Abstract
Dysfunction of dopamine neurons has been implicated in several neuropsychiatric disorders, including Parkinson's disease, addiction, bipolar disorder and depression. Recent elucidation of gene expression profiles in dopamine neuron subpopulations has shed light on the function of different groups of dopamine neurons in the CNS and on their dysfunction in disease states. In particular, concerted differences in gene expression appear to underlie the unique properties of distinct dopamine neurons. Specifically, dopamine neurons in the substantia nigra (SN), which are prone to degenerate in Parkinson's disease, express high levels of transcripts related to energy metabolism, mitochondria and phosphate signalling pathways. In contrast, ventral tegmental area (VTA) dopamine neurons prominently express genes related to synaptic plasticity and neuropeptides, suggesting intriguing mechanisms for the involvement of VTA dysfunction in addiction and mood disorders. As new functions of dopaminergic neurotransmission become clearer, continued exploration of the transcriptional neuroanatomy of these unique neurons will be vital for producing targeted, selective, and effective therapeutic agents.
Due to their diverse anatomy and disparate functions, different populations of dopamine neurons have been specifically implicated in a variety of neurological disorders. Parkinson's disease (PD), a common extrapyramidal movement disorder, is associated with selective neurodegeneration of substantia nigra (SN) dopamine neurons (Hirsch et al. 1988). Ventral tegmental area (VTA) neurons are much less affected, and other types of dopamine neurons are spared. This selective anatomic pattern appears to be conserved between PD patients and animal models of the disorder (Dawson et al. 2002). In contrast, dysfunction of VTA dopamine neurons is thought to play an important role in addiction, mood disorders, attention disorders, and schizophrenia (Bonci et al. 2003; Nestler & Carlezon, 2006). As one might suppose, mainstays of therapy for these disorders differ, an example being that dopamine replacement is the standard of care for PD while dopamine antagonism is an effective treatment for psychotic disorders.
One current drawback of dopaminergic therapy is that it cannot be targeted to a specific subpopulation of dopamine neurons. A consequence of this is significant dopamine-related side-effects, such as hallucinations or compulsive gambling during treatment for PD or extrapyramidal and sexual side-effects caused by antipsychotics. The ability to directly target symptoms of interest will be impossible until understanding of discriminators underlying specific dopamine neuron functions is more complete. In addition, from the standpoint of pathogenesis, it is clear that dopaminergic neurotransmission is not the sole underlying factor for selective neuronal dysfunction in these neurological diseases.
Although extrinsic factors such as circuitry differences assuredly are important in differential functions of dopamine neuron subpopulations, recent evidence suggests that there are intrinsic differences in transcriptional neuroanatomy between different groups of dopamine neurons. Further understanding of the significance of these inherent differences will go a long way toward explaining and preventing dopaminergic dysfunction in human diseases.
Dopamine neurons in the CNS
Dopamine neurons are compartmentalized into anatomically and functionally distinct groups in the mammalian central nervous system (Prakash & Wurst, 2006). The two largest groups are located in the midbrain (Fig. 1). SN neurons are functionally a component of the basal ganglia and provide ascending dopaminergic input to the neostriatum. They are involved in regulating voluntary movements and postural reflexes. VTA neurons located just medially to the SN give rise to mesolimbic and mesocortical ascending dopaminergic fibres projecting diffusely to multiple cortical and subcortical structures. VTA neurons are thought to be involved in reward, attention, and regulation of addictive or emotional behaviours. A third population of midbrain dopamine neurons resides in the retrorubral field and projects primarily to the dorsal striatum and the pontomedullary reticular formation; it is thought to play a role in orofacial movements. There are also connections between the retrorubral field and SN/VTA dopamine neurons.
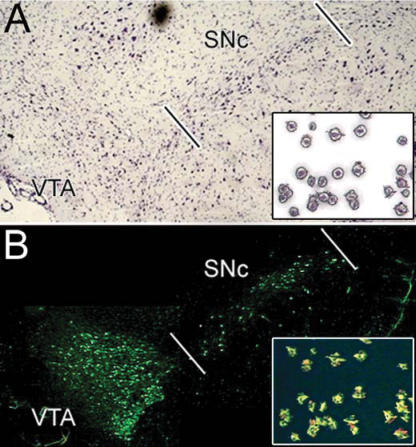
Figure 1. Isolation of dopamine neurons.
