Abstract
Transcription factors (TFs) play pivotal roles in directing the formation of neurons and glia. Here I will review the recent genome-scale analysis of the expression of TFs in the developing mouse nervous system and discuss the logic by which TFs control the establishment of neuronal phenotype. Accumulating evidence suggests that while combinatorial action of TFs is able to define the basic framework of the nervous system, other control mechanisms, such as stochastic and epigenetic regulation of gene expression, also contribute to the generation of nerve cell diversity.
The mammalian nervous system is thought to contain thousands of neuronal cell types that are distinguished on the basis of unique location and morphology (Masland, 2004). Moreover, recent molecular studies have started to reveal additional levels of complexity. For example, over 1000 subtypes of olfactory sensory neurons can be distinguished by monogenic expression of odour receptors (Buck, 2000). A fundamental question in neuroscience is to understand the mechanisms by which this remarkable cellular diversity is established during development.
In the past two decades, several key principles regarding neuronal cell type specification have emerged. First, extrinsic signals pattern the neural tube spatially and temporally, such that distinct types of neurons are formed at defined places and times (McConnell, 1995; Jessell, 2000; Anderson, 2001; Livesey & Cepko, 2001; Kiecker & Lumsden, 2005; Guillemot et al. 2006). Second, transcription factors (TFs) often act in combination to control neuronal cell type specification – the so-called TF combinatorial control mode (Lee & Pfaff, 2001). In this article, I will discuss to what degree and how TFs are used to establish various aspects of neuronal identity.
Genome-scale analysis of TF expression in the mouse nervous system
The mammalian genome encodes about 1500 TFs that contain known DNA-binding motifs (Gray et al. 2004). Since completion of the sequencing of the human and mouse genomes, my colleagues and I have been using high throughput in situ hybridization to compile a genome-scale map of TF expression in the developing mouse nervous system. With 80% screen coverage, we have identified over 350 TFs that show spatially and/or temporally restricted expression (Gray et al. 2004).
Several conclusions can be drawn from this genome-scale analysis, as well as from numerous independent studies. First, a given TF is often expressed in multiple brain regions. In other words, TFs that are expressed exclusively in one type of neuron, such as the restriction of Pet1 to serotonergic neurons (Hendricks et al. 1999), are extremely rare. This characteristic of TF expression patterns is consistent with the current view that TFs act in combination to control cell type specification (or a TF may control a feature shared by different types of neurons). Second, TF expression is sufficient to define basic brain anatomy, such as lamina-specific organization in the neocortex, retina and cerebellum, and nuclear organization in the thalamus and hypothalamus (Kandel et al. 2000; Nakagawa & O'Leary, 2001; Blackshaw et al. 2004; Gray et al. 2004; Guillemot et al. 2006) and the gradient expression of many TFs in the striatum may underlie the genetic basis for a topographic organization in this brain area (Kandel et al. 2000; Gray et al. 2004). Third, within a brain region, TF expression is able to mark the major populations of neurons, such as the Purkinje versus granule cells in the cerebellum, or ganglion versus amacrine neurons in the retina. Furthermore, TF expression can at least partially reveal additional heterogeneity within a defined population of neurons. For example, many TF genes are expressed with differential density within retinal ganglion or amacrine cell layers (Blackshaw et al. 2004; Gray et al. 2004).
However, combinatorial TF expression alone might be insufficient to account for the entirety of nerve cell diversity. For example, within a given cerebellar compartment, no TF expression is able to mark Purkinje cell diversity that was revealed by restricted expression of protocadherin molecules in small fractions of these neurons (Gray et al. 2004; Esumi et al. 2005; D. Rowitch, personal communication). TF expression also appears unable to mark the reported diversity of the peripheral nociceptive sensory neurons (C. Cen & Q. Ma, unpublished data). The emerging theme is that while combinatorial TF expression is sufficient to define the basic framework of the nervous system, other control mechanisms probably contribute to the generation of cell diversity (see below).
