Abstract
Intracellular Ca2+ regulates a variety of neuronal functions, including neurotransmitter release, protein phosphorylation, gene expression and synaptic plasticity. In a variety of cell types, including neurons, Ca2+ is involved in actin reorganization, resulting in either actin polymerization or depolymerization. Very little, however, is known about the relationship between Ca2+ and the actin cytoskeleton organization in retinal neurons. We studied the effect of high-K+-induced depolarization on F-actin organization in salamander retina and found that Ca2+ influx through voltage-gated L-type channels causes F-actin disruption, as assessed by 53 ± 5% (n = 23, P < 0.001) reduction in the intensity of staining with Alexa-Fluor488-phalloidin, a compound that permits visualization and quantification of polymerized actin. Calcium-induced F-actin depolymerization was attenuated in the presence of protein kinase C antagonists, chelerythrine or bis-indolylmaleimide hydrochloride (GF 109203X). In addition, phorbol 12-myristate 13-acetate (PMA), but not 4α-PMA, mimicked the effect of Ca2+ influx on F-actin. Activation of ionotropic AMPA and NMDA glutamate receptors also caused a reduction in F-actin. No effect on F-actin was exerted by caffeine or thapsigargin, agents that stimulate Ca2+ release from internal stores. In whole-cell recording from a slice preparation, light-evoked ‘off’ but not ‘on’ EPSCs in ‘on–off’ ganglion cells were reduced by 60 ± 8% (n = 8, P < 0.01) by cytochalasin D. These data suggest that elevation of intracellular Ca2+ during excitatory synaptic activity initiates a cascade for activity-dependent actin remodelling, which in turn may serve as a feedback mechanism to attenuate excitotoxic Ca2+ accumulation induced by synaptic depolarization.
The important roles of intracellular Ca2+ and of Ca2+-activated neuroregulatory processes in modulating ligand- and voltage-activated channels in retinal neurons have been extensively studied in recent years (reviewed by Akopian & Witkovsky, 2002). One of the major sources for intracellular Ca2+ elevation is calcium influx through voltage-activated Ca2+ channels, which are activated when cells receive excitatory drive through synaptic channels. Such changes in intracellular Ca2+ during synaptic transmission are associated with important physiological processes, such as synaptic plasticity, neuronal differentiation and neuromodulation (Chittajallu et al. 1998). Calcium is also implicated in neurodegeneration and cell death in such diseases as glaucoma and retinal ischaemia, in which neurotoxicity is caused by an excessive stimulation of glutamate receptors (reviewed by Sucher et al. 1997).
In a variety of cell types, one important action of Ca2+ influx is its ability to interfere with the structure of the actin cytoskeleton. The cytoskeleton is intimately involved in the regulation of cell motility, excitotoxicity, receptor activity and synaptic function (Neely & Gesemann, 1994; Furukawa et al. 1995; Oertner & Matus, 2005). Studies indicate that Ca2+ induces diverse changes in F-actin structure that in some cases appear contradictory. For instance, Ca2+ influx has been associated with increases in the F-actin network in goldfish retinal bipolar cell terminals (Job & Lagnado, 1998) and in the induction of filopodia in grasshopper neurons (Lau et al. 1999), whereas in hippocampal neurons, Ca2+ influx caused a depolymerization of F-actin and a dislocation of dendritic spines (Rosenmund & Westbrook, 1993; Furukawa et al. 1995; Halpain et al. 1998; Brunig et al. 2004). The reason for these apparent discrepancies is not clear. It is possible that the different effects of Ca2+ on the actin cytoskeleton are associated with the presence of diverse Ca2+-activated proteins, such as gelsolin and profilin, and their targeted substrates that produced differential actions on the actin cytoskeleton (Oertner & Matus, 2005).
In salamander retinal ganglion cells, actin depolymerization causes a reduction in Ca2+ influx through voltage-activated L-type channels (Schubert & Akopian, 2004) and glutamate receptor-activated channels (Akopian et al. 2006), but whether Ca2+ entry affected F-actin organization in third-order retinal neurons was unknown. In this study, we show that Ca2+ influx through high-voltage-activated L-type channels causes F-actin depolymerization in salamander retinal neurons by a protein kinase C (PKC)-mediated mechanism.
Methods
Tissue and cell preparation
The handling and maintenance of animals met the National Institute of Health guidelines and were approved by the Institutional Care and Use Committee at NYU School of Medicine. Salamanders (Ambystoma tigrinum) were anaesthetized in a bath of tricaine methanosulphonate (100 mg ml−1) until the animal no longer reacted to tactile stimulation, after which they were decapitated and double pithed. The eyes were cut in half; both retinas were removed and placed in control Ringer solution containing (mm): NaCl, 100; KCl, 3; CaCl2, 2; MgCl2, 2; and Hepes, 10; adjusted to pH 7.6 with NaOH. Eyecups were used for preparation of cryostat sections. To dissociate cells, retinas were incubated for 1 h at room temperature with oxygenated Ringer solution containing 14 i.u. ml−1 of papain (Worthington Biochemicals, Lakewood, NJ, USA), 0.5 mm Ca2+ per 25 mm NaHCO3, 6 mg cysteine, 1 mm sodium pyruvate and 16 mm d-glucose. After rinses, retinas were dissociated in DMEM medium (Gibco, Carlsbad, CA, USA) using a wide-bore 5 ml pipette. The resulting cell suspension was plated on poly l-lysine-coated coverslips, and the cells were allowed to settle for 30–45 min before treatments.
Materials
Compounds were obtained from the following sources: Alexa-Fluor488-phalloidin (Molecular Probes, Eugene, OR, USA), glutamate, amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), N-methyl-d-aspartate (NMDA), chelerythrine chloride, bis-indolylmaleimide hydrochloride (GF 109203X), ω-conotoxin GVIA, nifedipine, thapsigargin, phorbol 12-myristate 13-acetate (PMA) and 4α-PMA (all from Sigma, St Louis, MO, USA), latrunculin A and cytochalasin D (Biomol International, Plymouth Meeting, PA, USA).
F-Actin staining of retinal cryostat sections
Control and high-K+-treated eyecups were fixed for 30 min in an ice-cold solution of 4% paraformaldehyde inphosphate-buffered saline (PBS; pH 7.4). After a wash, eyecups were dehydrated with 30% sucrose (overnight at 4°C), embedded in Tissue-Tek and sectioned at 15 μm on a cryostat. To visualize actin filaments, sections were treated with 0.3% Triton X-100 for 5 min, pre-incubated for 30 min with 3% bovine serum albumin (BSA; with 0.1% Triton X-100 in PBS), and incubated for 2 h at room temperature (RT) with Alexa-Fluor488-phalloidin diluted in the BSA–Triton X-100 solution to a final phalloidin concentration of 0.2–0.3 μm. After extensive rinsing, the tissues were mounted with Vectashield (Vector, Burlingame, CA, USA) and observed with a confocal laser-scanning microscope (Nikon Eclipse C-1; Nikon, Japan). Single-plane fluorescence images were acquired using the ×60 or ×100 oil-immersion objective lens and EZ-C1 software (Nikon). Images from control and high-K+-treated samples were obtained with the same scanning parameters and were processed using Adobe Photoshop version 7.0 (Adobe Systems Inc., San Jose, CA, USA). To control for day-to-day variations in staining intensity, treated specimens were always compared with controls prepared the same day under identical fixation, permeabilization, staining and microscopy conditions, thus allowing meaningful comparisons among samples.
