Abstract
In many developing neuronal cell types, the resting membrane potential is relatively depolarized, then gradually hyperpolarizes during the early postnatal period. The regulatory roles of membrane potential changes in neuronal development and maturation have been extensively studied in developing cerebellar granule cells, using primary culture under depolarizing and non-depolarizing conditions in combination with in vivo analysis. Depolarization enhances calcium entry via voltage-sensitive Ca2+ channels (VSCCs) and activates Ca2+–calmodulin-dependent protein kinase (CaMK) and calcineurin phophatase (CaN). The activation of CaN induces many genes encoding extracellular and intracellular signalling molecules implicated in granule cell development. The inactivation of CaN in turn up-regulates many other genes characteristic of mature granule cells, including NR2C NMDA receptor and GABAAα1 and α6 receptors. The induction of NR2C also requires CaMK-up-regulated brain-derived neurotrophic factor (BDNF), indicating a convergence of signalling mechanism of the CaMK and CaN cascades. The inactivation of CaN maintains the phosphorylated and sumoylated form of a transcriptional myocyte enhances factor 2A (MEF2A) regulator. This form of MEF2A acts as a transcriptional repressor and is essential for the dendritic morphogenesis of differentiated granule cells. Collectively, the membrane potential change and the resulting Ca2+ signalling play a pivotal role in development and maturation of neuronal cells.
Neuronal cell development is controlled by a highly organized sequence of developmental events that consist of proliferation, differentiation, migration and maturation (Wang & Zoghbi, 2001; West et al. 2002). Each step in the developmental sequence results from an interplay between extracellular signalling and intracellular signalling. This intercellular communication is controlled not only by extracellular signalling molecules but also by the intrinsic responsiveness of neuronal cells. In many neuronal cells, the resting membrane potential has been shown to shift from a relatively depolarized state to a more hyperpolarized state at the early postnatal period. For example, the lateral geniculate nucleus (LGN) dramatically changes its morphology and exhibits extensive modifications in its circuit formation during the early postnatal period. Electrophysiological studies demonstrated that immature LGN relay neurons display relatively depolarized resting membrane potential (about −45 mV) with high input resistances and long membrane time constants, which becomes gradually more negative (about −60 mV) during the postnatal period (Ramoa & McCormick, 1994). Similar changes in resting membrane potentials have been reported in developing layer 1 cortical neurons and in many other neurons (Zhou & Hablitz, 1996; Tyzio et al. 2003, and references therein). Although there is an argument against the existence of a depolarized membrane potential in immature hippocampal pyramidal neurons (Tyzio et al. 2003), changes in membrane potentials should have a great influence on neuronal cell excitability and intracellular Ca2+ signalling in development and maturation of neuronal cells. However, little attention has been paid to involvement of altering intrinsic membrane properties in controlling neuronal cell development and maturation. In this article, we discuss how membrane potential-regulated Ca2+ signalling is involved in development and maturation of cerebellar granule cells.
Regulation of gene expression in cerebellar granule cells by membrane potential
The cerebellum develops by a hierarchical series of developmental events after birth (Hatten & Heintz, 1995) (Fig. 1). In the process of granule cell development, the resting membrane potential has been reported to gradually decrease from −25 mV in immature cells to −55 mV in mature cells (Rossi et al. 1998). In primary culture, granule cells are highly enriched and show many properties characteristic of developing granule cells in vivo (Gallo et al. 1987; Sato et al. 2005). Furthermore, the membrane potential of cultured granule cells is controlled by changing external KCl concentrations, being −35 mV with high KCl (25 mm) and −50 mV with low KCl (5 mm) (Mellor et al. 1998). Depolarization of granule cells enhances calcium entry (more than 3-fold) via voltage-sensitive Ca2+ channels (VSCCs) (Suzuki et al. 2005). This calcium entry activates Ca2+–calmodulin-dependent protein kinase (CaMK) and calcineurin phosphatase (CaN) (West et al. 2002) and mimics signalling mechanisms of developing granule cells.
