Abstract
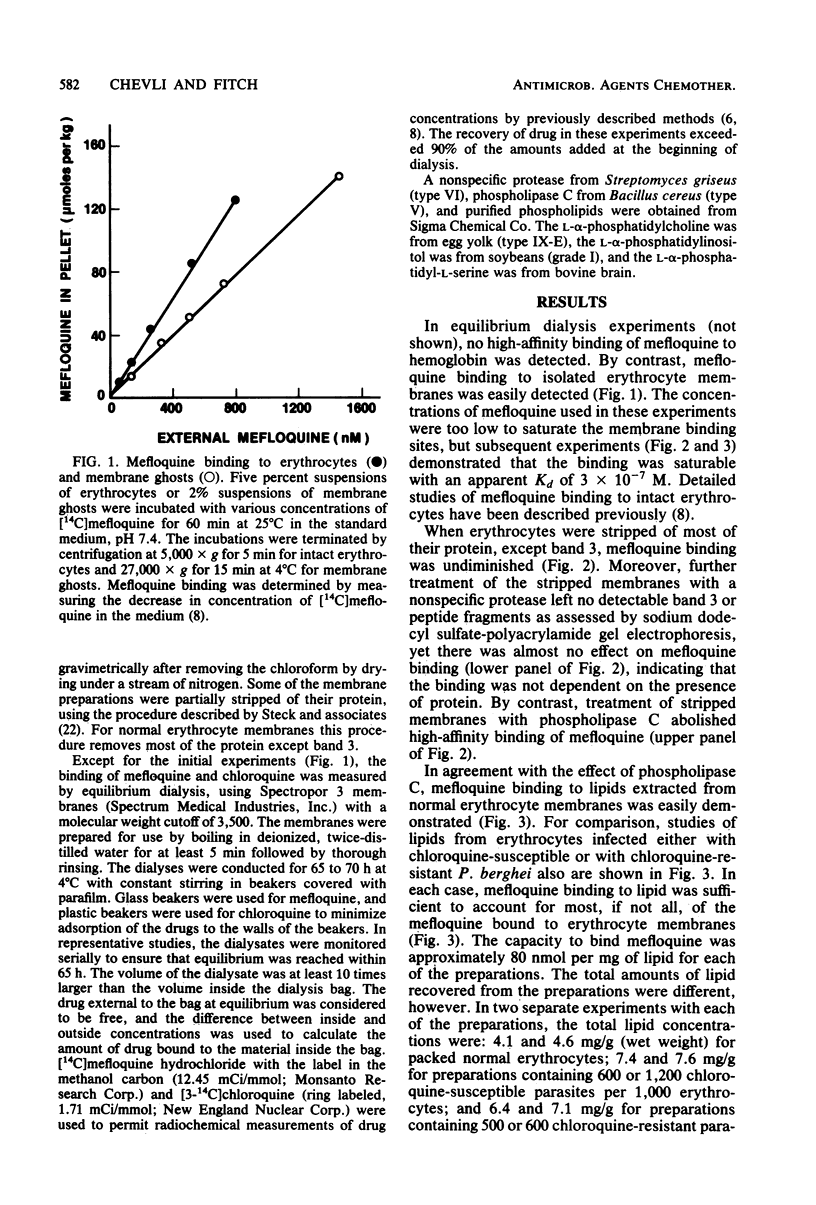
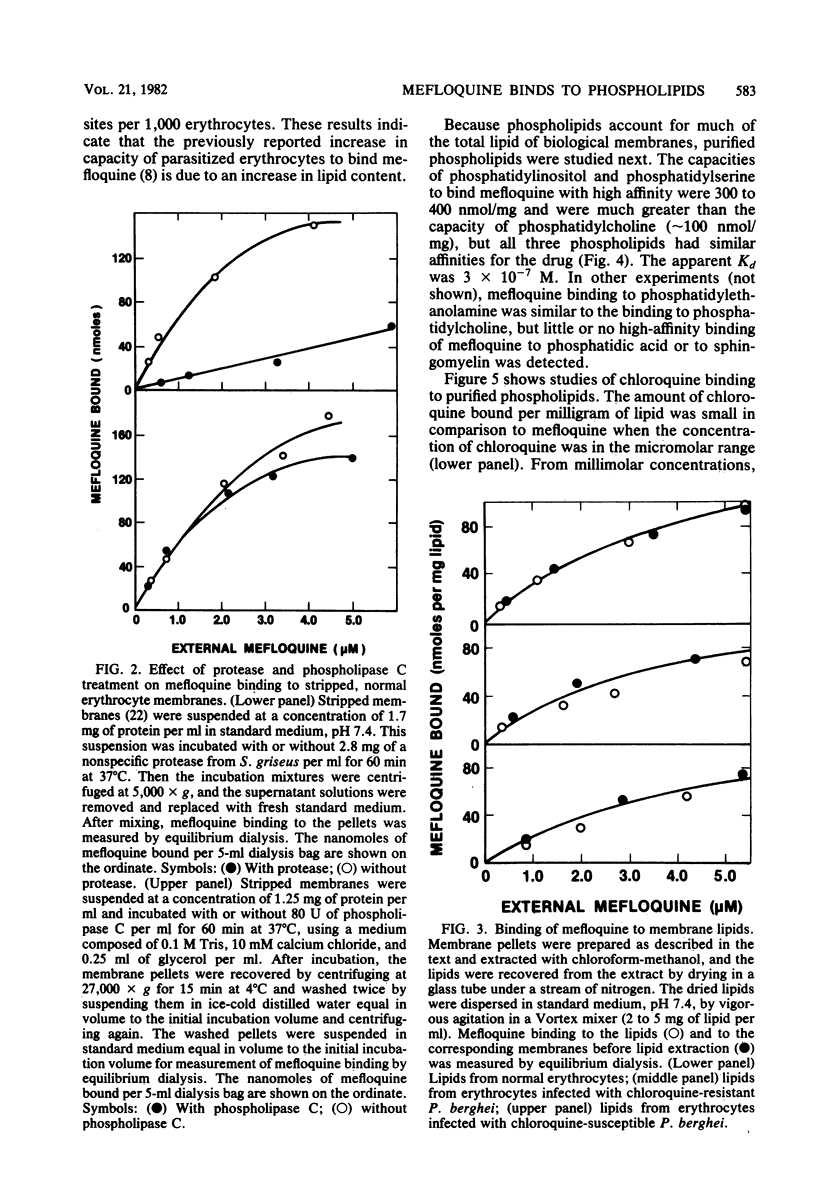
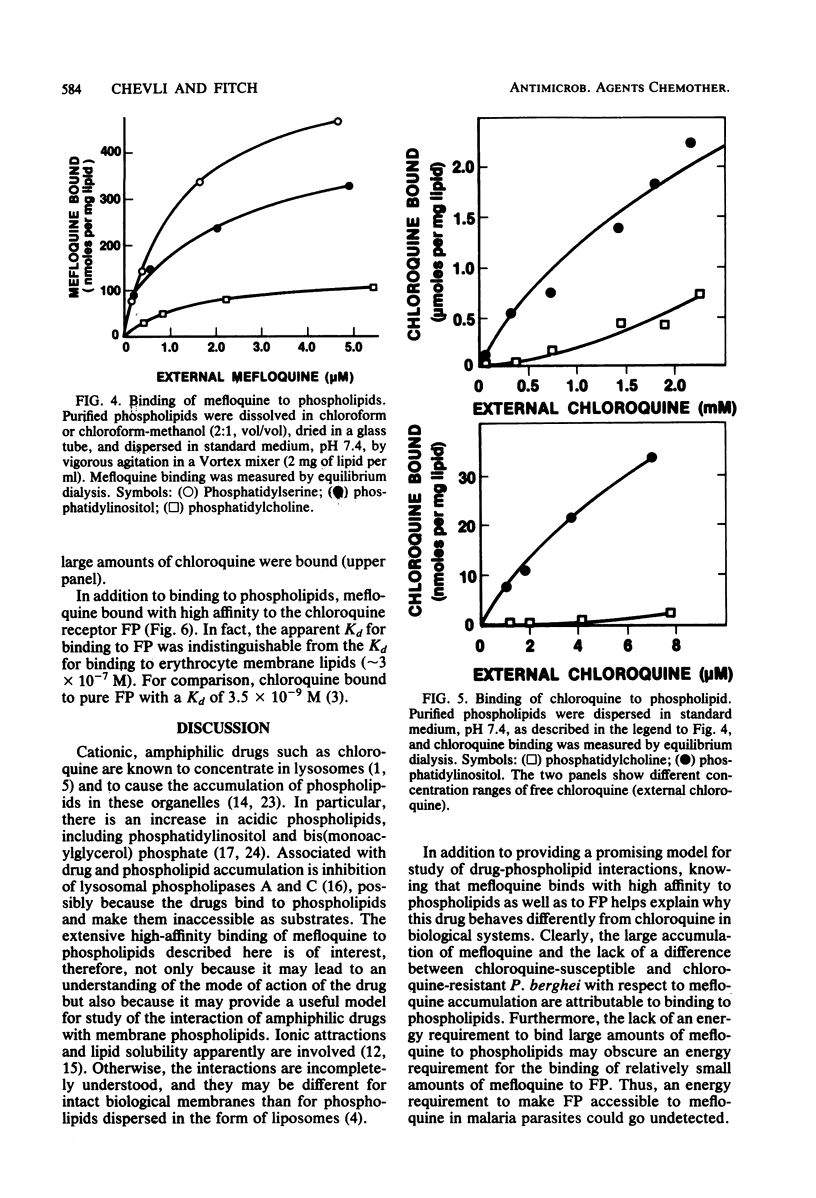
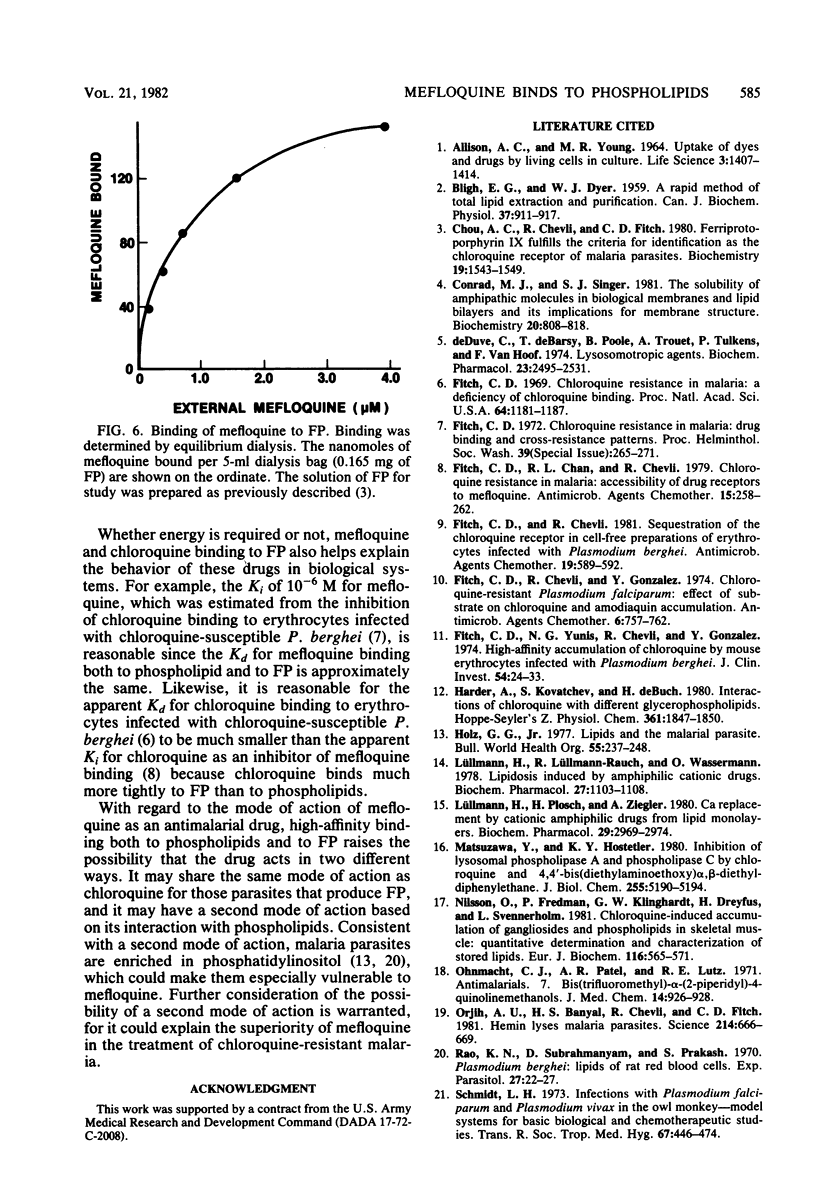
The new antimalarial drug mefloquine bound with high affinity (Kd approximately 3 X 10-7 M) to membrane lipids of normal mouse erythrocytes and of erythrocytes infected either with chloroquine-susceptible or chloroquine-resistant Plasmodium berghei. Approximately 80 nmol of mefloquine was bound per mg of total lipid. Mefloquine also bound to purified phospholipids with high affinity (Kd approximately 3 X 10-7 M). Phosphatidylinositol and phosphatidylserine bound 300 to 400 nmol of mefloquine per mg. Phosphatidylcholine and phosphatidylethanolamine bound approximately 100 nmol of mefloquine per mg. Mefloquine did not bind to hemoglobin with high affinity, but it bound to free ferriprotoporphyrin IX with a Kd of approximately 3 X 10-7 M. In comparison with mefloquine, chloroquine did not bind effectively to purified phospholipids, although it is known to bind with high affinity to free ferriprotoporphyrin IX. Greater binding to phospholipids may account for the superiority of mefloquine in the treatment of chloroquine-resistant malaria.