Abstract
Repetitive transcranial magnetic stimulation (rTMS) or repetitive electrical peripheral nerve stimulation (rENS) can induce changes in the excitability of the human motor cortex (M1) that is often short-lasting and variable, and occurs only after prolonged periods of stimulation. In 10 healthy volunteers, we used a new repetitive paired associative stimulation (rPAS) protocol to facilitate and prolong the effects of rENS and rTMS on cortical excitability. Sub-motor threshold 5 Hz rENS of the right median nerve was synchronized with submotor threshold 5 Hz rTMS of the left M1 at a constant interval for 2 min. The interstimulus interval (ISI) between the peripheral stimulus and the transcranial stimulation was set at 10 ms (5 Hz rPAS10ms) or 25 ms (5 Hz rPAS25ms). TMS was given over the hot spot of the right abductor pollicis brevis (APB) muscle. Before and after rPAS, we measured the amplitude of the unconditioned motor evoked potential (MEP), intracortical inhibition (ICI) and facilitation (ICF), short- and long-latency afferent inhibition (SAI and LAI) in the conditioned M1. The 5 Hz rPAS25ms protocol but not the 5 Hz rPAS10ms protocol caused a somatotopically specific increase in mean MEP amplitudes in the relaxed APB muscle. The 5 Hz rPAS25ms protocol also led to a loss of SAI, but there was no correlation between individual changes in SAI and corticospinal excitability. These after-effects were still present 6 h after 5 Hz rPAS25ms. There was no consistent effect on ICI, ICF and LAI. The 5 Hz rENS and 5 Hz rTMS protocols failed to induce any change in corticospinal excitability when given alone. These findings show that 2 min of 5 Hz rPAS25ms produce a long-lasting and somatotopically specific increase in corticospinal excitability, presumably by sensorimotor disinhibition.
In humans, repetitive transcranial magnetic stimulation (rTMS) has been used extensively to induce functional changes in the stimulated cortex and connected brain areas that last beyond the time of stimulation (Siebner & Rothwell, 2003). It has been shown that rTMS can influence many aspects of brain function, including changes in regional excitability, regional neuronal activity, interregional coupling and behaviour (Siebner & Rothwell, 2003). The after-effects of rTMS on cortical excitability have been mainly studied in the primary motor hand area (M1). In these studies, single-pulse or paired-pulse TMS was given to the M1 before and after rTMS conditioning, and the size of the motor-evoked potentials (MEPs) in contralateral hand muscles was used to assess rTMS-induced changes in corticospinal and intracortical excitability (Siebner & Rothwell, 2003).
In healthy individuals, rTMS can produce bi-directional changes in corticospinal excitability depending on the frequency of stimulation. High-frequency rTMS (at frequencies of about 5 Hz and more) usually induces a lasting facilitation at suprathreshold (Pascual-Leone et al. 1994) or subthreshold intensities of stimulation (Maeda et al. 2000; Peinemann et al. 2004; Quartarone et al. 2005). Conversely, low-frequency rTMS (at a rate of around 1 Hz) produces a lasting suppression of corticospinal excitability at suprathreshold (Chen et al. 1997) or subthreshold intensities of stimulation (Touge et al. 2001). In this context, ‘threshold’ refers to the motor threshold (MT). The MT is defined as the intensity needed to induce a minimal motor response in contralateral hand muscles.
Differences in the functional state of the stimulated cortex at the time of stimulation are supposed to have a substantial impact on the response to rTMS conditioning, and thus may contribute to the substantial variability of rTMS-induced after-effects (Siebner & Rothwell, 2003). If this hypothesis is correct, a promising strategy to render the after-effects of rTMS more consistent would be to standardize the functional state of the cortex at the time of stimulation. An effective way to experimentally manipulate the state of the cortex is to condition rTMS with electrical stimulation of afferent inputs to the stimulated cortical area (Tokimura et al. 2000; Kobayashi et al. 2003; Helmich et al. 2005).
Given the potent modulatory effects of afferent stimulation on cortical excitability, paired associative stimulation (PAS) has been introduced as an effective means of inducing a Hebbian-like associative plasticity in the stimulated M1 (Stefan et al. 2000, 2002; McKay et al. 2002; Pitcher et al. 2003). The PAS protocol introduced by Stefan et al. (2000) pairs a conditioning electrical stimulus to the right median nerve with suprathreshold single-pulse TMS of the contralateral M1 (Stefan et al. 2000) and produces bi-directional changes in cortical excitability in the stimulated M1 (Wolters et al. 2003; Ziemann, 2004). The direction of excitability changes induced by PAS critically depends on the interstimulus interval (ISI) between the peripheral and cortical stimulus, following a Hebbian rule (Wolters et al. 2003). If the peripheral stimulus is given approximately at the N20 latency of the somatosensory evoked potential (e.g. ISI of approximately 25 ms), PAS facilitates the excitability of corticospinal output neurons (Wolters et al. 2003; Ziemann, 2004). Conversely, PAS at shorter interstimulus intervals (e.g. ISI of approximately 10 ms) produces a long-lasting suppression of corticospinal excitability (Wolters et al. 2003; Ziemann, 2004). The after effects of PAS have been shown to be rapidly evolving (<30 min), long-lasting (duration >60 min), reversible and topographically specific (Stefan et al. 2000). N-methyl-d-aspartate (NMDA) receptor antagonist has been shown to block PAS-induced plastic changes (Stefan et al. 2002). Therefore, it has been proposed that the conditioning effects induced by PAS represent associative long-term potentiation (LTP) or long-term-depression (LTD)-like plasticity at a cell population level (Iriki et al. 1989; Donoghue & Sanes, 1994; Stefan et al. 2000)
In addition to the ISI between the peripheral and cortical stimulus, the repetition rate may influence the after-effects of PAS, presumably by producing rTMS-like effects. To minimize this confound, paired stimuli have been applied at very low frequencies (i.e. ≤0.25 Hz) (Stefan et al. 2000, 2002; McKay et al. 2002; Pitcher et al. 2003; Ziemann, 2004). However, rTMS-like effects may still occur even at a very low repetition rate of PAS (Murase et al. 2005).
While previous PAS studies have tried to minimize the effects of the repetition rate of TMS on the conditioning effects of PAS, the present study adopted a different approach. We wondered whether the efficacy of 5 Hz rTMS conditioning could be enhanced by appropriately pairing each transcranial stimulus with a conditioning peripheral stimulus. To test this hypothesis, we designed a new repetitive PAS (rPAS) protocol in which submotor-threshold electrical stimulation of the right median nerve was synchronized with submotor-threshold rTMS of the contralateral M1 at a constant rate of 5 Hz (referred to as 5 Hz rPAS). Our prediction was that afferent conditioning should boost the ‘facilitatory’ effects of 5 Hz rTMS if the electrical stimulus was given 25 ms before the transcranial stimulus (‘facilitatory’ PAS) but not at an ISI of 10 ms (i.e. ‘inhibitory’ PAS).
Methods
Participants
Ten right-handed healthy volunteers (six women), aged 26–38 years (mean age 30 ± 3 years), participated in the study. The experiment was approved by the local Ethics Committee. All subjects gave their written informed consent for the experiments. Subjects were seated in a comfortable reclining chair during the experiment. Both arms were supported by a pillow. They were asked to completely relax and to look straight ahead.
Main experiment
Figure 1 illustrates the timeline of the experimental procedures. The main experiment was designed to assess the conditioning effects of 5 Hz rPAS to the left M1 on various measures of motor cortical excitability. Measurements were performed in blocks immediately before (baseline) and after 5 Hz rPAS to the left M1 (post 0) as well as 30 and 60 min (post 30 and post 60) after 5 Hz rPAS. Each block of measurements lasted for approximately 15 min. In each block, we first measured MTs at rest and during tonic contraction. Threshold measurements were followed by measurements of corticospinal excitability and intracortical inhibition and facilitation at rest. We then assessed corticospinal excitability and the duration of the cortical silent period during tonic voluntary contraction. Finally, we measured intracortical sensorimotor inhibition at rest.