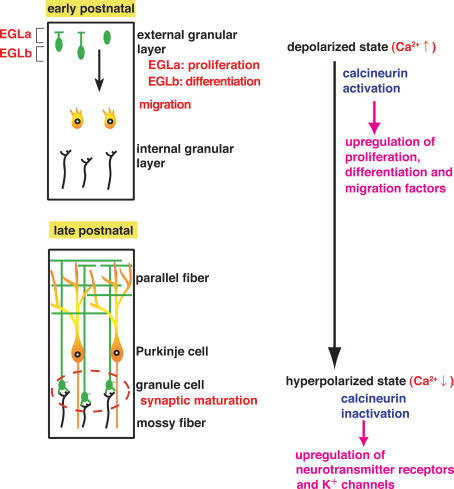
Figure 1. Development and maturation of cerebellar granule cells and a model of regulation of gene expression of developing granule cells.
Granule cells proliferate at the outer part of EGL (EGLa) and differentiate at the inner part of EGL (EGLb). These cells migrate inward and form synaptic connections at IGL. The resting membrane potential has been shown to be relatively high in immature EGL granule cells and become more negative in mature IGL granule cells. Many genes are up-regulated in immature EGL granule cells by the CaN activation and in turn down-regulated in mature IGL granule cells. The CaN inactivation up-regulates many other genes involved in synaptic transmission and modulation of mature IGL granule cells.
The genome-wide expression profiles of depolarization-responsive and CaN-responsive genes were investigated by microarray analysis of cultured granule cells under three different conditions: low KCl, high KCl and high KCl in the presence of the CaN inhibitor FK506 (Sato et al. 2005). The relevance of the identified genes to developmentally regulated genes in vivo was then analysed. These studies demonstrated that membrane potential-dependent changes in intracellular Ca2+ signals control gene expression in immature and mature granule cells via CaN signalling in distinct ways (Sato et al. 2005). CaN activation induces many genes implicated in cell proliferation, differentiation, migration and neurite growth of immature granule cells in the external granular layer (EGL). In contrast, the inactivation of CaN predominantly up-regulates genes encoding functional molecules involved in synaptic transmission and modulation of differentiated granule cells in the internal granular layer (IGL). The latter includes the α1 and α6 subunits of GABAA receptors, the NR2C subunit of NMDA receptors, the TASK1 K+ channel and the KCC2 co-transporter, all of which have been shown to be critical for synaptic transmission in mature granule cells (Sato et al. 2005). CaN signalling thus controls both development and maturation of granule cells during the postnatal period.
Receptor expression in synaptic maturation of granule cells
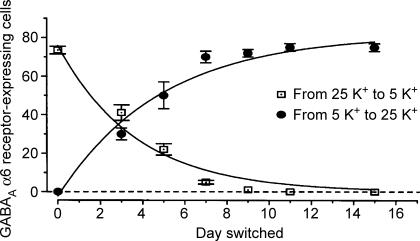
Switching of subunit composition of neurotransmitter receptors is a hallmark of the maturation of synaptic transmission. The regulatory mechanisms of expression of GABAA receptors and NMDA receptors were extensively studied in granule cells cultured in low and high KCl (Thompson et al. 1996; Vallano et al. 1996; Gault & Siegel, 1997; Jones et al. 1997; Brandoli et al. 1998; Lin et al. 1998; Mellor et al. 1998, 2000; Xie et al. 2004; Sato et al. 2005, 2006). In the cerebellum, the α2, α3, β3, γ1 and γ2 subunits of GABAA receptors are expressed in proliferating/premigratory granule cells (Wisden et al. 1996). Later, α2, α3 and γ1 are down-regulated and α1, α6 and δ are markedly up-regulated when granule cells arrive at the IGL (Wisden et al. 1996). In cultures of mouse granule cells in high KCl, the α6 gene was expressed at low levels up to at least 15 days, whereas it was highly expressed in low KCl (Mellor et al. 1998). Interestingly, culture in high KCl for more than 3 days curtailed the ability to induce the α6 gene on transfer to low KCl (Fig. 2). When culture started in low KCl, granule cells still expressed the α6 gene in high KCl. It has been discussed that this regulatory switching of the α6 expression at a critical time point reflects the terminal differentiation program of granule cells. Interestingly, the δ expression is differently regulated by membrane potential in rat granule cell culture (Gault & Siegel, 1997). The δ mRNA increased in granule cells cultured in high KCl, but not in low KCl. Furthermore, the δ expression was markedly reduced when the culture conditions were switched from high KCl to low KCl. The up-regulation of the δ gene was inhibited by both the L-type VSCC inhibitor and CaMK inhibitor. The depolarization effect of the δ gene expression thus seems to mimic the excitatory state of differentiated granule cells in vivo.