F-Actin staining of acutely dissociated retinal cells
Isolated cells treated with either control or drug-containing solutions were fixed for 30 min in an ice-cold solution of 4% paraformaldehyde, 4% sucrose in PBS (pH 7.4). To stain actin filaments with Alexa-Fluor488-phalloidin, we used the procedure described above. Coverslips were mounted with Prolong Antifade Mounting Medium (Molecular Probes) and observed with a confocal laser-scanning microscope. Viability of cells included in quantitative analysis was assessed by morphological criteria; only cells with intact processes (presumed amacrine or ganglion) and soma with a smooth appearance were considered. For each treatment, three to five independent experiments were carried out with 12 coverslips in each experiment, and at least 20 cells in each of the control and treated samples were monitored for phalloidin staining. To quantify the changes in the actin network, scans of pixel intensity were performed by linescan using Metavue software (Universal Imaging Co., Downington, PA, USA) and the intensity profile was obtained along a line drawn through the centre of the dissociated cells, or by means of a bar ranging from the outer limit of the outer plexiform layer (OPL) to the inner limit of the inner plexiform layer (IPL) in vertical sections. For statistical analysis, the intensity at two peaks representing F-actin at either side of the cell was measured under different experimental conditions and normalized to a control value for a given series of experiments. All pooled data are presented as n±s.e.m. with n indicating the number of neurons tested. Levels of significance were assessed using Student's paired t test. Fluorescence intensities are indicated in arbitrary units (a.u.) from 0 to 250. A P value of less than 0.05 was considered to indicate statistical significance in all of the analysis.
Electrophysiology
Under dim red light illumination, retinas were isolated from the eyecup and placed over a piece of Millipore filter paper (pore size, 0.45 μm), with the ganglion cell layer facing up, and were cut into 150–200 μm slices with a custom-made slicer. Whole-cell patch clamp recordings were made from ‘on–off’ ganglion cells in the slice preparation using ∼5 MΩ electrodes containing (mm): potassium gluconate, 100; MgCl2, 2; CaCl2, 0.2; EGTA, 2; Hepes, 10; ATP, 2; and GTP, 0.1; adjusted to pH 7.3 with KOH. The bath (Ringer) solution contained (mm): NaCl, 100; KCl, 3; CaCl2, 2; MgCl2, 2; and Hepes, 10; adjusted to pH 7.6 with NaOH. Strychnine (20 μm) and picrotoxin (80 μm) were added to the bath solution to block glycinergic and GABAergic transmissions, respectively. The voltage- and current-clamp recordings were made with an Axopatch 200B amplifier. Analog signals were filtered at 1 kHz and sampled at 2 kHz with the Digidata 1322A analog-to-digital board. The PClamp8 software package (Axon Instruments) was used for data acquisition and analysis. Summary data are presented as means ±s.e.m. Full-field, red (667 nm) light stimuli were used to evoke EPSCs and EPSPs. The intensity of the unattenuated light was 7.3 × 109 quanta cm−2 s−1, as measured by a photodiode referenced to a calibrated thermopile. A 2 s light stimulus was presented each 30–60 s.
Live/dead assay
Dissociated cells were exposed either for 30 min to 100 μm glutamate (in the presence of 100 μm Cd2+), or for 5 min to modified Ringer solution containing 100 mm KCl, and then washed for 2 h and loaded with fluorescent dyes calcein-AM (2 μm) and ethidium homodimer (4 μm), for 30 min (Live/Dead assay, Molecular Probes). Cells were visualized with laser scanning confocal microscope and counted directly. The percentage number of viable cells (normalized to control value) was calculated as: (number of live cells/total number of cells) × 100. All data are expressed as the means ±s.e.m. Significance of differences between groups was assessed by Student's paired t test.
Results
Depolarization-induced F-actin disruption in retinal vertical sections
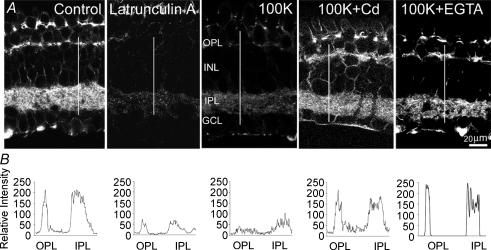
To investigate depolarization-induced changes in the organization of the actin cytoskeleton, one eyecup was treated with control Ringer solution while the second eyecup (from the same animal) was exposed for 5 min to modified Ringer solution containing 100 mm KCl. Both eyecups were then fixed and stained with Alexa-Fluor488-phalloidin. Under these conditions, the fluorescence intensity of phalloidin, used to stain, visualize and quantify the actin cytoskeleton (Dancker et al. 1975), was significantly reduced in the outer plexiform (OPL), the inner plexiform (IPL) and the ganglion cell layer (GCL), indicating that F-actin depolymerization had occurred. Representative confocal images of vertical cryostat sections (from 3 independent experiments), stained after exposure to control Ringer solution or after 5 min incubation with 100 mm KCl, show a depolarization-induced reduction in polymerized actin, as assessed by 60 ± 5% loss in Alexa-Fluor488-phalloidin intensity (Fig. 1A). To make sure that the loss of phalloidin staining was not the result of cell death after high-K+ treatment, we exposed retinal eyecups for 5 min to 100 mm KCl and then washed for 2 h; whole-cell voltage- and light-induced currents were then recorded from ganglion cells in a slice preparation. All cells responded to both voltage and light stimuli (not shown), indicating that they were still viable after exposure to high K+. The disruptive effect of high-K+-induced depolarization on F-actin closely resembled the action of 30 min exposure to 10 μm latrunculin A, an actin-depolymerizing agent (Spector et al. 1983). The effect of high K+ on phalloidin intensity was attenuated in the presence of CdCl2 (100 μm), which was applied 5 min prior to and during high-K+ treatment, indicating that Ca2+ influx through voltage-gated channels mediated the depolarization-induced F-actin disruption. The L-type Ca2+ channel blocker nifedipine (10 μm), applied 5 min before and during high-K+ treatment, eliminated the effect of K+-induced depolarization (not shown). In addition, when experiments were carried out in a nominally Ca2+-free Ringer solution (Ca2+ replaced with Mg2+), in the presence of 10 mm EGTA (applied 10 min before and during high-K+ treatment), the disrupting effect of high K+ on F-actin was significantly reduced. Fluorescence intensity profiles of the OPL and the IPL along the white line on each image are illustrated (Fig. 1B).
