Abstract
We addressed the hypothesis that single vagal afferent C-fibres can be stimulated via either the adenosine A1 or A2A receptor subtypes. The effect of adenosine on the nerve terminals of vagal sensory nerve subtypes was evaluated in an ex vivo perfused guinea pig lung preparation using extracellular recording techniques. Adenosine (10 μm) consistently evoked action potential discharge in lung C-fibre terminals arising from the nodose ganglia, but failed to evoke action potential discharge in most jugular ganglion C-fibres. Adenosine also failed to activate stretch-sensitive nodose A-fibres in the lungs. The selective A1 antagonist DPCPX (0.1 μm) or the selective A2A antagonist SCH 58261 (0.1 μm) partially inhibited the nodose C-fibre activation by adenosine, and the combination of both antagonists almost completely inhibited the response. The adenosine-induced action potential discharge in nodose C-fibres was mimicked by either the selective A1 agonist CCPA (1 μm) or the selective A2A agonist CGS 21680 (1 μm). Single cell PCR techniques revealed that adenosine A1 and A2A receptor mRNA was expressed in individual nodose neurons retrogradely labelled from the lungs. The gramicidin-perforated patch clamp technique on neurons retrogradely labelled from the lungs was employed to study the functional consequence of adenosine receptor agonists directly on neuronal membrane properties. Both the selective A1 agonist CCPA (1 μm) and the selective A2A agonist CGS 21680 (1 μm) depolarized the airway-specific, capsaicin-sensitive, nodose neurons to action potential threshold. The data support the hypothesis that adenosine selectively depolarizes vagal nodose C-fibre terminals in the lungs to action potential threshold, by stimulation of both adenosine A1 and A2A receptor subtypes located in the neuronal membrane.
Several studies indicate that extracellular adenosine may contribute to the symptoms of airway inflammatory diseases such as asthma and chronic obstructive pulmonary disease (COPD). The concentration of adenosine is increased in bronchoalveolar lavage (BAL) fluid in asthmatics and cigarette smokers compared with normal individuals (Driver et al. 1993). Adenosine concentration in exhaled breath condensate is higher in steroid-naïve asthmatics compared with healthy volunteers and steroid-treated patients (Huszar et al. 2002). From a physiological perspective, adenosine inhalation induces bronchoconstriction, dyspnoea and cough in asthmatic individuals (Cushley et al. 1983; Basoglu et al. 2005). Inhaled adenosine has been shown to cause acute sensations of dyspnoea, which are more pronounced than those observed with equi-effective bronchoconstricting doses of methacholine (Marks et al. 1996). Moreover, Burki et al. (2005) showed that intravenous adenosine induced dyspnoea in the absence of bronchospasm.
Evidence supports the hypothesis that some pulmonary responses to adenosine are secondary to sensory C-fibre nerve activation. The adenosine-induced bronchoconstriction in asthma appears to be due, in part, to neuronal reflex mechanisms (Polosa et al. 1992; Meade et al. 1996; Meade et al. 2001). In rats, adenosine evokes classic pulmonary C-fibre associated reflexes including apnoea, bradycardia and hypotension (Kwong et al. 1998). In addition, right atrial injection of adenosine leads to overt activation (Hong et al. 1998) and increases in excitability (Gu et al. 2003) of capsaicin-sensitive C-fibres in the rat lung.
Vagal C-fibres in guinea pig lungs can be subdivided into those that are derived from neurons situated in the nodose ganglia and those derived from neurons in the jugular ganglia (Undem et al. 2004). The intrapulmonary nodose C-fibres can be distinguished from intrapulmonary jugular C-fibres based on their embryological origins (placode and neural crest, respectively), as well as their neurochemical and pharmacological properties. Circumstantial evidence indicates that the placodal nodose C-fibres may be analogous to the so-called ‘pulmonary’ C-fibres according to the classification scheme proposed by the Coleridges (Coleridge & Coleridge, 1977; Undem et al. 2004). In addition to C-fibres, vagal sensory nodose ganglion nerves in guinea pig pulmonary tissue include slowly adapting stretch receptors (SARs) and rapidly adapting stretch receptors (RARs) and tracheal ‘touch-sensitive’ fibres (Canning et al. 2004). The direct effect of adenosine on the various subtypes of guinea pig vagal afferent nerves is unknown.
Four adenosine receptor subtypes, namely A1, A2A, A2B and A3, have been cloned in mammals (Palmer & Stiles, 1995; Ralevic & Burnstock, 1998; Fredholm et al. 2001). Electrophysiological and histological studies indicate that vagal sensory (nodose) ganglion neurons express functional A1 and A2A receptors (Castillo-Melendez et al. 1994; Lawrence et al. 1997). Studies in rat lungs indicate that the adenosine-induced activation of lung C-fibres is blocked by an A1 receptor antagonist (Hong et al. 1998; Kwong et al. 1998). In the present study we evaluated the response of vagal sensory nerve subtypes within guinea pig isolated lungs to adenosine. In addition we set out to characterize the adenosine receptor subtypes involved in vagal sensory nerve activation in this tissue.
Methods
All experiments were approved by the Johns Hopkins Animal Use and Care Committee.
Extracellular recording of action potentials
Male Hartley guinea pigs (Hilltop Laboratory Animals Inc., Scottsdale, PA, USA) weighing 100–200 g were intraperitoneally injected with anticoagulant heparin (2000 IU kg−1; diluted in saline 1000 IU ml−1) 20 min before killing with CO2 inhalation and exsanguination. Heparin prevents blood clot formation and was used to improve blood removal from the pulmonary circulation. The blood from the pulmonary circulation was washed out by in situ perfusion with Krebs bicarbonate solution (KBS); composed of (mm): 118 NaCl, 5.4 KCl, 1.0 NaH2PO4, 1.2 MgSO4, 1.9 CaCl2, 25.0 NaHCO3, 11.1 dextrose, gassed with 95% O2–5% CO2 (pH 7.4). Trachea and right lungs with intact right-side extrinsic vagal innervation including right jugular and nodose ganglia were dissected and placed in a two-compartment tissue bath. The right nodose and jugular ganglia along with rostral vagus nerve were placed in one compartment, lung and trachea in the second compartment. The two compartments were separately superfused with KBS (6 ml min−1, 37°C) containing indomethacin (3 μm). Indomethacin reduces the indirect influence of tissue prostanoids on C-fibre activation. The pulmonary artery and trachea were cannulated with PE tubing and continuously perfused with KBS (4 ml min−1 and 2 ml min−1, respectively). The tracheal perfusion pressure reflecting the airway smooth muscle contraction was measured with a pressure transducer (P23AA, Statham, Hata Rey, PR, USA) and the pressure was recorded by a chart recorder (TA240, Gould, Valley View, OH, USA). Prior to the perfusion, 10 punctures with a 26-gauge needle were made throughout the surface of the lung. The perfusing buffer solution therefore exits the lungs via both these puncture ports and the pulmonary veins.