Figure 2. Effects of switch of external KCl concentrations on the GABAAα6 gene expression in cultured granule cells.
Knock-in mice, in which the β-galactosidase (lacZ) transgenic expression was controlled by the GABAAα6 gene, were generated and granule cells were cultured by switching KCl concentrations either from 25 mm to 5 mm or from 5 mm to 25 mm at the indicated day. The expression of the GABAAα6 gene was measured by counting the number of lacZ-expressing granule cells in culture for 15 days. Data reproduced, with permission, from Mellor et al. (1998).
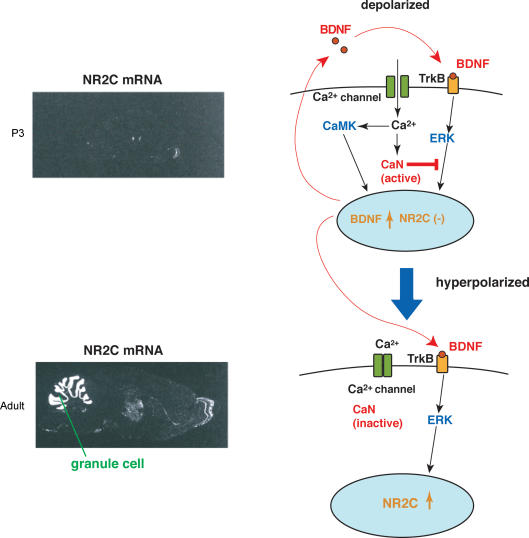
NMDA receptors are composed of a common NR1 subunit and distinct combinations of NR2 subunits (NR2A–NR2D) (Nakanishi, 1992). Switching of NR2B-containing receptors to NR2C-containing receptors is important for establishment of mature mossy fibre–granule cell transmission (Farrant et al. 1994). The mechanisms of depolarization-regulated NR2C expression were investigated in primary culture of mouse granule cells in combination with in vivo analysis (Suzuki et al. 2005) (Fig. 3). In granule cells in low KCl, BDNF up-regulated NR2C via the BDNF receptor (TrkB)-extracellular signal-regulated kinase (ERK) cascade, but failed to do so in cells cultured in high KCl. Upon switching to high KCl, the stimulation of L-type VSCCs activated CaN phosphatase and the resulting dephosphorylation blocked the TrkB/ERK-dependent NR2C mRNA up-regulation. The BDNF–TrkB–ERK pathway, however, has the potential to up-regulate NR2C when the calcium entry or CaN dephosphorylation is blocked under deporalizing conditions. Furthermore, the depolarization-induced Ca2+ increase simultaneously up-regulated BDNF via CaMK. Consequently, when CaN was inhibited by FK506 under depolarizing conditions, the endogenously up-regulated BDNF was capable of inducing NR2C via the common TrkB–ERK cascade. The importance of the BDNF–TrkB signal in vivo was verified by a significant reduction of NR2C in TrkB-deficient granule cells. These findings suggest that the depolarized membrane potential blocks the NR2C induction in immature granule cells via CaN, but the progressive reduction of membrane potential allows induction of NR2C in conjunction with the BDNF–TrkB–ERK signalling cascade. CaMK and CaN have been well documented to regulate intracellular signalling by phosphorylation and dephosphorylation of the same signalling target. Importantly, in the case of NR2C induction, CaMK and CaN act on different signalling cascades and ultimately converge for the regulation of NR2C gene expression.