Figure 1. Distribution of F-actin in control and depolarized retina.
A, confocal fluorescence images of F-actin distribution in the vertical cryostat sections of retina treated with control Ringer solution (Control), after 30 min incubation with 1 μm latrunculin A, after 5 min exposure to modified Ringer solution containing 100 mm KCl alone (100K), or in combination with 100 μm CdCl2 (100K+Cd) or 10 mm EGTA (100K+EGTA). Phalloidin fluorescence intensity profiles across the line from the outer border of the OPL to inner boarder of the IPL in each experimental condition are illustrated (B). OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer.
Calcium-induced F-actin depolymerization in dissociated cells
It is usually difficult to observe detailed changes in fluorescence intensity of individual neurons in retinal sections. Therefore, further experiments were conducted on acutely dissociated cells. In our observations, we focused primarily on large cells (presumed amacrine or ganglion cells) that retained one or more long processes after dissociation, and which were easily distinguished on morphological grounds from photoreceptors, horizontal, bipolar and Müller cells.
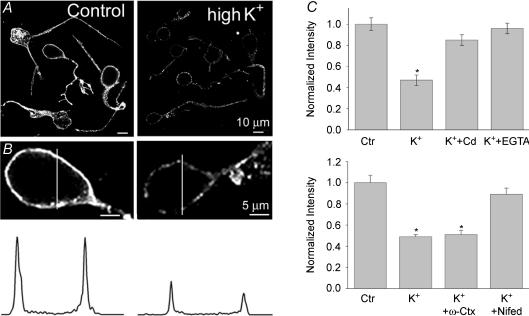
To test the effect of depolarization on F-actin organization, we briefly (5 min) exposed dissociated cells to modified Ringer solution containing 20–30 mm KCl and then fixed and stained them with Alexa-Fluor488-phalloidin. Figure 2A illustrates actin filament staining, which outlined the perimeter of the control cell body. Some phalloidin-stained actin filament granules were also detected in the cell's interior. High-K+-induced depolarization resulted in a loss of polymerized actin, as assessed by very faint phalloidin staining. To quantify the reorganization of the actin cytoskeleton, the intensity profile of F-actin staining along the indicated white lines was determined (Fig. 2B) and averaged for at least 15 cells from three to five independent experiments (each in control and high-K+ Ringer solution). The cytoskeleton organization in soma was characterized by two prominent peaks of F-actin staining, delineating the submembranous area, and a strong reduction in intensity between two peaks representing the interior of the cells. As observed in the vertical sections, block of voltage-activated Ca2+ channels by CdCl2 (100 μm), applied 5 min before and during the 5 min exposure to high K+, or removal of external free Ca2+ by EGTA (10 mm, applied 10 min before and during 5 min exposure to high K+), attenuated a disruptive effect of depolarization on F-actin (not illustrated). Quantitative analysis revealed a 53 ± 5% (n = 23, P < 0.001) reduction in phalloidin fluorescence intensity in cells treated with high K+ compared to untreated cells. In contrast, the mean reduction in fluorescence intensity elicited by depolarization in the presence of Cd2+ and EGTA were 4 ± 5% (n = 17, n.s.), and 5 ± 5% (n = 20, n.s.), respectively (Fig. 2C). Sincee a block of Ca2+ channels by Cd2+ eliminated depolarization-induced F-actin disruption, we next tested the specific contribution of high-voltage-activated N- and L-type channels. Dissociated cells were treated for 5 min with control Ringer solution containing 5 μm nifedipine, and then exposed for 5 min to high-K+ solution (in the continuous presence of nifedipine), prior to fixing and staining with Alexa-Fluor488-phalloidin. Blockade of L-type channels by nifedipine sharply attenuated the effect of depolarization, resulting in only 10 ± 6% (n = 15, P > 0.5) reduction in phalloidin intensity. In contrast, similar treatment with ω-conotoxin GVIA (1 μm), which blocks N-type Ca2+ channels, had no effect, since high K+ still reduced the fluorescence intensity of phalloidin staining by 50 ± 5% (n = 15, P < 0.001). The bar graph in Fig. 2C (lower panel) summarizes these results. Neither of these agents applied in control Ringer solution had any significant effect on the intensity of phalloidin staining (n = 10, not shown). These data, combined with those observed in retinal sections, indicate that depolarization-induced Ca2+ influx through high-voltage-activated L-type channels results in actin depolymerization in salamander retinal cells.
Figure 2. A depolarization-induced change in F-actin organization in retinal neurons is mediated by Ca2+ influx through L-type channels.
A, cells are stained with Alexa-Fluor488-phalloidin treated either in control Ringer solution (Control) or after 5 min exposure to modified Ringer solution containing 20 mm KCl (high K+). Magnified fluorescence confocal images of cell bodies and corresponding fluorescence intensity profiles across the indicated white lines are illustrated for control and high-K+-treated cells (B). C, the bar charts summarize the effects of 100 μm Cd2+ and 10 mm EGTA on high-K+-induced F-actin disruption. Nifedipine (10 μm), but not ω-conotoxin GVIA (1 μm) attenuated the disrupting effect when applied together with high K+. The change in F-actin content is expressed as a relative change in the mean phalloidin fluorescence intensity, and the fluorescence intensity of untreated control cells is expressed as 1. Quantification of F-actin changes was performed based on at least 15 cells from 3 independent experiments and the means ±s.e.m. are shown. *P < 0.0001 compared to control values.