Figure 1. Design of the main experiment.
Healthy volunteers received repetitive paired associative stimulation of the right median nerve and left M1 at a rate of 5 Hz (5 Hz rPAS). The interval between the peripheral and transcranial stimulus was set at 25 ms (5 Hz rPAS25ms) or 10 ms (5 Hz rPAS10ms). We recorded the motor evoked potential (MEPs) from the right abductor pollicis brevis (APB) muscle to assess change in corticospinal and intracortical excitability immediately before and after 2 min of 5 Hz rPAS. Measurements were performed in blocks. In each block, we assessed the active and resting motor thresholds (MTs), mean MEP amplitudes at rest (MEP-R) and during tonic contraction (MEP-C), short-latency intracortical inhibition (SICI) at an interstimulus interval (ISI) of 2 ms and intracortical facilitation (ICF) at an ISI of 12 ms, the duration of the cortically evoked silent period (CSP) and short-latency and long-latency afferent inhibition (SAI and LAI).
Conditioning protocol
5Hz rPAS consisted of 600 pairs of stimuli which were continuously delivered to the left M1 at a rate of 5 Hz for 2 min. Each pair of stimuli consisted of an electrical conditioning stimulus given to the right median nerve followed by a biphasic transcranial magnetic stimulus given to the left M1 in all subjects. In two separate sessions, 5 Hz rPAS used an ISI of 25 ms (referred to as 5 Hz rPAS25ms) or 10 ms (referred to as 5 Hz rPAS10ms) between the conditioning stimulus (CS) and the transcranial stimulus. We chose ISIs of 25 and 10 ms for 5 Hz rPAS because Wolters et al. (2003) have shown that when given at a very low frequency, PAS25ms and PAS10ms produce long-lasting facilitation or suppression of motor cortical excitability, respectively. The two 5 Hz rPAS sessions were given at least 1 week apart. The order of 5 Hz rPAS25ms and 5 Hz rPAS10ms was balanced among subjects.
A square wave pulse was applied to the right median nerve at the wrist through a bipolar electrode (Digitimer D-160 stimulator; Digitimer Ltd, Welwyn Garden City, Herts, UK). The cathode was located proximally and the pulse width was 500 μs. TMS was given through a standard figure-of-eight-shaped coil connected to a Magstim Rapid stimulator (Magstim Company, Whitland, Dyfed, UK). The mean loop diameters of the coil were 9 cm. The coil was placed tangentially at the optimum scalp position which consistently elicited the largest MEPs with the steepest initial slope in the relaxed right abductor pollicis brevis (APB) muscle (referred to as ‘motor hot spot’).
The intensity of the electrical stimulus was set at twice the sensory threshold, while the intensity of TMS was individually adjusted to 90% of active MT. In contrast to previous PAS studies, stimulus intensities of the peripheral and transcranial stimulus were always subthreshold for eliciting muscle twitches. We chose submotor threshold intensities to avoid any conditioning effect related to re-afferent feedback activation caused by muscle twitches during 5 Hz rPAS. The intensity of the transcranial stimulus was subthreshold for evoking descending volleys to the spinal cord (Di Lazzaro et al. 1998). The very low intensity of TMS also minimized the spread of excitation to adjacent premotor and sensory cortex (Takano et al. 2004).
The magnetic stimulus had a biphasic waveform with a pulse width of approximately 300 μs. The junction region of the coil pointed backwards and laterally at a 45 deg angle away from the midline, approximately perpendicular to the line of the central sulcus. During the first phase of the stimulus, the current in the centre of the coil flowed toward the handle and the first phase of the biphasic stimulus induced a posterior–anterior current in the brain. The biphasic stimulus configuration differed from the monophasic stimulus configuration that had been used in previous PAS studies. We used a biphasic stimulus configuration and a posterior coil orientation to ensure comparability with our previous work on the conditioning effects of subthreshold 5 Hz rTMS on motor cortical excitability (Quartarone et al. 2005).
Measures of cortical excitability
The conditioning effects of 5 Hz rPAS on motor cortical excitability were probed using TMS pulses with a monophasic pulse configuration. Single or paired pulses were given to the left M1 using a standard figure-of-eight coil connected with a single (for single-pulse TMS) or two (for paired-pulse TMS) high-power Magstim 200 stimulators. The coil had an external loop diameter of 9 cm. An identical coil position was used as for 5 Hz rPAS. The centre of the coil was located over the ‘motor hot spot’ for stimulation of the contralateral APB muscle and the handle of the coil pointed 45 deg postero-laterally. The monophasic magnetic stimulus had a rise time of approximately 100 μs, decaying back to zero over approximately 0.8 ms. The coil current during the rising phase of the magnetic field flowed toward the handle. Thus, the induced current in the cortex flowed in a posterior–anterior direction.
Threshold measurements
First we measured resting and active MTs. Resting MT was defined as the minimum intensity that evoked a peak-to-peak MEP of 50 μV in at least 5 out of 10 consecutive trials in the relaxed APB muscle. Active MT was defined as the minimum intensity that elicited a reproducible MEP of at least 200 μV in the tonically contracting APB muscle in at least 5 out of 10 consecutive trials. Participants maintained a force level of approximately 10–15% of maximum force during measurements of the active MT.
Intracortical paired-pulse excitability and corticospinal excitability at rest
Short-latency intracortical inhibition (SICI) and intracortical facilitation (ICF) were determined according to the paired-pulse method described by Kujirai et al. (1993). The intensity of the conditioning stimulus was set at 80% of active MT. The intensity of the test stimulus was adjusted to elicit MEPs with peak-to-peak amplitudes of 0.5–1.0 mV at baseline (∼115–125% of resting MT). Stimulus intensities were kept constant across the blocks of measurement. SICI and ICF were assessed at ISIs of 2 and 12 ms, respectively. Fifteen trials were recorded for each ISI and randomly intermingled with 15 trials in which MEPs were elicited by the test stimulus alone. The peak-to-peak amplitude of the unconditioned MEP was taken as a measure of corticospinal excitability. Mean amplitude of the conditioned MEP was expressed as percentage of the amplitude of the unconditioned MEP. The relative change in MEP amplitude induced by the conditioning stimulus characterized the strength of SICI and ICF. This approach enabled us to assess the after effects of 5 Hz rPAS on corticospinal excitability and at the same time, to test for specific effects on SICI and ICF over and above the conditioning effects on corticospinal excitability. Trials in which the APB muscle was not completely relaxed were discarded from analysis.
Cortical excitability during tonic contraction
We measured the peak-to-peak amplitude of 10 consecutive MEPs during slight tonic contraction of the right APB muscle at approximately 10–15% of maximum force level. Audiovisual feedback of ongoing EMG activity was provided to ensure a constant force level. Stimulus intensity was identical to the stimulus intensity used for the test stimulus during paired-pulse TMS (∼115–125% of resting MT). The peak-to-peak amplitude of the MEP was measured to characterize corticospinal excitability. The same trials were used to measure the duration of the cortically evoked silent period (CSP) which is a marker for the excitability of long-lasting (presumably GABABergic) intracortical inhibition (Siebner et al. 1998; Werhahn et al. 1999). For CSP measurements, EMG traces were rectified but not averaged. The duration of the CSP was measured in each trial and defined as the time from the onset of the MEP to reappearance of sustained EMG activity (Orth & Rothwell, 2004).
