some pontine descending fibres terminate directly on bladder preganglionic neurones (Blok & Holstege, 1997) but the bulk of the descending excitation seems to be relayed indirectly via yet unidentified segmental interneurones (De Groat et al. 1996; Shefchyk, 2001). The simplest explanation for the parallel recurrent control of the bladder Aδ and C fibre reflexes would be that all three pathways converge onto these excitatory prepreganglionic interneurones (Fig. 10). The recurrent inhibition of such interneurones could be either postsynaptic, as illustrated in Fig. 10, or presynaptic on their axon terminals. Further experiments would be required to differentiate between these and other possible alternatives.
Figure 10. Schematic diagram of proposed neuronal pathways.
Bladder Aδ and C fibre reflex pathways are shown to converge onto spinal prepreganglionic interneurones, which are also the target of enkephalinergic recurrent inhibitory connections from bladder preganglionic neurones (•).
Naloxone greatly enhanced the bladder Aδ and C fibre reflexes and essentially abolished the recurrent inhibition of both reflexes. It is unlikely that the inhibition was simply concealed by the heightened excitatory drive, as reflex discharges matching the prenaloxone response in size resisted the inhibition. Further, another type of inhibition of comparable magnitude, namely the inhibition of the bladder C fibre reflex evoked by stimulation of dorsal clitoris afferents, was unaffected by the drug. Thus, the observed effect of naloxone points to an opioid link in the recurrent inhibitory pathway. Bladder parasympathetic preganglionic neurones are known to synthesize leu-enkephalin (Glazer & Basbaum, 1980; Kawatani et al. 1983, 1989). Activity-dependant release of leu-enkephalin from their intraspinal axon collaterals could then account for the observed inhibition. The slow development and long duration of the recurrent inhibitory effect is consistent with the involvement of a peptidergic transmitter.
Feed-forward inhibition with similar characteristics – induction by repetitive stimulation of bladder parasympathetic preganglionic axons, long duration and elimination by naloxone – can also be observed at the level of bladder parasympathetic ganglia (De Groat & Kawatani, 1989). The effect is mimicked by topical applications of leu-enkephalin, presumably acting on presynaptic δ-opioid receptors on ganglionic terminals of preganglionic neurones. Thereby, the release of their fast excitatory transmitter, acetylcholine, onto the postganglionic cells is reduced (Simonds et al. 1983; De Groat & Kawatani, 1989). The spinal recurrent inhibition must be differently organized, since the intraspinal axon collaterals are not part of the main excitatory pathways to preganglionic neurones. In keeping, we found no evidence for a recurrent (cholinergic) facilitation of the bladder C fibre reflex. However, δ-opioid receptors seem to be the major opioid receptor also for bladder related activity in the sacral spinal cord (Hisamitsu & De Groat, 1983).
The enhancement of bladder Aδ and C fibre reflexes may not be explained by the drug effect on the recurrent inhibitory pathway. The two effects had somewhat different time courses with the reflex enhancement peaking at the time when the recurrent inhibition had already started to recover. Neither was there any ongoing efferent activity in bladder preganglionic neurones under our experimental conditions, activity that could produce a tonic inhibition to be removed by naloxone. Further, the sacral preganglionic neurones do not seem to release leu-enkephalin in the absence of spike activity, since peripheral ganglionic test responses were unchanged by naloxone (Fig. 4D). These observations suggest that the reflex enhancement after naloxone is due to removal of tonic opioid inhibition elsewhere, presumably at the level of the brain stem (Hisamitsu & De Groat, 1983).
When the bladder is filled to micturition threshold and the outlet closed, bladder parasympathetic preganglionic neurones typically exhibit rhythmic bursts of activity, causing phasic detrusor contractions (De Groat & Ryall, 1969; De Groat et al. 1982). It has been proposed that such phasic activity is largely shaped by periodic central inhibition (Lindström et al. 1984). From its properties, the discussed recurrent inhibition is perfectly tailored to produce such a patterning of the efferent discharge. This inference is supported by the present finding that naloxone transformed the phasic efferent activity into a continuous tonic discharge in parallel to the reduction in recurrent inhibition (see also Roppolo et al. 1983).