The single nerve activity was recorded with glass microelectrodes pulled with micropipette puller model P-87 (Sutter Instrument Company, Novato, CA, USA) and filled with 3 m sodium chloride (resistance ∼2 MΩ). The signal was amplified (Microelectrode AC amplifier 1800, A-M systems, Everett, WA, USA), filtered (low cut off, 0.3 kHz; high cut off, 1 kHz), displayed on an oscilloscope (TDS 340, Tektronix, Beaverton, OR, USA) and a chart recorder (TA240), and recorded (sampling frequency 33 kHz) onto a Macintosh computer for offline analysis (software: TheNerveOflt; PHOCIS, Baltimore, MD, USA).
The recording electrode was manipulated into either nodose or jugular ganglion. A mechanosensitive receptive field was identified when a mechanical stimulus (Von Frey hair, 1800–3000 mN) bluntly applied to the lung surface evoked a burst of action potentials. Once a mechanosensitive receptive field was identified, a brief (< 1 ms) electrical stimulus was delivered by a small concentric electrode positioned over this discrete mechanosensitive region to determine the conduction velocity of the fibre. The receptive field was stimulated electrically with a square pulse (0.5 ms) of increasing voltage (starting at 5 V) until an action potential was evoked. Conduction velocity was calculated by dividing the distance along the nerve pathway by the time between the shock artifact and the action potential evoked by electrical stimulation of the mechanosensitive receptive field. In the vast majority of our experiments we were able to record the activity of a single neuron. On those rare occasions where two units were recorded simultaneously, wave analysis software (TheNerveOflt; PHOCIS) was used to distinguish between the two peaks. This method has been previously described (Undem et al. 2004).
Nerve fibres with conduction velocities of < 1 m s−1 were considered to be C-fibres based on our previous analysis of the conduction velocity of vagal compound action potentials (Canning & Undem, 1993; Riccio et al. 1996). Each agonist was diluted to its final concentration in KBS and infused simultaneously into both the tracheal and pulmonary artery circuit in a volume of 1 ml at a rate of 50 μl s−1. Adenosine, selective A1 receptor agonists CPA (N6-cyclopentyladenosine) and CCPA 2-chloro-N6-cyclopentyladenosine, and the selective A2A receptor agonist CGS 21680 (2-p-(2-carboxyethyl)phenethyl-amino-5′-N-ethylcarboxamidoadenosine hydrochloride) were used to characterize adenosine receptor subtype(s). Capsaicin added at the end of the experiment provided a positive ‘control’ response (virtually all vagal C-fibres were responsive to capsaicin). The fibre was considered unresponsive to a particular agonist when administration of an agonist failed to evoke a response (less than 2-fold over baseline), but a positive ‘control’ response could be obtained.
The selective A1 receptor antagonist DPCPX (1,3-dipropyl-8-cyclopentylxanthine) and/or the selective A2A receptor antagonist SCH 58261 (7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazolo-[4,3-e]-1,2,4-triazolo[1,5-c]pyrimidine) was infused for 30 min into the lungs simultaneously via tracheal and pulmonary artery perfusion prior to agonist administration. Neither adenosine antagonist nor the vehicle administration evoked action potential discharge in either nodose or jugular C-fibres.
Gramicidin-perforated patch clamp recording
Gramicidin-perforated patch-clamp recordings from the nodose and jugular neurons retrogradely labelled from the lungs were performed. The retrograde tracer DiI (0.2%, 300 μl; dissolved in 10% dimethyl sulfoxide (DMSO) diluted in saline) was instilled into surgically exposed rostral trachea under anaesthesia (ketamine 50 mg kg−1 and xylazine 2.5 mg kg−1 intramuscular injection). Seven to nine days later, the nodose and jugular ganglia were harvested, dissociated with enzymes (collagenases type I (Sigma-Aldrich Co, St Louis, MO, USA) and dispase II (Boehringer Mannheim, Mannheim, Germany), both 1 mg ml−1 in Ca2+ and Mg2+-free Hanks' balanced salt solution) for 2 h at 37°C. The neurons were dissociated by trituration, washed and suspended in Leibovitz's L-15 medium (Gibco BRL, Rockville, MD, USA) containing 10% bovine serum albumin and transferred onto poly d-lysine (0.1 mg ml−1, Sigma) coated circular glass coverslips. The coverslips containing neurons were incubated at 37°C for 2 h to allow the neurons to adhere to them and then the debris and floating neurons were flooded out with 2 ml of serum containing L-15 medium. The neurons adhering on coverslips were used for the study within 24 h.