Sensorimotor intracortical inhibition
Short-latency afferent inhibition (SAI) and long-latency afferent inhibition (LAI) were studied using the conditioning-test protocol described by Tokimura et al. (2000). An electrical conditioning stimulus was given to the right median nerve at the wrist (cathode proximal) prior to a transcranial test stimulus given to the left M1 (Digitimer D-160 stimulator). The median nerve was stimulated through bipolar electrodes at the wrist (cathode proximal) using a square wave pulse with a pulse width of 500 μs. The intensity was set just above the threshold for evoking a visible twitch of the thenar muscles (approximately three times perceptual threshold). The intensity of the transcranial test stimulus was adjusted to evoke a muscle response in relaxed APB with a peak-to-peak amplitude of approximately 1 mV (approximately 115–125% of resting MT). SAI and LAI were probed at ISIs of 25 and 200 ms, respectively. Fifteen stimuli were delivered at each ISI and randomly intermingled with 15 trials in which MEPs were elicited by the test stimulus alone. The mean amplitude of the conditioned MEP was expressed as percentage of the mean amplitude of the unconditioned MEP. The relative change in MEP amplitude induced by the peripheral stimulus was taken as a measure of SAI and LAI. Trials in which the APB muscle was not fully relaxed were excluded from analysis.
Control experiments
In addition to the main experiment, we performed additional experiments to further characterize the conditioning effects of the 5 Hz rPAS25ms. The experiments were carried out in subgroups of the volunteers who had participated in the main experiment. The 5 Hz rPAS protocol was identical to the main experiment.
Influence of the amplitude of the test response on SAI
Since focal 5 Hz rPAS increased the amplitude of MEPs evoked by single-pulse TMS over the left M1, it is conceivable that an increase in test pulse efficacy, enlarging the size of the unconditioned test response, might have reduced the magnitude of SAI. In four subjects, we studied how the increase in amplitude of the test MEP affected measurements of the relative strength of SAI after 5 Hz rPAS25ms. SAI was assessed at baseline and 30 min after 5 Hz rPAS25ms. In contrast to the main experiment in which we had used a constant TMS intensity throughout the experiment, the stimulus intensity of TMS was adjusted after 5 Hz rPAS25ms. We reduced the intensity of TMS until stimulation evoked MEPs in the relaxed right APB muscle that were matched in amplitude to preintervention baseline. Otherwise, experimental procedures were identical to those used in the main experiment.
Duration of after effects
In six volunteers, measurements were performed at baseline and 1, 6 and 24 h after 5 Hz rPAS25ms conditioning. Apart from the timeline of measurements, experimental procedures were identical to the main experiment.
Somatotopy
In six participants, we examined whether the conditioning effects of 5 Hz rPAS25ms were topographically specific to the sensorimotor representations that were conditioned by the rPAS protocol. To this end, we recorded the MEP amplitudes at rest from the right APB, first dorsal interosseus (FDI) and extensor carpi radialis (ECR) muscle at baseline and up to 1 h after 5 Hz rPAS25ms conditioning.
Effects of repetitive nerve stimulation and rTMS alone
Since 5 Hz rPAS25ms consisted of 5 Hz repetitive electrical nerve stimulation (5 Hz rENS) and 5 Hz rTMS paired at a constant ISI (i.e. 25 ms), we probed the conditioning effects of 5 Hz rENS and 5 Hz rTMS when given alone. In separate sessions, five participants received 5 Hz rENS at 200% the perceptual threshold given to the right median nerve or 5 Hz rTMS at 90% AMT given to the left M1. The rENS and rTMS sessions were performed at least 1 week apart in a counterbalanced order. The blocks of measurements, as well as the timeline of the experiment, were identical to the main experiment.
Spinal excitability
In eight subjects, we tested the acute conditioning effects of 5 Hz rPAS25ms conditioning on the excitability of spinal motoneurons using the F-wave method. F-waves were evoked in the relaxed APB muscle by supramaximal electrical stimulation of the median nerve at the wrist (Digitimer D-160 stimulator). The electrical pulses had a square wave configuration and a pulse width of 200 μs 15 F-waves were recorded immediately before and 5 min after 5 Hz rPAS25ms, and the mean F-wave amplitude was taken as a measure of spinal motoneuronal excitability.
F-wave measurements have a limited sensitivity to detect changes in spinal excitability because the amplitude of F-waves depends on a subpopulation of the spinal motor neuronal pool. Therefore, we additionally performed electrical stimulation of the descending motor tracts in to monitor the after effects of 5 Hz rPAS25ms on the excitability of spinal motoneurons. Because electrical brain stem stimulation is rather painful, this procedure was only performed in three of the eight participants who participated in the F-wave measurements. We used the method described by Ugawa et al. (1991) to stimulate the brain stem at the level of the foramen magnum. Surface electrodes were positioned over each mastoid process on both sides of the inion, with the anode on the right and the cathode on the left. Electrical stimuli were delivered through a Digitimer D-180 stimulator. Changes in MEP amplitude were measured in the slightly precontracted APB muscle. Stimulus intensity was adjusted to evoke MEPs with a peak-to-peak amplitude of approximately 0.5 mV in the preactivated target muscle. Ten consecutive MEPs were recorded at baseline and approximately 5 min after 5 Hz rPAS25ms. Because of the small number of subjects, results are reported descriptively only.
Data acquisition and analysis
EMG was recorded with Ag–AgCl surface electrodes from the right APB muscle using a belly tendon montage. The signal was amplified and bandpass filtered (32 Hz to 1 KHz) by a Digitimer D-150 amplifier and stored at a sampling rate of 10 kHz on a personal computer for off-line analysis (Signal Software; Cambridge Electronic Design, Cambridge, UK). During the experiments EMG activity was continuously monitored with visual (oscilloscope) and auditory (speakers) feedback to ensure either complete relaxation at rest or a constant level of EMG activity during tonic contraction.
For each block of measurements, the peak-to-peak amplitudes of each MEP (mV) and the duration of the CSP were measured off-line and the mean MEP amplitudes and mean CSP duration were calculated for each stimulation condition. The effects of 5 Hz rPAS on motor thresholds (resting and active MT), peak-to-peak MEP amplitude, duration of CSP, paired-pulse intracortical excitability (SICI and ICF) and sensorimotor inhibition (SAI and LAI) were evaluated in separate repeated-measures analyses of variance (ANOVA). For each dependent variable, we computed a two-way repeated measures ANOVA with time (four levels: baseline, post 0, post 30 and post 60) and intervention (two levels: 5 Hz rPAS25msversus 5 Hz rPAS10ms) as within-subject factors. For the evaluation of motor thresholds, the state of the muscle (relaxation versus contraction) was included as a third factor in the ANOVA model. ISI was considered as an additional factor in the ANOVAs testing for changes in paired-pulse intracortical excitability and sensorimotor intracortical inhibition. To evaluate the topographical specificity of the after-effects induced by 5 Hz PAS25ms, we performed an additional two-way repeated measures ANOVA with time (three levels: baseline, post 0, post 60) and muscle (three levels: APB, FDI and ECR) as within subjects factor. The Greenhouse-Geisser method was used if necessary to correct for nonsphericity. Conditional on a significant F value, post hoc paired-sample t tests were performed to explore the strength of main effects and the patterns of interaction between experimental factors. A P value of <0.05 was considered significant. All data are given as means ±s.e.m. We also calculated a two-tailed bivariate Pearson's coefficient of correlation between individual changes in MEP amplitude and changes in the strength of SAI.
Results
No participants reported any adverse effects during or after the experiments. For the main experiment, the mean intensities were 2.1 ± 0.6 mA for peripheral CS and 42.4 ± 5% of maximal stimulator output for the transcranial stimulus. No EMG responses were evoked in the relaxed right APB muscles by the 120 s train of 5 Hz rPAS, regardless of the ISI between the peripheral and transcranial stimulus.
Main experiment
Single-pulse excitability
The mean data for each block of measurements are given in Table 1. For motor threshold, three-factorial ANOVA disclosed a strong main effect of motor state (F1,9 = 119, P < 0.001) because MTs were consistently lower during tonic contraction relative to rest. There was no main effect of time (F3,27 = 0.8, P = 0.4) and no interaction between time and the other experimental factors, indicating that 5 Hz rPAS had no effect on MTs.
Table 1.