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., YOUNG M. R. UPTAKE OF DYES AND DRUGS BY LIVING CELLS IN CULTURE. Life Sci. 1964 Dec;3:1407–1414. doi: 10.1016/0024-3205(64)90082-7. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Chou A. C., Chevli R., Fitch C. D. Ferriprotoporphyrin IX fulfills the criteria for identification as the chloroquine receptor of malaria parasites. Biochemistry. 1980 Apr 15;19(8):1543–1549. doi: 10.1021/bi00549a600. [DOI] [PubMed] [Google Scholar]
- Conrad M. J., Singer S. J. The solubility of amphipathic molecules in biological membranes and lipid bilayers and its implications for membrane structure. Biochemistry. 1981 Feb 17;20(4):808–818. doi: 10.1021/bi00507a024. [DOI] [PubMed] [Google Scholar]
- Fitch C. D., Chan R. L., Chevli R. Chloroquine resistance in malaria: accessibility of drug receptors to mefloquine. Antimicrob Agents Chemother. 1979 Feb;15(2):258–262. doi: 10.1128/aac.15.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch C. D., Chevli R., Gonzalez Y. Chloroquine-resistant Plasmodium falciparum: effect of substrate on chloroquine and amodiaquin accumulation. Antimicrob Agents Chemother. 1974 Dec;6(6):757–762. doi: 10.1128/aac.6.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch C. D., Chevli R. Sequestration of the chloroquine receptor in cell-free preparations of erythrocytes infected with Plasmodium berghei. Antimicrob Agents Chemother. 