The involvement of PKC in Ca2+-induced F-actin depolymerization
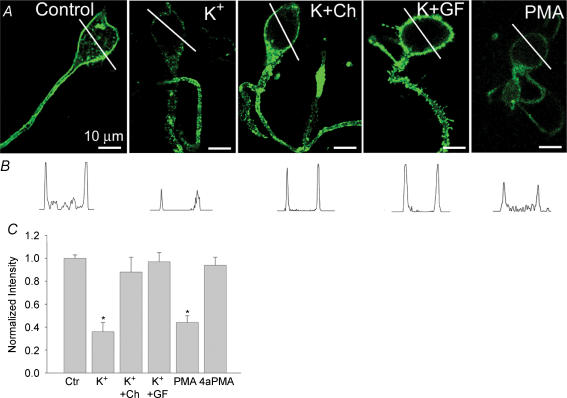
Increasing lines of evidence indicate that classical and novel isoforms of PKC have significant roles in regulating cytoskeleton-driven processes (reviewed by Keenan & Kelleher, 1998; Larsson, 2006). We used PKC antagonists to determine whether it is involved in the Ca2+-induced F-actin disruption. Chelerythrine and GF 109203X are highly specific PKC antagonists with a > 100-fold selectivity for PKC over either protein kinase A or calcium–calmodulin-dependent protein kinase (Herbert et al. 1990). To assess the ability of these agents to interfere with the depolarization-induced F-actin changes, dissociated cells were first incubated for 20 min in control Ringer solution containing either 50 μm chelerythrine or 1 μm GF 109203X, then for 5 min with modified solution containing 20 mm KCl in the continuous presence of PKC antagonist, before fixing and staining with Alexa-Fluor488-phalloidin. Figure 3A shows confocal fluorescence images of cells stained with Alexa-Fluor488-phalloidin in control Ringer solution, in modified solution containing 20 mm KCl alone, or in solution containing 20 mm KCl combined with either chelerythrine or GF 109203X. As observed by confocal laser scanning microscope analysis, both PKC antagonists attenuated a depolarization-induced F-actin disruption. The mean reduction in phalloidin fluorescence intensity induced by high K+ was 12 ± 13% (n = 13; P > 0.5) and 3 ± 8% (n = 15; P > 0.5) in cells pretreated with chelerythrine or GF 109203X, respectively, compared to a 63 ± 8% (n = 28; P < 0.001) reduction observed in cells treated with high K+ alone. Neither of these agents had any significant effect on F-actin when applied alone in control Ringer solution (n = 15, not illustrated). Further evidence for the involvement of PKC activation was provided by the use of the phorbol ester, phorbol 12-myristate 13-acetate (PMA), which activates PKC in a Ca2+-independent manner (Mosior & Newton, 1996). Exposure of cells for 20 min to control Ringer solution containing 0.5 μm PMA resulted in a loss of F-actin, as assessed by a 56 ± 6% (n = 20; P < 0.001) reduction in phalloidin fluorescence intensity. Similar treatment with 4α-PMA (0.5 μm), an inactive PMA analogue, was ineffective (n = 10; not illustrated). Fluorescence intensity profiles of corresponding cells across the marked white line are illustrated (Fig. 3B). The bar graph in Fig. 3C summarizes these results indicating that PKC plays a critical role in Ca2+-induced F-actin depolymerization in retinal neurons.
Figure 3. Inhibitors of PKC attenuate Ca22+-induced F-actin depolymerization.
A, confocal fluorescence images of F-actin distribution in cells treated with control Ringer solution (control), after depolarization with 20 mm KCl alone (K+), or in combination with either 50 μm chelerythrine (K+Ch) or 1 μm GF 109203X (K+GF), then fixed and stained with Alexa-Fluor488-phalloidin. PMA (0.5 μm) was applied to the control Ringer solution for 20 min prior to staining with phalloidin. B, intensity profiles of phalloidin staining for each cell across the marked white lines are illustrated. C, bar graph summarizing effects of PKC antagonists on Ca2+-induced F-actin depolymerization. Values represent mean fluorescence intensity and s.e.m. Data are based on 15–20 cells from 3–5 independent experiments. *P < 0.0001 compared to control values.
Effect of the other sources of Ca2+ on F-actin
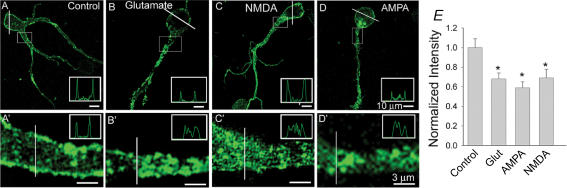
We next tested whether a rise in the intracellular Ca2+ concentration that triggers F-actin disruption results specifically from Ca2+ entering the cell through voltage-gated channels or may also result either from Ca2+ entry through glutamate receptor-activated channels or from Ca2+ being released from intracellular stores. In salamander third-order retinal neurons, activation of ionotropic glutamate receptors is associated with a Ca2+ influx (Shen & Slaughter, 1999; Akopian et al. 2006). To examine the effect of Ca2+ entry through glutamate receptors, cells were incubated for 30 min in Ringer solution containing 100 μm glutamate and 100 μm CdCl2 (to exclude Ca2+ entry through voltage-gated channels), prior to fixing and staining with Alexa-Fluor488-phalloidin. Blockade of voltage-gated Ca2+ channels will also significantly prevent excitotoxicity in cells caused by glutamate (Sucher et al. 1997). In these conditions, glutamate caused a disruption of F-actin in the perimeter of cell somata (Fig. 4B) as assessed by a reduction in the intensity of phalloidin staining around the soma. A significant change in pattern of phallodin staining (described below) was observed in processes. Quantification revealed a 34 ± 6% (n = 35, P < 0.001) reduction in fluorescence intensity of phalloidin in the submembranous area of cells treated with glutamate. Intensity profiles in cell somata and processes along the indicated white lines are illustrated in the inset of each image. To determine the type(s) of ionotropic glutamate receptor involved in F-actin disruption, we treated cells separately with AMPA (50 μm, in normal medium) and NMDA (100 μm, in Mg2+-free medium) for 10–15 min, then fixed and stained the cells with Alexa-Fluor488-phalloidin. Activation of either AMPA or NMDA receptors resulted in a reduction of phalloidin fluoresence intensity by 42 ± 6% (n = 24, P < 0.01) and 31 ± 9% (n = 24, P < 0.01), respectively, compared to control cells. In some cells (Fig. 4C and C′), treatment with NMDA had little effect on the intensity of phalloidin staining in the cell soma, but caused a dramatic change in the pattern of staining in processes. In contrast to control cell processes, where pronounced phalloidin-positive labelling of the submembranous area and a more diffuse, granular staining within the cytoplasmic compartment was observed (Fig. 4A′), cells treated with glutamate agonists displayed a much reduced intensity of phalloidin labelling along the membrane and more intense fluorescent patches in the centre of the dendritic shaft (Fig. 4B′, C′ and D′). Changes in phalloidin staining could be the result of permanent damage to cells caused by glutamate excitotoxicity. In our experiments, we applied 100 μm Cd2+ to exclude any contribution from voltage-gated Ca2+ channels, which would also significantly reduce glutamate-induced excitotoxicity (Sucher et al. 1997). Nevertheless, to exclude this possibility, dissociated cells were treated with 100 μm glutamate for 30 min and then washed for 2 h with normal Ringer solution, and whole-cell voltage-gated currents were recorded from cells with a smooth appearance of soma and intact processes. All cells (n = 10) generated currents in response to depolarizing voltage steps from −50 to +30 mV evoked from a holding potential of −70 mV (not shown). The bar graph in Fig. 4E summarizes the effects of ionotropic glutamate receptor activation on F-actin organization.
Figure 4. Activation of glutamate receptors causes F-actin disruption in third-order retinal neurons.