Specification of generic neuronal identity
Each neuron shares a set of features, such as the expression of pan-neural markers, elaboration of dendritic and axonal processes, formation of synaptic connections, and the ability to generate and transduce electrical signals. Specification of these generic neuronal features (or initiation of the process leading to the establishment of these features) is controlled by a group of neuronal determination genes that encode basic-helix–loop-helix (bHLH) class TFs (Anderson et al. 1997; Lee, 1997; Schuurmans & Guillemot, 2002). Harold Weintraub and colleagues first established bHLH genes as master regulators in muscle cell fate determination (Weintraub, 1993). Genetic studies in Drosophila then showed that several bHLH genes, called proneural genes, determine a neural versus an epidermal cell fate (Jan & Jan, 1993). Subsequently, mammalian homologs of the fly proneural genes, including Mash1, Neurogenin1–3 (Ngn1–3), and Math1/3, serve as vertebrate neuronal determination genes (Anderson et al. 1997; Lee, 1997; Schuurmans & Guillemot, 2002).
An important feature of determination factors is their capacity to convert other types of cells into neurons. For example, Ngn1/2 and their downstream target NeuroD are able to drive multipotent neural precursors, ectodermal cells and even muscle precursors into ‘differentiated’ neurons (Lee et al. 1995; Ma et al. 1996; Perez et al. 1999; Sun et al. 2001). In the case of the muscle determination factor MyoD, the determination activity is conferred by its ability to bind and activate target genes at repressed chromatin states, thereby orchestrating a de novo muscle differentiation program (Gerber et al. 1997; Tapscott, 2005). Neuronal determination factors probably contain similar capacities, by recruiting chromatin-remodelling complexes and activating High Mobility Group (HMG) class TFs (Koyano-Nakagawa et al. 1999; Sun et al. 2001; Sandberg et al. 2005). However, to date surprisingly little has been shown about how the expression of neuronal determination genes leads to the eventual establishment of generic neuronal identity.
Neurogenesis coupled with neuronal cell type specification
Vertebrate neuronal determination genes are expressed in largely non-overlapping populations of neural precursors (Fig. 1) (Gradwohl et al. 1996; Sommer et al. 1996; Ma et al. 1997; Gowan et al. 2001; Helms & Johnson, 2003). One important consequence of this expression pattern is the coupling of neurogenesis with neuronal cell type specification (Anderson & Jan, 1997; Brunet & Ghysen, 1999; Bertrand et al. 2002; Helms & Johnson, 2003). Indeed, elegant genetic manipulations show that Ngn2 and Mash1 can replace each other in driving generic neuronal differentiation, but cannot do that in specifying neuronal subtypes (Parras et al. 2002).
Figure 1. Expression and function of bHLH genes in developing dorsal spinal cord (E10.5–E11.5).
Along the dorsal–ventral axis, neural precursors in the ventricular zone (VZ) are divided into five domains (dP1–5) that can be distinguished by combinatorial expression of bHLH genes. Ngn1/2, Math1, Olig3 and Mash1 are expressed in dividing precursors, whereas Ptf1a is expressed in cells exiting from the cell cycle. Each progenitor domain gives rise to distinct types of dorsal horn neurons (called DI1–5, simplified as 1–5) that can be distinguished by combinatorial expression of TFs. In mice carrying the mutation of each of these bHLH genes, the identity of a subset of dorsal horn neurons is changed (grey circles). It needs to be pointed out that the expansion of DI2 and DI4 neurons in Mash1 mutants is caused through a non-autonomous mechanism (Helms et al. 2005).
What is surprising is that the same neuronal determination genes are actively involved with specification of quite distinct neuronal cell types. For example, Ngn1/2 are required to specify peripheral sensory neurons, glutamatergic projection neurons in the cerebral cortex, the dopaminergic neurons in the midbrain, and a variety of spinal cord neurons (Fode et al. 1998, 2000; Ma et al. 1998, 1999; Gowan et al. 2001; Parras et al. 2002; Schuurmans et al. 2004; Andersson et al. 2006a, b; Jeong et al. 2006; Kele et al. 2006). Similarly, Mash1 is constructively involved with specification of a variety of neuronal subtypes (Guillemot et al. 1993; Hirsch et al. 1998; Casarosa et al. 1999; Cau et al. 2002; Parras et al. 2002; Pattyn et al. 2004; McNay et al. 2006). In fact, the CNS mutation of a neuronal determination gene often does not cause a defect in neurogenesis, due to a derepression of another determination gene; however, the identity of the neurons is almost invariably changed to the type of neurons that are normally formed in adjacent precursor domains (Fig. 1) (Fode et al. 2000; Gowan et al. 2001; Glasgow et al. 2005; Helms et al. 2005; Muller et al. 2005; Mizuguchi et al. 2006; Wildner et al. 2006). The functional versatility of neuronal determination genes is in many ways analogous to the involvement of the Hoxgenes in controlling the development of very distinct types of cells, tissues, and organs (Mann & Carroll, 2002). It is possible that like Hox genes, neuronal determination genes act as selector genes (Mann & Carroll, 2002) that are capable of activating and repressing hundreds or even thousands of target genes, including many TFs that are expressed in newly formed neurons. The context of precursors, such as the expression of distinct TFs or cofactors, or the existence of distinct chromatin states, might then dictate which differentiation pathway is to be activated.