A, Nissl staining of a midbrain section depicting SN and VTA. Inset, SN neurons isolated by LCM, Nissl-staining. B, rapid tyrosine hydroxylase immunofluorescence (THIF) in a midbrain section. Note: TH-positive neurons in SN and VTA. Inset, SN neurons isolated by LCM, TH-staining. Reprinted from Neurobiology of Disease Greene et al. (2005), with permission from Elsevier.
There are at least four groups of dopamine neurons in the hypothalamus that are intimately involved in neuroendocrine, hormonal and arousal processes.
Finally, there are populations of dopaminergic amacrine cells in the retina that contribute to neural adaptation to light, and also some dopamine cells in the olfactory bulb.
Identification of dopamine neurons
Identification of these subpopulations of dopamine neurons has been possible for years using immunohistochemical and, to a lesser extent, electrophysiological, techniques, but only in the last decade have techniques developed to take advantage of specific identification on a transcriptional level. Immunohistochemistry for tyrosine hydroxylase (TH), the rate-limiting enzyme for dopamine synthesis, is the classic method for identification of dopamine neurons. Actually, TH staining identifies all catecholaminergic neurons, including noradrenergic neurons. Since noradrenergic neurons in the CNS are limited to the lower brainstem, identification of dopamine neurons is made easily on anatomic grounds.
Due to its relative speed and reliability, coupling immunological identification procedures with laser capture microdissection (LCM) has become the preferred method for obtaining homogeneous populations of dopamine neurons for subsequent expression profiling (Fig. 1) (Fasulo & Hemby, 2003).
This review will focus on data obtained from relatively homogeneous dopaminergic populations. While data from macro-dissected tissue can provide interesting information, the heterogeneous cell composition of brain tissue makes interpretation vastly more complex.
Transcriptional neuroanatomy of dopamine neurons
The question of what makes different populations of dopamine neurons unique lends itself well to large-scale gene expression profiling techniques. Dopamine neurons are easily identifiable and have been extensively studied, providing a substantial foundation for interpretation of results. Recent experiments have not only confirmed results previously obtained using candidate-mechanism approaches, but have also suggested new avenues of investigation.
Experiments to date have focused almost exclusively on SN and VTA dopamine neurons, predominantly in rodents. Several studies have spotlighted effects of experimental manipulations on dopamine neurons, but recent experiments have explored in detail baseline differences between SN and VTA dopamine neurons. These studies are important because keys to selective vulnerability and unique functions of dopamine neuron subpopulations are likely to be expressed at baseline, prior to any experimental insult or manipulation. Understanding the ‘normal’ situation will be vital to provide a backbone for interpretation of experimental results.
One thing that appears clear is that SN and VTA dopamine neurons have similar gene expression profiles such that relative to the total number of genes expressed in these neurons, the number of genes differentially expressed is low (< 5%) (Grimm et al. 2004; Chung et al. 2005; Greene et al. 2005). Confirming this, unsupervised hierarchical clustering of four catecholaminergic cell populations, including SN and VTA dopamine neurons, based on gene expression profile has indicated that SN and VTA gene expression is highly correlated. That experiment also revealed that hypothalamic dopamine neurons were distinct from their midbrain counterparts, and that noradrenergic neurons in the locus coeruleus are even more dissimilar (Grimm et al. 2004).
Despite their similarities, there are some critical differences in gene expression between SN and VTA neurons, both at the level of individual genes and in several potentially relevant cellular pathways. In general, unbiased gene expression profiling has successfully confirmed nearly every previously noted difference in gene expression between the regions (Table 1), proving that the large datasets produced by profiling experiments are reliable ‘jumping-off points’ for developing testable hypotheses (Grimm et al. 2004; Chung et al. 2005; Greene et al. 2005).
Table 1.
Several known differentially expressed genes confirmed using large scale expression profiling
| Higher in SN | Reference |
|---|---|
| D2 receptor | Hurd et al. 1994 |
| mGluR1 | Shigemoto et al. 1992 |
| Parvalbumin | Alfahel-Kakunda & Silverman, 1997 |
| Glutamate decarboxylase | Hedou et al. 2000 |
| IGF1 | Garcia-Segura et al. 1991 |
| GIRK2 | Schein et al. 1998 |
| Aldehyde dehydrogenase 1A7 | McCaffery & Drager, 1994 |
| Higher in VTA | |
| Calbindin | Liang et al. 1996 |
| BDNF | Seroogy et al. 1994 |
| Neurotrophin 3 | Seroogy et al. 1994 |
| Neuromedin K receptor | Massi et al. 2000 |
| α1B Adrenergic receptor | Andersson et al. 1994 |
| Cholecystokinin | Seroogy et al. 1989 |
| GTP cyclohydrolase I | Dassesse et al. 1997 |
In general, the large amount of data obtained from a microarray experiment can be approached either by ‘cherry-picking’ individual genes of interest or by systems analysis of the data. Data from dopamine neurons have been considered both ways.