Patch clamp recordings were performed with DiI-labelled neuronal cells isolated from the jugular or nodose ganglion as described before (Undem et al. 2004; Lee et al. 2005). The labelled cells were identified under a fluorescence microscope equipped with a filter set of 500–570 nm excitation band and 555–655 nm emission band. Gramicidin-perforated patch clamp recordings in current clamp mode were carried out using a Multiclamp 700A amplifier and the Axograph 4.9 program (Axon Instruments, Union City, CA, USA). Gramicidin is a polypeptide antibiotic that forms pores in the membrane which allows only ions to pass through the patch pipette-attached membrane. Gramicidin was dissolved in DMSO and added into pipette solution with a final concentration of 1 μg ml−1 (containing 0.1% DMSO). Since gramicidin activity decreases over time, the pipette solution containing gramicidin was used within 1 h for the optimum activity. The patch pipette (1.5–3 MΩ) pulled with micropipette puller model P-87 (Sutter Instrument Co., Novato, CA, USA) was filled with the pipette solution containing gramicidin (1 μg ml−1), KCL (140 mm), CaCl2 (1 mm), MgCl2 (2 mm), EGTA (11 mm), Hepes (10 mm) and dextrose (10 mm) titrated to pH 7.3 with KOH (304 mosmol l−1). The cells were continuously superfused (6 ml min−1) with Locke's solution (35°C); composed of (mm): 136 NaCl, 5.6 KCl, 1.2 MgCl2, 2.2 CaCl2, 1.2 NaH2PO4, 14.3 NaHCO3 and 10 dextrose gassed with 95% O2–5% CO2 (pH 7.3–7.4). After forming a gigaohm seal, the membrane potential was held at −60 mV and the pipette capacitance was measured. It usually takes 10–20 min for gramicidin to make pores in the patched membrane. We started the recordings when series resistance (Rs) became less than 20 MΩ. The membrane properties were measured by using the ‘Test Membrane’ protocol of Axograph 4.9 software. During current clamp mode, the pipette capacitance was neutralized by 90% of measured value and Rs was compensated using bridge balance by 60% of measured Rs. The membrane current was held at 0 pA and the membrane potential change was measured at 2 kHz sampling rate (1 kHz of bessel filter applied). Adenosine (10 μm), CCPA (0.1 μm), CGS 21680 (0.1 μm) and capsaicin (0.3 μm) were diluted in Locke's solution. Drugs were applied directly into the recording bath (∼200 μl bath volume) for 30 s by fast switching the perfusion flow to the drug-containing Locke's solution. For each experiment adenosine, CCPA or CGS 21680 was chosen for the study and capsaicin was added at the end of the experiment. Since virtually all C-fibre neurons selectively respond to capsaicin, only lung-labelled capsaicin-sensitive neurons were included in the study. The interval between each drug application was at least 5 min for the cell to recover.
RT-PCR and single cell RT-PCR
RNA isolation and RT-PCR
The RNA from nodose and jugular ganglia was isolated using TRISOL reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions and treated with DNAse I (Ambion, Austin, TX, USA). Specific primers were designed based on the sequence of the guinea pig β-actin (accession number AF508792; forward primer: 259–278, GATCTGGCACCACACCTTTT; reverse primer: 505–523 GGCGTAGCCTCGTAGATG; product length 265 bp), the adenosine A1 receptor (accession number U04279; forward primer: 718–737 CATTGGGCCACAGACCTACT; reverse primer: 925–944, AACCAAACATAGGCGTCAGG; product length 227 bp) and the adenosine A2A receptor (accession number D63674; forward primer: 200–218, CATCCCCTTCGCTATCACC; reverse primer: 647–666 GCTGGCTTTCCATTTGTTTC; product size: 467). The reverse transcription was performed using SuperScript III reverse transcriptase (Invitrogen) according to the manufacturer's instructions. Briefly, 100–200 ng of total RNA was denaturated, primed with random hexamers (2.5 ng μl−1) and reverse transcribed for 50 min at 50°C. The reverse transcriptase or template RNA was omitted in the negative control experiments. The reaction product was then used for PCR amplification of each target sequence (β-actin, A1, A2A). After initial denaturation (94°C, 2 min), the cDNA was amplified by 30 cycles of denaturation at 94°C for 30 s, annealing at 53°C for 30 s, and extension at 72°C for 30 s followed by final elongation at 72°C for 10 min using Platinum Taq polymerase (Invitrogen). Products were visualized in ethidium bromide-stained 2% agarose gels or using FlashGel System (1.2% agarose) (Cambrex Bioscience Rockland Inc., Rockland, ME, USA).
Single cell RT-PCR
Single cell RT-PCR was performed using the SuperScript III CellsDirect kit (Invitrogen) according to the manufacturer's instructions. As described above for the patch clamp recording experiments, the vagal sensory ganglia neurons (nodose, jugular) were enzymatically dissociated, plated on the poly d-lysine-coated coverslips, and constantly superfused with Locke's solution (4–6 ml min−1), and the neurons retrogradely labelled from the lung and airway were identified using fluorescence microscopy. A single cell was harvested into the glass pipette (tip diameter 50–150 μm) by applying negative pressure, the pipette tip was broken in the PCR tube containing resuspension buffer (1 μl) with RNAse inhibitor (RNAseOUT, 2 U μl−1), immediately snap-frozen and stored in liquid nitrogen. From one coverslip, one to four labelled cells were collected. The sample of bath solution (1–3 μl) from the vicinity of a labelled neuron was also collected from each coverslip for no-template experiments (negative control). Later, the samples were defrosted, lysed (10 min at 75°C), treated with DNAse I (Invitrogen) and reverse transcribed. RT was omitted in the negative control experiments. Aliquots (2 μl) of the reaction product were then used for PCR amplification of guinea pig β-actin and adenosine A1 receptor, and of β-actin and adenosine A2A receptor. After initial denaturation (94°C, 2 min), the cDNA was amplified by 45 cycles of denaturation at 94°C for 30 s, annealing at 53°C for 30 s, and extension at 72°C for 30 s followed by final elongation at 72°C for 10 min using Platinum Taq polymerase (Invitrogen). Samples with superfusing fluid as a template and template from RT-free reactions were used as negative controls. Products were visualized in ethidium bromide-stained 2% agarose gels or using FlashGel System (1.2% agarose) (Cambrex).
Drugs and chemicals
In the extracellular recording study, adenosine and 2-chloro-N6-cyclopentyladenosine (CCPA) were dissolved in distilled water to concentrations of 10 mm and 1 mm, respectively. N6-cyclopentyladenosine (CPA), CGS 21680, DPCPX and SCH 58261 were dissolved in DMSO making a concentration of 10 mm. Adenosine was made fresh on the day of the experiment, and the other drugs were kept at −20°C and diluted with KBS into the assigned concentration on the day of experiment. All drugs were purchased from Sigma-Aldrich Co. (St Louis, MO, USA).
Data analysis
All the activity evoked by a given concentration of agonist was recorded in 1 s bins and analysed off-line. A response was defined as the action potential discharge after agonist administration that was higher than 2 times the baseline activity. The response to a given stimulus was deemed to have terminated when the action potential discharge ceased or at such time that the discharge returned to the defined threshold level (≤ 2 times that observed at baseline). The total number of action potentials and the peak frequency over the baseline was counted from fibres that responded. The data are expressed as means ±s.e.m. The analysis used Student's paired or unpaired t test for the response fibres from the same or different fibres, respectively. P < 0.05 was considered to be significant.