Specification of neurotransmitter phenotype
One of the best-studied neuronal features in the mammalian nervous system is the specification of neurotransmitter phenotypes. Interested readers are referred to several recent reviews and articles that describe the development of three major modulator neurotransmitters, including dopamine, serotonin and noradrenaline, as well as the establishment of cholinergic transmitter phenotype (Goridis & Rohrer, 2002; Spitzer et al. 2004; Howard, 2005; Thiel, 2006). In this article, I only discuss the specification of two principal excitatory and inhibitory transmitters, glutamate and GABA, which are established in a largely mutually exclusive manner in the vertebrate brain (Bennett & Balcar, 1999; Fremeau et al. 2004). A set of studies suggests that the choice of these two transmitter phenotypes is regulated at several levels.
First, neuronal determination genes are actively involved in determining transmitter phenotypes. As mentioned above, Ngn1/2 and Mash1 determine the glutamatergic versus the GABAergic cell fates in the forebrain and spinal cord spinal neurons (Fode et al. 2000; Schuurmans et al. 2004; Mizuguchi et al. 2006), and they also determine different types of monoaminergic neurons in the midbrain and the hindbrain (Hirsch et al. 1998; Goridis & Rohrer, 2002; Pattyn et al. 2004; Andersson et al. 2006a, b Jeong et al. 2006; Kele et al. 2006). The involvement of neuronal determination genes explains why transmitter phenotype is among the earliest neuronal features established in differentiating neurons (Goridis & Rohrer, 2002). Part of the logic behind this early specification is the involvement of neurotransmitters in controlling a variety of neuronal differentiation processes (Vitalis & Parnavelas, 2003; Represa & Ben-Ari, 2005).
Second, there are binary genetic switches that operate in early post-mitotic neurons. In the dorsal spinal cord, three sets of post-mitotic TFs are involved in choosing an excitatory versus an inhibitory cell fate. First, the homeobox gene Lbx1 defines a basal GABAergic differentiation state (Cheng et al. 2005). Second, the homeobox genes Tlx1 and Tlx3 antagonize Lbx1 to promote a glutamatergic cell fate (Cheng et al. 2004, 2005). Finally, the bHLH gene Ptf1a acts to suppress Tlx3 expression, which in turns allows Lbx1 to promote GABAergic differentiation (Glasgow et al. 2005; Hoshino et al. 2005). Loss or gain of all these genes is sufficient to cause a cell fate switch, from glutamatergic to GABAergic or vice versa (Cheng et al. 2004, 2005; Glasgow et al. 2005).
Third, neuronal activity can regulate excitatory and inhibitory transmitter phenotype in a homeostatic fashion (Spitzer et al. 2004). In the developing frog spinal cord, an increase in neuronal activity increases the number of neurons expressing inhibitory neurotransmitters, whereas a decrease in neuronal activity results in an exactly inverse result: more excitatory and fewer inhibitory neurons (Borodinsky et al. 2004). In the dentate gyrus of rat hippocampus, enhanced excitability under seizures can promote GABAergic transmitter phenotype development in presumably glutamatergic granule cells (Schwarzer & Sperk, 1995; Sloviter et al. 1996; Ramirez & Gutierrez, 2001; Gutierrez, 2003). It will be interesting to determine if neuronal activity acts to modulate the expression or function of those post-mitotic switch factors, such as Tlx3 and Lbx1.
