Abstract
We have reported that epoxyeicosatrienoic acids (EETs), the cytochrome P450 (CYP) epoxygenase metabolites of arachidonic acid (AA), are potent sarcolemmal ATP-sensitive K+ (KATP) channel activators. However, activation of cardiac and vascular KATP channels by endogenously produced EETs under physiological intracellular conditions has not been demonstrated and direct comparison of the mechanisms whereby EETs activate the KATP channels in cardiac myocytes versus vascular smooth muscle cells has not been made. In this study, we examined the effects of AA on KATP channels in freshly isolated cardiac myocytes from rats, wild-type (WT) and transgenic mice overexpressing CYP2J2 cDNA, and mesenteric arterial smooth muscle cells from rats. We also compared the activation of cardiac and vascular KATP channels by extracellularly and intracellularly applied 11,12-EET. We found that 1 μm AA enhanced KATP channel activities in both cardiac and vascular smooth muscle cells, and the AA effects were inhibited by preincubation with CYP epoxygenase inhibitors. Baseline cardiac KATP current densities in CYP2J2 transgenic mice were 190% higher than those of WT mice, and both were reduced to similar levels by CYP epoxygenase inhibition. Western blot analysis showed that expression of Kir6.2 and SUR2A was similar between WT and CYP2J2 transgenic hearts. 11,12-EET (5 μm) applied intracellularly enhanced the KATP currents by 850% in cardiac myocytes, but had no effect in vascular smooth muscle cells. In contrast, 11,12-EET (5 μm) applied extracellularly increased KATP currents by 520% in mesenteric arterial smooth muscle cells, but by only 209% in cardiac myocytes. Preincubation with 100 μmm-iodobenzylguanidine or 5 μm myristoylated PKI amide did not alter the activation of cardiac KATP channels by 5 μm 11,12-EET, but significantly inhibited activation of vascular KATP channels. Moreover, EET only enhanced the inward component of cardiac KATP currents, but activated both the inward and outward components of vascular KATP currents. Our results indicate that endogenously derived CYP metabolites of AA potently activate cardiac and vascular KATP channels. EETs regulate cardiac electrophysiology and vascular tone by KATP channel activation, albeit through different mechanisms: the cardiac KATP channels are directly activated by EETs, whereas activation of the vascular KATP channels by EETs is protein kinase A dependent.
Sarcolemmal ATP-sensitive K+ (KATP) channels are hetero-octameric complexes, composed of four pore-forming subunits (Kir6.x) and four sulphonylurea receptor (SUR) subunits (Aguilar-Bryan et al. 1998). KATP channels are robustly expressed in cardiac myocytes at 1–10 channels per square micrometre (Yokoshiki et al. 1998), but are present at lower density in vascular smooth muscle cells (0.1–0.2 channels per square micometre), populating both conduit and resistance vessels (Quayle et al. 1997). KATP channels are inhibited by intracellular ATP, but can be activated during ischaemia and hypoxia in response to changes of the intracellular metabolic state. Cardiac KATP channels are activated by intracellular nucleotide diphosphates (Tung & Kurachi, 1991), by protons (Koyano et al. 1993), and by G protein-mediated signalling pathways, including protein kinase A (PKA) (Lin et al. 2000), protein kinase C (Ito et al. 2001) and G-protein subunits (Terzic et al. 1994). Opening of the cardiac KATP channel leads to shortening of the cardiac action potential, reducing intracellular Ca2+ overload, and is thought to be a crucial mediator of cardiac preconditioning and postischaemic cardiac protection (Seino & Miki, 2003). Activation of the vascular KATP channel leads to membrane hyperpolarization, reduces Ca2+ influx, and is a key determinant of vascular tone (Yokoshiki et al. 1998). Studies with Kir6.2 knock-out and Kir6.2 overexpressor mice have demonstrated that cardiac KATP channels are important regulators of cardiac electrophysiology and contractility (Suzuki et al. 2001; Rajashree et al. 2002; Saito et al. 2005), and are critical elements for cardiac adaptation to stress (Zingman et al. 2002; Hodgson et al. 2003). Mice with ablation of the Kir6.1 gene, which encodes vascular KATP channels, developed spontaneous coronary spasm that leads to lethal atrioventricular block and sudden death (Miki et al. 2002). In addition, KATP channels are implicated in cardiac arrhythmogenesis (Liu et al. 2004) and in the development of dilated cardiomyopathy (Bienengraeber et al. 2004).
Cytochrome P450 (CYP) enzymes are widely distributed in mammalian tissues and play crucial roles in the metabolism of endogenous substrates and exogenous chemicals. More than 500 CYP genes and 78 CYP families have been identified (Roman, 2002). The CYP2Js are the primary CYP epoxygenase isoforms in cardiac myocytes, whereas the CYP2Cs are dominant in vascular beds. These enzymes convert arachidonic acid (AA) into four epoxyeicosatrienoic acid (EET) regioisomers: 5,6-, 8,9-, 11,12- and 14,15-EET (Wu et al. 1996; Zeldin, 2001; Roman, 2002). EETs are abundant in the rat heart, with 70 ng of total EETs per gram of heart (Wu et al. 1997). Most of AA is stored in membrane phospholipids, and upon phospholipase A2 activation can be released and converted into EETs (Wu et al. 1997; Zeldin, 2001). Moreover, EETs are rapidly taken up by cardiac myocytes and incorporated into membrane phospholipids, which serve as an important EET source (Lee et al. 1999). These mechanisms contribute to the significant increase in cardiac EET concentrations during ischaemia and reperfusion (Nithipatikom et al. 2001).
EETs are known to regulate multiple cellular functions including inflammation (Node et al. 1999), cell proliferation (Potente et al. 2002), vasoreactivity (Zhang et al. 2001), and cardiac protection from ischaemia/reperfusion injury (Wu et al. 1997; Seubert et al. 2004; Gross et al. 2005; Nithipatikom et al. 2006). EETs are ion channel regulators, modulating the activities of vascular smooth muscle Ca2+-activated K+ channels (Campbell et al. 1996; Lu et al. 2001b; Weston et al. 2005), cardiac Na+ channels (Lee et al. 1999), cardiac L-type Ca2+ channels (Xiao et al. 1998, 2004; Chen et al. 1999), epithelial Na+ channels (Wei et al. 2004), transient receptor potential (TRP) channels (Watanabe et al. 2003) and KATP channels including those in blood vessels (Ye et al. 2005), in sarcolemma (Lu et al. 2001a) and in cardiac mitochondria (Seubert et al. 2004). Most of these studies used exogenously applied EETs. Whether endogenously produced EETs would activate KATP channels in cardiovascular cells under physiological conditions is unknown.
The four EET regioisomers are equipotent activators of cardiac and vascular KATP channels (Lu et al. 2002; Ye et al. 2005). Activation of KATP channels by EETs may be mediated by different mechanisms, which may depend on the specific cellular organelle and cell type. In rat mesenteric arterial smooth muscle cells, activation of KATP channels by EETs was dependent on PKA activity (Ye et al. 2005, 2006). In cardiac mitochondria, activation of KATP channels by EETs is mediated through the p42/p44 mitogen activated protein (MAP) kinase signalling pathway (Seubert et al. 2004). In rat cardiac ventricular myocytes, activation of KATP channels by EETs involves direct interactions with specific positively charged residues, R192/R195, on the C-terminus of the Kir6.2 subunits, resulting in reduced channel sensitivity to ATP (Lu et al. 2005). However, it is unknown whether the PKA signalling pathway is also involved in the activation of cardiac KATP channels by EETs. In the present study, we tested the hypothesis that CYP epoxygenase activity is important for the maintenance of KATP channel function under physiological conditions and that endogenously derived EETs from AA metabolism could activate KATP channels in cardiac myocytes and in vascular smooth muscle cells. We further hypothesized that activation of the cardiac and vascular KATP channels by EETs is mediated through different signalling mechanisms. We compared the effects of 11,12-EET with another K+ channel opener (pinacidil) on cardiac KATP channel activation, and also the difference between cardiac and vascular KATP channel activities in response to 11,12-EET applied intracellularly and extracellularly. We found that AA activated KATP channels through CYP epoxygenase conversion to EETs in cardiac myocytes from normal rats, wild-type (WT) mice, transgenic mice overexpressing CYP2J2 cDNA, and rat mesenteric arterial smooth muscle cells. Cardiac KATP channels were preferentially activated by intracellularly applied EETs, whereas vascular KATP channels were only activated when EETs were applied extracellularly. In addition, PKA activity was crucial for EET-mediated KATP channel activation in vascular smooth muscle cells, but not in cardiac ventricular myocytes.
Methods
Animals
The use of animals and the procedures were approved by the Institutional Animal Care and Use Committee at Mayo Clinic. Male Sprague-Dawley rats (200–250 g) were used for isolation of cardiac myocytes and mesenteric arterial smooth muscle cells. Transgenic mice with cardiac myocyte-specific overexpression of CYP2J2 and age/sex-matched WT controls were bred and genotyped as previously described (Seubert et al. 2004; Xiao et al. 2004).
Isolation of cardiac ventricular myocytes
Single ventricular myocytes were isolated enzymatically as previously described (Lu et al. 1999). Briefly, male Sprague-Dawley rats (200–250 g body weight) were anaesthetized with pentobarbital (100 mg kg−1i.p.). The rat hearts were rapidly excised and perfused with nominally Ca2+-free Tyrode solution using a modified Langendorff apparatus at 8 ml min−1 for 5 min. The nominally Ca2+-free Tyrode solution contained (mm): NaCl 138, KCl 4.5, MgCl2 0.5, Na2HPO4 0.33, glucose 5.5, Hepes 10, and bovine serum albumin 0.1% (w/v), adjusted to pH 7.4 with NaOH. The perfusate was switched to a nominally Ca2+-free Tyrode solution containing 0.6 mg ml−1 collagenase (Worthington, CLS-2, 347 units mg−1) for 10 min. The ventricle was transferred into fresh 0.6 mg ml−1 collagenase solution, cut into small pieces (∼2 mm3) and incubated at room temperature (22°C) for 5 min. Dissociated cells were filtered through a medium mesh, centrifuged and stored in KB solution which contained (mm): KOH 70, KCl 40, l-glutamic acid 50, taurine 20, MgCl2 0.5, K2HPO4 1, EGTA 0.5, Hepes 10, creatine 5, pyruvic acid 5, and Na2ATP 5, adjusted to pH 7.38 with KOH. Mice were anaesthetized by isofluorane inhalation, and isolated hearts were retrogradely perfused on the Langendorff apparatus (1 ml min−1) for 5 min with Krebs-Ringer solution containing (mm): NaCl 35, KCl 4.75, KH2PO4 1.2, Na2HPO4 16, sucrose 134, Hepes 10, glucose 10, NaHCO3 25, pH 7.4 with NaOH and equilibrated with 5% CO2. Then, the mouse hearts were perfused with Krebs-Ringer solution containing 1 mg ml−1 collagenase for another 8 min. Dissociated cells were stored in KB solution and used within 24 h.