Results
Extracellular recordings of action potentials
C-Fibres
The conduction velocities of vagal bronchopulmonary C-fibres derived from nodose and jugular ganglia averaged 0.62 ± 0.03 m s−1 (n = 25) and 0.59 ± 0.05 m s−1 (n = 9), respectively. Both nodose and jugular C-fibres similarly responded to capsaicin (1 μm) with the action potential discharge of peak frequencies (maximum spikes per any 1 s bin) averaging 13 ± 2 Hz (n = 18), and 10 ± 2 Hz (n = 9), respectively (P > 0.1).
Administration of adenosine (10 μm, 1 ml perfused over 20 s) into the lungs did not change the tracheal perfusion pressure suggesting that adenosine did not appreciably contract bronchial smooth muscle. For comparison, methacholine (10 μm) caused a rapid increase in tracheal perfusion pressure that averaged 68 ± 19 cmH2O above the baseline (n = 3).
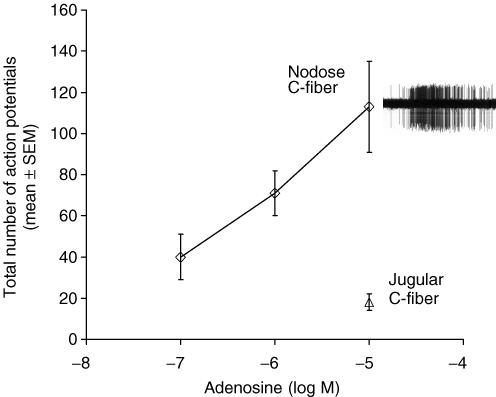
Adenosine evoked action potential discharge in 12/13 intrapulmonary nodose C-fibres in a concentration-dependent manner (Fig. 1). The onset from the time the drug reached the tissue to action potential discharge averaged 16 ± 2 s, and persisted an average of 45 ± 5 s, at which time the action potential discharged either ceased, or diminished to a frequency within 2 times baseline activity. The number of action potentials evoked by adenosine (10 μm, 1 ml) averaged 113 ± 22 with a peak frequency averaging 6 ± 1 Hz, n = 12.
Figure 1. Concentration–response curve of adenosine in vagal nodose C-fibres in the guinea pig lungs.
Nodose C-fibres responded to adenosine in a concentration-dependent manner (n = 5–12), whereas the jugular C-fibres did not respond (6 of 9) or poorly responded (3 of 9) to 10 μm adenosine. The potencies should be interpreted in light of the fact that the agonists were perfused for 20 s and were unlikely to have reached equilibrium conditions. Inset, representative trace of an extracellular recording of action potential shows discharge from a nodose C-fibre responding to 10 μm adenosine.
By contrast to the nodose C-fibres, adenosine (10 μm) caused little action potential discharge in jugular C-fibres (zero response in 6 fibres, slight response in 3 fibres, Fig. 1). In three experiments in which 10 μm adenosine had no effect on the intrapulmonary jugular C-fibre under study, increasing the concentration of adenosine to 30 μm also failed to activate the C-fibres. In separate experiments we found that adenosine (10 μm) also failed to activate (zero action potentials) extrapulmonary jugular C-fibres with nerve terminals located in the isolated trachea, whereas these fibres were effectively stimulated by capsaicin (n = 3).
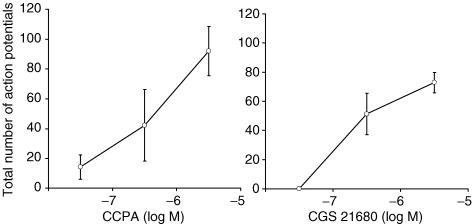
The selective adenosine A1 receptor agonist CPA (10 μm) mimicked the effect of adenosine in evoking action potential discharge in 4 of 4 nodose C-fibres with the total number of action potentials averaging 69 ± 9. CCPA is an agonist that is even more selective than CPA for the A1 receptor subtype. We found that CCPA (1 μm) also evoked action potential discharge in nodose C-fibres (Fig. 2A).
Figure 2. Concentration–response curves of the selective A1 receptor agonist CCPA (n = 4) and the selective A2A receptor agonist CGS 21680 (n = 5) for action potential discharge in vagal bronchopulmonary nodose C-fibres in guinea pigs.
Each point represents the mean ± s.e.m.
That CCPA was acting selectively on adenosine A1 fibres is supported by the observation that the response to CCPA (1 μm) was abolished by the adenosine A1 receptor antagonist DPCPX (0.1 μm), but was not inhibited by a concentration of SCH 58261 (0.1 μm) that is expected to be selective for the adenosine A2A receptor antagonist (control, 72 ± 10; SCH 58261, 67 ± 12; SCH 58261 + DPCPX, 1 ± 1, P < 0.05 compared with control; n = 6).
The selective adenosine A2A receptor agonist CGS 21680 (1 μM) also mimicked the effect of adenosine in evoking action potential discharge in nodose C-fibres (Fig. 2B). That CGS 21680 was acting selectively on A2A receptors to evoke the response is supported by the findings that responses were unaffected by a selective concentration of DPCPX (0.1 μm), but was abolished by SCH 58261 (0.1 μm) (control, 99 ± 20; DPCPX, 177 ± 41; DPCPX + SCH 58261, 25 ± 17, P < 0.05 compared with control; n = 6).
In most studies, a fibre was used to investigate either the selective A1 or A2A receptor agonist, but in two experiments, both the selective A1 agonist CCPA and the selective A2A agonist CGS 21680 were studied in the same fibre. In both experiments we found that the same nodose C-fibre responded robustly to both the A1 and A2A agonist.
Based on the data presented above, we chose 0.1 μm DPCPX and 0.1 μm SCH 58261 to selectively block the A1 receptors and the A2A receptors, respectively, and then analysed the effect of 10 μm adenosine. This concentration of adenosine was chosen as it provides a consistent but not a supramaximal response with respect to evoking action potential discharge in nodose C-fibres (Fig. 1). Based on published affinity constants, these selective antagonists should cause ∼2–3 log rightward shift in the adenosine concentration–response curve if only the respective receptor type was involved (i.e. we would expect to see near abolition of the response of 10 μm adenosine). We found that neither DPCPX nor SCH 58261 alone was able to abolish the action potential discharge evoked by adenosine (10 μm); however, the combination of the two antagonists caused ∼90% blockage (Table 1).