Confocal fluorescence images of cells incubated in the absence (A) or presence (B) of 100 μm glutamate in control Ringer solution for 30 min, with 100 μm NMDA (in Mg2+-free medium) for 10 min (C) or 50 μm AMPA for 10 min (D) prior to fixing and staining with Alexa-Fluor488-phalloidin. Glutamate and its agonists were applied in the presence of 100 μm CdCl2. Panels A′–D′ show magnified fluorescence images of the dendritic region adjacent to cell somata shown in panels A–D, as indicated by the boxes. Fluorescence intensity profiles across the indicated white lines for individual cell somata and dendrites are illustrated (insets). Note that cells treated with glutamate agonists displayed reduced intensity of phalloidin labelling along the membrane and more discrete fluorescent patches inside the dendritic shaft compared to control cells. E, bar graph summarizing the effect of ionotropic glutamate receptor agonists on F-actin content. Values represent the mean phalloidin fluorescence intensity and s.e.m. measured in at least 25 cells from 3 independent experiments. *P < 0.05 compared to control values.
We next tested the possible effect of a rise in intracellular Ca2+ released from intracellular stores on F-actin. In the first series of experiments, retina was treated with caffeine, which stimulates Ca2+ release from ryanodine-sensitive stores. Cells were treated with Ringer solution containing 10 mm caffeine for 5–10 min prior to fixing and staining with Alexa-Fluor488-phalloidin. Caffeine had no effect on the intensity of phalloidin staining (n = 5, not shown). Lack of an effect of caffeine on actin organization in our study might result from a small and transient rise in intracellular Ca2+, in contrast to the sustained rise in Ca2+ evoked by high K+ (Krizaj et al. 2003), which might therefore not be sufficient to depolymerize actin filaments. In the next approach, we stimulated Ca2+ release by incubating dissociated cells for 20–30 min with thapsigargin (1 μm in Ringer solution) prior to phalloidin staining. Thapsigargin also had no effect on phalloidin fluorescence intensity (n = 10; not illustrated). These data indicate that, in contrast to Ca2+ influx through voltage- and glutamate-activated channels, the Ca2+ released from internal stores is unable to affect actin organization, suggesting a crucial role for a source of intracellular [Ca2+] elevation for its action.
Viability of cells treated with glutamate and high K+
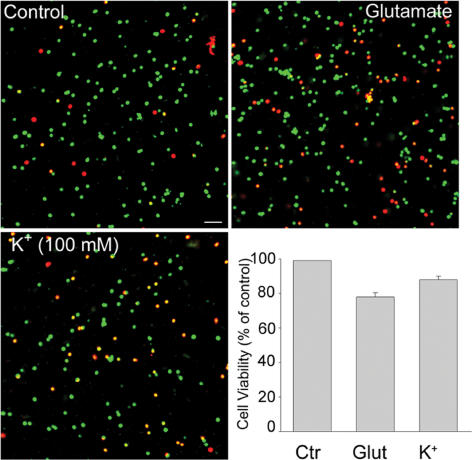
To assure that changes in phalloidin fluorescence intensity was not associated with cell death caused by various treatments (high K+, glutamate), we conducted two-dye colour live/dead assay on dissociated cells treated and subsequently washed for 2 h. For comparison, control cells were loaded with dyes following 2 h incubation in control Ringer solution. Viable cells were labelled by the green fluorescence generated by the esterase hydrolysis of a membrane-permeant dye, calcein-AM. Dead cells were marked by the red fluorescence of a membrane-impermeant DNA marker, ethidium homidimer. The results of three separate experiments (each provided in duplicate), showed that, compared to control cells, 78 ± 2% of glutamate-treated cells and 88 ± 2% of KCl-treated cells were viable (Fig. 5A, B and C). The bar graph in Fig. 5D summarizes the percentage number of viable cells in each experimental condition. As a further test of the fluorescence viability assay, whole-cell currents were measured in treated dissociated cells and slices. All recorded cells exhibited voltage-gated currents and light-induced EPSCs characteristic of normal functional cells. The results indicate that in the presence of Ca2+ channel blocker (Cd2+ in the external solution), glutamate-induced cell damage was relatively low and that changes in phalloidin fluorescence intensity was not associated with cell death.
Figure 5. Live/dead fluorescence assay of dissociated cells.
Confocal images of dissociated cells treated with either 100 μm glutamate (for 30 min; B), or 100 mm KCl (for 5 min; C) and subsequently washed for 2 h before double labelling with the fluorescent dyes ethidium homodimer (red) and calcein-AM (green). Control cells (A) were labelled after 2 h incubation in control Ringer solution. Viable cells were marked by green fluorescence, whereas dead cells were marked by red fluorescence. D, bar graph represents cell viability (% of control) calculated from 3 separate experiments. The ratios of live cells to total cells (viability) were determined for control and treated cells by directly counting 300–600 cells in randomly chosen microscopic fields of each sample. All data represent means +s.e.m. Calibration bar, 100 μm.
Implication in the regulation of light-evoked EPSCs
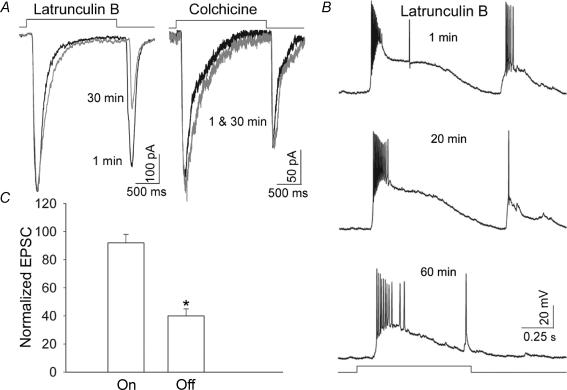
Postsynaptic actin serves as a key mediator between structural plasticity and signal transmission at excitatory synapses (reviewed by Matus, 1999; Dillon & Goda, 2005). The disruption of F-actin by glutamate-induced Ca2+ influx (present study) and the subsequent effect of actin depolymerization on Ca2+ influx through glutamate receptor-activated channels (Akopian et al. 2006) prompted us to examine the possible physiological significance of these findings. We tested whether glutamatergic synaptic responses were affected by the actin cytoskeleton reorganization. In a slice preparation, ‘on–off’ ganglion cells were held at −70 mV, and whole-cell EPSCs in response to 2 s light stimulation were recorded with the patch pipette containing either latrunculin B (5 μm) or cytochalasin B (10 μm). To assess the effect of F-actin disruption, EPSCs were recorded within 1 min of membrane rupture and after 30 min incubation with latrunculin B (Fig. 6A). A disruption of F-actin resulted in a 60 ± 8% (n = 8, P < 0.01) reduction in ‘off’ EPSCs, remarkably having no significant effect on ‘on’ EPSCs. In contrast, similar treatment with colchicine (50 μm), a microtubule-disrupting agent, had no significant effect on either ‘on’ or ‘off’ EPSCs (n = 4). Whole-cell recordings in current-clamp mode revealed qualitatively similar attenuation of light-evoked ‘off’ EPSPs by latrunculin B, without significant changes in ‘on’ EPSPs. In the cell illustrated in Fig. 6B, a longer (60 min) incubation with latrunculin B resulted in a complete inhibition of the ‘off’ EPSPs with only a slight reduction in the ‘on’ EPSPs. The bar graph in Fig. 6C summarizes the effect of actin depolymerization on the amplitudes of light-evoked ‘on’ and ‘off’ EPSCs in retinal ganglion cells.