Isolation of mesenteric arterial smooth muscle cells
Mesenteric smooth muscle cells were dissociated enzymatically as previously reported (Ye et al. 2005). Third- and fourth-ordered branches of rat mesenteric arteries were carefully dissected. The small arteries were then placed in 1 ml of physiological saline solution which contained (mm): NaCl 145, KCl 4, CaCl2 0.05, MgCl2 1, Hepes 10, glucose 10, pH 7.2 with bovine serum albumin 0.1% (w/v). After 10 min incubation at 37°C in a shaking water bath, the vessels were treated with 1.75 mg papain and 1.25 mg dithiothreitol in 1 ml saline solution for 10 min. The vessels were further digested with 1.25 mg collagenase and 1.25 mg of trypsin inhibitor in 1 ml saline solution at 37°C for 10 min, then washed three times with 1 ml aliquots of saline solutions, and gently triturated with a fire-polished glass pipette until the cells were completely dissociated.
Whole-cell KATP current recordings
Whole-cell K+ currents in freshly isolated cardiac ventricular myocytes and mesenteric arterial smooth muscle cells were recorded using an Axopatch 200B integrating amplifier (Axon Instruments, Union City, CA, USA), filtered at 2 kHz and digitized at 50 kHz. In cardiac myocytes, K+ currents were elicited at test potentials (TP) from −120 mV to +40 mV at 10 mV increments from a holding potential (HP) of 0 mV to inactivate Na+ and Ca2+ channels. To further eliminate Na+ and Ca2+ current contaminations, the following bath solution was used (mm): NaCl 20, KCl 4.5, choline chloride 130, CaCl2 1, CoCl2 2, MgCl2 2, Hepes 10, and glucose 5.5, pH 7.4, as previously reported (Lu et al. 1999). Pipette resistance was 0.5–0.8 MΩ when filled with the pipette solution which contained (mm): KCl 140, CaCl2 0.465 (200 nm free Ca2+), MgCl2 0.5, Na2ATP 5, Na2GTP 0.5, Hepes 1, EGTA 1, pH 7.3. Cardiac KATP currents were obtained as the HMR-1098-sensitive (3 μm), or the glyburide-sensitive (5 μm) K+ currents by digital subtraction. In vascular smooth muscle cells, K+ currents were recorded (TP from −100 mV to 40 mV, HP = 0 mV) in symmetrical K+ with the bath solution containing (mm) KCl 140, MgCl2 1, CaCl2 0.1, Hepes 10, glucose 10.0, IBTX 0.1, pH 7.4, and the pipette solution containing (mM) KCl 110, KOH 30, MgCl2 1, CaCl2 1 (20 nm free Ca2+), Na2ATP 0.1, Na2ADP 0.1 and Na2GTP 0.5, Hepes 10, EGTA 10, pH 7.35. Vascular KATP currents were obtained as the glyburide-sensitive (10 μm) K+ currents. Current–voltage (I–V) relationships were fitted using equations previously described (Lu et al. 2001a).
Single channel recordings
Single channel KATP and the strong inwardly rectifying K+ (IK1) currents in inside-out membrane patches of cardiac myocytes (TP of −60 and +60 mV) were recorded in symmetrical K+ (140 mm) as previously described (Lu et al. 2001a). The output signals were filtered with an eight-pole Bessel filter (902 LPF, Frequency Devices Inc., Haverhill, MA, USA) at 1 kHz and digitized at 20 kHz. Patch pipettes were coated with Sylgard 184 (Dow Corning, Midland, MI, USA) and had a typical tip resistance of 10 MΩ when filled with the pipette solution that contained (mM): KCl 140, MgCl2 1, CaCl2 0.1, Hepes 10. pH 7.4. The bath solution contained (mm): KCl 70, l-aspartic acid monopotassium salt 70, EGTA 1, Hepes 10, MgCl2 1, pH to 7.35. Data were acquired and further analysed using pCLAMP 8 software (Axon Instruments). All experiments were conducted at room temperature (22°C).
Western blot analysis
Polyclonal rabbit anti-Kir6.2 and anti-SUR2A antibodies were custom prepared against synthetic peptides corresponding to antigenic sites in rat Kir6.2 (16-PLRKRSVAVAKAKPKFSISPDSLS-39) and SUR2A (263-LKEAYEEQKKKAADHPNRTPS-284). Kir6.2 and SUR2A antibodies bind to cardiac proteins of 40 kDa and 140 kDa, respectively, consistent with previous results (Crawford et al. 2002; Morrissey et al. 2005). Kir6.2 and SUR2A protein expression in hearts were determined using Western blots. Left ventricles from three pairs of WT and CYP2J2 transgenic mice were homogenized in 300 μl of T-PER tissue protein-extraction reagent containing protease inhibitors (Pierce, Biotechnology Inc.), resolved on a 4–15% Tris-HCl polyacrylamide gradient gel, and transferred electrophoretically onto polyvinylidene difluoride membranes (Wang et al. 2005). The membranes were blocked for 2 h with 10% milk in phosphate-buffered saline (PBS), followed by incubation with rabbit anti-Kir6.2 or anti-SUR antibodies (1:300 dilution). After extensive washes with 0.1% PBS–Tween, the primary antibodies were detected with horseradish peroxidase-conjugated secondary antibodies, and the signals were developed by an Immune-Star HRP Chemiluminescence Kit (Bio-Rad Laboratories Inc., Hercules, CA, USA). Optical densities of the bands were analysed using Scion Image software (Scion, Frederick, MD, USA).
Immunoblotting of heart microsomal fractions to confirm the presence of the CYP2J2 transgene was performed using polyclonal antibodies against the recombinant human CYP2J2 protein (anti-CYP2J2rec) and against the CYP2J2-specific peptide HMDQNF-GNRPVTPMR (anti-CYP2J2pep1) as previously described (Wu et al. 1996; King et al. 2002).
Chemicals
11,12-EET was obtained from Cayman Chemical Co. (Ann Arbor, MI, USA), prepared in 100% ethanol (5 mm) and stored at −80°C. Myristoylated PKI amide and β-diethyl-aminoethyldiphenylpropylacetate (SKF525A) were purchased from BIOMOL (Plymouth Meeting, PA, USA). HMR-1098 was a gift from Aventis Pharmaceuticals Co. (Frankfurt, Germany). Unless otherwise mentioned, all other chemicals were purchased from Sigma-Aldrich Co. (St Louis, MO, USA).
Statistical analysis
Data were presented as the mean ± s.e.m. Student's t test was used to compare data between two groups. One-way ANOVA followed by Tukey's test was used to compare data from multiple groups using SigmaStat software (Systat Software Inc., Point Richmond, CA, USA).
Results
Effects of AA on KATP channels in rat cardiac ventricular myocytes
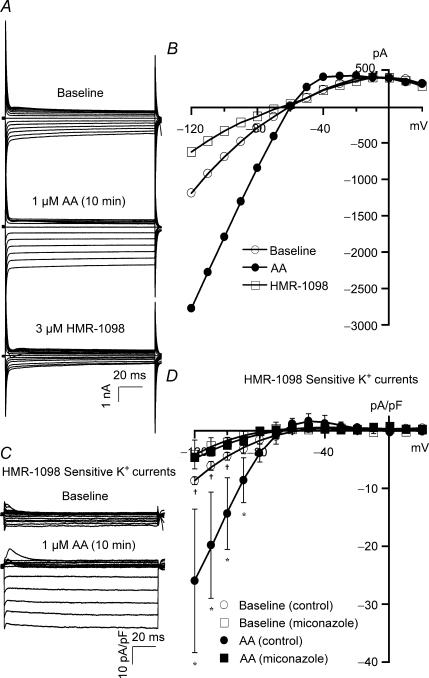
In freshly isolated rat ventricular myocytes, whole-cell K+ currents (HP = 0 mV, TP from −120 mV to +20 mV) were activated on exposure to 1 μm AA, reaching a maximal effect in 10–15 min without altering the current reversal potential (EK). The effects of AA were blocked by 3 μm HMR-1098, a specific sarcolemmal KATP channel inhibitor that was added after the AA effects have reached steady state. Tracings of a typical recording are shown in Fig. 1A, and the K+ current I–V relationships before and after application of AA and HMR-1098 are plotted in Fig. 1B. The HMR-sensitive K+ currents, obtained by digital subtraction of the residual currents after exposure to HMR-1098 (3 μm) from the total K+ currents, are defined as the cardiac KATP currents and shown in Fig. 1C. Group data summarizing the effects of AA on the I–V relationships of cardiac KATP channels are shown in Fig. 1D. Interestingly, AA significantly increased cardiac KATP currents at all voltages negative to EK; for example, at a TP of −100 mV, AA increased the KATP currents from a baseline of −4.50 ± 0.66 pA pF−1 to −14.37 ± 6.18 pA pF−1 (n = 5, P < 0.05). In contrast, AA had no effects on KATP currents at voltages positive to EK; for example, at a TP of −30 mV, baseline KATP current density was 0.38 ± 0.02 pA pF−1 and was 0.66 ± 0.76 pA pF−1 after exposure to AA (n = 5, N.S.). Hence, the effects of AA on cardiac KATP channels were voltage dependent, and AA activated only the inward but not the outward currents. These findings confirmed our previous results on single KATP channel recordings (Lu et al. 2001a). However, after incubation with 10 μm miconazole or 5 μm SKF525A (60 min), which are CYP epoxygenase inhibitors that block the conversion of AA to EETs, the baseline cardiac KATP currents were significantly reduced to −2.65 ± 0.11 pA pF−1 (TP = −100 mV, n = 6, P < 0.05 versus control), and AA failed to activate KATP currents (−2.10 ± 0.91 pA pF−1, n = 5, N.S. versus baseline) (Fig. 1D). These results indicate that the effects of AA on the cardiac KATP currents are mainly mediated through metabolites of CYP epoxygenase.
Figure 1. Effects of AA metabolites on cardiac KATP channel activities in rat ventricular myocytes.