It follows that recurrent inhibition may contribute to the termination of normal voidings. A complete emptying of the bladder is ascertained by the micturition reflex being organized as a positive feed-back system, driven by tension sensitive mechanoreceptor Aδ afferents (Iggo, 1955; De Groat & Ryall, 1969; Fall & Lindström, 1991). The afferent activity will decline as the tension in the bladder wall is reduced by bladder emptying. The recurrent inhibition, developed through the prolonged preganglionic activity of a normal voiding, would further ensure that the intense activity of these neurones is curtailed. It is well established that the capillary blood flow is much reduced during maintained detrusor contractions (Greenland & Brading, 1996). Thus, with a positive feed-back drive, an activity dependent recurrent inhibitory mechanism might be required to limit the duration and frequency of detrusor contractions. Such a mechanism might be particularly important in situations when the micturition reflex is facilitated by ongoing activity in bladder C afferents.
Acknowledgments
This study was supported by the County of Östergötland (project LIO-4904) and the Swedish Medical Research Council (project 04767).
References
- Blok BF, Holstege G. Ultrastructural evidence for a direct pathway from the pontine micturition center to the parasympathetic preganglionic motoneurons of the bladder of the cat. Neuroscience Lett. 1997;222:195–198. doi: 10.1016/s0304-3940(97)13384-5. [DOI] [PubMed] [Google Scholar]
- Cruz F. Mechanisms involved in new therapies for overactive bladder. Urology. 2004;63:65–73. doi: 10.1016/j.urology.2003.11.001. [DOI] [PubMed] [Google Scholar]
- De Groat WC. Mechanisms underlying recurrent inhibition in the sacral parasympathetic outflow to the urinary bladder. J Physiol. 1976;257:503–513. doi: 10.1113/jphysiol.1976.sp011381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat WC, Booth AM, Milne RJ, Roppolo JR. Parasympathetic preganglionic neurons in the sacral spinal cord. J Autonomic Nervous System. 1982;5:23–43. doi: 10.1016/0165-1838(82)90087-x. [DOI] [PubMed] [Google Scholar]
- De Groat WC, Kawatani M. Enkephalinergic inhibition in parasympathetic ganglia of the urinary bladder of the cat. J Physiol. 1989;256:13–29. doi: 10.1113/jphysiol.1989.sp017639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat WC, Kawatani M, Hisamitsu T, Cheng CL, Ma CP, Thor K, Steers W, Roppolo JR. Mechanisms underlying the recovery of urinary bladder function following spinal cord injury. J Autonomic Nervous System. 1990;30(Suppl.):S71–S77. doi: 10.1016/0165-1838(90)90105-r. [DOI] [PubMed] [Google Scholar]
- De Groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. J Auton Nerv Syst. 1981;39:135–160. doi: 10.1016/0165-1838(81)90059-x. [DOI] [PubMed] [Google Scholar]
- De Groat WC, Ryall RW. The identification and characteristics of sacral parasympathetic preganglionic neurones. J Physiol. 1968a;196:563–577. doi: 10.1113/jphysiol.1968.sp008523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat WC, Ryall RW. Recurrent inhibition in sacral parasympathetic pathways to the bladder. J Physiol. 1968b;196:579–591. doi: 10.1113/jphysiol.1968.sp008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat WC, Ryall RW. Reflexes to sacral parasympathetic neurones concerned with micturition in the cat. J Physiol. 1969;200:87–108. doi: 10.1113/jphysiol.1969.sp008683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat WC, Vizzard MA, Araki I, Roppolo J. Spinal interneurons and preganglionic neurons in sacral autonomic reflex pathways. Prog Brain Res. 1996;107:97–111. doi: 10.1016/s0079-6123(08)61860-9. [DOI] [PubMed] [Google Scholar]
- De Groat WC, Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog Brain Res. 2006;152:59–84. doi: 10.1016/S0079-6123(05)52005-3. [DOI] [PubMed] [Google Scholar]
- Fall M, Lindström S. Electrical stimulation. A physiologic approach to the treatment of urinary incontinence. Urologic Clinics North Am. 1991;18:393–407. [PubMed] [Google Scholar]
- Fall M, Lindström S, Mazières L. A bladder-to-bladder cooling reflex in the cat. J Physiol. 1990;427:281–300. doi: 10.1113/jphysiol.1990.sp018172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer EJ, Basbaum AI. Leucine enkephalin: localization in and axoplasmic transport by sacral parasympathetic preganglionic neurons. Science. 1980;208:1479–1481. doi: 10.1126/science.6155697. [DOI] [PubMed] [Google Scholar]