It is noteworthy that the establishment of the excitatory and inhibitory transmitter phenotypes is controlled by a set of region-specific TFs, rather than through a unitary transcriptional program. For example, the Dlx class homeobox genes are implicated in specification of forebrain GABAergic neurons, but not in other brain areas (Anderson et al. 1999). The bHLH gene Heslike controls GABAergic differentiation in the midbrain (Miyoshi et al. 2004), whereas Tlx3, Lbx1 and Ptf1a operate in the cerebellum, the hindbrain and/or the spinal cord (Gross et al. 2002; Müller et al. 2002; Cheng et al. 2004; Glasgow et al. 2005; Hoshino et al. 2005). One advantage for the evolvement of these TFs is to couple the specification of generic transmitter phenotype with that of region-specific neuronal identity (Cheng et al. 2004; Guillemot et al. 2006), similar to the function of the neuronal determination genes.
Specification of neuronal morphology and other neuronal identity
The identity of a mature neuron includes a defined axonal trajectory pattern and a unique pattern of dendritic arborization. Many TFs have been shown to control axon pathfinding or pruning. For example, the lim-homeobox TFs and hox proteins control motor neuron innervation to specific muscle targets (Lee & Pfaff, 2001; Dasen et al. 2005), Fezl and Ctip2 control long-range descending projections of cortico-spinal motor neurons (Arlotta et al. 2005; Chen et al. 2005; Molyneaux et al. 2005), Tbx5, Vax2 and Islet2 determine topographic projections of retinal ganglion neurons (Schulte et al. 1999; Koshiba-Takeuchi et al. 2000; Mui et al. 2002; Pak et al. 2004), the runt-domain transcription factors (Runx1 and Runx3) and the Ets-domain protein (Er81) control central target selections by somatic sensory afferents (Arber et al. 2003; Chen et al. 2006a, b; Kramer et al. 2006), and the homeobox protein Otx1 controls axonal pruning (Weimann et al. 1999). In Drosophila, a recent genome-scale analysis has identified a large set of TFs that control dendritic morphogenesis (Parrish et al. 2006). However, in mammals only a few TFs that control dendritic morphology have been characterized (Aizawa et al. 2004; Gaudilliere et al. 2004; Cobos et al. 2005; Hand et al. 2005).
The function of a neuron also depends on appropriate expression of a variety of functional proteins, such as neurotransmitter receptors, and voltage-gated calcium, sodium and potassium channels. Surprisingly, to date only a few TFs that control the expression of these types of functional genes have been reported (Cheng et al. 2004, 2006b; Eng et al. 2004; Li et al. 2006). One notable exception is the extensive characterization of TFs that control the expression of a variety of hormone genes in the pituitary gland (Zhu et al. 2005). To promote this area of research, it is becoming critically important to compile a genome-scale map of the spatial and temporal distribution patterns of ion channels and receptors in the developing nervous system, and to correlate this map with the aforementioned TF map.
Coordinated regulation of neuronal identity
In principle, the various aspects of neuronal features could be independently regulated, in a piecemeal manner. Surprisingly, a number of studies suggest a considerable coordinated regulation of neuronal phenotype in a given neuron. For example, HB9/MNR2 coordinates motor neuron development, including the establishment of cholinergic transmitter phenotype and axonal trajectory patterns (Tanabe et al. 1998). Pet1 and Lmx1b regulate the expression of a set of genes that define the serotonergic transmitter phenotype, including the enzymes for serotonin (5-HT) synthesis and the transporters that package 5-HT to synaptic vesicles or re-uptake 5-HT after synaptic release (Cheng et al. 2003; Ding et al. 2003; Hendricks et al. 2003). Crx controls the expression of a large set of functional genes in photoreceptors (Furukawa et al. 1997). Phox2a and Phox2b specify distinct classes of neurons and features within the autonomic circuitry (Brunet & Pattyn, 2002; Thiel, 2006). Finally, our genome-scale TF screen so far only identified a few TFs that distinguish nociceptors from proprioceptors or mechanoceptors (C. Cen & Q. Ma, unpublished data). Indeed, one nociceptor-specific TF Runx1 is able to coordinate the expression of neurotrophin receptors, neuropetides, and two-dozen ion channels and receptors, as well as the establishment of specific afferent central target selections (Chen et al. 2006b; Kramer et al. 2006; Marmigere et al. 2006). These findings have practical implications as the coordinated control of neuronal phenotype by a small number of TFs has been exploited to drive embryonic stem cells to differentiate into defined neuronal cell types, including dopaminergic and motor neurons, thereby opening the door for future cell replacement therapy for certain neurological disorders (Kim et al. 2002; Wichterle et al. 2002; Andersson et al. 2006b).