Parameters of corticospinal excitability before and after 5 Hz rPAS25ms and 5 Hz rPAS10ms
| Measure | Intervention | Baseline | Post 0 | Post 30 | Post 60 |
|---|---|---|---|---|---|
| rMT (%) | 5 Hz rPAS25ms | 43.3 ± 4.8 | 42.9 ± 4.2 | 42.5 ± 4.3 | 42.5 ± 3.8 |
| 5 Hz rPAS10ms | 43.0 ± 4.1 | 43.1 ± 3.1 | 42.8 ± 4.1 | 43.1 ± 4.3 | |
| aMT (%) | 5 Hz rPAS25ms | 37.1 ± 4.5 | 37.3 ± 4.4 | 36.7 ± 4.1 | 37.2 ± 4.2 |
| 5 Hz rPAS10ms | 37.7 ± 3.7 | 37.8 ± 4.1 | 37.4 ± 4 | 37.7 ± 3.4 | |
| MEP at rest (mV) | 5 Hz rPAS25ms | 0.65 ± 0.28 | 0.90 ± 0.37 | 0.90 ± 0.28 | 0.9 ± 0.27 |
| 5 HzrPAS10ms | 0.75 ± 0.30 | 0.64 ± 0.30 | 0.70 ± 0.20 | 0.66 ± 0.2 | |
| MEP active (mV) | 5 Hz rPAS25ms | 3.5 ± 0.9 | 3.6 ± 0.9 | 3.5 ± 0.9 | 3.2 ± 1.0 |
| 5 Hz rPAS10ms | 3.7 ± 0.8 | 3.6 ± 1.0 | 3.7 ± 1.0 | 3.7 ± 1.0 | |
| ICI 2 ms (%) | 5 Hz rPAS25ms | 52 ± 20 | 54 ± 17 | 54 ± 16 | 59 ± 21 |
| 5 Hz rPAS10ms | 65 ± 24 | 58 ± 21 | 66 ± 26 | 52 ± 20 | |
| ICF 12 ms (%) | 5 Hz rPAS25ms | 140 ± 47 | 114 ± 28 | 111 ± 22 | 135 ± 31 |
| 5 Hz rPAS10ms | 134 ± 35 | 117 ± 21 | 110 ± 30 | 110 ± 28 | |
| SAI 25 ms (%) | 5 Hz rPAS25ms | 64 ± 10 | 97 ± 9 | 99 ± 12 | 95 ± 12 |
| 5 Hz rPAS10ms | 72 ± 11 | 76 ± 18 | 69 ± 16 | 71 ± 14 | |
| LAI 200 ms (%) | 5 Hz rPAS25ms | 68 ± 17 | 66 ± 23 | 67 ± 28 | 62 ± 9 |
| 5 Hz rPAS10ms | 73 ± 15 | 73 ± 14 | 69 ± 22 | 71 ± 17 | |
| CSP (ms) | 5 Hz rPAS25ms | 167 ± 32 | 167 ± 35 | 170 ± 32 | 172 ± 38 |
| 5 Hz rPAS10ms | 156 ± 42 | 164 ± 36 | 160 ± 42 | 167 ± 49 |
rMT, resting motor threshold; aMT, active motor threshold; MEP, motor evoked potential; ICI, intracortical inhibition; SAI, short-latency afferent inhibition; LAI, long-latency afferent inhibition; CSP, cortically evoked silent period. Each value corresponds to the mean (±s.d.).
In contrast, 5 Hz rPAS modified mean peak-to-peak amplitudes for the unconditioned MEPs (Fig. 2). The ANOVA showed an interaction between time and intervention (F3,27 = 7.07, P = 0.001). Separate follow-up ANOVAs with time as a within-subject factor were performed to characterize time-dependent changes in MEP amplitude for 5 Hz rPAS25ms and 5 Hz rPAS10ms. Only 5 Hz rPAS25ms induced a consistent change in MEP amplitude at rest as there was a prominent time effect for 5 Hz rPAS25ms(F3,27= 7.01, P = 0.001) but not for 5 Hz rPAS10ms (P > 0.3). 5Hz rPAS25ms caused a lasting facilitation of MEP amplitudes (Fig. 2A). Post hoc t tests revealed an increase in MEP amplitude at post 0 (t1,9= 2.6, P = 0.02), post 30 (t1,9= 3.6, P = 0.005) and post 60 (t1,9= 4.7, P = 0.001) relative to baseline. In all participants, 5 Hz rPAS25ms induced at least some MEP facilitation. Relative increases in MEP amplitude ranged from +10 to +150% after conditioning, and this is comparable to the magnitude of MEP facilitation induced by conventional low-frequency PAS at 25 ms (Stefan et al. 2000).
Figure 2. Time course of mean MEP amplitudes.
The MEPs of the relaxed right APB muscle showed a significant increase in mean amplitude that lasted for at least 1 h after 5 Hz rPAS25ms (A). No differences in MEP size were found after 5 Hz rPAS10ms (A). No significant increase of MEP amplitude occurred during tonic contraction of the APB muscle after 5 Hz rPAS25ms or 5 Hz rPAS10ms (B). Error bars indicate s.e.m. *Significant difference, as revealed by paired t test compared with baseline (P≤ 0.05).
The 5 Hz rPAS25ms protocol induced a lasting facilitation in the relaxed but not in the active APB muscle. For the preactivated APB muscle, ANOVA showed no time effect (F3,27= 0.2, P = 0.8) on the MEP amplitude. There was also no interaction between time and intervention (F3,27= 2.4, P = 0.09). Neither 5 Hz rPAS25ms nor 5 Hz rPAS10ms increased the MEP amplitude in the preactivated APB muscle (Fig. 2B). Measurements during tonic contraction also revealed no consistent effect of 5 Hz rPAS on the duration of the CSP (Table 1). ANOVA only revealed a weak statistical trend towards a main effect of time (F3,27= 2.3, P = 0.09) reflecting a gradual increase in duration of the CSP over time. There was no indication for a time by intervention interaction (P = 0.6).
Intracortical paired-pulse excitability
At baseline, paired-pulse stimulation consistently produced SICI at an ISI of 2 ms and ICF at an interval of 12 ms (Fig. 3). The 5 Hz rPAS protocol induced similar changes in amplitude of unconditioned and conditioned MEPs. Conditioned MEP amplitudes increased after 5 Hz rPAS25ms, but were unchanged after 5 Hz rPAS10ms. However, the ratio between unconditioned and conditioned MEP amplitudes did not differ among the blocks of measurements (Fig. 3). Accordingly, ANOVA only disclosed a strong main effect of ISI (F1,9= 80, P < 0.00001), but there was no main effect of time(F3,27= 2.06, P = 0.1) and intervention (F1,9= 0.01, P = 0.9) and no interaction between the factors.
Figure 3. Time course of intracortical inhibition (ICI) and ICF.
ICI at an ISI of 2 ms and ICF at an ISI of 12 ms are unchanged after 5 Hz rPAS25ms (A) or 5 Hz rPAS10ms (B).
We computed separate ANOVA s for SICI and ICF with the factors time and intervention, as SICI and ICF are mediated through different neuronal circuits. For ICI, there were no main effects of time or intervention and no interaction between these factors. For ICF, ANOVA showed a main effect for the factor time (F3,27= 2.5, P = 0.02) with no interaction between the factors time and intervention (F3,27 = 1, P = 0.3). Post hoc t tests revealed no relative change in ICF compared with baseline values. ICF tended to decrease after 5 Hz rPAS25ms (post 0 versus baseline: t1,9= 1.6, P = 0.1; post 30 versus baseline: t1,9= 1.9, P = 0.09) and after 5 Hz rPAS10ms (post 0 versus baseline: t1,9= 2.1, P = 0.06; post 30 versus baseline: t1,9= 1.9, P = 0.09; post 60 versus baseline: t1,9= 2, P = 0.07).