- Greenland JE, Brading AF. Urinary bladder blood flow changes during the micturition cycle in a conscious pig model. J Urol. 1996;156:1858–1861. [PubMed] [Google Scholar]
- Häbler HJ, Jänig W, Koltzenburg M. Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol. 1990;425:545–562. doi: 10.1113/jphysiol.1990.sp018117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisamitsu T, de Groat WC. The inhibitory effect of opioid peptides and morphine applied intrathecally and intracerebroventricularly on the micturition reflex in the cat. Brain Res. 1983;42:51–65. doi: 10.1016/0006-8993(84)91146-6. [DOI] [PubMed] [Google Scholar]
- Iggo A. Tension receptors in the stomach and the urinary bladder. J Physiol. 1955;128:593–607. doi: 10.1113/jphysiol.1955.sp005327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang CH, Lindström S. Prolonged enhancement of the micturition reflex in the cat by repetitive stimulation of bladder afferents. J Physiol. 1999;517:599–605. doi: 10.1111/j.1469-7793.1999.0599t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang CH, Mazières L, Lindström S. Cold- and menthol-sensitive C afferents of cat urinary bladder. J Physiol. 2002;543:211–220. doi: 10.1113/jphysiol.2002.019042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawatani M, Lowe IP, Booth AM, Backes MG, Erdman SL, De Groat WC. The presence of leucine-enkephalin in the sacral preganglionic pathway to the urinary bladder of the cat. Neuroscience Lett. 1983;271:143–148. doi: 10.1016/0304-3940(83)90067-8. [DOI] [PubMed] [Google Scholar]
- Kawatani M, Shioda S, Nakai Y, Takeshige C, de Groat WC. Ultrastructural analysis of enkephalinergic terminals in parasympathetic ganglia innervating the urinary bladder of the cat. J Comparative Neurol. 1989;257:81–91. doi: 10.1002/cne.902880107. [DOI] [PubMed] [Google Scholar]
- Kruse MN, Mallory BS, Noto H, Roppolo JR, de Groat WC. Modulation of the spinobulbospinal micturition reflex pathway in cats. Am J Physiol. 1992;262:R478–R484. doi: 10.1152/ajpregu.1992.262.3.R478. [DOI] [PubMed] [Google Scholar]
- Lindström S, Fall M, Carlsson CA, Erlandson BE. Rhythmic activity in pelvic efferents to the bladder: an experimental study in the cat with reference to the clinical condition ‘unstable bladder’. Urologia Intis. 1984;39:272–279. doi: 10.1159/000280992. [DOI] [PubMed] [Google Scholar]
- Mazières L, Jiang C, Lindström S. The C fibre reflex of the cat urinary bladder. J Physiol. 1998;513:531–541. doi: 10.1111/j.1469-7793.1998.531bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CW. Axons of sacral preganglionic neurons in the cat. II. Axon collaterals. J Neurocytol. 2001;30:767–787. doi: 10.1023/a:1019637304031. [DOI] [PubMed] [Google Scholar]
- Morgan CW, de Groat WC, Felkins LA, Zhang SJ. Axon collaterals indicate broad intraspinal role for sacral preganglionic neurons. Proc Natl Acad Sci U S A. 1991;126:6888–6892. doi: 10.1073/pnas.88.15.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renshaw B. Influence of discharge of motoneurons upon excitation of neighboring motoneurons. J Neurophysiol. 1941;4:167–183. [Google Scholar]
- Roppolo JR, Booth AM, De Groat WC. The effects of naloxone on the neural control of the urinary bladder of the cat. Brain Res. 1983;7:355–358. doi: 10.1016/0006-8993(83)90841-7. [DOI] [PubMed] [Google Scholar]
- Ryall RW, Piercey MF. Visceral afferent and efferent fibers in sacral ventral roots in cats. Brain Res. 1970;33:57–65. doi: 10.1016/0006-8993(70)90349-5. [DOI] [PubMed] [Google Scholar]
- Shefchyk SJ. Sacral spinal interneurones and the control of urinary bladder and urethral striated sphincter muscle function. J Physiol. 2001;533:57–63. doi: 10.1111/j.1469-7793.2001.0057b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonds WF, Booth AM, Thor KB, Ostrowski NL, Nagel JR, de Groat WC. Parasympathetic ganglia: naloxone antagonizes inhibition by leucine-enkephalin and GABA. Brain Res. 1983;271:365–370. doi: 10.1016/0006-8993(83)90303-7. [DOI] [PubMed] [Google Scholar]
- Yoshimura N, Seki S, Chancellor MB, de Groat WC, Ueda T. Targeting afferent hyperexcitability for therapy of the painful bladder syndrome. Urology. 2002;59:61–67. doi: 10.1016/s0090-4295(01)01639-9. [DOI] [PubMed] [Google Scholar]