A, whole-cell total K+ currents were elicited from −120 mV to +20 mV at a holding potential of 0 mV at increments of 10 mV in control rat ventricular myocytes. K+ currents were activated by 1 μm AA, reaching steady state in 10 min, and then exposed to 3 μm HMR-1098. B, I–V relationships of total K+ currents at baseline, in response to 1 μm AA, and 3 μm HMR-1098. C, HMR-1098-sensitive K+ currents (cardiac KATP currents) were obtained from the current tracings in A by digital subtraction of the residual currents after exposure to HMR-1098 (3 μm) from the total K+ currents. KATP currents were activated by AA. D, the KATP channel I–V relationships show inward rectification with a reversal potential of −70 mV. AA (1 μm) enhanced KATP currents at all voltages negative to reversal potential (inward currents). Treatment with 10 μm miconazole reduced KATP current densities at baseline and abolished the response to AA. Each point represents the mean ± s.e.m. *P < 0.05 for AA versus baseline (n = 6). †P < 0.05 for baseline currents with versus without miconazole treatment (n = 5).
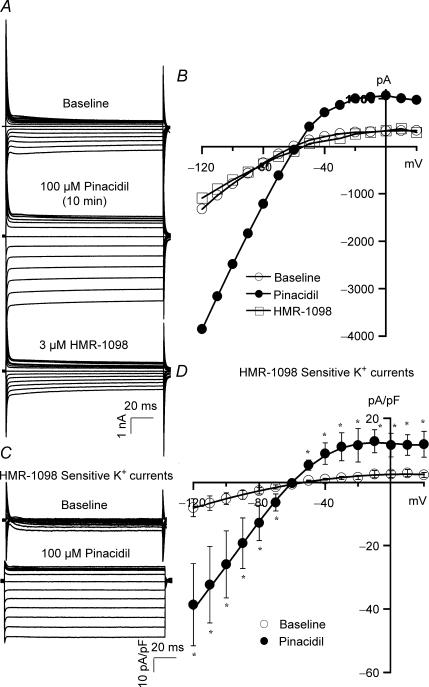
We compared the effects of AA with pinacidil, a K+ channel opener, on cardiac KATP channel activation. Raw tracings of total K+ currents at baseline, with pinacidil (100 μm), and then with HMR-1098 (3 μm) are shown in Fig. 2A, while the corresponding I–V relationships are plotted in Fig. 2B. Figure 2C shows the HMR-1098-sensitive KATP currents from the same recordings of Fig. 2A after digital subtraction. Pinacidil increased both inward (at TP of −100 mV, from −6.7 ± 2.3 pA pF−1 at baseline to −32.4 ± 12.0 pA pF−1, n = 3, P < 0.05) and outward KATP currents (at TP of −30 mV, from −1.4 ± 0.6 pA pF−1 at baseline to 11.5 ± 5.3 pA pF−1, n = 3, P < 0.05). These results are similar to previous reports that pinacidil activates both the inward and outward components of cardiac KATP currents (Terzic et al. 1995; Lawson, 2000); hence, the CYP epoxygenase metabolites of AA are unique KATP channel activators.
Figure 2. Effects of pinacidil on cardiac KATP channel activities.
Whole-cell total K+ currents (A) and the I–V relationships (B), in a rat ventricular myocyte, before and after exposure to 100 μm pinacidil, and then 3 μm HMR-1098, using the same protocol as described in Fig. 1. KATP currents (C) and I–V curves (D) were obtained from the current tracings of A by subtraction of the HMR-1098-insensitive K+ currents. Unlike the effect of AA, 100 μm pinacidil enhanced both the inward and outward KATP currents. *P < 0.05 versus baseline (n = 3).
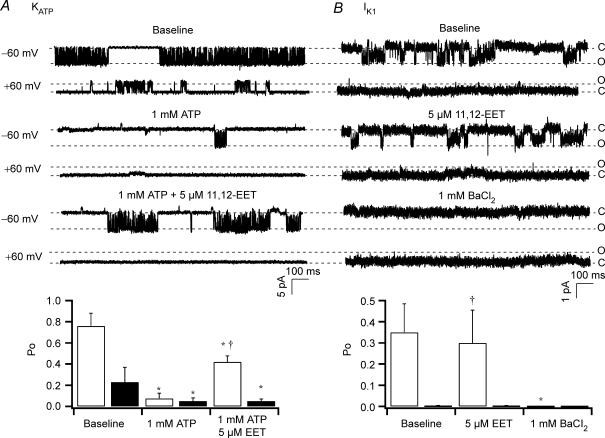
To further confirm the unique voltage dependence of KATP channel activation by the CYP epoxygenase metabolites of AA in cardiac myocytes, we examined the effects of 11,12-EET (5 μm) on inside-out single KATP and IK1 channel activities. In the presence of symmetrical K+ (140 mm), the single KATP current amplitude was −5 pA at −60 mV and +2.5 pA at +60 mV, similar to what we have previously described (Lu et al. 2001a) (Fig. 3A). Opening probability (Po) of KATP channels at baseline (0 mm ATP) was 0.76 ± 0.12 at −60 mV and 0.23 ± 0.14 at +60 mV (n = 3), and on exposure to 1 mm ATP was reduced to 0.08 ± 0.05 at −60 mV (n = 3, P < 0.05 versus baseline) and to 0.05 ± 0.03 at +60 mV (n = 3, P < 0.05 versus baseline). However, in the presence of 1 mm ATP, application of 5 μm 11,12-EET significantly activated the KATP channels at − 60 mV with a robust Po of 0.42 ± 0.06 (n = 3, P < 0.05 versus baseline and 1 mm ATP alone). In contrast, there was no change in Po at +60 mV by 11,12-EET (Fig. 3A). Hence, 11,12-EET activated specifically the inward component of KATP currents, converting the channel rectification properties from weakly inward to strongly inward. In the presence of symmetrical K+ (140 mm), the single cardiac IK1 current amplitudes were −1.0 pA at −60 mV and +0.2 pA at +60 mV, and exposure to 5 μm 11,12-EET had no effect on channel activity, which was completely blocked by 1 mm BaCl2 (Sakmann & Trube, 1984; Masuda & Sperelakis, 1993; Nakamura et al. 1998) (Fig. 3B). Baseline Po of IK1 was 0.35 ± 0.14 at −60 mV and 0.03 ± 0.02 at +60 mV (n = 2). There was no change in Po by 5 μm 11,12-EET (n = 2, 0.3 ± 0.1 at −60 mV and 0.003 ± 0.002 at + 60 mV, N.S. versus baseline). Group data on the effects of 11,12-EET on KATP and IK1 channel Po are summarized in bar graphs (Fig. 3, lower panels). These results indicate that enhancement of inward rectifying K+ currents by the AA metabolites of CYP epoxygenase pathway is mediated through the activation of KATP and not IK1 channels in rat ventricular myocytes.
Figure 3. Effects of 11,12-EET on cardiac KATP and IK1 channels in rat ventricular myocytes.
A, inside-out single cardiac KATP currents were elicited from −60 mV and +60 mV in symmetrical K+ (140 mm) at baseline (0 mm ATP), on exposure to 1 mm ATP, and 1 mm ATP plus 5 μm 11,12-EET. Cardiac KATP channel activities were robustly activated by 11,12-EET even in the presence of 1 mm ATP. B, inside-out single IK1 currents were recorded from −60 mV and +60 mV in symmetrical K+ (140 mm) at baseline, on exposure to 5 μm 11,12-EET, and 1 mm BaCl2. 11,12-EET had no effect on IK1 channel activities, which were totally inhibited by 1 mm BaCl2. Dashed lines indicate the levels of channel open state (o) and closed state (c). Bar graphs (lower panels) summarizing the effects of 5 μm 11,12-EET on Po of KATP and IK1 channels. Open bars represent the Po at −60 mV and filled bars the Po at +60 mV. *P < 0.05 versus baseline. †P < 0.05 versus 1 mm ATP or 1 mm BaCl2. n = 3 for each group.
Effects of AA on rat vascular KATP channels in mesenteric arterial smooth muscle cells
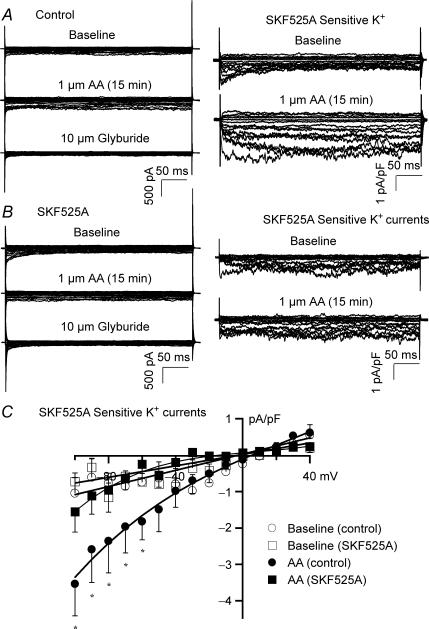
Figure 4A (left column) shows recordings of whole-cell total K+ currents (TP = −100 mV to +40 mV, HP = 0 mV), from rat mesenteric arterial smooth muscle cells at baseline, with 1 μm AA, and then 10 μm glyburide. Vascular KATP currents were obtained by digital subtraction of the glyburide-insensitive K+ currents (Fig. 4A, right column). Vascular KATP current densities were enhanced from −1.09 ± 0.35 pA pF−1 to −3.54 ± 0.78 pA pF−1 by 1 μm AA (TP = −100 mV, n = 6, P < 0.05 versus baseline). After incubation with 5 μm SKF525A (60 min), AA failed to activate vascular KATP channels (Fig. 4B). Figure 4C shows the vascular KATP channel I–V relationships in response to 1 μm AA, with and without pretreatment with SKF525A. AA (1 μm) only had small but significant effects on vascular KATP channels at negative voltages, and the effects were completely abolished by SKF525A. These results indicate that AA could activate vascular KATP channels through CYP metabolites, but the response is modest compared to that in cardiac ventricular myocytes.
Figure 4. Effects of AA metabolites on KATP channel activities in rat mesenteric arterial smooth muscle cells.
A, whole-cell total K+ currents in rat mesenteric arterial smooth muscle cells were elicited from −100 mV to + 40 mV at a holding potential of 0 mV at 10 mV increments under control conditions. Total K+ currents were enhanced by 1 μm AA and blocked by 10 μm glyburide (left column). Glyburide-sensitive KATP currents were obtained by digital subtraction of glyburide-insensitive components of K+ currents. AA (1 μm) increased vascular KATP currents (right column). B, whole cell total K+ currents were recorded from cells preincubated with 5 μm SKF525A for 60 min. AA failed to activate total K+ currents (left column). AA had no effect on vascular KATP currents (right column). C, vascular KATP channel I–V relationships showing the current densities before and after exposure to AA, with and without preincubation with SKF525A. n = 6 for all groups; *P < 0.05, AA versus baseline.