Sensorimotor intracortical inhibition
The unconditioned MEPs showed a prominent increase in MEP size after 5 Hz rPAS25ms, but not after 5 Hz rPAS10ms (Fig. 4). Using the conditioned MEP amplitudes as a dependent variable, the ANOVA showed an interaction between time, intervention and ISI (F3,27 = 3.4, P = 0.03). The interaction was caused by a selective reduction in SAI but not LAI, and this only occurred after 5 Hz rPAS25ms but not after 5 Hz rPAS10ms (Fig. 4). There was also a main effect of ISI (F1,9 = 14, P = 0.004) and time (F3,27 = 3.7, P = 0.02).
Figure 4. Time course of mean afferent inhibition.
Short-latency afferent inhibition at an ISI of 25 ms (SAI) was abolished for at least 1 h after 5 Hz rPAS25ms, whereas the magnitude of long-latency afferent inhibition at 200 ms (LAI) was unchanged following 5 Hz rPAS25ms (A). 5 Hz rPAS10ms had no effect on afferent intracortical inhibition at 25 ms and 200 ms (B). Error bars indicate s.e.m. *Significant difference as revealed by paired t test compared with baseline (P ≤ 0.05).
A differential effect of the type of intervention on SAI and LAI was confirmed by separate follow-up ANOVA s for SAI and LAI that used time of measurement and intervention as within-subject factors. For SAI, there was a prominent main effect for time (F3,27 = 15.7, P < 0.0001) and a significant interaction between time and intervention (F3,27 = 6.1, P < 0.002). Figure 4 shows that 5 Hz rPAS25ms and 5 Hz rPAS10ms decreased the relative strength of SAI, accounting for the main effect of time. The interaction was caused by a marked disinhibition of SAI after 5 Hz rPAS25ms (Fig. 4). SAI was only slightly reduced by 5 Hz rPAS10ms, whereas SAI was completely abolished after 5 Hz rPAS25ms. Accordingly, post hoc t tests only revealed significant reductions in SAI after 5 Hz rPAS25ms but not after 5 Hz rPAS10ms. The reduction in SAI was significant at post 0 (t1,9 = 5.57, P = 0.008), post 30 (t1,9 = 6.03, P = 0.0001) and post 60 (t1,9 = 4.5, P = 0.001) relative to baseline. Correlational analysis yielded no correlation between individual changes in MEP amplitude and changes in the strength of SAI (r = 0.2, P = 0.5). For LAI, ANOVA demonstrated no main effect of time (F3,27 = 0.2, P = 0.8) and intervention (F1,9 = 0.5, P = 0.5) and no interaction (F3,27 = 0.2, P < 0.8).
Control experiments
Influence of the amplitude of the test response on SAI
In four subjects, we adjusted the intensity of the transcranial stimulus after 5 Hz rPAS25ms to evoke unconditioned MEPs of the same magnitude as before 5 Hz rPAS25ms. When matching the test MEP amplitudes before and 30 min after 5 Hz rPAS25ms, we observed a marked reduction of SAI after 5 Hz rPAS25ms (before 5 Hz rPAS25ms 57.3 ± 2.5% versus 30 min after 5 Hz rPAS25ms 91.6 ± 9%; t1,3 = −9.0, P = 0.002).
Long-lasting changes in corticospinal excitability after 5 Hz rPAS25ms
In six subjects, we investigated the long-term effects of 5 Hz rPAS25ms immediately after as well as 1, 6 and 24 h after conditioning. In this experiment, we only assessed the mean amplitudes of unconditioned MEP amplitudes at rest and the relative strength of SAI because the main experiment had revealed significant changes of these measures after 5 Hz rPAS25ms. The results are summarized in Fig. 5. For the unconditioned MEP amplitude and SAI, separate one-way ANOVAs showed a significant effect of time (MEP amplitudes: F4,20 = 6.56, P = 0.001; SAI: F4,20 = 3.5, P = 0.02). Post hoc t tests revealed significant increase in resting MEP amplitudes at post 0 (t1,5 = 2.9, P = 0.03), post 60 (t1,5 = 6.3, P = 0.001), and post 6 h (t1,5 = 6.97, P = 0.0009). MEP amplitudes returned to almost baseline levels after 24 h (t1,5 = 1.19, P = 0.28). The same pattern of time-dependent changes was observed for SAI. Post hoc t tests indicated significant reduction in SAI at post 0 (t1,5 = −3.7, P = 0.01), post 60 (t1,5 = −6.3, P = 0.001), and post 6 h (t1,5 = −3.4, P = 0.001), and this returned to baseline levels at post 24 h (t(1,5) = −1.2, P = 0.27).
Figure 5. Long-term effects of 5 Hz rPAS25ms on corticospinal excitability and SAI at 25 ms.
The 5 Hz rPAS25ms protocol induced a long-lasting increase in mean MEP amplitude at rest (A) that was paralleled by a marked decrease in SAI (B). Six hours after the end of the intervention the changes in excitability were still present and tended to return baseline values after 24 h. Error bars indicate s.e.m. *Significant difference as revealed by paired t test compared with baseline (P ≤ 0.05).
Topography of the changes in MEP amplitude
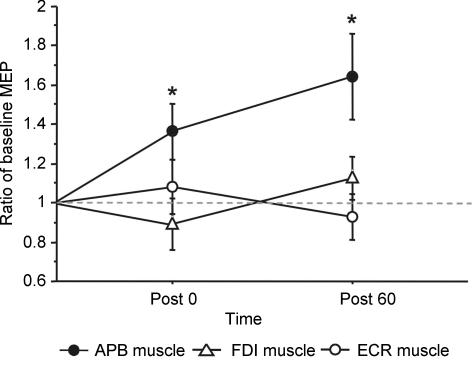
The facilitatory after-effect of 5 Hz rPAS25ms on mean MEP amplitude was specific to the relaxed APB muscle as no such change was found for the relaxed FDI and ECR muscle (Fig. 6). A muscle-specific effect was confirmed by the ANOVA. There was a significant interaction between muscle and time (F4,20 = 3.36, P = 0.029). Post hoc t tests showed that mean MEP amplitudes were increased in the APB muscle at post 0 (t1,5 = −2.5, P = 0.04) and post 60 (t1,5 = −2.6, P = 0.04) but not in the other muscles.
Figure 6. Topographical specificity of 5 Hz rPAS25ms conditioning.
The 5Hz rPAS25ms protocol induced a relative increase in mean MEP amplitude in the right APB muscle, whereas the MEP amplitudes of the right FDI and ECR muscle were not modified by 5 Hz rPAS25ms. Mean MEP amplitudes are normalized to baseline values. Error bars indicate s.e.m. *Significant difference as revealed by paired t test compared with baseline (P ≤ 0.05).
No effects of rENS and rTMS alone
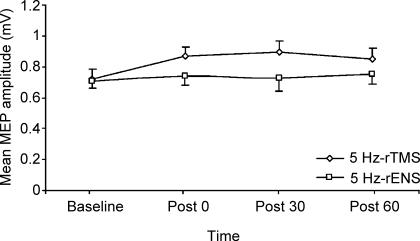
In five subjects, we examined the after effects of 600 pulses of 5 Hz rTMS at 90% of active MT or 600 pulses of 5 Hz rENS on the amplitude of unconditioned MEPs. While 5 Hz rTMS induced a modest increase in MEP amplitude, it was unchanged after 5 Hz rENS (Fig. 7). For both interventions, one-way ANOVA revealed no effect of time (P > 0.2). Hence, neither 5 Hz rTMS at 90% of aMT nor 5 Hz rENS induced a consistent change in corticospinal excitability when given alone.
Figure 7. Time course of mean MEP amplitudes after 5 Hz rTMS and 5 Hz rENS alone.
MEP amplitudes in the right APB muscle at rest are unchanged after 5 Hz rTMS at 90% of active motor threshold (aMT), and after 5 Hz rENS when given alone.
No modulation of spinal excitability
Neither F-wave measurements nor brain stem stimulation at the level of the foramen magnum revealed a change in spinal excitability. In eight subjects, mean F-wave amplitude of the right median nerve remained unchanged after 5 Hz rPAS25ms (mean amplitude at baseline 650 ± 30 μV versus mean amplitude after 5 Hz rPAS25ms 690 ± 30 μV; P = 0.46).