Figure 6. Regulation of the light-evoked EPSCs by actin cytoskeleton in ‘on–off’ ganglion cells.
A, F-actin depolymerization by latrunculin B caused a reduction in ‘off’ but not ‘on’ EPSCs. In contrast, disruption of microtubules by colchicine had no effect on either type of EPSCs. Traces shown are recorded within 1 min of membrane rupture (black lines) and after 30 min in the whole-cell configuration (grey lines) with the patch pipette containing either 5 μm latrunculin B or 50 μm colchicine. B, in current clamp mode, F-actin disruption by latrunculin B resulted in a dramatic reduction in ‘off’ EPSPs but only a small effect on ‘on’ EPSPs. C, bar graph summarizes the effect of 30 min incubation with cytochalasin B on ‘on’ and ‘off’ EPSCs. Values represent the mean and s.e.m. of determinations made in 8 cells in three separate preparations. *P < 0.01 compared to values obtained within 1 min of membrane rupture.
Discussion
The principal finding of our study is that in salamander inner retinal neurons, Ca2+ influx through high-voltage-activated L-type channels causes F-actin depolymerization, and PKC activation plays a critical role in this process.
The importance of the relationship between Ca2+ and the cytoskeletal architecture in the vertebrate retina stems from the role that Ca2+ plays in signal transduction, and in its regulation of numerous cellular processes through the activation of multiple intracellular cascades (reviewed by Akopian & Witkovsky, 2002; Krizaj & Copenhagen, 2002). Thus, in salamander retina, intracellular Ca2+ elevation through diverse sources, including influx through L-type channels, regulates the activity of GABA receptors in ganglion cells (Shen & Slaughter, 1999), closes non-selective cation channels activated by mGluR6 in bipolar cells (Nawy, 2000), reduces the light-evoked EPSCs in ganglion cells (Akopian & Witkovsky, 2001) and appears to play an important role in mediating transmitter release in amacrine cells (Bieda & Copenhagen, 2004). Calcium influx from different sources induces diverse changes in actin cytoskeleton organization that in some cases are difficult to reconcile or even appear contradictory (Oertner & Matus, 2005). We found that Ca2+ influx through voltage- and glutamate-activated channels, but not by release from internal stores, caused F-actin disruption. Quantification of F-actin on cell somata revealed a much stronger (∼60%) disruption when Ca2+ entered through voltage-gated channels compared to glutamate receptor-activated channels (30–40%). At the same time, more dramatic changes in the pattern of phalloidin staining by glutamate receptor agonists was observed in the cell processes (Fig. 4B′, C′ and D′), where fluorescence appears as discrete patches inside the dendritic shaft, compared to more continuous labelling along the membrane in control cells (Fig. 4A′). This may reflect the higher amount of Ca2+ entering through voltage-gated L-type channels, which are densely localized on the surface of cell somata (M. Cristofanilli & A. Akopian, unpublished observation), compared to the amount of Ca2+ influx stimulated by AMPA or NMDA, whose receptors are preferentially expressed by amacrine and ganglion cell dendrites (Grunert et al. 2002). In addition, the greater effect of Ca2+ influx through voltage-gated channels may reflect a longer time available for Ca2+ entry through sustained L-type channels compared to desensitizing glutamate receptors. Alternatively, the temporal and spatial regulation of intracellular [Ca2+] may also be critical for cytoskeleton rearrangement. The fact that Ca2+ release from internal stores had no effect on actin organization supports this idea. A multiple spatiotemporal mode of actin reorganization by NMDA receptors and voltage-gated Ca2+ channels has been demonstrated in hippocampal neurons (Furuyashiki et al. 2002).
The idea that PKC could be an important regulator of cytoskeletal organization has been supported by numerous studies (reviewed by Larsson, 2006). Block of Ca2+-induced F-actin disruption in the presence of specific PKC inhibitors suggests that the PKC activation mediates the effect of Ca2+ entering through voltage-gated channels. Our results are consistent with those observed in other cell types, in which the activation of PKC has been shown to mediate actin depolymerization (Schliwa et al. 1984; Brandt et al. 2002), but contrast strikingly with the Ca2+- and PKC-mediated polymerization of F-actin in bipolar cell terminals of the goldfish retina (Job & Lagnado, 1998). The reason for such apparent discrepancy might be the differential localization and expression of distinct isoenzymes of protein kinase C in the retina (Kosaka et al. 1998; Fyk-Kolodziej et al. 2002), and the family of substrate proteins that have been suggested as candidates for mediating PKC effects on the cytoskeleton (reviewed by Larsson, 2006). These substrates include a group of actin-binding proteins that link actin with the surface membrane and promote diverse changes in actin filament structure when phosphorylated by PKC (reviewed by Keenan & Kelleher, 1998; Calabrese & Halpain, 2005). In general, classical (PKCα, βI, βII and γ), and novel isoforms (PKCɛ, δ, η, and Θ) both contain binding domains for phorbol esters, whereas only the classic isoforms contain a domain for Ca2+ binding (Larsson, 2006). The fact that the PKC antagonists, chelerythrine and GF 109230X, attenuated the effect of depolarization-induced Ca2+ influx on F-actin disruption, and that PMA mimicked this effect in the absence of KCl-induced depolarization, suggests an involvement of both Ca2+-dependent and -independent pathways of cytoskeleton regulation by PKC in retinal neurons. In contrast, only the PKCα (Ca2+-dependent) isoform is expressed in the synaptic terminals of depolarizing bipolar cells, and no other isoform of PKC was detected (Osborne et al. 1992). Accordingly, in this preparation, phorbol esters had no effect on actin network in the absence of depolarization-induced Ca2+ influx (Job & Lagnado, 1998). The lack of a common effect of PKC on F-actin in different cells suggests that a wide range of pathways may mediate the PKC effect, including direct phosphorylation of cytoskeletal regulators and influence on other intracellular signalling pathways.
Two major proteins that mediate Ca2+-dependent changes in the actin cytoskeleton, namely gelsolin (Forscher, 1989; Furukawa et al. 1997) and myristoylated alanine-rich C kinase substrate (MARCKS; Arbuzova et al. 2002), might be involved in Ca2+-induced actin depolymerization. There is an apparent link between PKC activation and function of these proteins. That is, antagonists of PKC have been shown to inhibit the induction of galsolin in Human Leulzemia (HL)60 cells (Miyamoto & Wu, 1990), whereas phosphorylation of MARCKS, an F-actin cross-linking protein, by PKC induced spine loss and shrinkage, accompanied by a reduced F-actin content in hippocampal neurons (Calabrese & Halpain, 2005). We observed antigelsolin immunoreactivity in cell bodies in the inner nuclear layer and the ganglion cell layer of salamander retina (M. Cristofanilli and A. Akopian, unpublished observations), but whether gelsolin is involved in Ca2+-induced F-actin depolymerization in the retina remains to be determined.