Effects of AA on cardiac KATP channels in transgenic mice overexpressing CYP2J2
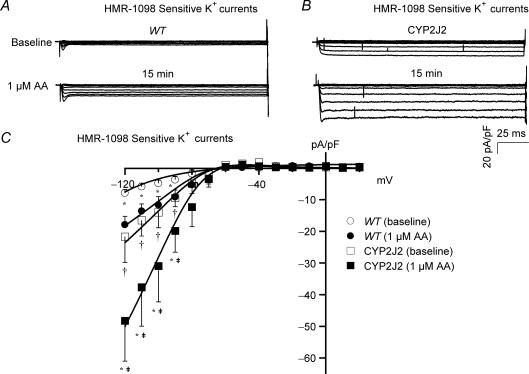
To further determine the role of CYP epoxygenase on cardiac KATP channel activation, we examined the effects of AA on cardiac myocytes from transgenic mice overexpressing the CYP2J2 gene. Figure 5A and B shows the HMR-1098-sensitive KATP channels from WT and CYP2J2 transgenic mice. The baseline cardiac KATP currents in WT mice were −4.85 ± 1.03 pA pF−1 (HP = 0 mV, TP = − 100 mV, n = 5), but those in the CYP2J2 transgenic mice were 190% higher at −14.01 ± 4.84 pA pF−1 (n = 6, P < 0.05 versus WT). Exposure to 1 μm AA increased the KATP currents in WT to −11.66 ± 2.60 pA pF−1 (n = 5, P < 0.05 versus baseline) and produced a further substantial increase in CYP transgenic mice to −30.96 ± 11.34 pA pF−1 (n = 6, P < 0.05 versus baseline, and versus WT with AA). The cardiac KATP channel I–V relationships of WT and CYP2J2 transgenic mice are shown in Fig. 5C. Similar to that observed in rats, AA only enhanced the inward components of cardiac KATP currents in mice.
Figure 5. Effects of AA metabolites on cardiac KATP channel activities in transgenic mice overexpressing CYP2J2.
Representative tracings of cardiac KATP currents (after subtraction of HMR-1098-insensitive K+ currents) were recorded from ventricular myocytes of WT mice (A) and transgenic mice overexpressing CYP2J2 (B), before (upper panels) and 15 min after (lower panels) the application of AA (1 μm). Baseline KATP current densities were 2-fold higher in the CYP2J2 transgenic mice than in WT. Exposure to 1 μm AA enhanced the KATP current densities in both WT and transgenic mice. C, KATP channel I–V relationships showing significant increase in current densities at voltages negative to EK on exposure to AA in both WT and CYP2J2 transgenic mice. *P < 0.05 AA versus baseline for both WT and CYP2J2 transgenic mice. n = 5 for WT and n = 6 for CYP2J2 transgenic mice; †P < 0.05 baseline of CYP2J2 versus baseline of WT; ‡P < 0.05 CYP2J2 transgenic with AA versus WT with AA.
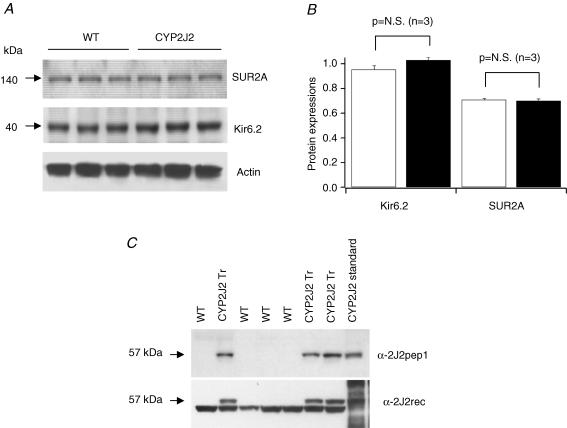
To determine whether KATP channels are up-regulated in the CYP2J2 transgenic hearts, we determined the expression of channel proteins, Kir6.2 and SUR2A, by Western blotting. There was no significant difference in Kir6.2 and SUR2A expression between CYP2JA transgenic and WT hearts (Fig. 6A and B). Hence, the higher KATP current densities in the CYP2J2 transgenic myocardium are likely to represent more robust KATP channel activation at baseline. Exposure to AA further enhanced the KATP currents, suggesting that CYP activity might be a rate-limiting step.
Figure 6. Immunoblot analysis of Kir6.2, SUR2A, and CYP2Js expression in hearts of WT and CYP2J2 transgenic mice.
A, Western blot showing that the expression of Kir6.2 and SUR2A is similar between WT and CYP2J2 transgenic hearts. Actin serves as the loading control. B, group data from three pairs of WT and CYP2J2 transgenic mice. There is no difference in SUR2A and Kir6.2 protein expression between WT and CYP2J2 transgenic hearts. C, Western blots showing that expression of CYP2J2 in cardiac myocytes of WT and CYP2J2 transgenic mice. Anti-CYP2J2pep1 is specific for CYP2J2 and anti-CYP2J2rec is a polyclonal antibody against human recombinant CYP2J2 and cross reacts with murine cardiac CYP2J.
Immunoblotting with two different CYP2J2 antibodies revealed that CYP2J2 transgenic mice had abundant cardiac-specific expression of the transgene, whereas WT littermates did not (Fig. 6C). Densitometry of the immunoblots performed with anti-CYP2J2rec antibody revealed an approximately 2-fold increase in CYP2J protein expression in CYP2J2 transgenic versus WT hearts. Previous work has confirmed that microsomes from CYP2J2 transgenic hearts had approximately 3-fold higher AA epoxygenase activity than microsomes from WT hearts (Seubert et al. 2004). Moreover, CYP2J2 transgenic cardiac myocytes released significantly more stable EET metabolites into culture medium than did WT cardiac myocytes (Seubert et al. 2004).
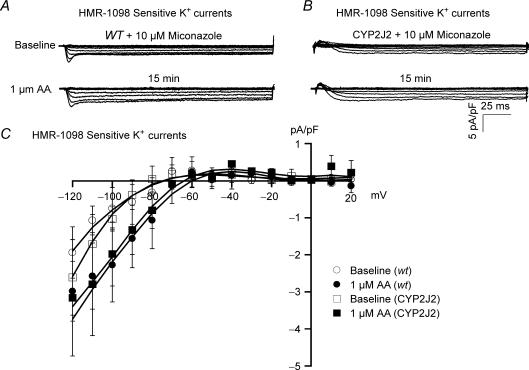
To determine whether the higher baseline level of cardiac KATP currents in CYP2J2 transgenic mice is due to enhanced CYP activities, we inhibited CYP activities with miconazole. Incubation with 10 μm miconazole for 60 min not only abolished the AA effects on KATP channel activation in both WT and CYP2J2 transgenic mice (Fig. 7A), but also reduced the current densities in CYP2J2 transgenic mice to those of WT mice (−0.75 ± 0.56 pA pF−1 in WT versus−1.03 ± 0.32 pA pF−1 in transgenic mice, n = 6 for both, N.S., Fig. 7B). KATP channel I–V relationships of WT and CYP2J2 transgenic mice pretreated with miconazole, before and after exposure to AA are shown in Fig. 7C. These results indicate that enhanced baseline KATP channel activities in CYP2J2 transgenic mice are due to the increased formation of CYP epoxygenase metabolites.
Figure 7. Effects of AA on cardiac KATP channel activities are abolished by treatment with miconazole.
HMR-1098-sensitive KATP currents were recorded in ventricular myocytes from WT (A) and CYP2J2 transgenic mice (B) preincubated with 10 μm miconazole (60 min), before (upper panels) and 15 min after (lower panels) the application of 1 μm AA. Treatment with miconazole not only abolished the effects of AA on KATP channel activities in both WT and CYP2J2 transgenic mice, but also reduced the baseline current densities of the CYP2J2 transgenic myocytes to that of WT. C, after treatment with miconazole, KATP channel I–V relationships showed no significant difference in KATP channel activities between WT and CYP2J2 transgenic cardiac myocytes before and after exposure to AA. n = 6 for both groups.
Activation of KATP channels by EETs is mediated through different mechanisms in rat ventricular myocytes and arterial smooth muscle cells
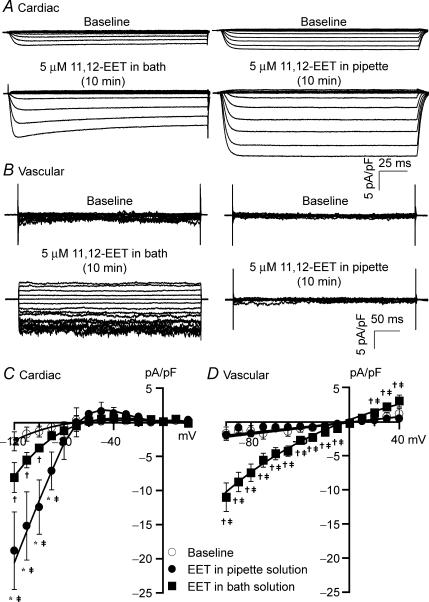
To further determine the effects of EETs on KATP channel activation in cardiac myocytes and vascular smooth muscle cells, we compared the effects of intracellular and extracellular applications of 11,12-EET. In rat cardiac myocytes, 5 μm 11,12-EET applied extracellularly (in the bath solution) increased KATP currents from −2.17 ± 0.83 pA pF−1 to −4.53 ± 0.56 pA pF−1 (HP = 0 mV, TP =−100 mV, n = 6, P < 0.05), and from −0.32 ± 0.21 pA pF−1 to −0.53 ± 0.16 pA pF−1 (TP = −30 mV, n = 6, N.S.) (Fig. 8A, left column). In contrast, 5 μm 11,12-EET applied intracellularly (in the pipette solution) enhanced KATP currents by 850% from −1.37 ± 0.80 pA pF−1 to −12.46 ± 3.98 pA pF−1 (TP = − 100 mV, n = 6, P < 0.05 versus baseline and versus EET applied extracellularly), and −0.20 ± 0.14 pA pF−1 to −0.53 ± 0.16 pA pF−1 (TP = −30 mV, n = 6, N.S. versus baseline and EET applied extracellularly) (Fig. 8A, right column). Hence, similar to AA, 11,12-EET only enhanced the inward currents of cardiac KATP channels. The effects of 11,12-EET reached a steady-state about 10 min after the whole-cell configuration was established with membrane break-through. This may reflect the time course of buffer equilibration between the pipette solution and the intracellular milieu.