The 5 Hz rPAS25ms protocol also had no effect on mean MEP amplitudes evoked by electrical brain stem stimulation in the isometrically contracting APB muscle (mean amplitude at baseline 0.40 ± 0.1 mV versus mean amplitude after 5 Hz rPAS25ms 0.39 ± 0.1 mV). Compared with baseline, none of the three subjects showed a relative change in MEP amplitude of more than 20% after 5 Hz rPAS25ms.
Discussion
Two minutes of subthreshold 5 Hz rPAS25ms induced a long-lasting increase in the excitability of the homotopic corticospinal output from the stimulated M1 as indexed by a persistent increase in MEP amplitudes in the relaxed right APB muscle. Corticospinal facilitation was paralleled by a selective loss of SAI at an ISI of 25 ms. The conditioning effects of 5 Hz rPAS critically depended on the ISI between the peripheral and transcranial stimulus, because 5 Hz rPAS only produced a marked facilitation of corticospinal excitability at an ISI of 25 ms (5 Hz rPAS25ms) but not at an ISI of 10 ms (5 Hz rPAS10ms). We discuss the relevance of these results to current research that has used transcranial stimulation to induce rapid motor reorganization in the human brain.
Rationale behind the associative stimulation paradigm
Our prediction was that concurrent peripheral stimulation will potentiate the ‘facilitatory’ high-frequency (5 Hz) rTMS if the peripheral stimulus is given 25 ms before the transcranial stimulus. Consistent with our hypothesis, 600 paired stimuli of 5 Hz rPAS25ms were sufficient to induce a consistent and long-lasting increase in resting excitability of homotopic corticospinal output neurons. In contrast, 600 stimuli of 5 Hz rTMS at 90% of active MT failed to induce a significant increase in MEP amplitude. The failure of 5 Hz rTMS alone to produce consistent after effects is in accordance with previous 5 Hz rTMS studies in which 150–1500 stimuli of 5 Hz rTMS given at 90% of resting or active MT failed to produce a persistent change in corticospinal excitability (Rizzo et al. 2004; Takano et al. 2004; Quartarone et al. 2005). We infer that the synchronization of rENS and rTMS at an ISI of 25 ms potentiated the facilitatory effect of 5 Hz rTMS on corticospinal excitability. The same conclusion applies to rENS of the median nerve because 600 stimuli of 5 Hz rENS at 200% perceptual threshold had no effect on corticospinal excitability. Indeed previous rENS studies have already demonstrated that more than 10 min of high-frequency rENS are required to provoke consistent increases in corticospinal excitability. (Ridding et al. 2000, 2001; Pyndt & Ridding, 2004).
Changes in corticospinal excitability
Compared with the excitability changes evoked by conventional PAS (Stefan et al. 2000), 5 Hz rPAS25ms evoked a similar pattern of changes in corticospinal excitability as indexed by the mean MEP amplitude. The magnitude of MEP facilitation was comparable with previous reports that used conventional PAS (Stefan et al. 2000; Wolters et al. 2003; Ziemann, 2004). In analogy to conventional PAS protocols, facilitation of corticospinal excitability was confined to the homotopic motor representation that corresponded to the stimulated sensory afferents and was only found at rest.
Several lines of evidence suggest that the site of excitability changes lies in the stimulated M1. Previous studies using conventional PAS (Stefan et al. 2000) or subthreshold 5 Hz rTMS (Di Lazzaro et al. 2002; Takano et al. 2004; Quartarone et al. 2005) have shown that these interventions induce lasting changes in cortical excitability of M1. The stimulus intensity used for TMS during 5 Hz rPAS25ms was below active MT which is subthreshold for inducing descending corticospinal volleys (Di Lazzaro et al. 1998). Moreover, the increase in MEP amplitude was associated with a concurrent attenuation of SAI at 25 ms which has been shown to be of cortical origin (Tokimura et al. 1996). Finally, the notion of a cortical origin of the excitability changes is further corroborated by the present study. Mean F-wave amplitude of the relaxed right APB muscle, as well as mean MEP amplitude evoked by electrical brain stem stimulation in the preactivated APB muscle, were unchanged after 5 Hz rPAS25ms. However, the results obtained with brain stem stimulation need to be interpreted with caution, as the main experiment revealed no change in corticospinal excitability in the preactivated APB muscle after 5 Hz rPAS25ms.
In support of the notion of spike-time-dependent plasticity, the efficacy of 5 Hz rPAS conditioning was critically dependent on the interval between the peripheral and transcranial stimulus because 5 Hz rPAS25ms but not 5 Hz rPAS10ms induced a lasting facilitation of corticospinal excitability. The present finding suggests that 5 Hz rTMS boosts LTP-like aspects of PAS-induced plasticity if 5 Hz rTMS is combined with ‘facilitatory’ PAS at an ISI of 25 ms. Conversely, 5 Hz rTMS appears to block LTD-like aspects of PAS-induced plasticity if 5 Hz rTMS is coupled with ‘inhibitory’ PAS at an ISI of 10 ms.
For LTP and LTD induction, the critical variable in determining the sign of the synaptic modification appears to be the level of postsynaptic depolarization during conditioning stimulation and the resulting pattern of postsynaptic increases in Ca2+ levels. A transient inflow of high amounts of Ca2+ into the neuron triggers LTP, while LTD is induced by a modest but more sustained elevation in postsynaptic Ca2+ levels (Kirkwood & Bear, 1995; Bi & Poo, 2001). Hence, it is conceivable that 5 Hz rPAS10ms caused a moderate postsynaptic Ca2+ elevation that lasted too short for inducing LTD and was too small for inducing LTP-like plasticity. If so, a more sustained stimulation (e.g. 1 Hz rPAS10ms) or intermittent bursts of 5 Hz rPAS25ms stand a better chance to induce a LTD-like suppression of corticospinal excitability. A second explanation for the failure of 5 Hz rPAS10ms to reduce corticospinal excitability is that the critical time window for inducing LTD-like plasticity differs from conventional rPAS. If this was the case, an ISI of 10 ms might have been inappropriate for inducing LTD-like plasticity. Whatever the mechanism, the lack of a lasting inhibition after 5 Hz rPAS10ms shows that 5 Hz rPAS produce qualitatively different effects in the stimulated M1 compared with rPAS at very low frequencies.
Changes in intracortical excitability
The 5 Hz rPAS25ms protocol induced a different pattern of changes in intracortical excitability compared with the changes that were reported after conventional 0.05 Hz rPAS25ms (Stefan et al. 2000; Quartarone et al. 2003, 2005). While conventional PAS has been shown to increase the duration of the CSP, 5 Hz rPAS25ms had no consistent effect on the duration of the CSP. Conversely, 5 Hz rPAS25ms specifically abolished SAI at 25 ms, whereas no such change was found after conventional low-frequency PAS (Stefan et al. 2002). The selective attenuation of SAI at 25 ms after 5 Hz rPAS25ms corroborates the notion that LAI and SAI are mediated through different sensorimotor circuits (Sanger et al. 2001). More importantly, these differences in the conditioning effect between 5 Hz rPAS25ms and conventional low-frequency PAS25ms indicate that both interventions produce qualitatively different effects in terms of intracortical reorganization, and highlight the complexity of possible interactions of PAS conditioning on the various neuronal subpopulations in human M1.