Table 1.
The ability of the selective A1 receptor antagonist DPCPX and the selective A2A receptor antagonist SCH 58261 to inhibit the action potential discharge from vagal nodose C-fibres evoked by adenosine in the guinea pig lungs: mean ±sem total number of action potentials evoked by adenosine (10 μm)
| Antagonist | Control | Treatment | % inhibition |
|---|---|---|---|
| DPCPX (n = 5) | 116 ± 26 | 69 ± 12 | 30 ± 13%* |
| SCH 58261 (n = 4) | 101 ± 24 | 46 ± 17 | 56 ± 11%* |
| DPCPX & SCH 58261 (n = 8) | 112 ± 19 | 8 ± 5 | 90 ± 6%* |
P < 0.01 when comparing the difference between control and treated values.
A-fibres
A total of five intrapulmonary nodose stretch-sensitive A-fibres with conduction velocities of 12 ± 2 m s−1 were evaluated for their responsiveness to adenosine. None of these fibres responded with action potential discharge in response to adenosine (10 μm). All of these fibres responded to lung distention and to smooth muscle contraction evoked by methacholine (10 μm). Some of the characteristics of these intrapulmonary stretch receptors have recently been described elsewhere (Canning et al. 2004).
Adenosine receptor mRNA expression studies
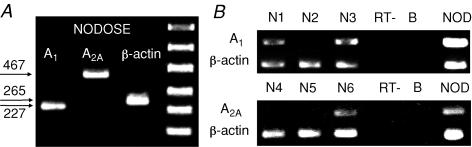
Cell suspensions were obtained from enzymatically dissociated nodose ganglia. In three separate experiments we noted that nodose ganglion cells expressed both adenosine A1 and A2A receptor mRNA (Fig. 3A). To more critically examine whether the receptor mRNA was expressed specifically in neurons projecting fibres to the lungs, we evaluated mRNA expression in single nodose ganglion neurons retrogradely labelled from the lungs. A total 34 neurons from 10 ganglia were studied. We detected the mRNA transcripts for the adenosine A1 and A2A receptors in a subset of individual nodose neurons (Fig. 3B). In total we found A1 receptor mRNA in 9/15 retrogradely labelled nodose neurons, and A2A receptor mRNA in 6/12 lung-specific nodose neurons. In another set of experiments coexpression of A1 and A2A receptors was investigated. Out of seven lung-specific nodose neurons studied from two ganglia, simultaneous expression of A1 and A2A was noted in two neurons, while one neuron expressed A1 only, and two neurons A2A only.
Figure 3.
A, RT-PCR of adenosine A1 and A2A receptors in nodose and jugular ganglia. Three distinct bands (227 bp, 467 bp and 265 bp) represent A1, A2A and β-actin (positive control), respectively. Omission of reverse transcriptase or the template resulted in no amplification product (not shown). B, single cell RT-PCR of adenosine A1 and A2A receptors in individual nodose ganglion neurons retogradely labelled from the lungs. N1–N6 represent six individual lung-specific nodose neurons. In this experiment, A1 receptor expression was detected in 2 of 3 neurons, whereas A2A receptor was expressed in 1 of 3 neurons. Omission of the reverse transcriptase (RT-, n = 5) or using a sample of the bath solution as the template (B, n = 6) resulted in no amplification products (negative controls). NOD = total RNA from multiple nodose neurons (positive control).
Gramicidin-perforated patch clamp studies
The gramicidin-perforated patch clamp technique was used to evaluate the responsiveness of 42 vagal sensory neurons retrogradely labelled from the lungs to adenosine A1 and A2A receptor agonists. A response to an adenosine receptor agonist was quantified, in current clamp mode, as a change in membrane potential. Capsaicin sensitivity, determined at the end of the experiment, was used as a means to detect the nociceptive nature of the neuron.
Lung-specific, capsaicin-sensitive nodose neurons
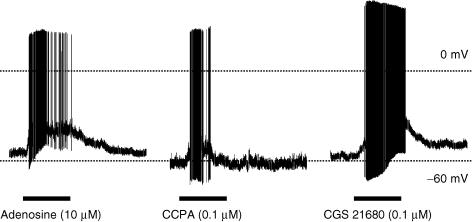
We evaluated nine lung-specific, capsaicin-sensitive nodose neurons for their response to the adenosine A1 selective agonist CCPA (0.1 μm). CCPA stimulated 6 of 9 neurons. The CCPA-induced membrane depolarization, 16 ± 2 mV, reached threshold for action potential discharge occurring in all six neurons. An additional seven lung-specific, capsaicin-sensitive neurons were evaluated for their response to the adenosine A2a selective agonist CGS 21680 (0.1 μm). Four of these seven neurons responded with a robust depolarization (22 ± 6 mV) that reached action potential threshold. As in the extracellular recording of action potentials arising from the nerve terminals, we found that both CGS 21680 and CCPA were capable of stimulating the same neuron. An example of adenosine-, CPPA- and CGS 21680-induced membrane depolarization of current clamped nodose neurons labelled from the lungs is shown in Fig. 4.
Figure 4. Representative action potential discharge evoked by adenosine (10 μm), the selective A1 receptor agonist CCPA (0.1 μm) and the selective A2A receptor agonist CGS 21680 (0.1 μm) in gramicidin-perforated patch clamp recordings in current clamp mode of lung-labelled capsaicin-sensitive nodose neurons.
Filled bars under the tracings are the duration for the perfusion of each agonist.
Lung-specific, capsaicin-sensitive jugular neurons
A total of 10 lung-specific, capsaicin-sensitive, jugular neurons were studied. CCPA (0.1 μm) evoked membrane depolarizations that averaged 11 mV in 2 of 5 labelled jugular neurons, and CGS 21680 (0.1 μm) evoked membrane depolarizations (7 ± 3 mV) in 3 of 5 labelled jugular neurons.