The contribution of the actin cytoskeleton to the regulation of synaptic transmission and plasticity has been a matter of extensive study in recent years (reviewed by Dillon & Goda, 2005). Disruption of postsynaptic F-actin (latrunculin B applied to the patch pipette) in our study caused a significant reduction in light-evoked ‘off’ EPSCs, remarkably having no effect on ‘on’ EPSCs in ‘on–off’ ganglion cells. We did not investigate mechanism(s) underlying the selective regulation of ‘off’ EPSCs by F-actin, since it was beyond the scope of the present study. It is possible, however, that the regulatory pathways involving different kinases and their substrates, a group of actin-binding proteins that promote diverse changes in actin filament structure when phosphorylated, are different for receptors in sublaminae 1 and 5, where ‘on–off’ ganglion cell processes are stratified. Disruption of microtubules with colchicines, in contrast, had no effect on either ‘on’ or ‘off’ EPSCs, indicating that in the vertebrate retina, as in the hippocampus, the actin cytoskeleton plays an important role in the regulation of excitatory postsynaptic responses. Further studies are needed to explore the mechanism(s) underlying cytoskeletal regulation of synaptic responses and the role of various enzymes and actin-binding proteins in the retina.
Physiological implications
In the vertebrate retina, actin reorganization causes a variety of structural (Mandell et al. 1993; Weiler & Janssen-Bienhold, 1993; Job & Lagnado, 1998; Gallo et al. 2002) and functional changes (Maguire et al. 1998; Schmidt, 2004). Our data suggest that the activity-dependent increase of postsynaptic Ca2+ levels beyond a particular threshold may trigger a disruption of actin filaments, which in turn will tend to reduce the Ca2+ influx through voltage-activated (Schubert & Akopian, 2004) or glutamate receptor-activated channels (Akopian et al. 2006), thereby influencing the light-evoked excitatory postsynaptic responses (present study). For example, the build up of internal Ca2+ resulting from the light-‘on’ response might initiate F-actin disruption and subsequent reduction in the ‘off’ response. Depending on the source of Ca2+ increase, the result may be the local, and transient or long-lasting changes in F-actin organization and postsynaptic cell function. Calcium-dependent cytoskeleton reorganization observed in our study may also underlie the mechanism of Ca2+-induced Ca2+ inactivation (Johnson & Byerly, 1993).
Taking together, our data suggest a reciprocal relationship between glutamate receptors, Ca2+ and F-actin in regulating synaptic function in the salamander retina. Such a relationship may underlie a negative feedback pathway, which serves to attenuate the excessive intracellular Ca2+ accumulation that plays a primary role in excitotoxicity, a form of neuronal injury caused by overstimulation of glutamate receptors (Sucher et al. 1997).
Acknowledgments
We thank Dr P. Witkovsky for critically reading the manuscript. This research was supported by the NIH grant EY 12497 to A.A.
References
- Akopian A, Szikra T, Cristofanilli M, Krizaj D. Glutamate-induced Ca2+ influx in third-order neurons of salamander retina is regulated by the actin cytoskeleton. Neuroscience. 2006;138:17–24. doi: 10.1016/j.neuroscience.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopian A, Witkovsky P. Intracellular calcium reduces light-induced excitatory post-synaptic responses in salamander retinal ganglion cells. J Physiol. 2001;532:43–53. doi: 10.1111/j.1469-7793.2001.0043g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopian A, Witkovsky P. Calcium and retinal function. Mol Neurobiol. 2002;25:113–132. doi: 10.1385/MN:25:2:113. [DOI] [PubMed] [Google Scholar]
- Arbuzova A, Schmitz AA, Vergeres G. Cross-talk unfolded: MARCKS proteins. Biochem J. 2002;362:1–12. doi: 10.1042/0264-6021:3620001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieda MC, Copenhagen DR. N-Type and L-type calcium channels mediate glycinergic synaptic inputs to retinal ganglion cells of tiger salamanders. Vis Neurosci. 2004;21:545–550. doi: 10.1017/S0952523804214055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt D, Gimona M, Hillmann M, Haller H, Mischak H. Protein kinase C induces actin reorganization via a Src- and Rho-dependent pathway. J Biol Chem. 2002;277:20903–20910. doi: 10.1074/jbc.M200946200. [DOI] [PubMed] [Google Scholar]
- Brunig I, Kaech S, Brinkhaus H, Oertner TG, Matus A. Influx of extracellular calcium regulates actin-dependent morphological plasticity in dendritic spines. Neuropharmacology. 2004;47:669–676. doi: 10.1016/j.neuropharm.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Calabrese B, Halpain S. Essential role for the PKC target MARCKS in maintaining dendritic spine morphology. Neuron. 2005;48:77–90. doi: 10.1016/j.neuron.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Alford S, Collingridge GL. Ca2+ and synaptic plasticity. Cell Calcium. 1998;24:377–385. doi: 10.1016/s0143-4160(98)90061-6. [DOI] [PubMed] [Google Scholar]
- Dancker P, Low I, Hasselbach W, Wieland T. Interaction of actin with phalloidin: polymerization and stabilization of F-actin. Biochim Biophys Acta. 1975;400:407–414. doi: 10.1016/0005-2795(75)90196-8. [DOI] [PubMed] [Google Scholar]
- Dillon C, Goda Y. The actin cytoskeleton: integrating form and function at the synapse. Annu Rev Neurosci. 2005;28:25–55. doi: 10.1146/annurev.neuro.28.061604.135757. [DOI] [PubMed] [Google Scholar]
- Forscher P. Calcium and polyphosphoinositide control of cytoskeletal dynamics. Trends Neurosci. 1989;12:468–474. doi: 10.1016/0166-2236(89)90098-2. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Fu W, Li Y, Witke W, Kwiatkowski DJ, Mattson MP. The actin-severing protein gelsolin modulates calcium channel and NMDA receptor activities and vulnerability to excitotoxicity in hippocampal neurons. J Neurosci. 1997;17:8178–8186. doi: 10.1523/JNEUROSCI.17-21-08178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K, Smith-Swintosky VL, Mattson MP. Evidence that actin depolymerization protects hippocampal neurons against excitotoxicity by stabilizing [Ca2+]i. Exp Neurol. 1995;133:153–163. doi: 10.1006/exnr.1995.1018. [DOI] [PubMed] [Google Scholar]