SAI is controlled by muscarinic neurotransmission because blockade of muscarinic receptors with scopolamine reduces the amount of SAI (Di Lazzaro et al. 2000). A single dosage of lorazepam also causes a reduction in the magnitude of SAI, presumably through a GABAAergic inhibitory effect on acetylcholine release (Vazquez & Baghdoyan, 2003; Di Lazzaro et al. 2005). Therefore, it is conceivable that 5 Hz rPAS25ms reduced the amount of SAI by inducing LTP-like effects in glutamatergic synaptic inputs of cortical interneurons to GABAAergic neurons that control the magnitude of SAI. Hence, 5 Hz rPAS25ms may result in a lasting potentiation of a facilitatory sensorimotor pathway that is distinct from the inhibitory pathways mediating the SAI. According to this scenario, the increase in facilitatory sensorimotor drive onto the corticospinal output neurons will shift the balance between short-latency sensorimotor facilitation and inhibition towards facilitation. At present, we can only speculate why 5 Hz rPAS25ms was more efficient in reducing SAI in the M1, yet failed to modify the inhibitory circuits that mediate the CSP. There were several important differences in the PAS protocol compared with conventional PAS. Our 5 Hz rPAS25ms protocol used a lower intensity (90% of active MT) and a biphasic stimulus configuration for TMS and we gave a higher number of paired stimuli at a rapid rate. We favour the hypothesis that the very low intensity of cortical stimulation accounts for the differential effect of 5 Hz rPAS25ms on intracortical excitability, but this remains to be clarified in future studies.
We found no evidence for a sustained effect of 5 Hz rPAS on the relative strength in SICI at an ISI of 2 ms or LAI at an ISI of 200 ms. There was some evidence for a modest attenuation of ICF at 12 ms and a trend towards a slight increase in the duration of the CSP after 5 Hz rPAS. Since these changes were found after 5 Hz rPAS25ms and 5 Hz rPAS10ms, they were not specifically related to the timing of the afferent stimulus. Paired-pulse measurements of intracortical excitability were only assessed at a single ISI, and no measurements were performed in which the intensity of the test stimulus was adjusted to match the amplitudes of the unconditioned test response among all blocks of measurements before and after 5 Hz rPAS25ms conditioning. Therefore, we cannot exclude subtle after effects on SICI or LAI. However, any effect on SICI or LAI should be relatively subtle compared with the marked attenuation of SAI.
Neuronal substrate of PAS-induced plasticity
It has been proposed that during PAS, the peripheral and cortical stimuli activate distinct excitatory inputs that converge onto the same postsynaptic neurons in the M1 and, if appropriately timed, can induce spike-time-dependent associative plasticity (Stefan et al. 2000, 2002; Wolters et al. 2003).
Because the intensity of the transcranial stimulus was below active MT, 5 Hz rPAS25ms induced no relevant postsynaptic activity in fast-conducting corticospinal output cells. Therefore, we argue that 5 Hz rPAS25ms induced associative plasticity in glutamatergic sensorimotor inputs to cortical interneurons that generate an excitatory drive on pyramidal output neurons. Within the framework of spike-time-dependent plasticity, we propose that the transcranial stimulus induced postsynaptic activity (i.e. action potentials) in this population of cortical interneurons, while the peripheral stimulus produced presynaptic activity through activation of sensorimotor inputs onto these interneurons. Experimental evidence in primates suggests that the sensory inputs to the M1 mainly terminate in superficial cortical layers (layers II–III) and project on dendrites of a discrete population of interneurons that have modulatory effects on corticofugal neurones located in layers V and VI (Porter et al. 1990). These interneurons are the most likely neuroanatomical target for associative plasticity induced by low-intensity subthreshold 5 Hz rPAS25ms.
We did not assess the conditioning effects of 5 Hz rPAS on facilitatory I-wave interactions (Rothwell, 1999). However, previous paired-pulse investigations of facilitatory I–wave interactions reported an increase of the first peak of I-wave facilitation following 600 subthreshold stimuli of 5 Hz rTMS (Quartarone et al. 2005) and after PAS of the motor point and the M1 (Ridding et al. 2001). Hence, it is conceivable that 5 Hz rPAS25ms also caused a lasting increase in synaptic efficacy within intracortical circuits mediating the first I-wave, but this issue needs to be addressed in future studies.
Short-afferent inhibition and PAS-induced facilitation of corticospinal excitability
Though the increase in MEP amplitude and the attenuation of SAI shared a similar time course, we found no correlation between individual changes in SAI and MEP amplitudes after 5 Hz rPAS25ms. In addition, the conventional PAS protocol at an ISI of 25 ms produces MEP facilitation without a consistent effect on the strength of SAI (Stefan et al. 2002). Hence, it is questionable whether sensorimotor disinhibition (as indexed by a reduction of SAI) contributed to the lasting increase in excitability of corticospinal output neurons after 5 Hz rPAS25ms.
There is another issue which deserves some discussion. Electrical stimulation of the median nerve produces a consistent suppression of the MEP amplitude if TMS is given to the M1 25 ms after the conditioning electrical stimulus at the wrist (Di Lazzaro et al. 2000, 2005; Tokimura et al. 2000). The acute inhibitory effects of afferent conditioning on corticospinal excitability beg the question why 5 Hz rPAS and conventional PAS at an ISI of 25 ms (Stefan et al. 2000; Ziemann, 2004) result in a lasting facilitation of corticospinal excitability. A solution to this apparent contradiction is that 5 Hz rPAS25ms increases corticospinal excitability by potentiating a facilitatory sensorimotor pathway that is not involved in the generation of SAI. According to this hypothesis, PAS at an ISI of 25 ms increases synaptic efficacy of a facilitatory sensorimotor pathway that projects onto corticospinal output neurons in M1, producing a lasting potentiation of corticospinal excitability. We speculate that this facilitatory pathway cannot be assessed using a conditioning-test protocol (Di Lazzaro et al. 2000, 2005; Tokimura et al. 2000) because the preponderant effects of the ‘SAI pathway’ masks any concurrent facilitatory effects conveyed to M1 via the facilitatory sensorimotor pathway.
Potential significance
In contrast to other protocols that use paired stimulation, such as conventional PAS (Stefan et al. 2000; Wolters et al. 2003), repetitive paired-pulse TMS (Khedr et al. 2004; Thickbroom et al. 2006) and paired rENS (McKay et al. 2002), 5 Hz rPAS25ms is a very rapid method of conditioning the human M1. Another protocol that can rapidly induce a marked increase in corticospinal excitability is intermittent theta burst stimulation (iTBS). iTBS consists of three-pulse trains of 50 Hz rTMS given at a rate of 5 Hz and an intensity below active MT (Huang et al. 2005). iTBS has been shown to produce a lasting attenuation in SICI (Huang et al. 2005), whereas the present study shows a prominent effect of 5 Hz rPAS25ms on SAI. These findings indicate that 5 Hz rPAS25ms and iTBS appear to induce different patterns of motor reorganization in the intracortical circuits. They also suggest that a stimulation-induced acute disinhibition within distinct intracortical circuits may be important for a rapid induction of LTP-like effects in the M1.
An interesting feature of 5 Hz rPAS25ms is the somatotopic specificity of the conditioning effects which may help to shape the regional pattern of reorganization. Concurrent afferent conditioning of several peripheral nerves may produce a more generalized enhancement of the corticospinal output to the limb, whereas the stimulation of a single nerve is more suited to condition a distinct population of corticospinal output neurons.
It has been proposed that the reorganization induced by afferent stimulation may have a therapeutic application for the rehabilitation of hand function (Conforto et al. 2002). Since 5 Hz rPAS25ms is highly efficient in enhancing the excitability of the corticospinal output to the stimulated limb, 5 Hz rPAS25ms may be a useful tool for motor neurorehabilitation.