Discussion
Our results demonstrate that adenosine evokes action potential discharge in vagal C-fibres in guinea pig lungs, but this effect is selective for C-fibres arising from nodose ganglion neurons. Adenosine fails to consistently evoke action potential discharge in jugular C-fibres or pulmonary stretch receptors in the lungs. The activation of nodose C-fibres is best explained by direct stimulation of both adenosine A1 and A2A receptors located in the nodose C-fibre terminals.
Vagal C-fibre sensory nerves in the guinea pig respiratory system can be subdivided into two phenotypically distinct subtypes based on the ganglionic location of their cell bodies (Riccio et al. 1996; Undem et al. 2004). The cell bodies of one subtype are situated in the nodose ganglia (derived from the embryonic placode), whereas the cell bodies of the other subtype are located in the jugular ganglia (derived from the embryonic neural crest). In addition to being different with respect to anatomical location and neuropeptide content (Undem et al. 2004), the nodose and jugular C-fibres in the lungs are different with respect to their responsiveness to chemical stimuli. The present data considered with previous work (Undem et al. 2004; Chuaychoo et al. 2005) indicate that nodose C-fibres and jugular C-fibres both respond to capsaicin and bradykinin, but the nodose phenotype is much more responsive than jugular C-fibres to 5-HT3 receptor agonists, purinergic P2X receptor agonists, and adenosine receptors agonists. The observation that adenosine activated pulmonary C-fibres is consistent with the findings of Hong et al. (1998), who found that, in rats studied in vivo, adenosine effectively evoked action potential discharge in C-fibres most accessible by the pulmonary circulation (‘pulmonary C-fibers’ based on the classification of Coleridge & Coleridge (1977).
The lack of effect of adenosine on pulmonary stretch receptors and jugular C-fibres is unlikely to be explained by diffusion barriers between the infused adenosine and the receptive fields of jugular C-fibre or stretch receptors. We found that jugular C-fibres and nodose C-fibres in the lung preparation equally responded to capsaicin (1 μm), and we have previously noted that the stretch receptors are strongly and directly stimulated by P2X receptor agonists using the same experimental design (Canning et al. 2004). Moreover, adenosine also failed to activate jugular C-fibres in the isolated trachea where the drug can be applied directly to the receptive field.
Adenosine induces several pulmonary reflexes consistent with C-fibre activation (Kwong et al. 1998; Keir et al. 2006). The present findings indicate that the extent to which capsaicin-sensitive nerves are involved in adenosine-induced pulmonary reflexes in guinea pigs, they are likely to be of the nodose C-fibre subtype. Adenosine causes dyspnoeic sensations in humans (Verani et al. 1990; Mosqueda-Garcia, 1992; Rankin et al. 1992; Cerqueira et al. 1994; Marks et al. 1996; Wilbur & Marchlinski, 1997). Recent studies have led to the hypothesis that this may involve the activation of pulmonary C-fibres (Burki et al. 2005). Since adenosine only activated nodose C-fibres (not jugular C-fibres or pulmonary stretch receptors), it is tempting to conclude that activation of nodose C-fibres in lungs may contribute to dyspnoeic sensations.
We found that adenosine did not change the tracheal perfusion pressure. This indicates that adenosine did not significantly induce airway smooth muscle contraction in our isolated nerve–lung preparation. This finding is consistent with other studies showing adenosine does not cause substantive bronchoconstriction in unsensitized guinea pig lungs (in vitro and in vivo studies) (Thorne & Broadley, 1992, 1994) and did not have any effect on airway calibres in normal mouse lung slices (Bergner & Sanderson, 2002). We have found that capsaicin causes a large increase in the tracheal perfusion pressure in this preparation, via the release of tachykinins acting on NK1 and NK2 receptors (Chuaychoo et al. 2005). That adenosine is capable of mimicking the action potential discharge evoked by capsaicin in nodose but not jugular C-fibres is consistent with the hypothesis that the jugular C-fibres, but not nodose C-fibres, are responsible for capsaicin evoked tachykinergic airway contraction in this species. This would be in agreement with our findings that the jugular C-fibre neurons contain tachykinins and project fibres that innervate the large airways (Undem et al. 2004). Lagente et al. (1997) have also noted that adenosine fails to evoke tachykinergic contractions in guinea pig isolated bronchi (Lagente et al. 1997).
The data support the following conclusions regarding the nature of the adenosine receptor subtypes involved in nodose C-fibre activation. First, capsaicin-sensitive nodose neurons retrogradely labelled from the lungs express both A1 and A2A receptor mRNA. Second, selective A1 and A2A receptor agonists are capable of evoking membrane depolarization in capsaicin-sensitive nodose neurons retrogradely labelled from the lungs. Third, either A1 or A2A receptor activation can lead to action potential discharge in nodose C-fibre terminals within the lungs. Although studies on single sensory nerve fibres were not subjected to rigorous quantitative pharmacological analysis, this conclusion is supported by the results from studies using receptor selective agonists and antagonists. We used DPCPX and SCH 58261 to selectively antagonize A1 and A2A receptors, respectively. DPCPX has a reported Ki of about 4 nm against A1 receptors and > 100 nm against A2A receptors; whereas SCH 58261 has a reported Ki against A2A of < 1 nm and > 100 nm against A1 receptors (see Fredholm et al. 2001). At a concentration of 1 μm the selective A1 agonist CCPA caused action potential discharge that was not inhibited by SCH 58261 when studied at a concentration capable of blocking the response to the A2A agonist. Likewise, the action potential discharge evoked by the A2A receptor selective agonist CGS 21680 was unaffected by a concentration of a DPCPX that blocked the response to the A1 selective agonist. Fourth, based on studies with selective concentrations of the abovementioned antagonists, the overall adenosine response of a given nodose C-fibre appears to be due to the combined result of A1 and A2A receptor activation. In the rat lung studied in vivo, the C-fibre discharge and the increases in excitability in response to adenosine were abolished by an A1 receptor antagonist, implicating a sole role for this receptor subtype in the response (Hong et al. 1998). This may reveal a species difference between rats and guinea pigs.