- Furuyashiki T, Arakawa Y, Takemoto-Kimura S, Bito H, Narumiya S. Multiple spatiotemporal modes of actin reorganization by NMDA receptors and voltage-gated Ca2+ channels. Proc Natl Acad Sci U S A. 2002;99:14458–14463. doi: 10.1073/pnas.212148999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyk-Kolodziej B, Caj W, Pourcho RG. Distribution of protein kinase C isoforms in the cat retina. Vis Neurosci. 2002;19:549–562. doi: 10.1017/s0952523802195010. [DOI] [PubMed] [Google Scholar]
- Gallo G, Yee HF, Letourneau PC. Actin turnover is required to prevent axon retraction driven by endogenous actomyosin contractility. J Cell Biol. 2002;158:1219–1228. doi: 10.1083/jcb.200204140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunert U, Haverkamp S, Fletcher EL, Wassle H. Synaptic distribution of ionotropic glutamate receptors in the inner plexiform layer of the primate retina. J Comp Neurol. 2002;447:138–151. doi: 10.1002/cne.10220. [DOI] [PubMed] [Google Scholar]
- Halpain S, Hipolito A, Saffer L. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J Neurosci. 1998;18:9835–9844. doi: 10.1523/JNEUROSCI.18-23-09835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert JM, Augerean JM, Gleye J, Maffrard JP. Chelerythrine is a patent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1999;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- Job C, Lagnado L. Calcium and protein kinase C regulate the actin cytoskeleton in the synaptic terminal of retinal bipolar cells. J Cell Biol. 1998;143:1661–1672. doi: 10.1083/jcb.143.6.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, Byerly Y. A cytoskeletal mechanism for Ca2+ channel metabolic dependence and inactivation by intracellular Ca2+ Neuron. 1993;10:797–804. doi: 10.1016/0896-6273(93)90196-x. [DOI] [PubMed] [Google Scholar]
- Keenan C, Kelleher D. Protein kinase C and the cytoskeleton. Cell Signal. 1998;10:225–232. doi: 10.1016/s0898-6568(97)00121-6. [DOI] [PubMed] [Google Scholar]
- Kosaka J, Suzuki A, Morii E, Nomura S. Differential localization and expression of alpha and beta isoenzymes of protein kinase C in the rat retina. J Neurosci Res. 1998;54:655–663. doi: 10.1002/(SICI)1097-4547(19981201)54:5<655::AID-JNR10>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Krizaj D, Copenhagen DR. Calcium regulation in photoreceptors. Front Biosci. 2002;7:2023–2044. doi: 10.2741/a896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizaj D, Lai FA, Copenhagen DR. Ryanodine stores and calcium regulation in the inner segments of salamander rods and cones. J Physiol. 2003;547:761–774. doi: 10.1113/jphysiol.2002.035683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson C. Protein kinase C and the regulation of the actin cytoskeleton. Cell Signal. 2006;18:276–284. doi: 10.1016/j.cellsig.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Lau P, Zucker RS, Bentley D. Induction of filopodia by direct local elevation of intracellular calcium ion concentration. J Cell Biol. 1999;145:1265–1275. doi: 10.1083/jcb.145.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire G, Connaughton V, Prat AG, Jackson GR, Cantiello HR. Actin cytoskeleton regulates ion channel activity in retinal neurons. Neuroreport. 1998;9:665–670. doi: 10.1097/00001756-199803090-00019. [DOI] [PubMed] [Google Scholar]
- Mandell JW, MacLeish PR, Townes-Anderson E. Process outgrowth and synaptic varicosity formation by adult photoreceptors in vitro. J Neurosci. 1993;13:3533–3548. doi: 10.1523/JNEUROSCI.13-08-03533.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus A. Postsynaptic actin and neuronal plasticity. Curr Opid Neurobiol. 1999;9:561–565. doi: 10.1016/S0959-4388(99)00018-5. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Wu JM. Effect of staurosporine on the induction of actin/gelsolin in PMA-treated HL-60 cells. Biochem Int. 1990;22:427–433. [PubMed] [Google Scholar]
- Mosior M, Newton AC. Calcium-independent binding to interfacial phorbol esters causes protein kinase C to associate with membranes in the absence of acidic lipids. Biochemistry. 1996;35:1612–1623. doi: 10.1021/bi952031q. [DOI] [PubMed] [Google Scholar]
- Nawy S. Regulation of the on bipolar cell mGluR6 pathway by Ca2+ J Neurosci. 2000;20:4471–4479. doi: 10.1523/JNEUROSCI.20-12-04471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neely MD, Gesemann D. Disruption of microfilaments in growth cones following depolarization and calcium influx. J Neurosci. 1994;14:7511–7520. doi: 10.1523/JNEUROSCI.14-12-07511.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertner TG, Matus A. Calcium regulation of actin dynamics in dendritic spines. Cell Calcium. 2005;37:477–482. doi: 10.1016/j.ceca.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Osborne NN, Barnett NL, Morris NJ, Huang FL. The occurrence of three isoenzymes of protein kinase C (α, β and γ) in retinas of different species. Brain Res. 1992;570:161–166. doi: 10.1016/0006-8993(92)90577-v. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Westbrook G. Calcium-induced actin depolymerization reduces NMDA channel activity. Neuron. 1993;10:805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- Schliwa M, Nakamura T, Porter KR, Euteneuer U. A tumor promoter induces rapid and coordinated reorganization of actin and vinculin in cultured cells. J Cell Biol. 1984;99:1045–1059. doi: 10.1083/jcb.99.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt JT. Activity-driven sharpening of the retinotectal projection: the search for retrograde synaptic signaling pathways. J Neurobiol. 2004;59:114–133. doi: 10.1002/neu.10343. [DOI] [PubMed] [Google Scholar]
- Schubert T, Akopian A. Actin filaments regulate voltage-gated ion channels in salamander retinal ganglion cells. Neuroscience. 2004;125:583–590. doi: 10.1016/j.neuroscience.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Shen W, Slaughter MM. Internal calcium modulates apparent affinity of metabotropic GABA receptors. J Neurophysiol. 1999;82:3298–3306. doi: 10.1152/jn.1999.82.6.3298. [DOI] [PubMed] [Google Scholar]
- Spector I, Shochet NR, Kashman Y, Groweiss A. Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. Science. 1983;219:493–495. doi: 10.1126/science.6681676. [DOI] [PubMed] [Google Scholar]
- Sucher NJ, Lipton SA, Dryer EB. Molecular basis of glutamate toxicity in retinal ganglion cells. Vision Res. 1997;37:3483–3493. doi: 10.1016/S0042-6989(97)00047-3. [DOI] [PubMed] [Google Scholar]
- Weiler R, Janssen-Bienhold U. Spinule-type neurite outgrowth from horizontal cells during light adaptation in the carp retina: an actin-dependent process. J Neurocytol. 1993;22:129–139. doi: 10.1007/BF01181576. [DOI] [PubMed] [Google Scholar]