Individual genes
Several interesting individual genes are differentially expressed between SN and VTA neurons in addition to those previously identified. Table 2 list five genes independently confirmed as differentially expressed in three separate large-scale microarray experiments (Grimm et al. 2004; Chung et al. 2005; Greene et al. 2005). Considering that these experiments were performed on different species, with different techniques, and on different microarray platforms, this is an extremely stringent list.
Table 2.
Recent independently identified genes expressed differently between SN and VTA neurons
| Gene symbol | Gene name | Higher region |
|---|---|---|
| MARCKS | Myristolated alanine-rich C-kinase substrate | VTA |
| GSYN | Gamma-synuclein | SN |
| ADCYAP1 | Pituitary adenylate cyclase activating polypeptide | VTA |
| NMDAR2C | N-methyl-d-aspartate receptor subunit 2C | SN |
| LPL | Lipoprotein lipase | VTA |
MARCKS (myristoylated, alanine-rich C-kinase substrate) has recently been found to be an important regulator of dendritic spine plasticity through an activity- and PKC-dependent pathway (Calabrese & Halpain, 2005). These functions of MARCKS are important for learning and memory and the plastic synaptic events that underlie them, such as long-term potentiation (LTP) (Matus, 2005). Interestingly, MARCKS gene expression is altered in postmortem brains from suicide patients, and its expression is down-regulated by the mood stabilizer lithium (McNamara et al. 1999; Wang et al. 2001). Since dysfunctional VTA dopamine neurons are thought to be involved in addiction behaviour and mood disorders, higher MARCKS expression in this neuronal population provides a tantalizing clue for further investigation.
Pituitary adenylate cyclase activating polypeptide (ADCYAP1 or PACAP) is known to support survival of dopamine neurons, and lipoprotein lipase may protect cells from damage caused by oxidized lipoproteins (Takei et al. 1998; Paradis et al. 2003; Reglodi et al. 2004). Both are intriguing in that VTA dopamine neurons are relatively protected from degeneration in parkinsonism. The neuronal role of γ-synuclein is not clear, but it has been reported to be involved in regulation of the cell cycle, abnormalities of which have been implicated in neurodegeneration (Inaba et al. 2005). NMDAR2C may possibly be involved in excitatory neurotoxicity in SN dopamine neurons (Kress & Reynolds, 2005).
Several single gene products discovered by expression profiling to be higher in the VTA have been successfully tested in vitro against dopaminergic neurotoxins, including the aforementioned PACAP, as well as gastrin-releasing peptide (GRP) and calcitonin/calcitonin gene-related peptide alpha (CGRP), providing further verification of the quality of experimental leads generated (Chung et al. 2005).
Categorical pathways
The discussion above highlights several interesting individual genes in the context of functional differences between dopamine neuron subtypes. However, even though it is small, the number of individual transcript differences between subtypes makes it unlikely that single gene differences are responsible for the uniqueness of different neuronal populations. In fact, it seems intuitively obvious that it is not a single gene, but multiple genes, all differentially expressed, that accounts in the majority for functional differences.
Recently, ‘systems’ approaches have been applied to expression analysis of dopamine neurons whereby the data were not examined on an individual level, but on a categorical one, with startling results. There are concerted differences in gene expression between different subpopulations of dopamine neurons (Fig. 2), and it is likely that these broad categorical and pathway differences account for the distinctiveness of the neuron types (Chung et al. 2005; Greene et al. 2005).
Figure 2. Schematic of transcriptional neuroanatomy of midbrain dopamine neurons in rodents.
Note that SN dopamine neurons that degenerate in parkinsonism project primarily to the motor striatum, where as mesolimbic and mesocortical fibres involved in addiction and mood disorders emerge from the VTA. The lists highlight substantial categorical differences in gene expression between the two regions. Abbreviations: SN, substantia nigra; VTA, ventral tegmental area; FC, frontal cortex; STR, striatum, NAc, nucleus accumbens; H, hippocampus.