Figure 3. A model of induction of the NR2C NMDA receptor.
Left, developmentally regulated NR2C mRNA expression in cerebellar granule cells, as analysed by in situ hybridization, is shown. Right, the BDNF–TrkB signal has the potential to induce NR2C expression through the common ERK cascade in both depolarizing and non-depolarizing conditions. In immature granule cells, the Ca2+–calmodulin-activated CaN inhibits the ERK cascade and blocks the induction of NR2C. The Ca2+–calmodulin-activated CaMK simultaneously up-regulates BDNF. Consequently, BDNF becomes operative in inducing NR2C when the calcium entry is reduced by hyperpolarization or blocked by the VSCC or CaN inhibitor.
A CaN signalling mechanism in granule cell maturation
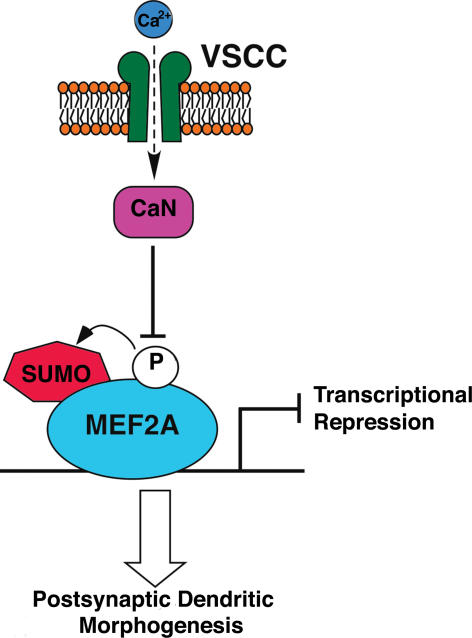
Understanding of the molecular mechanism of CaN-regulated granule cell maturation has been greatly advanced by the finding that a transcriptional repressor, myocyte enhancer factor 2A (MEF2A), is regulated by CaN dephosphorylation and is essential for the dendritic maturation of granule cells (Shalizi et al. 2006) (Fig. 4). MEF2A is progressively up-regulated in the IGL during development. MEF2A is not only phosphorylated but also secondarily modified by sumoylation, a post-translational modification with a sumo polypeptide covalently attached to a lysine residue. This phosphylated and sumoylated MEF2A primarily acts as a transcriptional repressor and promotes the synapse assembly. Importantly, it has been shown that L-type VSCC-dependent activation of CaN dephosphorylates and in turn non-sumoylates MEF2A and promotes synapse disassembly. The data of Shalizi et al. (2006) suggest that the Ca2+-activated CaN prevents the MEF2A-dependent synapse differentiation, and the progressive inactivation of CaN by hyperpolarization promotes synapse maturation by switching the unmodified MEF2A to the phophorylated and sumoylated transcriptional repressor. This mechanism was elucidated by both orgnotypic culture and in vivo analysis. Since the developmental expression of postsynaptic receptors parallels the dendritic morphogenesis, it is tempting to speculate that the CaN-regulated MEF2A serves as a key regulator in induction of mature postsynaptic receptors and ion channels.
Figure 4. A model of the MEF2A-mediated dendritic morphogenesis of granule cells.
The phosphorylated and sumoylated form of MEF2A acts as a transcriptional repressor that promotes synapse assembly. CaN dephosphorylates and in turn secondarily non-sumoylates MEF2A. The inactivation of CaN by hyperpolarization could thus play a key role in generating the phosphorylated and sumoylated form of MEF2A that leads to the dendritic morphogenesis of granule cells. Data reproduced, with permission, from Shalizi et al. (2006).