Beyond TF combinatorial codes
One important principle emerging from studies over the past two-decades is that combinatorial TF codes are able to define the major populations of neuronal cells. This control mechanism emphasizes the precision in genetic control of nervous system development. However, previously unrecognized cellular complexity has been revealed in systems where neuronal morphology or functional genes have been extensively characterized. For example, over two dozen subtypes of retinal amacrine cells are distinguished by their unique morphology, and olfactory neurons show a monogenic expression of over 1000 odour receptors (Buck, 2000; Masland, 2004). This level of complexity raises the question about whether the spatial and temporal patterning has enough resolution to provide a combinatorial code of TFs (and cofactors) for individual neuronal subtypes. Indeed, increasing evidence suggests that cell diversity can arise from a population of neurons that might share the same TF (and cofactor) codes (Shykind, 2005). Here I only discuss a few of many possible mechanisms.
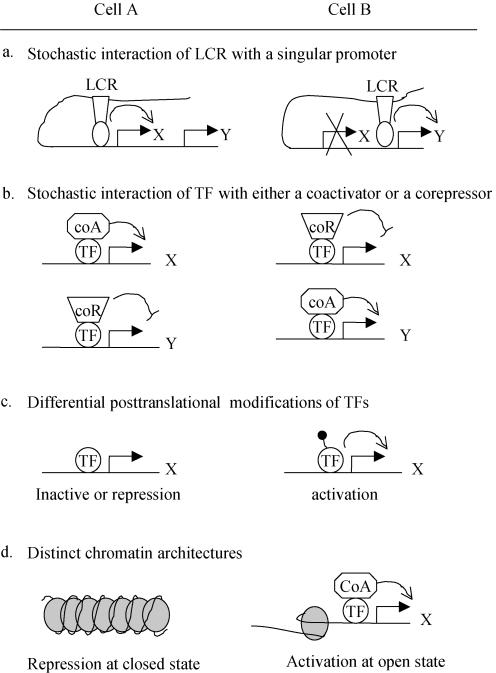
One is the involvement of the locus control region (LCR) that was first shown to control beta-globin gene expression (Li et al. 1999). In this model TFs that bind to LCR interact with only one of many target promoters clustered in the same chromosome (Fig. 2A). In the nervous system, this control mechanism has been proposed for a stochastic activation of either red or green cone pigment gene in a given photoreceptor (Smallwood et al. 2002), or the choice of one olfactory receptor (OR) from a cluster of OR genes (Serizawa et al. 2003). A variation of this control model is the involvement of a specific locus in the nucleus for the activation of a group of target genes, as recently demonstrated by the generation of monoallelic expression of variant surface glycoprotein genes in Trypanosoma brucei (Navarro & Gull, 2001).
Figure 2. Generation of neuronal cell diversity from a group of neurons that share the same TF codes.
LCR, locus control region; coA, coactivator; coR, corepressor.
The second scenario is that the TF activity is limited, such that only a small fraction of target genes can be activated in a given neuron. TF activity can be limited by low expression levels, or squelching by inhibitors. In addition, many TFs can function as either a transcription activator or a repressor. If a group of cells simultaneously express coactivator and corepressor, a competitive interaction with these two opposing cofactors will limit a TF to stochastically activate a fraction of targets in a given cell (Fig. 2B). If positive or negative feedback exists, the initial stochastic choice can be stabilized, leading to an effective way in generating cell diversity (Kaern et al. 2005; Raser & O'Shea, 2005; Shykind, 2005).
Third, cell diversity can be generated after axons reach distinct targets. For example, the sympathetic neurons that innervate the sweat gland make a switch in transmitter phenotypes, from noradrenergic to cholinergic (Francis & Landis, 1999). Similarly, the identity of postsynaptic neurons can be re-defined by distinct presynaptic inputs (Sur & Rubenstein, 2005). Target-derived signalling could change TF/cofactor codes, or modulate TF activity through post-translational modifications (Fig. 2C).