In examining Table 1, one can note at least five genes potentially related to ‘neuroprotection’ are higher in VTA dopamine neurons, including four neuropeptide-related genes, and the calcium binding protein, calbindin. In fact, profiling experiments have confirmed that neuropeptide genes as a category are more highly expressed in VTA dopamine neurons than in those from the SN (Chung et al. 2005; Greene et al. 2005). This suggests that large differences in a single neuropeptide may not be as important or as protective as a concerted difference in multiple genes in the pathway. This concept may have significant consequences for attempts at development of effective neuroprotective therapies, in that a cocktail of compounds may be much more effective than any one alone. It also has ramifications for mood disorders, suggesting that neuropeptide modulation may be an effective targeting strategy to regulate dopaminergic neurotransmission from the VTA.
The other most prominent categorical difference between SN and VTA dopamine neurons is energy metabolism (Fig. 3). Multiple genes related to energy pathways, electron transport and mitochondria are more highly expressed in the SN than the VTA (Chung et al. 2005; Greene et al. 2005). This is particularly interesting because mitochondrial dysfunction may be critical to the pathogenesis of PD. These profiles suggest that SN dopamine neurons are more metabolically active than their counterparts in the VTA, that they rely more on oxidative energy metabolism, and/or that they have less energetic reserve capacity available to them. These data provide circumstantial evidence that SN dopamine neurons are under greater metabolic stress, and potentially provide an explanation for why these neurons are selectively susceptible to mitochondrial toxins and why they are more likely to degenerate in PD (Greene et al. 2005).
Figure 3. Categorical enhancement of energy metabolism transcripts in SN.
A, hierarchical clustering analysis using 15 significantly different energy metabolism genes sorts SN and VTA samples separately. Red depicts higher expression levels and blue lower; deeper shade indicates larger difference. B, expression ratios determined by microarray analysis (mean ± s.e.m.) for significantly different energy metabolism transcripts. † Difference in transcript was confirmed using quantitative RT-PCR. C, Q-RT-PCR of selected energy metabolism transcripts indicates that expression in dentate gyrus granule cells (DG) is markedly lower than in both types of midbrain dopamine neurons. * DG is significantly lower than SN and VTA (P < 0.05, ANOVA with post hoc Bonferroni test). # Significantly lower than SN, but not VTA. D, frequency distribution of expression ratios for 75 energy metabolism transcripts (filled bars) and 17 proteasome transcripts (open bars) categorized by Kyoto Encyclopedia of Genes and Genomes (KEGG) categories. The dashed line is the distribution of all expressed genes, which is normal. Note that while the distribution of proteasome genes is normal (P < 0.05, Shapiro-Wilk normality test), the distribution of energy metabolism genes is skewed toward SN. Reprinted from Neurobiology of Disease, Greene et al. (2005) with permission from Elsevier.
A recent experiment in postmortem human SN appears to confirm the pathophysiological relevance of differences in energy metabolism gene expression in dopamine neuron vulnerability in that one significantly altered category of gene expression in PD was nuclear-encoded mitochondrial genes (Hauser et al. 2005).
Another report profiling individual dopamine neurons from PD patients revealed potential differences between DA neurons containing Lewy bodies (a pathological hallmark of PD) and those without (Lu et al. 2005). Furthermore, analysis of midbrain dopamine neurons from cocaine-overdose victims has revealed selective changes of gene expression patterns in the VTA (Tang et al. 2003). Gene expression profiles of postmortem human disease remain in the early stages, especially since gene expression profiles from normal human dopamine neurons have not been described in detail.
Conclusions
Expression profiling of dopamine neurons in the CNS remains incomplete, but acquiring detailed information about dopamine neuron transcriptional neuroanatomy is critical for elucidating the functions of dopamine neurons in health and disease.
Acknowledgments
This work was supported by K08 NS048858 from the NIH and a Cotzias Fellowship from the American Parkinson Disease Association.