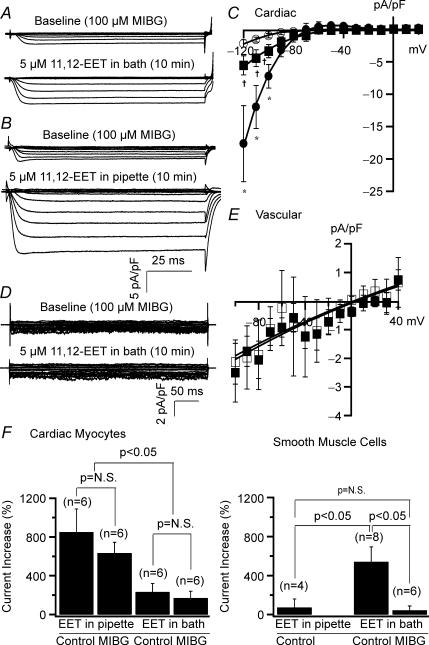
Figure 8. Cardiac and vascular KATP channels are activated by 11,12-EET through different mechanisms.
A, recordings of whole-cell KATP currents (after subtraction of glyburide-insensitive K+ currents) from rat ventricular myocytes. Extracellularly applied 11,12-EET (5 μm) produced small enhancement of KATP currents after 10 min of exposure (left panel), while intracellularly applied 11,12-EET resulted in a much greater stimulation of current activities (right panel). Cardiac KATP currents were recorded in the presence of 140 mm intracellular K+ and 4.5 mm extracellular K+. B, vascular KATP currents recorded from rat mesentery arterial smooth muscle cells. Extracellularly applied 11,12-EET (5 μm) produced significant KATP current activation (left panel), while intracellularly applied 11,12-EET had no effect (right panel). Vascular KATP currents were recorded in the presence of symmetrical K+ concentration at 140 mm. I–V relationships of cardiac (C) (n = 6 for all groups) and vascular KATP channels (D) (n = 4 for intracellularly applied 11,12-EET and n = 8 for extracellularly applied 11,12-EET), showing the effects of 5 μm 11,12-EET applied intracellularly or extracellularly. *P < 0.05, intracellularly applied 11,12-EET versus baseline. †P < 0.05, extracellularly applied 11,12-EET versus baseline. ‡P < 0.05, intracellularly applied versus extracellularly applied 11,12-EET.
In rat mesenteric arterial smooth muscle cells, 5 μm 11,12-EET applied extracellularly markedly increased KATP currents from −2.00 ± 0.54 pA pF−1 to −11.10 ± 2.08 pA pF−1 (HP = 0 mV, TP = −100 mV, n = 8, P < 0.05) (Fig. 8B). In contrast, 5 μm 11,12-EET applied intracellularly had no effects (from −1.11 ± 0.14 pA pF−1 to −1.78 ± 1.04 pA pF−1, n = 4, N.S.) (Fig. 8B). The I–V relationships of cardiac and vascular KATP channels are shown in Fig. 8C and D, respectively. Unlike cardiac KATP channels, which were activated by 11,12-EET only at voltages negative to EK, vascular KATP channels were enhanced by extracellular 11,12-EET at almost all voltages including both the inward and outward components. These results suggest that activation of cardiac and vascular KATP channels by EETs is mediated through different mechanisms.
Role of PKA in the activation of cardiac and vascular KATP channels by EET
It is unknown whether cAMP-dependent signalling is involved in the activation of cardiac KATP channels by EETs. We examined the role of PKA on the activation of cardiac and vascular KATP channels by EETs by treating the cells for 60 min with 5 μm myristoylated PKI amide, a membrane-permeant PKA inhibitor. After PKI treatment in rat ventricular myocytes, 5 μm 11,12-EET applied extracellularly had a very small effect on the KATP currents, from −1.41 ± 0.40 pA pF−1 to −2.86 ± 0.67 pA pF−1 (HP = 0 mV, TP = −100 mV, n = 5, P < 0.05) (Fig. 9A). However, 5 μm 11,12-EET applied intracellularly produced a marked increase in activation of KATP currents, from −1.76 ± 0.67 pA pF−1 at baseline to −11.46 ± 4.50 pA pF−1 (n = 5, P < 0.05 versus baseline, and versus EET applied extracellularly) (Fig. 9B). Hence, treatment with PKI did not alter the activation of cardiac KATP channel by 11,12-EET, indicating that the effects of EET were not mediated through PKA-dependent mechanisms. However, the baseline cardiac KATP currents were significantly reduced by PKI, suggesting that PKA may modulate the basal activities of the channel. Figure 9C shows the KATPI–V relationships for these studies.
Figure 9. Effects of 11,12-EET on cardiac KATP channels are not inhibited by PKI.
Recordings of HMR-1098-sensitive cardiac KATP currents in rat cardiac myocytes after treatment with 5 μm myristoylated PKI amide, showing the effects of extracellularly applied (A) and intracellular applied (B) 11,12-EET (5 μm). Pretreatment with myristoylated PKI amide had no effects on cardiac KATP channel sensitivity to 11,12-EET. C, cardiac KATP channel I–V relationships with no EET treatment (○), and with 11,12-EET applied extracellularly (▪) or intracellularly (•). n = 5 for all groups; *P < 0.05, intracellularly applied 11,12-EET versus baseline; †P < 0.05, extracellular 11,12-EET versus baseline; ‡P < 0.05, intracellular application versus extracellular application. D, recordings of glyburide-sensitive vascular KATP currents in rat mesenteric smooth muscle cells after treatment with 5 μm myristoylated PKI amide showing that the effects of extracellularly applied 11,12-EET were significantly attenuated. E, vascular KATP channel I–V relationship after treatment with PKI at baseline (□) and after exposure to 11,12-EET (▪). The effects of 11,12-EET were significantly inhibited by PKI (compare with Fig. 8D). n = 5; *P < 0.05, effect of 11,12-EET versus baseline. F, bar graphs comparing the percentage KATP current increase in cardiac myocytes (left panel) and vascular smooth muscle cells (right panel) by 11,12-EET applied intracellularly and extracellularly with and without (control) treatment with PKI. Group data showing the cardiac and vascular KATP current densities measured at −100 mV. Incubation with PKI had no effects on the activation of cardiac KATP channels by 11,12-EET, but significantly inhibited that in vascular KATP channels.
In contrast, after PKI treatment in mesenteric arterial smooth muscle cells, the effects of extracellularly applied 5 μm 11,12-EET on the KATP currents were significantly reduced (TP = −100 mV, −1.28 ± 0.42 pA pF−1 at baseline to −3.94 ± 0.52 pA pF−1, n = 5, P < 0.05), producing only 34% of the effects in control (Fig. 9D). The channel I–V relationships are shown in Fig. 9E. These results suggest that the PKA signalling pathway plays an important role in the activation of KATP channels by EETs in vascular smooth muscle cells. Comparison between the effects of 11,12-EET on activations of cardiac and vascular KATP channels, in the presence and absence of PKI are summarized in Fig. 9F.
Since the activation of vascular BK channels by EETs involves ADP-ribosylation of Gsα (Li et al. 1999), we tried to further delineate the signalling mechanism by examining the effects of 11,12-EET on the activation of cardiac and vascular KATP channels after treatment with 100 μmm-iodobenzylguanidine (MIBG), an ADP-ribosyltransferase inhibitor. In rat ventricular myocytes after treatment with MIBG, exposure to 5 μm 11,12-EET in the bath solution produced a 234% increase in KATP currents, from a baseline of −0.99 ± 0.33 pA pF−1 to −3.34 ± 1.02 pA pF−1 (TP = − 100 mV, n = 6, P < 0.05) (Fig. 10A). 11,12-EET (5 μm) applied intracellularly produced a 689% increase in KATP currents, from −1.07 ± 0.70 pA pF−1 to −7.34 ± 1.82 pA pF−1 (n = 6, P < 0.05 versus baseline, and versus EET applied extracellularly) (Fig. 10B). Figure 10C shows the cardiac KATP channel I–V relationships before and after 5 μm 11,12-EET applied intracellularly or extracellularly in the presence of MIBG. These results were similar to those obtained without MIBG incubation, suggesting that the EET effects were independent of ADP-ribosylation.
Figure 10. Effects of 11,12-EET on cardiac KATP channels are not inhibited by MIBG.
Recordings of HMR-1098-sensitive KATP currents recorded in rat ventricular myocytes after incubation with 100 μm MIBG (60 min), showing the effects of extracellularly (A) and intracellularly (B) applied 11,12-EET (5 μm). Incubation with MIBG (60 min) did not alter the activation of cardiac KATP channels by 11,12-EET. C, cardiac KATP channel I–V relationships with no EET treatment (○), and with 11,12-EET (5 μm) applied extracellularly (▪) or intracellularly (•). n = 6 for all groups; *P < 0.05, intracellularly applied 11,12-EET versus baseline; †P < 0.05, extracellularly applied 11,12-EET versus baseline; ‡P < 0.05, intracellularly applied versus extracellularly applied 11,12-EET. D, recordings of glyburide-sensitive KATP currents in rat mesenteric smooth muscle cells after treatment with 100 μm MIBG. E, vascular KATP channel I–V relationships after treatment with MIBG at baseline (□) and after exposure to 11,12-EET (▪), showing that the effects of extracellularly applied 11,12-EET were abolished, n = 6. F, bar graphs comparing the percentage KATP current increase in cardiac myocytes (left panel) and vascular smooth muscle cells (right panel) by 11,12-EET applied intracellularly and extracellularly with and without treatment with MIBG. Group data showing the cardiac and vascular KATP current densities measured at −100 mV.
In contrast, incubation with MIBG totally abolished the effects of 11,12-EET on vascular KATP channel activation (TP = −100 mV, −1.73 ± 0.51 pA pF−1 at baseline and −2.50 ± 1.02 with 11,12-EET, n = 6, N.S.) (Fig. 10D), suggesting that ADP-ribosyltransferase activity is crucial in mediating the activation of vascular KATP channels by EETs. Figure 10E shows the I–V relationships and illustrates that 11,12-EET applied extracellularly had no effect on vascular KATP channel activities at all voltages examined. We did not examine the effects of intracellularly applied 11,12-EET after MIBG incubation since there was no vascular KATP channel activation by EETs at baseline (see the right column of Fig. 8B). Comparison between the effects of 11,12-EET on cardiac and vascular KATP channels, with and without MIBG incubation are summarized in Fig. 10F.