Future work
The membrane potential-regulated mechanisms of gene expression discussed here are mainly based on studies of cultured granule cells. It is thus important to provide compelling in vivo evidence that membrane potential shifts from a depolarized state to a hypolarized state in developing granule cells and this shifting influences the VSCC-mediated Ca2+ signalling during development. Granule cells in culture are composed of a heterogeneous cell population at different developmental stages, and their properties are influenced by culture conditions, medium components, animal sources and so on. Studies of cultured cells thus need to be carefully interpreted by combination with in vivo work. The calcium entry is enhanced by excitatory transmitters at the late stage of differentiation of granule cells. It has thus been thought that the KCl-induced depolarization represents responses of differentiated granule cells to excitatory transmitters. A number of lines of evidence discussed in this article, however, have also indicated that KCl-induced depolarization mimics the developing stage of immature granule cells, and the inactivation of CaN plays a more important role in controlling gene expression at the terminal differentiation of granule cells. Interestingly, Bonni's group has revealed the specialized roles of CaMK and CaN in the differentiation of granule cells; the activation of CaMK stimulates dendritic growth of granule cells, whereas the inactivation of CaN promotes synapse assembly without any effect on dentritic growth (Gaudillière et al. 2004; Shalizi et al. 2006). These findings further support the view that CaMK and CaN have specific roles in regulation of granule cell differentiation and maturation.
Several questions remain to be answered. (1) A diversity of types of VSCCs is expressed in both immature and mature granule cells, but Ca2+ currents are low in immature granule cells (Cull-Candy et al. 1989; Rossi et al. 1994; Randall & Tsien, 1995; D'Angelo et al. 1997). L-type VSCCs are responsible for CaN-mediated suppression of both NR2C expression and MEF2A-regulated synaptogenesis (Suzuki et al. 2005; Shalizi et al. 2006), whereas N-type VSCCs are critical for radial migration of granule cells (Komuro & Rakic, 1998). It remains, however, elusive whether low levels of VSCCs are effective in controlling CaMK- or CaN-mediated gene expression in immature granule cells and how different types of VSCCs are involved in distinct processes of granule cell development. (2) Developing granule cells inducibly express different types of K+ channels in a CaN-dependent manner (Sato et al. 2005). Among these, the mutation of the GIRK2 K+ channel in the weaver mutant displays impairment of cerebellar development, but this abnormality results from the secondary effect of persistently activated Na+ channels (Kofuji et al. 1996; Slesinger et al. 1996). TASK1, a leak K+ channel, is up-regulated not only in developing granule cells but also compensatorily in GABAAα6-deficient granule cells, indicating that TASK1 is important for homeostatic, tonic inhibition of granule cells (Brickley et al. 2001). However, the TASK1 knockout mice show no impairment of cerebellar development (Aller et al. 2005). What type of K+ channels and/or inhibitory receptors is involved in regulation of resting membrane potentials needs to be clarified. (3) It will be intriguing to find out whether membrane potentials contribute to controlling gene expression in other developing neuronal cells in the early postnatal period. This question is of great importance for substantiating the potential mechanisms that underlie the activity-dependent regulation of neuronal cell development and maturation. The combination of genomic approaches and physiology is the way forward for elucidating the mechanisms of development and maturation of neuronal cells.