References
- Alfahel-Kakunda A, Silverman WF. Calcium-binding proteins in the substantia nigra and ventral tegmental area during development: correlation with dopaminergic compartmentalization. Brain Res Dev Brain Res. 1997;103:9–20. doi: 10.1016/s0165-3806(97)00101-6. [DOI] [PubMed] [Google Scholar]
- Andersson JL, Marcus M, Nomikos GG, Svensson TH. Prazosin modulates the changes in firing pattern and transmitter release induced by raclopride in the mesolimbic, but not in the nigrostriatal dopaminergic system. Naunyn Schmiedebergs Arch Pharmacol. 1994;349:236–243. doi: 10.1007/BF00169289. [DOI] [PubMed] [Google Scholar]
- Bonci A, Bernardi G, Grillner P, Mercuri NB. The dopamine-containing neuron: maestro or simple musician in the orchestra of addiction? Trends Pharmacol Sci. 2003;24:172–177. doi: 10.1016/S0165-6147(03)00068-3. [DOI] [PubMed] [Google Scholar]
- Calabrese B, Halpain S. Essential role for the PKC target MARCKS in maintaining dendritic spine morphology. Neuron. 2005;48:77–90. doi: 10.1016/j.neuron.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Chung CY, Seo H, Sonntag KC, Brooks A, Lin L, Isacson O. Cell type-specific gene expression of midbrain dopaminergic neurons reveals molecules involved in their vulnerability and protection. Hum Mol Genet. 2005;14:1709–1725. doi: 10.1093/hmg/ddi178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassesse D, Hemmens B, Cuvelier L, Resibois A. GTP-cyclohydrolase-I like immunoreactivity in rat brain. Brain Res. 1997;777:187–201. doi: 10.1016/s0006-8993(97)01111-6. [DOI] [PubMed] [Google Scholar]
- Dawson T, Mandir A, Lee M. Animal models of PD: pieces of the same puzzle? Neuron. 2002;35:219–222. doi: 10.1016/s0896-6273(02)00780-8. [DOI] [PubMed] [Google Scholar]
- Fasulo WH, Hemby SE. Time-dependent changes in gene expression profiles of midbrain dopamine neurons following haloperidol administration. J Neurochem. 2003;87:205–219. doi: 10.1046/j.1471-4159.2003.01986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Segura LM, Perez J, Pons S, Rejas MT, Torres-Aleman I. Localization of insulin-like growth factor I (IGF-I) -like immunoreactivity in the developing and adult rat brain. Brain Res. 1991;560:167–174. doi: 10.1016/0006-8993(91)91228-s. [DOI] [PubMed] [Google Scholar]
- Greene JG, Dingledine R, Greenamyre JT. Gene expression profiling of rat midbrain dopamine neurons: implications for selective vulnerability in parkinsonism. Neurobiol Dis. 2005;18:19–31. doi: 10.1016/j.nbd.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Grimm J, Mueller A, Hefti F, Rosenthal A. Molecular basis for catecholaminergic neuron diversity. Proc Natl Acad Sci U S A. 2004;101:13891–13896. doi: 10.1073/pnas.0405340101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser MA, Li YJ, Xu H, Noureddine MA, Shao YS, Gullans SR, et al. Expression profiling of substantia nigra in Parkinson disease, progressive supranuclear palsy, and frontotemporal dementia with parkinsonism. Arch Neurol. 2005;62:917–921. doi: 10.1001/archneur.62.6.917. [DOI] [PubMed] [Google Scholar]
- Hedou G, Chasserot-Golaz S, Kemmel V, Gobaille S, Roussel G, Artault JC, et al. Immunohistochemical studies of the localization of neurons containing the enzyme that synthesizes dopamine, GABA, or gamma-hydroxybutyrate in the rat substantia nigra and striatum. J Comp Neurol. 2000;426:549–560. doi: 10.1002/1096-9861(20001030)426:4<549::aid-cne4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Graybiel AM, Agid YA. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson's disease. Nature. 1988;334:345–348. doi: 10.1038/334345a0. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Pristupa ZB, Herman MM, Niznik HB, Kleinman JE. The dopamine transporter and dopamine D2 receptor messenger RNAs are differentially expressed in limbic- and motor-related subpopulations of human mesencephalic neurons. Neuroscience. 1994;63:357–362. doi: 10.1016/0306-4522(94)90535-5. [DOI] [PubMed] [Google Scholar]
- Inaba S, Li C, Shi YE, Song DQ, Jiang JD, Liu J. Synuclein gamma inhibits the mitotic checkpoint function and promotes chromosomal instability of breast cancer cells. Breast Cancer Res Treat. 2005;94:25–35. doi: 10.1007/s10549-005-6938-0. [DOI] [PubMed] [Google Scholar]