Discussion
We have made a number of key observations in this study. First, we have demonstrated that AA activates cardiac and vascular KATP channels through CYP metabolites. Second, EETs activate cardiac and vascular KATP channels through different mechanisms. Specifically, EETs potently activated cardiac KATP channels when applied intracellularly but activated vascular KATP channels when applied extracellularly. Third, activation of cardiac KATP channels by EETs does not involve c-AMP dependent signalling, whereas activation of vascular KATP channels by EETs is dependent on PKA and ADP-ribosyltransferase activities. Fourth, cardiac KATP channel activities in rat and mice are tightly coupled to CYP epoxygenase activities.
EETs are endothelium-derived hyperpolarizing factors (EDHFs) that modulate vascular tone in various vessel beds including human coronary arteries (Fisslthaler et al. 1999; Archer et al. 2003). Inhibition of CYP epoxygenases attenuates EDHF-mediated vasodilatation in resistance arteries (Bolz et al. 2000). Products of CYP epoxygenases are among the most potent activators of the large conductance Ca2+-activated K+ (BK) channels, producing vasodilatation through hyperpolarization of membrane potentials in vascular smooth muscle cells (Lu et al. 2001b; Ye et al. 2002; Weston et al. 2005). In isolated rat hearts, application of 5 μm 11,12-EET significantly improved postischaemic cardiac contractility (Wu et al. 1997). Transgenic mice overexpressing cardiac CYP2J2 exhibit an improvement in the postischaemic recovery of left ventricular function (Seubert et al. 2004). The importance of these findings is underscored by a recent report that a polymorphism in the proximal promoter of human CYP2J2 leads to reduced gene expression and is associated with an increase risk of coronary artery disease, indicating a cardioprotective role of CYP2J2 in humans (Spiecker et al. 2004).
KATP channels are activated by various lipid metabolites including the long chain acyl-CoA esters (Schulze et al. 2003), phosphatidylinositol 4,5-bisphosphate (Baukrowitz et al. 1998; Shyng & Nichols, 1998) and l-palmitoylcarnitine (Haruna et al. 2000). We have previously reported that action potentials in cardiac myocytes can be modulated by EETs through activation of KATP channels. The effects of EET on cardiac KATP channels have an EC50 of 30 nm and do not require the presence of intracellular signalling agents such as GTP (Lu et al. 2001a). Unlike other lipid metabolites, the effects of EET on cardiac KATP channels are stereospecific (Lu et al. 2002). Using site-directed mutagenesis, we have determined that R192 and R195 on the C-terminus of Kir6.2 are critical sites for the EET effects (Lu et al. 2005). However, the physiological relevance of this finding is still not fully understood. In particular, the ability of endogenously produced EETs to activate cardiac KATP channels in the presence of physiological concentrations of cytoplasmic ATP has not been previously demonstrated. The results of the present study show that CYP metabolites of AA could enhance cardiac KATP currents severalfold. Application of 1 μm AA produced a 219% increase in KATP channel activities. The AA effects were mediated by CYP epoxygenase products, because they could be completely abolished by CYP epoxygenase inhibitors, miconazole or SKF525A. These inhibitors also reduced the baseline KATP currents suggesting that the steady-state production of EETs in cardiac myocytes is important in regulating the basal KATP currents in these cells. In CYP2J2 transgenic mice, cardiac KATP current densities were 190% higher than that of WT, suggesting that basal KATP channel activities are closely regulated by CYP2J2 products. Moreover, exposure to 1 μm AA resulted in further stimulation of KATP channel activities to levels that were 520% higher than that of WT at baseline. The enhanced KATP channel activities in the transgenic animals are not due to increased channel expression but are the result of enhanced CYP activities since miconazole reduced channel activities to that of WT and abolished the AA effects. Our results from the CYP2J2 transgenic animals have important physiological and clinical implications since CYP enzymes are known to be induced by chemicals and drugs, some of which are commonly used in clinical practice (Fisslthaler et al. 2003). Hence, AA can modulate the cardiac action potential through activation of KATP channels by its CYP metabolites.
Another important observation is that the effects of CYP products of AA, mainly EETs, on cardiac KATP channels are voltage dependent, which is consistent with our previous observation in single channel studies (Lu et al. 2001a). Cardiac KATP currents were activated preferentially at voltages negative to EK, hence potentiating the inward rectifying properties of the channel, converting it from a weak into a relatively strong inward rectifier. This has important physiological and clinical implications because a strong inward rectifier K+ current is potentially antiarrhythmic, maintaining a hyperpolarized membrane potential and preventing the development of automaticity (Dhamoon & Jalife, 2005). Indeed, transgenic animals with ablation of the Kir6.2 gene are predisposed to catecholamine-induced ventricular arrhythmia (Liu et al. 2004). We have also found that 11,12-EET had no effect on the activities of cardiac IK1, the major inward rectifier K+ channel in the heart (Fig. 3). Hence, during cardiac ischaemia when EET production is enhanced (Nithipatikom et al. 2001) and IK1 activities are reduced (Aimond et al. 1999), activation of KATP channels by EETs may provide antiarrhythmic protection in addition to the cardioprotection effects against reperfusion injuries (Nithipatikom et al. 2006). EETs are unique cardiac KATP channel activators because of the voltage dependence of channel activation (Figs 1, 2 and 3) and the stereo-specific effects (Lu et al. 2002). These unusual properties could potentially be exploited for therapeutic strategies. Currently, the mechanism of voltage dependence of EET effects on cardiac KATP channels is not fully understood. Whether the modulation of KATP currents by EETs plays a role in mediating cardioprotection from antiarrhythmic effects deserves further investigation.
In addition to the direct effects on ion channels, EETs are known to activate various kinase signalling pathways, including tyrosine kinases, MAP kinases, extracellular signal regulated kinases, as well as protein kinase B/Akt (Fleming et al. 2001), and have been shown to modulate ion channels through second messenger signalling pathways. Enhancement of bovine coronary BK channel activities by EETs is mediated through ADP-ribosylation of Gsα (Li et al. 1999). EETs have been shown to activate rat cardiac L-type Ca2+ currents through increased intracellular cAMP (Xiao et al. 1998, 2004). The MAP kinase signalling pathway has been shown to mediate mitochondria KATP channel activation by EETs (Seubert et al. 2004). Recently, we have reported that activation of mesenteric smooth muscle KATP channels by EETs is dependent on PKA activities and this contributes to 50% of the total vasodilatation by EETs in these vessels (Ye et al. 2005,2006). However, the molecular steps through which EETs activate vascular KATP channels are still unclear. Specific cell surface EET ‘receptors’ have been postulated to exist. The specific high affinity binding sites for 14,15-EET in U-937 cells may be associated with an EET receptor (Wong et al. 1997). The regio- and enantio-specific effects of EETs on vasoreactivity and ionic channel regulation strongly suggests that they may involve a ‘receptor’ mechanism. In renal arterial smooth cells, only the 11(R),12(S)-EET enantiomer, but not the 11(S),12(R)-EET enantiomer nor the 5,6–8,9- and 14,15-EET regioisomers, could activate BK currents (Zou et al. 1996). In rat cortical collecting ducts, only 11,12-EET could inhibit epithelial Na+ channel activity, while the other EET regioisomers had no effects (Wei et al. 2004). In bovine coronary arterial smooth muscle cells, pretreatment with 14,15-EEZE-mSI preferentially inhibited the 14,15-EET effects on BK channel activation and vasodilatation (Gauthier et al. 2003), suggesting specific structural requirements of the activities of EETs. Recently, cAMP-induced aromatase activities in cultured aortic smooth muscle cells were found to be inhibited by 14,15-EET, by the non-metabolized N-methylsulfanilamide derivative of 14,15-EET (14,15-EET-SI), and by the membrane-impermeant 14,15-EET-SI that was covalently tethered to silica beads. These results suggest the presence of a specific EET binding site, possibly a receptor, in the plasma membrane (Snyder et al. 2002). Our results on the effects of EETs on vascular KATP channels are consistent with the presence of a cell surface EET receptor since EETs exert their effects mainly from the extracellular side of the cell membrane. Intracellular application of EETs has minimal effect on vascular KATP channels and the EET effects were dependent on ADP-ribosyltransferase and PKA activities. However, definitive mechanisms have to await direct identification of the putative EET receptor(s). We have also noted that exposure to AA produced a small but significant increase in the vascular KATP current at very negative voltages. The mechanism of this increase is not entirely clear at present but could be due to formation of CYP products other than EETs or possible synergistic effects of the EET regioisomers.
In the present study, the contrast between the mechanisms through which EETs activate cardiac and vascular KATP channels is quite impressive. 11,12-EET activates cardiac KATP channels most effectively from the cytoplasmic side, whereas activation of the vascular KATP channels occurs from the extracellular surface of the membrane. In addition, treatments with PKI and MIBG did not alter the sensitivity of KATP channels to 11,12-EET in cardiac myocytes, but totally abolished it in vascular smooth muscle cells. The mechanisms that underlie such disparity can be partly explained by the inherent structural and pharmacological difference between these channels. First, cardiac KATP channels are composed of SUR2A and Kir6.2 subunits, and vascular channels of SUR2B and Kir6.1 (Seino & Miki, 2003). Second, cardiac and vascular KATP channels have different sensitivity to ATP. The ATP inhibition curve of cardiac KATP channels is sigmoidal with an IC50 of 30 μm, while that of vascular KATP channels is bell-shaped with channel stimulation by 0.1–1 mm ATP and channel inhibition by 3–5 mm ATP (Yokoshiki et al. 1998). Third, the electrophysiological properties of the two channels are different. The cardiac KATP channel is weakly inwardly rectifying with a conductance of 80 pS in symmetrical K+, whereas the vascular KATP channel I–V relationship is almost linear with a conductance of 33 pS. Fourth, their pharmacological properties are different. Nucleotide diphosphate (NDP) stimulation by ADP, UDP and GDP is critical for vascular but not cardiac KATP channel activation (Quayle et al. 1997; Yokoshiki et al. 1998). Vascular KATP channels are known to be more sensitive to sulphonylurea drugs such as glyburide and tolbutamide, as well as to the K+ channel opener diazoxide than cardiac KATP channels, implying that the properties of the SUR subunit may contribute to the diverse functions of the KATP channel isoforms. The physiological inference from our results is that KATP channels in vascular smooth muscle cells can be readily regulated by EETs through paracrine functions, whereas KATP channels in cardiac myocytes are tightly coupled to endogenous production of EETs which depend on the CYP epoxygenase activities. Since EETs are important signalling agents in the cardiovascular system, regulating a multitude of physiological functions, our findings in this study help delineate the mechanism of KATP channel regulation by these lipid metabolites and aid our understanding of the role of EETs in the regulation of cardiovascular physiology.