1981 Apr;19(4):589–592. doi: 10.1128/aac.19.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch C. D. Chloroquine resistance in malaria: a deficiency of chloroquine binding. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1181–1187. doi: 10.1073/pnas.64.4.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch C. D., Yunis N. G., Chevli R., Gonzalez Y. High-affinity accumulation of chloroquine by mouse erythrocytes infected with Plasmodium berghei. J Clin Invest. 1974 Jul;54(1):24–33. doi: 10.1172/JCI107747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder A., Kovatchev S., Debuch H. Interactions of chloroquine with different glycerophospholipids. Hoppe Seylers Z Physiol Chem. 1980 Dec;361(12):1847–1850. [PubMed] [Google Scholar]
- Holz G. G., Jr Lipids and the malarial parasite. Bull World Health Organ. 1977;55(2-3):237–248. [PMC free article] [PubMed] [Google Scholar]
- Lüllmann H., Lüllmann-Rauch R., Wassermann O. Lipidosis induced by amphiphilic cationic drugs. Biochem Pharmacol. 1978;27(8):1103–1108. doi: 10.1016/0006-2952(78)90435-5. [DOI] [PubMed] [Google Scholar]
- Lüllmann H., Plösch H., Ziegler A. Ca replacement by cationic amphiphilic drugs from lipid monolayers. Biochem Pharmacol. 1980 Nov 1;29(21):2969–2974. doi: 10.1016/0006-2952(80)90046-5. [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y., Hostetler K. Y. Inhibition of lysosomal phospholipase A and phospholipase C by chloroquine and 4,4'-bis(diethylaminoethoxy) alpha, beta-diethyldiphenylethane. J Biol Chem. 1980 Jun 10;255(11):5190–5194. [PubMed] [Google Scholar]
- Nilsson O., Fredman P., Klinghardt G. W., Dreyfus H., Svennerholm L. Chloroquine-induced accumulation of gangliosides and phospholipids in skeletal muscles. Quantitative determination and characterization of stored lipids. Eur J Biochem. 1981 Jun 1;116(3):565–571. doi: 10.1111/j.1432-1033.1981.tb05373.x. [DOI] [PubMed] [Google Scholar]
- Ohnmacht C. J., Patel A. R., Lutz R. E. Antimalarials. 7. Bis(trifluoromethyl)- -(2-piperidyl)-4-quinolinemethanols. J Med Chem. 1971 Oct;14(10):926–928. doi: 10.1021/jm00292a008. [DOI] [PubMed] [Google Scholar]
- Rao K. N., Subrahmanyam D., Prakash S. Plasmodium berghei: lipids of rat red blood cells. Exp Parasitol. 1970 Feb;27(1):22–27. doi: 10.1016/s0014-4894(70)80005-4. [DOI] [PubMed] [Google Scholar]