References
- Aller MI, Veale EL, Linden AM, Sandu C, Schwaninger M, Evans LJ, Korpi ER, Mathie A, Wisden W, Brickley SG. Modifying the subunit composition of TASK channels alters the modulation of a leak conductance in cerebellar granule neurons. J Neurosci. 2005;25:11455–11467. doi: 10.1523/JNEUROSCI.3153-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandoli C, Sanna A, De Bernardi MA, Follesa P, Brooker G, Mocchetti I. Brain-derived neurotrophic factor and basic fibroblast growth factor downregulate NMDA receptor function in cerebellar granule cells. J Neurosci. 1998;18:7953–7961. doi: 10.1523/JNEUROSCI.18-19-07953.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Marshall CG, Ogden D. Voltage-activated membrane currents in rat cerebellar granule neurones. J Physiol. 1989;414:179–199. doi: 10.1113/jphysiol.1989.sp017683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo E, De Filippi G, Rossi P, Taglietti V. Synaptic activation of Ca2+ action potentials in immature rat cerebellar granule cells in situ. J Neurophysiol. 1997;78:1631–1642. doi: 10.1152/jn.1997.78.3.1631. [DOI] [PubMed] [Google Scholar]
- Farrant M, Feldmeyer D, Takahashi T, Cull-Candy SG. NMDA-receptor channel diversity in the developing cerebellum. Nature. 1994;368:335–339. doi: 10.1038/368335a0. [DOI] [PubMed] [Google Scholar]
- Gallo V, Kingsbury A, Balázs R, Jørgensen OS. The role of depolarization in the survival and differentiation of cerebellar granule cells in culture. J Neurosci. 1987;7:2203–2213. doi: 10.1523/JNEUROSCI.07-07-02203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudillière B, Konishi Y, de la Iglesia N, Yao G, Bonni A. A CaMKII-NeuroD signaling pathway specifies dendritic morphogenesis. Neuron. 2004;41:229–241. doi: 10.1016/s0896-6273(03)00841-9. [DOI] [PubMed] [Google Scholar]
- Gault LM, Siegel RE. Expression of the GABAA receptor δ subunit is selectively modulated by depolarization in cultured rat cerebellar granule neurons. J Neurosci. 1997;17:2391–2399. doi: 10.1523/JNEUROSCI.17-07-02391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatten ME, Heintz N. Mechanisms of neural patterning and specification in the developing cerebellum. Annu Rev Neurosci. 1995;18:385–408. doi: 10.1146/annurev.ne.18.030195.002125. [DOI] [PubMed] [Google Scholar]
- Jones A, Korpi ER, McKernan RM, Pelz R, Nusser Z, Makela R, et al. Ligand-gated ion channel subunit partnerships: GABAA receptor α6 subunit gene inactivation inhibits δ subunit expression. J Neurosci. 1997;17:1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P, Hofer M, Millen KJ, Millonig JH, Davidson N, Lester HA, Hatten ME. Functional analysis of the weaver mutant GIRK2 K+ channel and rescue of weaver granule cells. Neuron. 1996;16:941–952. doi: 10.1016/s0896-6273(00)80117-8. [DOI] [PubMed] [Google Scholar]
- Komuro H, Rakic P. Orchestration of neuronal migration by activity of ion channels, neurotransmitter receptors, and intracellular Ca2+ fluctuations. J Neurobiol. 1998;37:110–130. [PubMed] [Google Scholar]
- Lin X, Cui H, Bulleit RF. BDNF accelerates gene expression in cultured cerebellar granule neurons. Brain Res Dev Brain Res. 1998;105:277–286. doi: 10.1016/s0165-3806(97)00193-4. [DOI] [PubMed] [Google Scholar]
- Mellor JR, Merlo D, Jones A, Wisden W, Randall AD. Mouse cerebellar granule cell differentiation: electrical activity regulates the GABAA receptor α6 subunit gene. J Neurosci. 1998;18:2822–2833. doi: 10.1523/JNEUROSCI.18-08-02822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor JR, Wisden W, Randall AD. Somato-synaptic variation of GABAA receptors in cultured murine cerebellar granule cells: investigation of the role of the α6 subunit. Neuropharmacology. 2000;39:1495–1513. doi: 10.1016/s0028-3908(00)00007-1. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Ramoa AS, McCormick DA. Developmental changes in electrophysiological properties of LGNd neurons during reorganization of retinogeniculate connections. J Neurosci. 1994;14:2089–2097. doi: 10.1523/JNEUROSCI.14-04-02089.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall A, Tsien RW. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J Neurosci. 1995;15:2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi P, D'Angelo E, Magistretti J, Toselli M, Taglietti V. Age-dependent expression of high-voltage activated calcium currents during cerebellar granule cell development in situ. Pflugers Arch. 1994;429:107–116. doi: 10.1007/BF02584036. [DOI] [PubMed] [Google Scholar]