References
- Bi G, Poo M. Synaptic modification by correlated activity: Hebb's postulate revisited. Annu Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Conforto AB, Kaelin-Lang A, Cohen LG. Increase in hand muscle strength of stroke patients after somatosensory stimulation. Ann Neurol. 2002;51:122–125. doi: 10.1002/ana.10070. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Mazzone P, Pilato F, Saturno E, Dileone M, Insola A, Tonali PA, Rothwell JC. Short-term reduction of intracortical inhibition in the human motor cortex induced by repetitive transcranial magnetic stimulation. Exp Brain Res. 2002;147:108–113. doi: 10.1007/s00221-002-1223-5. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Di Giovanni S, Zito G, Tonali P, Rothwell JC. Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Exp Brain Res. 2000;135:455–461. doi: 10.1007/s002210000543. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Saturno E, Dileone M, Pilato F, Nardone R, Ranieri F, Musumeci G, Fiorilla T, Tonali P. Effects of lorazepam on short latency afferent inhibition and short latency intracortical inhibition in humans. J Physiol. 2005;564:661–668. doi: 10.1113/jphysiol.2004.061747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res. 1998;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Sanes JN. Motor areas of the cerebral cortex. J Clin Neurophysiol. 1994;11:382–396. [PubMed] [Google Scholar]
- Helmich RC, Baumer T, Siebner HR, Bloem BR, Munchau A. Hemispheric asymmetry and somatotopy of afferent inhibition in healthy humans. Exp Brain Res. 2005;167:211–219. doi: 10.1007/s00221-005-0014-1. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Iriki A, Pavlides C, Keller A, Asanuma H. Long-term potentiation in the motor cortex. Science. 1989;245:1385–1387. doi: 10.1126/science.2551038. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Gilio F, Rothwell J. Effects of low frequency and low intensity repetitive paired pulse stimulation of the primary motor cortex. Clin Neurophysiol. 2004;115:1259–1263. doi: 10.1016/j.clinph.2003.08.025. [DOI] [PubMed] [Google Scholar]
- Kirkwood A, Bear MF. Elementary forms of synaptic plasticity in the visual cortex. Biol Res. 1995;28:73–80. [PubMed] [Google Scholar]
- Kobayashi M, Ng J, Theoret H, Pascual-Leone A. Modulation of intracortical neuronal circuits in human hand motor area by digit stimulation. Exp Brain Res. 2003;149:1–8. doi: 10.1007/s00221-002-1329-9. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111:800–805. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- McKay D, Brooker R, Giacomin P, Ridding M, Miles T. Time course of induction of increased human motor cortex excitability by nerve stimulation. Neuroreport. 2002;13:1271–1273. doi: 10.1097/00001756-200207190-00011. [DOI] [PubMed] [Google Scholar]
- Murase N, Rothwell JC, Kaji R, Urushihara R, Nakamura K, Murayama N, Igasaki T, Sakata-Igasaki M, Mima T, Ikeda A, Shibasaki H. Subthreshold low-frequency repetitive transcranial magnetic stimulation over the premotor cortex modulates writer's cramp. Brain. 2005;128:104–115. doi: 10.1093/brain/awh315. [DOI] [PubMed] [Google Scholar]
- Orth M, Rothwell JC. The cortical silent period: intrinsic variability and relation to the waveform of the transcranial magnetic stimulation pulse. Clin Neurophysiol. 2004;115:1076–1082. doi: 10.1016/j.clinph.2003.12.025. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Reimer B, Loer C, Quartarone A, Munchau A, Conrad B, Siebner HR. Long-lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5 Hz repetitive TMS to the primary motor cortex. Clin Neurophysiol. 2004;115:1519–1526. doi: 10.1016/j.clinph.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Pitcher JB, Ridding MC, Miles TS. Frequency-dependent, bi-directional plasticity in motor cortex of human adults. Clin Neurophysiol. 2003;114:1265–1271. doi: 10.1016/s1388-2457(03)00092-0. [DOI] [PubMed] [Google Scholar]
- Porter LL, Sakamoto T, Asanuma H. Morphological and physiological identification of neurons in the cat motor cortex which receive direct input from the somatic sensory cortex. Exp Brain Res. 1990;80:209–212. doi: 10.1007/BF00228864. [DOI] [PubMed] [Google Scholar]
- Pyndt HS, Ridding MC. Modification of the human motor cortex by associative stimulation. Exp Brain Res. 2004;159:123–128. doi: 10.1007/s00221-004-1943-9. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Bagnato S, Rizzo V, Morgante F, Sant'angelo A, Battaglia F, Messina C, Siebner HR, Girlanda P. Distinct changes in cortical and spinal excitability following high-frequency repetitive TMS to the human motor cortex. Exp Brain Res. 2005;161:114–124. doi: 10.1007/s00221-004-2052-5. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Bagnato S, Rizzo V, Siebner HR, Dattola V, Scalfari A, Morgante F, Battaglia F, Romano M, Girlanda P. Abnormal associative plasticity of the human motor cortex in writer's cramp. Brain. 2003;126:2586–2596. doi: 10.1093/brain/awg273. [DOI] [PubMed] [Google Scholar]
- Ridding MC, Brouwer B, Miles TS, Pitcher JB, Thompson PD. Changes in muscle responses to stimulation of the motor cortex induced by peripheral nerve stimulation in human subjects. Exp Brain Res. 2000;131:135–143. doi: 10.1007/s002219900269. [DOI] [PubMed] [Google Scholar]
- Ridding MC, McKay DR, Thompson PD, Miles TS. Changes in corticomotor representations induced by prolonged peripheral nerve stimulation in humans. Clin Neurophysiol. 2001;112:1461–1469. doi: 10.1016/s1388-2457(01)00592-2. [DOI] [PubMed] [Google Scholar]
- Rizzo V, Siebner HR, Modugno N, Pesenti A, Munchau A, Gerschlager W, Webb RM, Rothwell JC. Shaping the excitability of human motor cortex with premotor rTMS. J Physiol. 2004;554:483–495. doi: 10.1113/jphysiol.2003.048777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC. Paired-pulse investigations of short-latency intracortical facilitation using TMS in humans. Electroencephalogr Clin Neurophysiol Suppl. 1999;51:113–119. [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Dressnandt J, Auer C, Conrad B. Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve. 1998;21:1209–1212. doi: 10.1002/(sici)1097-4598(199809)21:9<1209::aid-mus15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res. 2003;148:1–16. doi: 10.1007/s00221-002-1234-2. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Takano B, Drzezga A, Peller M, Sax I, Schwaiger M, Lee L, Siebner HR. Short-term modulation of regional excitability and blood flow in human motor cortex following rapid-rate transcranial magnetic stimulation. Neuroimage. 2004;23:849–859. doi: 10.1016/j.neuroimage.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Thickbroom GW, Byrnes ML, Edwards DJ, Mastaglia FL. Repetitive paired-pulse TMS at I-wave periodicity markedly increases corticospinal excitability: a new technique for modulating synaptic plasticity. Clin Neurophysiol. 2006;117:61–66. doi: 10.1016/j.clinph.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Inolsa A, Mazzone P, Tonali P, Rothwell JC. Short latency inhibition of human hand motor cortex by somatosensory input from the hand. J Physiol. 2000;523:503–513. doi: 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touge T, Gerschlager W, Brown P, Rothwell JC. Are the after-effects of low-frequency rTMS on motor cortex excitability due to changes in the efficacy of cortical synapses? Clin Neurophysiol. 2001;112:2138–2145. doi: 10.1016/s1388-2457(01)00651-4. [DOI] [PubMed] [Google Scholar]
- Ugawa Y, Rothwell JC, Day BL, Thompson PD, Marsden CD. Percutaneous electrical stimulation of corticospinal pathways at the level of the pyramidal decussation in humans. Ann Neurol. 1991;29:418–427. doi: 10.1002/ana.410290413. [DOI] [PubMed] [Google Scholar]
- Vazquez J, Baghdoyan HA. Muscarinic and GABAA receptors modulate acetylcholine release in feline basal forebrain. Eur J Neurosci. 2003;17:249–259. doi: 10.1046/j.1460-9568.2003.02451.x. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J. Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol. 1999;517:591–597. doi: 10.1111/j.1469-7793.1999.0591t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, Benecke R, Classen J. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–2345. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- Ziemann U. TMS induced plasticity in human cortex. Rev Neurosci. 2004;15:253–266. doi: 10.1515/revneuro.2004.15.4.253. [DOI] [PubMed] [Google Scholar]