Acknowledgments
This work was supported by grants from the American heart Association (0265472Z to T.L.) and from the National Institute of Health (HL-63754, HL-74180 to H.L.). This research was also supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences. We thank Aventis Pharmaceuticals Co. (Frankfurt, Germany) for HMR-1098.
References
- Aguilar-Bryan L, Clement JPT, Gonzalez G, Kunjilwar K, Babenko A, Bryan J. Toward understanding the assembly and structure of KATP channels. Physiol Rev. 1998;78:227–245. doi: 10.1152/physrev.1998.78.1.227. [DOI] [PubMed] [Google Scholar]
- Aimond F, Alvarez JL, Rauzier JM, Lorente P, Vassort G. Ionic basis of ventricular arrhythmias in remodeled rat heart during long-term myocardial infarction. Cardiovasc Res. 1999;42:402–415. doi: 10.1016/s0008-6363(99)00070-x. [DOI] [PubMed] [Google Scholar]
- Archer SL, Gragasin FS, Wu X, Wang S, McMurtry S, Kim DH, Platonov M, Koshal A, Hashimoto K, Campbell WB, Falck JR, Michelakis ED. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BKCa channels. Circulation. 2003;107:769–776. doi: 10.1161/01.cir.0000047278.28407.c2. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- Bienengraeber M, Olson TM, Selivanov VA, Kathmann EC, O'Cochlain F, Gao F, Karger AB, Ballew JD, Hodgson DM, Zingman LV, Pang YP, Alekseev AE, Terzic A. ABCC9 mutations identified in human dilated cardiomyopathy disrupt catalytic KATP channel gating. Nat Genet. 2004;36:382–387. doi: 10.1038/ng1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolz SS, Fisslthaler B, Pieperhoff S, De Wit C, Fleming I, Busse R, Pohl U. Antisense oligonucleotides against cytochrome P450 2C8 attenuate EDHF-mediated Ca2+ changes and dilation in isolated resistance arteries. FASEB J. 2000;14:255–260. doi: 10.1096/fasebj.14.2.255. [DOI] [PubMed] [Google Scholar]
- Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- Chen J, Capdevila JH, Zeldin DC, Rosenberg RL. Inhibition of cardiac L-type calcium channels by epoxyeicosatrienoic acids. Mol Pharmacol. 1999;55:288–295. doi: 10.1124/mol.55.2.288. [DOI] [PubMed] [Google Scholar]
- Crawford RM, Ranki HJ, Botting CH, Budas GR, Jovanovic A. Creatine kinase is physically associated with the cardiac ATP-sensitive K+ channel in vivo. Faseb J. 2002;16:102–104. doi: 10.1096/fj.01-0466fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamoon AS, Jalife J. The inward rectifier current (IK1) controls cardiac excitability and is involved in arrhythmogenesis. Heart Rhythm. 2005;2:316–324. doi: 10.1016/j.hrthm.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Fisslthaler B, Michaelis UR, Randriamboavonjy V, Busse R, Fleming I. Cytochrome P450 epoxygenases and vascular tone: novel role for HMG-CoA reductase inhibitors in the regulation of CYP 2C expression. Biochim Biophys Acta. 2003;1619:332–339. doi: 10.1016/s0304-4165(02)00492-0. [DOI] [PubMed] [Google Scholar]
- Fisslthaler B, Popp R, Kiss L, Potente M, Harder DR, Fleming I, Busse R. Cytochrome P450, 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- Fleming I, Fisslthaler B, Michaelis UR, Kiss L, Popp R, Busse R. The coronary endothelium-derived hyperpolarizing factor (EDHF) stimulates multiple signalling pathways and proliferation in vascular cells. Pflugers Arch. 2001;442:511–518. doi: 10.1007/s004240100565. [DOI] [PubMed] [Google Scholar]
- Gauthier KM, Jagadeesh SG, Falck JR, Campbell WB. 14,15-Epoxyeicosa-5(Z)-enoic-mSI: a 14,15- and 5,6-EET antagonist in bovine coronary arteries. Hypertension. 2003;42:555–561. doi: 10.1161/01.HYP.0000091265.94045.C7. [DOI] [PubMed] [Google Scholar]
- Gross GJ, Falck JR, Gross ER, Isbell M, Moore J, Nithipatikom K. Cytochrome P450 and arachidonic acid metabolites: Role in myocardial ischemia/reperfusion injury revisited. Cardiovasc Res. 2005;68:18–25. doi: 10.1016/j.cardiores.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Haruna T, Horie M, Takano M, Kono Y, Yoshida H, Otani H, Kubota T, Ninomiya T, Akao M, Sasayama S. Alteration of the membrane lipid environment by 1-palmitoylcarnitine modulates KATP channels in guinea-pig ventricular myocytes. Pflugers Arch. 2000;441:200–207. doi: 10.1007/s004240000428. [DOI] [PubMed] [Google Scholar]
- Hodgson DM, Zingman LV, Kane GC, Perez-Terzic C, Bienengraeber M, Ozcan C, Gumina RJ, Pucar D, O'Coclain F, Mann DL, Alekseev AE, Terzic A. Cellular remodeling in heart failure disrupts KATP channel-dependent stress tolerance. EMBO J. 2003;22:1732–1742. doi: 10.1093/emboj/cdg192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Sato T, Arita M. Protein kinase C isoform-dependent modulation of ATP-sensitive K+ channels during reoxygenation in guinea-pig ventricular myocytes. J Physiol. 2001;532:165–174. doi: 10.1111/j.1469-7793.2001.0165g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LM, Ma J, Srettabunjong S, Graves J, Bradbury JA, Li L, Spiecker M, Liao JK, Mohrenweiser H, Zeldin DC. Cloning of CYP2J2 gene and identification of functional polymorphisms. Mol Pharmacol. 2002;61:840–852. doi: 10.1124/mol.61.4.840. [DOI] [PubMed] [Google Scholar]
- Koyano T, Kakei M, Nakashima H, Yoshinaga M, Matsuoka T, Tanaka H. ATP-regulated K+ channels are modulated by intracellular H+ in guinea-pig ventricular cells. J Physiol. 1993;463:747–766. doi: 10.1113/jphysiol.1993.sp019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson K. Potassium channel openers as potential therapeutic weapons in ion channel disease. Kidney Int. 2000;57:838–845. doi: 10.1046/j.1523-1755.2000.00923.x. [DOI] [PubMed] [Google Scholar]
- Lee H, Lu T, Weintraub NL, VanRollins M, Spector AA, Shibata EF. Effects of epoxyeicosatrienoic acids on the cardiac sodium channels in isolated rat ventricular myocytes. J Physiol. 1999;519:153–168. doi: 10.1111/j.1469-7793.1999.0153o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li PL, Chen CL, Bortell R, Campbell WB. 11,12-Epoxyeicosatrienoic acid stimulates endogenous mono-ADP-ribosylation in bovine coronary arterial smooth muscle. Circ Res. 1999;85:349–356. doi: 10.1161/01.res.85.4.349. [DOI] [PubMed] [Google Scholar]
- Lin YF, Jan YN, Jan LY. Regulation of ATP-sensitive potassium channel function by protein kinase A-mediated phosphorylation in transfected HEK293 cells. EMBO J. 2000;19:942–955. doi: 10.1093/emboj/19.5.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XK, Yamada S, Kane GC, Alekseev AE, Hodgson DM, O'Cochlain F, Jahangir A, Miki T, Seino S, Terzic A. Genetic disruption of Kir6.2, the pore-forming subunit of ATP-sensitive K+ channel, predisposes to catecholamine-induced ventricular dysrhythmia. Diabetes. 2004;53(Suppl. 3):S165–S168. doi: 10.2337/diabetes.53.suppl_3.s165. [DOI] [PubMed] [Google Scholar]
- Lu T, Hong MP, Lee H. Molecular determinants of cardiac KATP channel activation by epoxyeicosatrienoic acids. J Biol Chem. 2005;280:19097–19104. doi: 10.1074/jbc.M414065200. [DOI] [PubMed] [Google Scholar]
- Lu T, Hoshi T, Weintraub NL, Spector AA, Lee H. Activation of ATP-sensitive K+ channels by epoxyeicosatrienoic acids in rat cardiac ventricular myocytes. J Physiol. 2001a;537:811–827. doi: 10.1111/j.1469-7793.2001.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Katakam PV, VanRollins M, Weintraub NL, Spector AA, Lee H. Dihydroxyeicosatrienoic acids are potent activators of Ca2+-activated K+ channels in isolated rat coronary arterial myocytes. J Physiol. 2001b;534:651–667. doi: 10.1111/j.1469-7793.2001.t01-1-00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Lee H, Kabat JA, Shibata EF. Modulation of rat cardiac sodium channel by the stimulatory G protein α subunit. J Physiol. 1999;518:371–384. doi: 10.1111/j.1469-7793.1999.0371p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, VanRollins M, Lee H. Stereospecific activation of cardiac ATP-sensitive K+ channels by epoxyeicosatrienoic acids: a structural determinant study. Mol Pharmacol. 2002;62:1076–1083. doi: 10.1124/mol.62.5.1076. [DOI] [PubMed] [Google Scholar]
- Masuda H, Sperelakis N. Inwardly rectifying potassium current in rat fetal and neonatal ventricular cardiomyocytes. Am J Physiol. 1993;265:H1107–H1111. doi: 10.1152/ajpheart.1993.265.4.H1107. [DOI] [PubMed] [Google Scholar]
- Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, Koseki H, Iwanaga T, Nakaya H, Seino S. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med. 2002;8:466–472. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- Morrissey A, Rosner E, Lanning J, Parachuru L, Dhar Chowdhury P, Han S, Lopez G, Tong X, Yoshida H, Nakamura TY, Artman M, Giblin JP, Tinker A, Coetzee WA. Immunolocalization of KATP channel subunits in mouse and rat cardiac myocytes and the coronary vasculature. BMC Physiol. 2005;5:1. doi: 10.1186/1472-6793-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura TY, Artman M, Rudy B, Coetzee WA. Inhibition of rat ventricular IK1 with antisense oligonucleotides targeted to Kir2.1 mRNA. Am J Physiol. 1998;274:H892–H900. doi: 10.1152/ajpheart.1998.274.3.H892. [DOI] [PubMed] [Google Scholar]