- Kress GJ, Reynolds IJ. Dopaminergic neurotoxins require excitotoxic stimulation in organotypic cultures. Neurobiol Dis. 2005;20:639–645. doi: 10.1016/j.nbd.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Liang CL, Sinton CM, Sonsalla PK, German DC. Midbrain dopaminergic neurons in the mouse that contain calbindin-D28k exhibit reduced vulnerability to MPTP-induced neurodegeneration. Neurodegeneration. 1996;5:313–318. doi: 10.1006/neur.1996.0042. [DOI] [PubMed] [Google Scholar]
- Lu L, Neff F, Alvarez-Fischer D, Henze C, Xie Y, Oertel WH, Schlegel J, Hartmann A. Gene expression profiling of Lewy body-bearing neurons in Parkinson's disease. Exp Neurol. 2005;195:27–39. doi: 10.1016/j.expneurol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- McCaffery P, Drager UC. High levels of a retinoic acid-generating dehydrogenase in the meso-telencephalic dopamine system. Proc Natl Acad Sci U S A. 1994;91:7772–7776. doi: 10.1073/pnas.91.16.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara RK, Hyde TM, Kleinman JE, Lenox RH. Expression of the myristoylated alanine-rich C kinase substrate (MARCKS) and MARCKS-related protein (MRP) in the prefrontal cortex and hippocampus of suicide victims. J Clin Psychiatry. 1999;60(Suppl. 2):21–26. Discussion 40-1, 113–116. [PubMed] [Google Scholar]
- Massi M, Panocka I, de Caro G. The psychopharmacology of tachykinin NK-3 receptors in laboratory animals. Peptides. 2000;21:1597–1609. doi: 10.1016/s0196-9781(00)00291-6. [DOI] [PubMed] [Google Scholar]
- Matus A. MARCKS for maintenance in dendritic spines. Neuron. 2005;48:4–5. doi: 10.1016/j.neuron.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;12:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Paradis E, Clement S, Julien P, Ven Murthy MR. Lipoprotein lipase affects the survival and differentiation of neural cells exposed to very low density lipoprotein. J Biol Chem. 2003;278:9698–9705. doi: 10.1074/jbc.M208452200. [DOI] [PubMed] [Google Scholar]
- Prakash N, Wurst W. Development of dopaminergic neurons in the mammalian brain. Cell Mol Life Sci. 2006;63:187–206. doi: 10.1007/s00018-005-5387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reglodi D, Lubics A, Tamas A, Szalontay L, Lengvari I. Pituitary adenylate cyclase activating polypeptide protects dopaminergic neurons and improves behavioral deficits in a rat model of Parkinson's disease. Behav Brain Res. 2004;151:303–312. doi: 10.1016/j.bbr.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Schein JC, Hunter DD, Roffler-Tarlov S. Girk2 expression in the ventral midbrain, cerebellum, and olfactory bulb and its relationship to the murine mutation weaver. Dev Biol. 1998;204:432–450. doi: 10.1006/dbio.1998.9076. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, Lundgren KH, Tran TM, Guthrie KM, Isackson PJ, Gall CM. Dopaminergic neurons in rat ventral midbrain express brain-derived neurotrophic factor and neurotrophin-3 mRNAs. J Comp Neurol. 1994;342:321–334. doi: 10.1002/cne.903420302. [DOI] [PubMed] [Google Scholar]
- Seroogy K, Schalling M, Brene S, Dagerlind A, Chai SY, Hokfelt T, et al. Cholecystokinin and tyrosine hydroxylase messenger RNAs in neurons of rat mesencephalon: peptide/monoamine coexistence studies using in situ hybridization combined with immunocytochemistry. Exp Brain Res. 1989;74:149–162. doi: 10.1007/BF00248288. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: an in situ hybridization study in adult and developing rat. J Comp Neurol. 1992;322:121–135. doi: 10.1002/cne.903220110. [DOI] [PubMed] [Google Scholar]
- Takei N, Skoglosa Y, Lindholm D. Neurotrophic and neuroprotective effects of pituitary adenylate cyclase-activating polypeptide (PACAP) on mesencephalic dopaminergic neurons. J Neurosci Res. 1998;54:698–706. doi: 10.1002/(SICI)1097-4547(19981201)54:5<698::AID-JNR15>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Tang WX, Fasulo WH, Mash DC, Hemby SE. Molecular profiling of midbrain dopamine regions in cocaine overdose victims. J Neurochem. 2003;85:911–924. doi: 10.1046/j.1471-4159.2003.01740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Liu X, Lenox RH. Transcriptional down-regulation of MARCKS gene expression in immortalized hippocampal cells by lithium. J Neurochem. 2001;79:816–825. doi: 10.1046/j.1471-4159.2001.00631.x. [DOI] [PubMed] [Google Scholar]