- Schmidt L. H. Infections with Plasmodium falciparum and Plasmodium vivax in the owl monkey--model systems for basic biological and chemotherapeutic studies. Trans R Soc Trop Med Hyg. 1973;67(4):446–474. doi: 10.1016/0035-9203(73)90077-1. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Ramos B., Strapazon E. Proteolytic dissection of band 3, the predominant transmembrane polypeptide of the human erythrocyte membrane. Biochemistry. 1976 Mar 9;15(5):1153–1161. doi: 10.1021/bi00650a030. [DOI] [PubMed] [Google Scholar]
- Yamamoto A., Adachi S., Ishikawa K., Yokomura T., Kitani T. Studies on drug-induced lipidosis. 3. Lipid composition of the liver and some other tissues in clinical cases of "Niemann-Pick-like syndrome" induced by 4,4'-diethylaminoethoxyhexestrol. J Biochem. 1971 Nov;70(5):775–784. doi: 10.1093/oxfordjournals.jbchem.a129695. [DOI] [PubMed] [Google Scholar]
- Yamamoto A., Adachi S., Matsuzawa Y., Kitani T., Hiraoka A., Seki K. Studies on drug-induced lipidosis: VII. Effects of bis-beta-diethyl-aminoethylether of hexestrol, chloroquine, homochlorocyclizine, prenylamine, and diazacholesterol on the lipid composition of rat liver and kidney. Lipids. 1976 Aug;11(8):616–622. doi: 10.1007/BF02532875. [DOI] [PubMed] [Google Scholar]
- de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem Pharmacol. 1974 Sep 15;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]