The hypothesis that adenosine evoked action potential discharge by interacting with receptors expressed in the C-fibre terminal per se, as opposed to an indirect mechanism of action, is supported by the RT-PCR results on receptor expression in single neurons and by the electrophysiological patch clamp studies on lung-specific nerve cell bodies. Nearly all nodose C-fibre terminals in the lungs responded to adenosine, yet only a subset of labelled nodose neurons expressed mRNA for adenosine receptors. This is likely to be explained by the fact that the retrogradely labelled neurons dissociated from the nodose ganglia and used in the RT-PCR studies represented both C-fibre and A-fibre neurons, and nodose A-fibres were found to be adenosine insensitive. These data support a direct mechanism of action for adenosine, but do not rule out the possibility that in the physiological setting, adenosine may also evoke the release of neuro-active mediators from non-neuronal cells that may amplify the adenosine response.
The patch clamp data indicate that some jugular neurons may express functional A1 and A2A receptors, even though adenosine failed to evoke action potential discharge in most jugular C-fibre terminals. It is possible that the density of receptors at the jugular terminals is insufficient to cause responses that reach action potential threshold. It is also possible that adenosine receptor stimulation may alter the function of respiratory jugular C-fibres in a manner other than overt activation. In this context it is perhaps noteworthy that neurokinin-containing fibres in guinea pig trachea are jugular C-fibres (Riccio et al. 1996) and both adenosine A1 and A2A receptor stimulation have been linked to inhibition of evoked neurokinin release from this tissue (Lagente et al. 1997).
Our results indicate that both A1 and A2A receptors coexist in nodose neurons that project C-fibres to the lungs. Coexisting A1 and A2 adenosine receptors have been reported in a number of studies on different cell types including smooth muscle cells, neutrophils, mesangial cells, and vagal afferent neurons. These two receptor subtypes are typically linked to different types of G-proteins and their activation within these cells is associated with opposing responses (Ramkumar et al. 1990; Mills & Gewirtz, 1990; Cronstein et al. 1992; Olivera et al. 1992; Castillo-Melendez et al. 1994). It is therefore somewhat surprising that these typically opposing receptor subtypes lead to membrane depolarization and action potential discharge in individual nodose C-fibres. It would seem likely that the signalling mechanisms by which A1 and A2A receptor stimulation lead to action potential discharge in nodose C-fibres are dissimilar. We have previously reported that stimulation of bradykinin B2 receptors (commonly linked with Gq proteins) leads to action potential discharge in tracheal vagal C-fibres via activating TRPV1 and an unidentified chloride channel (Lee et al. 2005). Whether adenosine A1 or A2A receptor activation shares this mechanism or uses yet other means to evoke generator potentials and action potential discharge in nodose C-fibres awaits further investigation.
Acknowledgments
This work was supported by the National Institute on Aging-Intramural Research Program and The National Heart Lung and Blood Institute of the National Institutes of Health.
References
- Basoglu OK, Pelleg A, Essilfie-Quaye S, Brindicci C, Barnes PJ, Kharitonov SA. Effects of aerosolized adenosine 5′-triphosphate vs adenosine 5′-monophosphate on dyspnea and airway caliber in healthy nonsmokers and patients with asthma. Chest. 2005;128:1905–1909. doi: 10.1378/chest.128.4.1905. [DOI] [PubMed] [Google Scholar]
- Bergner A, Sanderson MJ. ATP stimulates Ca2+ oscillations and contraction in airway smooth muscle cells of mouse lung slices. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1271–L1279. doi: 10.1152/ajplung.00139.2002. [DOI] [PubMed] [Google Scholar]
- Burki NK, Dale WJ, Lee LY. Intravenous adenosine and dyspnea in humans. J Appl Physiol. 2005;98:180–185. doi: 10.1152/japplphysiol.00913.2004. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol. 2004;557:543–558. doi: 10.1113/jphysiol.2003.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Undem BJ. Evidence that distinct neural pathways mediate parasympathetic contractions and relaxations of guinea-pig trachealis. J Physiol. 1993;471:25–40. doi: 10.1113/jphysiol.1993.sp019889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo-Melendez M, Krstew E, Lawrence AJ, Jarrott B. Presynaptic adenosine A2a receptors on soma and central terminals of rat vagal afferent neurons. Brain Res. 1994;652:137–144. doi: 10.1016/0006-8993(94)90327-1. [DOI] [PubMed] [Google Scholar]
- Cerqueira MD, Verani MS, Schwaiger M, Heo J, Iskandrian AS. Safety profile of adenosine stress perfusion imaging: results from the Adenoscan Multicenter Trial Registry. J Am Coll Cardiol. 1994;23:384–389. doi: 10.1016/0735-1097(94)90424-3. [DOI] [PubMed] [Google Scholar]
- Chuaychoo B, Lee MG, Kollarik M, Undem BJ. Effect of 5-hydroxytryptamine on vagal C-fiber subtypes in guinea pig lungs. Pulm Pharmacol Ther. 2005;18:269–276. doi: 10.1016/j.pupt.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JC. Impulse activity in afferent vagal C-fibres with endings in the intrapulmonary airways of dogs. Respir Physiol. 1977;29:125–142. doi: 10.1016/0034-5687(77)90086-x. [DOI] [PubMed] [Google Scholar]
- Cronstein BN, Levin RI, Philips M, Hirschhorn R, Abramson SB, Weissmann G. Neutrophil adherence to endothelium is enhanced via adenosine A1 receptors and inhibited via adenosine A2 receptors. J Immunol. 1992;148:2201–2206. [PubMed] [Google Scholar]