- Nithipatikom K, DiCamelli RF, Kohler S, Gumina RJ, Falck JR, Campbell WB, Gross GJ. Determination of cytochrome P450 metabolites of arachidonic acid in coronary venous plasma during ischemia and reperfusion in dogs. Anal Biochem. 2001;292:115–124. doi: 10.1006/abio.2001.5044. [DOI] [PubMed] [Google Scholar]
- Nithipatikom K, Moore JM, Isbell MA, Falck JR, Gross GJ. Epoxyeicosatrienoic acids (EETs) in cardioprotection: Ischemic versus reperfusion injury. Am J Physiol Heart Circ Physiol. 2006 doi: 10.1152/ajpheart.00071.2006. in press. [DOI] [PubMed] [Google Scholar]
- Node K, Huo Y, Ruan X, Yang B, Spiecker M, Ley K, Zeldin DC, Liao JK. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potente M, Michaelis UR, Fisslthaler B, Busse R, Fleming I. Cytochrome P450 2C9-induced endothelial cell proliferation involves induction of mitogen-activated protein (MAP) kinase phosphatase-1, inhibition of the c-Jun N-terminal kinase, and up-regulation of cyclin D1. J Biol Chem. 2002;277:15671–15676. doi: 10.1074/jbc.M110806200. [DOI] [PubMed] [Google Scholar]
- Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- Rajashree R, Koster JC, Markova KP, Nichols CG, Hofmann PA. Contractility and ischemic response of hearts from transgenic mice with altered sarcolemmal KATP channels. Am J Physiol Heart Circ Physiol. 2002;283:H584–H590. doi: 10.1152/ajpheart.00107.2002. [DOI] [PubMed] [Google Scholar]
- Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- Saito T, Sato T, Miki T, Seino S, Nakaya H. Role of ATP-sensitive K+ channels in electrophysiological alterations during myocardial ischemia: a study using Kir6.2-null mice. Am J Physiol Heart Circ Physiol. 2005;288:H352–H357. doi: 10.1152/ajpheart.00695.2004. [DOI] [PubMed] [Google Scholar]
- Sakmann B, Trube G. Voltage-dependent inactivation of inward-rectifying single-channel currents in the guinea-pig heart cell membrane. J Physiol. 1984;347:659–683. doi: 10.1113/jphysiol.1984.sp015089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze D, Rapedius M, Krauter T, Baukrowitz T. Long-chain acyl-CoA esters and phosphatidylinositol phosphates modulate ATP inhibition of KATP channels by the same mechanism. J Physiol. 2003;552:357–367. doi: 10.1113/jphysiol.2003.047035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol. 2003;81:133–176. doi: 10.1016/s0079-6107(02)00053-6. [DOI] [PubMed] [Google Scholar]
- Seubert J, Yang B, Bradbury JA, Graves J, Degraff LM, Gabel S, Gooch R, Foley J, Newman J, Mao L, Rockman HA, Hammock BD, Murphy E, Zeldin DC. Enhanced postischemic functional recovery in CYP2J2 transgenic hearts involves mitochondrial ATP-sensitive K+ channels and p42/p44 MAPK pathway. Circ Res. 2004;95:506–514. doi: 10.1161/01.RES.0000139436.89654.c8. [DOI] [PubMed] [Google Scholar]
- Shyng SL, Nichols CG. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science. 1998;282:1138–1141. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- Snyder GD, Krishna UM, Falck JR, Spector AA. Evidence for a membrane site of action for 14,15-EET on expression of aromatase in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2002;283:H1936–H1942. doi: 10.1152/ajpheart.00321.2002. [DOI] [PubMed] [Google Scholar]
- Spiecker M, Darius H, Hankeln T, Soufi M, Sattler AM, Schaefer JR, Node K, Borgel J, Mugge A, Lindpaintner K, Huesing A, Maisch B, Zeldin DC, Liao JK. Risk of coronary artery disease associated with polymorphism of the cytochrome P450 epoxygenase CYP2J2. Circulation. 2004;110:2132–2136. doi: 10.1161/01.CIR.0000143832.91812.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Li RA, Miki T, Uemura H, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, Ogura T, Seino S, Marban E, Nakaya H. Functional roles of cardiac and vascular ATP-sensitive potassium channels clarified by Kir6.2-knockout mice. Circ Res. 2001;88:570–577. doi: 10.1161/01.res.88.6.570. [DOI] [PubMed] [Google Scholar]
- Terzic A, Jahangir A, Kurachi Y. Cardiac ATP-sensitive K+ channels: regulation by intracellular nucleotides and K+ channel-opening drugs. Am J Physiol. 1995;269:C525–C545. doi: 10.1152/ajpcell.1995.269.3.C525. [DOI] [PubMed] [Google Scholar]
- Terzic A, Tung RT, Inanobe A, Katada T, Kurachi Y. G proteins activate ATP-sensitive K+ channels by antagonizing ATP- dependent gating. Neuron. 1994;12:885–893. doi: 10.1016/0896-6273(94)90340-9. [DOI] [PubMed] [Google Scholar]
- Tung RT, Kurachi Y. On the mechanism of nucleotide diphosphate activation of the ATP-sensitive K+ channel in ventricular cell of guinea-pig. J Physiol. 1991;437:239–256. doi: 10.1113/jphysiol.1991.sp018593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XL, Ye D, Peterson TE, Cao S, Shah VH, Katusic ZS, Sieck GC, Lee H. Caveolae targeting and regulation of large conductance Ca2+-activated K+ channels in vascular endothelial cells. J Biol Chem. 2005;280:11656–11664. doi: 10.1074/jbc.M410987200. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- Wei Y, Lin DH, Kemp R, Yaddanapudi GS, Nasjletti A, Falck JR, Wang WH. Arachidonic acid inhibits epithelial Na channel via cytochrome P450 (CYP) epoxygenase-dependent metabolic pathways. J General Physiol. 2004;124:719–727. doi: 10.1085/jgp.200409140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston AH, Feletou M, Vanhoutte PM, Falck JR, Campbell WB, Edwards G. Bradykinin-induced, endothelium-dependent responses in porcine coronary arteries: involvement of potassium channel activation and epoxyeicosatrienoic acids. Br J Pharmacol. 2005;145:775–784. doi: 10.1038/sj.bjp.0706256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PY, Lai PS, Shen SY, Belosludtsev YY, Falck JR. Post-receptor signal transduction and regulation of 14(R),15(S)-epoxyeicosatrienoic acid (14,15-EET) binding in U-937 cells. J Lipid Med Cell Signal. 1997;16:155–169. doi: 10.1016/s0929-7855(97)00005-9. [DOI] [PubMed] [Google Scholar]
- Wu S, Chen W, Murphy E, Gabel S, Tomer KB, Foley J, Steenbergen C, Falck JR, Moomaw CR, Zeldin DC. Molecular cloning, expression, and functional significance of a cytochrome P450 highly expressed in rat heart myocytes. J Biol Chem. 1997;272:12551–12559. doi: 10.1074/jbc.272.19.12551. [DOI] [PubMed] [Google Scholar]
- Wu S, Moomaw CR, Tomer KB, Falck JR, Zeldin DC. Molecular cloning and expression of CYP2J2, a human cytochrome P450 arachidonic acid epoxygenase highly expressed in heart. J Biol Chem. 1996;271:3460–3468. doi: 10.1074/jbc.271.7.3460. [DOI] [PubMed] [Google Scholar]
- Xiao YF, Huang L, Morgan JP. Cytochrome P450: a novel system modulating Ca2+ channels and contraction in mammalian heart cells. J Physiol. 1998;508:777–792. doi: 10.1111/j.1469-7793.1998.777bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao YF, Ke Q, Seubert JM, Bradbury JA, Graves J, Degraff LM, Falck JR, Krausz K, Gelboin HV, Morgan JP, Zeldin DC. Enhancement of cardiac L-type Ca2+ currents in transgenic mice with cardiac-specific overexpression of CYP2J2. Mol Pharmacol. 2004;66:1607–1616. doi: 10.1124/mol.104.004150. [DOI] [PubMed] [Google Scholar]
- Yokoshiki H, Sunagawa M, Seki T, Sperelakis N. ATP-sensitive K+ channels in pancreatic, cardiac, and vascular smooth muscle cells. Am J Physiol. 1998;274:C25–C37. doi: 10.1152/ajpcell.1998.274.1.C25. [DOI] [PubMed] [Google Scholar]
- Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- Ye D, Zhang D, Oltman C, Dellsperger K, Lee H, VanRollins M. Cytochrome p-450 epoxygenase metabolites of docosahexaenoate potently dilate coronary arterioles by activating large-conductance calcium-activated potassium channels. J Pharmacol Exp Ther. 2002;303:768–776. doi: 10.1124/jpet.303.2.768. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Oltman CL, Lu T, Lee H, Dellsperger KC, VanRollins M. EET homologs potently dilate coronary microvessels and activate BKCa channels. Am J Physiol Heart Circ Physiol. 2001;280:H2430–H2440. doi: 10.1152/ajpheart.2001.280.6.H2430. [DOI] [PubMed] [Google Scholar]
- Ye D, Zhou W, Lee H. Activation of rat mesenteric arterial KATP channels by 11,12-epoxyeicosatrienoic acid. Am J Physiol Heart Circ Physiol. 2005;288:H358–H364. doi: 10.1152/ajpheart.00423.2004. [DOI] [PubMed] [Google Scholar]
- Ye D, Zhou W, Lu T, Jagadeesh SG, Falck JR, Lee H. Mechanism of rat mesenteric arterial KATP channel activation by 14,15-epoxyeicosatrienoic acid. Am J Physiol Heart Circ Physiol. 2006;290:H1326–H1336. doi: 10.1152/ajpheart.00318.2005. [DOI] [PubMed] [Google Scholar]
- Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, Pucar D, Bienengraeber M, Dzeja PP, Miki T, Seino S, Alekseev AE, Terzic A. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci U S A. 2002;99:13278–13283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. Stereospecific effects of epoxyeicosatrienoic acids on renal vascular tone and K+-channel activity. Am J Physiol. 1996;270:F822–F832. doi: 10.1152/ajprenal.1996.270.5.F822. [DOI] [PubMed] [Google Scholar]