- Rossi P, De Filippi G, Armano S, Taglietti V, D'Angelo E. The weaver mutation causes a loss of inward rectifier current regulation in premigratory granule cells of the mouse cerebellum. J Neurosci. 1998;18:3537–3547. doi: 10.1523/JNEUROSCI.18-10-03537.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Suzuki K, Nakanishi S. Expression profile of BDNF-responsive genes during cerebellar granule cell development. Biochem Biophys Res Commun. 2006;341:304–309. doi: 10.1016/j.bbrc.2005.12.184. [DOI] [PubMed] [Google Scholar]
- Sato M, Suzuki K, Yamazaki H, Nakanishi S. A pivotal role of calcineurin signaling in development and maturation of postnatal cerebellar granule cells. Proc Natl Acad Sci U S A. 2005;102:5874–5879. doi: 10.1073/pnas.0501972102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalizi A, Gaudillière B, Yuan Z, Stegmüller J, Shirogane T, Ge Q, Tan Y, Schulman B, Harper JW, Bonni A. A calcium-regulated MEF2 sumoylation switch controls postsynaptic differentiation. Science. 2006;311:1012–1017. doi: 10.1126/science.1122513. [DOI] [PubMed] [Google Scholar]
- Slesinger PA, Patil N, Liao YJ, Jan YN, Jan LY, Cox DR. Functional effects of the mouse weaver mutation on G protein-gated inwardly rectifying K+ channels. Neuron. 1996;16:321–331. doi: 10.1016/s0896-6273(00)80050-1. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Sato M, Morishima Y, Nakanishi S. Neuronal depolarization controls brain-derived neurotrophic factor-induced upregulation of NR2C NMDA receptor via calcineurin signaling. J Neurosci. 2005;25:9535–9543. doi: 10.1523/JNEUROSCI.2191-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CL, Pollard S, Stephenson FA. Developmental regulation of expression of GABAA receptor alpha 1 and alpha 6 subunits in cultured rat cerebellar granule cells. Neuropharmacology. 1996;35:1337–1346. doi: 10.1016/s0028-3908(96)00114-1. [DOI] [PubMed] [Google Scholar]
- Tyzio R, Ivanov A, Bernard C, Holmes GL, Ben-Ari Y, Khazipov R. Membrane potential of CA3 hippocampal pyramidal cells during postnatal development. J Neurophysiol. 2003;90:2964–2972. doi: 10.1152/jn.00172.2003. [DOI] [PubMed] [Google Scholar]
- Vallano ML, Lambolez B, Audinat E, Rossier J. Neuronal activity differentially regulates NMDA receptor subunit expression in cerebellar granule cells. J Neurosci. 1996;16:631–639. doi: 10.1523/JNEUROSCI.16-02-00631.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang VY, Zoghbi HY. Genetic regulation of cerebellar development. Nat Rev Neurosci. 2001;2:484–491. doi: 10.1038/35081558. [DOI] [PubMed] [Google Scholar]
- West AE, Griffith EC, Greenberg ME. Regulation of transcription factors by neuronal activity. Nat Rev Neurosci. 2002;3:921–931. doi: 10.1038/nrn987. [DOI] [PubMed] [Google Scholar]
- Wisden W, Korpi ER, Bahn S. The cerebellum: a model system for studying GABAA receptor diversity. Neuropharmacology. 1996;35:1139–1160. doi: 10.1016/s0028-3908(96)00076-7. [DOI] [PubMed] [Google Scholar]
- Xie F, Raetzman LT, Siegel RE. Neuregulin induces GABAA receptor β2 subunit expression in cultured rat cerebellar granule neurons by activating multiple signaling pathways. J Neurochem. 2004;90:1521–1529. doi: 10.1111/j.1471-4159.2004.02685.x. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Hablitz JJ. Postnatal development of membrane properties of layer I neurons in rat neocortex. J Neurosci. 1996;16:1131–1139. doi: 10.1523/JNEUROSCI.16-03-01131.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]