- Cushley MJ, Tattersfield AE, Holgate ST. Inhaled adenosine and guanosine on airway resistance in normal and asthmatic subjects. Br J Clin Pharmacol. 1983;15:161–165. doi: 10.1111/j.1365-2125.1983.tb01481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver AG, Kukoly CA, Ali S, Mustafa SJ. Adenosine in bronchoalveolar lavage fluid in asthma. Am Rev Respir Dis. 1993;148:91–97. doi: 10.1164/ajrccm/148.1.91. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Ap IJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Ruan T, Hong JL, Burki N, Lee LY. Hypersensitivity of pulmonary C fibers induced by adenosine in anesthetized rats. J Appl Physiol. 2003;95:1315–1324. doi: 10.1152/japplphysiol.00107.2003. discussion 1314. [DOI] [PubMed] [Google Scholar]
- Hong JL, Ho CY, Kwong K, Lee LY. Activation of pulmonary C fibres by adenosine in anaesthetized rats: role of adenosine A1 receptors. J Physiol. 1998;508:109–118. doi: 10.1111/j.1469-7793.1998.109br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszar E, Vass G, Vizi E, Csoma Z, Barat E, Molnar Vilagos G, Herjavecz I, Horvath I. Adenosine in exhaled breath condensate in healthy volunteers and in patients with asthma. Eur Respir J. 2002;20:1393–1398. doi: 10.1183/09031936.02.00005002. [DOI] [PubMed] [Google Scholar]
- Keir S, Boswell-Smith V, Spina D, Page C. Mechanism of adenosine-induced airways obstruction in allergic guinea pigs. Br J Pharmacol. 2006;147:720–728. doi: 10.1038/sj.bjp.0706663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong K, Hong JL, Morton RF, Lee LY. Role of pulmonary C fibers in adenosine-induced respiratory inhibition in anesthetized rats. J Appl Physiol. 1998;84:417–424. doi: 10.1152/jappl.1998.84.2.417. [DOI] [PubMed] [Google Scholar]
- Lagente V, Barlinski J, Cano E, Frossard N. Adenosine reduces airway excitatory non-cholinergic (e-NC) contraction through both A1 and A2 adenosine receptor activation in the guinea pig. Fundam Clin Pharmacol. 1997;11:494–500. doi: 10.1111/j.1472-8206.1997.tb00853.x. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Krstew E, Jarrott B. Complex interactions between nitric oxide and adenosine receptors in the rat isolated nodose ganglion. Eur J Pharmacol. 1997;328:83–88. doi: 10.1016/s0014-2999(97)83032-4. [DOI] [PubMed] [Google Scholar]
- Lee MG, Macglashan DW, Jr, Undem BJ. Role of chloride channels in bradykinin-induced guinea pig airway vagal C-fibre activation. J Physiol. 2005;566:205–212. doi: 10.1113/jphysiol.2005.087577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks GB, Yates DH, Sist M, Ceyhan B, De Campos M, Scott DM, Barnes PJ. Respiratory sensation during bronchial challenge testing with methacholine, sodium metabisulphite, and adenosine monophosphate. Thorax. 1996;51:793–798. doi: 10.1136/thx.51.8.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade CJ, Dumont I, Worrall L. Why do asthmatic subjects respond so strongly to inhaled adenosine? Life Sci. 2001;69:1225–1240. doi: 10.1016/s0024-3205(01)01231-0. [DOI] [PubMed] [Google Scholar]
- Meade CJ, Mierau J, Leon I, Ensinger HA. In vivo role of the adenosine A3 receptor: N6-2-(4-aminophenyl) ethyladenosine induces bronchospasm in BDE rats by a neurally mediated mechanism involving cells resembling mast cells. J Pharmacol Exp Ther. 1996;279:1148–1156. [PubMed] [Google Scholar]
- Mills I, Gewirtz H. Cultured vascular smooth muscle cells from porcine coronary artery possess A1 and A2 adenosine receptor activity. Biochem Biophys Res Commun. 1990;168:1297–1302. doi: 10.1016/0006-291x(90)91170-w. [DOI] [PubMed] [Google Scholar]
- Mosqueda-Garcia R. Adenosine as a therapeutic agent. Clin Invest Med. 1992;15:445–455. [PubMed] [Google Scholar]
- Olivera A, Tomas M, Lopez-Novoa JM. Effect of adenosine A1 and A2 agonists and antagonists on cAMP and Ca2+ in cultured rat mesangial cells. Am J Physiol. 1992;262:C840–C844. doi: 10.1152/ajpcell.1992.262.4.C840. [DOI] [PubMed] [Google Scholar]
- Palmer TM, Stiles GL. Adenosine receptors. Neuropharmacology. 1995;34:683–694. doi: 10.1016/0028-3908(95)00044-7. [DOI] [PubMed] [Google Scholar]
- Polosa R, Rajakulasingam K, Church MK, Holgate ST. Repeated inhalation of bradykinin attenuates adenosine 5′-monophosphate (AMP) induced bronchoconstriction in asthmatic airways. Eur Respir J. 1992;5:700–706. [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Ramkumar V, Barrington WW, Jacobson KA, Stiles GL. Demonstration of both A1 and A2 adenosine receptors in DDT1 MF-2 smooth muscle cells. Mol Pharmacol. 1990;37:149–156. [PMC free article] [PubMed] [Google Scholar]
- Rankin AC, Brooks R, Ruskin JN, McGovern BA. Adenosine and the treatment of supraventricular tachycardia. Am J Med. 1992;92:655–664. doi: 10.1016/0002-9343(92)90784-9. [DOI] [PubMed] [Google Scholar]
- Riccio MM, Kummer W, Biglari B, Myers AC, Undem BJ. Interganglionic segregation of distinct vagal afferent fibre phenotypes in guinea-pig airways. J Physiol. 1996;496:521–530. doi: 10.1113/jphysiol.1996.sp021703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne JR, Broadley KJ. Adenosine-induced bronchoconstriction of isolated lung and trachea from sensitized guinea-pigs. Br J Pharmacol. 1992;106:978–985. doi: 10.1111/j.1476-5381.1992.tb14445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne JR, Broadley KJ. Adenosine-induced bronchoconstriction in conscious hyperresponsive and sensitized guinea pigs. Am J Respir Crit Care Med. 1994;149:392–399. doi: 10.1164/ajrccm.149.2.8306036. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Chuaychoo B, Lee MG, Weinreich D, Myers AC, Kollarik M. Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol. 2004;556:905–917. doi: 10.1113/jphysiol.2003.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verani MS, Mahmarian JJ, Hixson JB, Boyce TM, Staudacher RA. Diagnosis of coronary artery disease by controlled coronary vasodilation with adenosine and thallium-201 scintigraphy in patients unable to exercise. Circulation. 1990;82:80–87. doi: 10.1161/01.cir.82.1.80. [DOI] [PubMed] [Google Scholar]
- Wilbur SL, Marchlinski FE. Adenosine as an antiarrhythmic agent. Am J Cardiol. 1997;79:30–37. doi: 10.1016/s0002-9149(97)00261-0. [DOI] [PubMed] [Google Scholar]