Finally, neuronal cell type specification is also subject to epigenetic control, through histone modifications, DNA methylation and the remodelling of chromatin architectures (Hsieh & Gage, 2005). While some TFs are capable of recruiting enzymatic cofactors to modify chromatin structures, other TFs only bind target promoters at open chromatin states (Maier et al. 2004). Therefore, cell diversity can emerge if a group of neurons inherit distinct chromatin states, even if they share the same TF codes (Fig. 2D). Recent years have also started to recognize the important roles of non-coding RNA, including microRNA and small interfering RNA (siRNA), in controlling gene expression at both chromatin and post-transcription levels, and these new regulatory molecules will most probably contribute to the generation of neuronal cell diversity (Mattick & Makunin, 2005).
Concluding remarks
Generation of nerve cell diversity is a complex process and involves multiple control mechanisms. At early stages of neural development, spatial and temporal patterning leads to the specification of distinct groups of precursors and neurons, each of which is often defined and controlled by a combinatorial expression of TFs. This deterministic control mechanism ensures that the framework of the nervous system is subject to precise ‘hardwired’ genetic control, a feature certainly critical for proper brain function. However, cell diversity can be generated through epigenetic or stochastic regulation of gene expression in a group of functionally related neurons that may share the same TF codes. These latter control mechanisms suggest the existence of an intrinsic variability in vertebrate nervous system development. In other words, individual difference may arise even before nurturing starts to reshape the brain.
Acknowledgments
I thank Jane Johnson and Martyn Goulding for their comments on this manuscript. The work performed in my lab was supported by NIH grants from NINDS and NIDCR, the Pew Scholar Award, and the Claudia Adams Barr Investigator Awards.
References
- Aizawa H, Hu SC, Bobb K, Balakrishnan K, Ince G, Gurevich I, Cowan M, Ghosh A. Dendrite development regulated by CREST, a calcium-regulated transcriptional activator. Science. 2004;303:197–202. doi: 10.1126/science.1089845. [DOI] [PubMed] [Google Scholar]
- Anderson DJ. Stem cells and pattern formation in the nervous system: the possible versus the actual. Neuron. 2001;30:19–35. doi: 10.1016/s0896-6273(01)00260-4. [DOI] [PubMed] [Google Scholar]
- Anderson DJ, Groves A, Lo L, Ma Q, Rao M, Shah NM, Sommer L. Cell lineage determination and the control of neuronal identity in the neural crest. Cold Spring Harb Symp Quant Biol. 1997;62:493–504. [PubMed] [Google Scholar]
- Anderson DJ, Jan YN. The determination of the neuronal phenotype. In: Cowan WM, Jessell TM, Zipursky SL, editors. Molecular and Cellular Approaches to Neural Development. New York: Oxford University Press; 1997. pp. 26–63. [Google Scholar]
- Anderson S, Mione M, Yun K, Rubenstein JL. Differential origins of neocortical projection and local circuit neurons: role of Dlx genes in neocortical interneuronogenesis. Cereb Cortex. 1999;9:646–654. doi: 10.1093/cercor/9.6.646. [DOI] [PubMed] [Google Scholar]
- Andersson E, Jensen JB, Parmar M, Guillemot F, Bjorklund A. Development of the mesencephalic dopaminergic neuron system is compromised in the absence of neurogenin 2. Development. 2006a;133:507–516. doi: 10.1242/dev.02224. [DOI] [PubMed] [Google Scholar]
- Andersson E, Tryggvason U, Deng Q, Friling S, Alekseenko Z, Robert B, Perlmann T, Ericson J. Identification of intrinsic determinants of midbrain dopamine neurons. Cell. 2006b;124:393–405. doi: 10.1016/j.cell.2005.10.037. [DOI] [PubMed] [Google Scholar]
- Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell. 2003;101:485–498. doi: 10.1016/s0092-8674(00)80859-4. [DOI] [PubMed] [Google Scholar]
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Bennett MR, Balcar VJ. Forty years of amino acid transmission in the brain. Neurochem Int. 1999;35:269–280. doi: 10.1016/s0197-0186(99)00068-6. [DOI] [PubMed] [Google Scholar]
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neuroscience. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- Blackshaw S, Harpavat S, Trimarchi J, Cai L, Huang H, Kuo WP, Weber G, Lee K, Fraioli RE, Cho SH, Yung R, Asch E, Ohno-Machado L, Wong WH, Cepko CL. Genomic analysis of mouse retinal development. Plos Biol. 2004;2:E247. doi: 10.1371/journal.pbio.0020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodinsky LN, Root CM, Cronin JA, Sann SB, Gu X, Spitzer NC. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 2004;429:523–530. doi: 10.1038/nature02518. [DOI] [PubMed] [Google Scholar]
- Brunet J, Ghysen A. Deconstructing cell determination: proneural genes and neuronal identity. Bioessays. 1999;21:313–318. doi: 10.1002/(SICI)1521-1878(199904)21:4<313::AID-BIES7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Brunet JF, Pattyn A. Phox2 genes – from patterning to connectivity. Curr Opin Genet Dev. 2002;12:435–440. doi: 10.1016/s0959-437x(02)00322-2. [DOI] [PubMed] [Google Scholar]
- Buck LB. The molecular architecture of odor and pheromone sensing in mammals. Cell. 2000;100:611–618. doi: 10.1016/s0092-8674(00)80698-4. [DOI] [PubMed] [Google Scholar]
- Casarosa S, Fode C, Guillemot F. Mash1 regulates neurogenesis in the ventral telencephalon. Development. 1999;126:525–534. doi: 10.1242/dev.126.3.525. [DOI] [PubMed] [Google Scholar]
- Cau E, Casarosa S, Guillemot F. Mash1 and Ngn1 control distinct steps of determination and differentiation in the olfactory sensory neuron lineage. Development. 2002;129:1871–1880. doi: 10.1242/dev.129.8.1871. [DOI] [PubMed] [Google Scholar]
- Chen AI, de Nooij JC, Jessell TM. Graded activity of transcription factor Runx3 specifies the laminar termination pattern of sensory axons in the developing spinal cord. Neuron. 2006a;49:395–408. doi: 10.1016/j.neuron.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Chen B, Schaevitz LR, McConnell SK. Fezl regulates the differentiation and axon targeting of layer 5 subcortical projection neurons in cerebral cortex. Proc Natl Acad Sci U S A. 2005;102:17184–17189. doi: 10.1073/pnas.0508732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CL, Broom DC, Liu Y, de Nooij JC, Li Z, Cen C, Samad OA, Jessell TM, Woolf CJ, Ma Q. Runx1 determines nociceptive sensory neuron phenotype and is required for thermal and neuropathic pain. Neuron. 2006b;49:365–377. doi: 10.1016/j.neuron.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Cheng L, Arata A, Mizuguchi R, Qian Y, Karunaratne A, Gray PA, Arata S, Shirasawa S, Bouchard M, Luo P, Chen CL, Busslinger M, Goulding M, Onimaru H, Ma Q. Tlx3 and Tlx1 are post-mitotic selector genes determining glutamatergic over GABAergic cell fates. Nat Neurosci. 2004;7:510–517. doi: 10.1038/nn1221. [DOI] [PubMed] [Google Scholar]
- Cheng L, Chen CL, Luo P, Tan M, Qiu M, Johnson R, Ma Q. Lmx1b, Pet-1, and Nkx2.2 coordinately specify serotonergic neurotransmitter phenotype. J Neurosci. 2003;23:9961–9967. doi: 10.1523/JNEUROSCI.23-31-09961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Samad OA, Xu Y, Mizuguchi R, Luo P, Shirasawa S, Goulding M, Ma Q. Lbx1 and Tlx3 are opposing switches in determining GABAergic versus glutamatergic transmitter phenotypes. Nat Neurosci. 2005;8:1510–1515. doi: 10.1038/nn1569. [DOI] [PubMed] [Google Scholar]
- Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, Rubenstein JL. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci. 2005;8:1059–1068. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Tice BC, Brenner-Morton S, Jessell TM. A Hox regulatory network establishes motor neuron pool identity and target-muscle connectivity. Cell. 2005;123:477–491. doi: 10.1016/j.cell.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Marklund U, Yuan W, Yin J, Wegman L, Ericson J, Deneris E, Johnson RL, Chen ZF. Lmx1b is essential for the development of serotonergic neurons. Nat Neurosci. 2003;6:933–938. doi: 10.1038/nn1104. [DOI] [PubMed] [Google Scholar]
- Eng SR, Lanier J, Fedtsova N, Turner EE. Coordinated regulation of gene expression by Brn3a in developing sensory ganglia. Development. 2004;131:3859–3870. doi: 10.1242/dev.01260. [DOI] [PubMed] [Google Scholar]
- Esumi S, Kakazu N, Taguchi Y, Hirayama T, Sasaki A, Hirabayashi T, Koide T, Kitsukawa T, Hamada S, Yagi T. Monoallelic yet combinatorial expression of variable exons of the protocadherin-alpha gene cluster in single neurons. Nat Genet. 2005;37:171–176. doi: 10.1038/ng1500. [DOI] [PubMed] [Google Scholar]
- Fode C, Gradwohl G, Morin X, Dierich A, LeMeur M, Goridis C, Guillemot F. The bHLH protein NEUROGENIN2 is a detemination factor for epibranchial placode-derived sensory neurons. Neuron. 1998;120:483–494. doi: 10.1016/s0896-6273(00)80989-7. [DOI] [PubMed] [Google Scholar]
- Fode C, Ma Q, Casarosa S, Ang S-L, Anderson DJ, Guillemot F. A role for neural determination genes in specifying the dorsoventral identity of telencephalic neurons. Genes Dev. 2000;14:67–80. [PMC free article] [PubMed] [Google Scholar]
- Francis NJ, Landis SC. Cellular and molecular determinants of sympathetic neuron development. Annu Rev Neurosci. 1999;22:541–566. doi: 10.1146/annurev.neuro.22.1.541. [DOI] [PubMed] [Google Scholar]
- Fremeau RTJ, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Morrow EM, Cepko CL. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell. 1997;91:531–541. doi: 10.1016/s0092-8674(00)80439-0. [DOI] [PubMed] [Google Scholar]
- Gaudilliere B, Konishi Y, de la Iglesia N, Yao G, Bonni A. A CaMKII-NeuroD signaling pathway specifies dendritic morphogenesis. Neuron. 2004;41:229–241. doi: 10.1016/s0896-6273(03)00841-9. [DOI] [PubMed] [Google Scholar]
- Gerber AN, Klesert TR, Bergstrom DA, Tapscott SJ. Two domains of MyoD mediate transcriptional activation of genes in repressive chromatin: a mechanism for lineage determination in myogenesis. Genes Dev. 1997;11:436–450. doi: 10.1101/gad.11.4.436. [DOI] [PubMed] [Google Scholar]
- Glasgow SM, Henke RM, Macdonald RJ, Wright CV, Johnson JE. Ptf1a determines GABAergic over glutamatergic neuronal cell fate in the spinal cord dorsal horn. Development. 2005;132:5461–5469. doi: 10.1242/dev.02167. [DOI] [PubMed] [Google Scholar]
- Goridis C, Rohrer H. Specification of catecholaminergic and serotonergic neurons. Nat Rev Neurosci. 2002;3:531–541. doi: 10.1038/nrn871. [DOI] [PubMed] [Google Scholar]
- Gowan K, Helms AW, Hunsaker TL, Collisson T, Ebert PJ, Odom R, Johnson JE. Crossinhibitory activities of Ngn1 and Math1 allow specification of distinct dorsal interneurons. Neuron. 2001;31:219–232. doi: 10.1016/s0896-6273(01)00367-1. [DOI] [PubMed] [Google Scholar]
- Gradwohl G, Fode C, Guillemot F. Restricted expression of a novel murine atonal-related bHLH protein in undifferentiated neural precursors. Dev Biol. 1996;180:227–241. doi: 10.1006/dbio.1996.0297. [DOI] [PubMed] [Google Scholar]
- Gray PA, Fu H, Luo P, Zhao Q, Yu J, Ferrari A, et al. Mouse brain organization revealed through direct genome-scale TF expression analysis. Science. 2004;306:2255–2257. doi: 10.1126/science.1104935. [DOI] [PubMed] [Google Scholar]
- Gross MK, Dottori M, Goulding M. Lbx1 specifies somatosensory association interneurons in the dorsal spinal cord. Neuron. 2002;34:535–549. doi: 10.1016/s0896-6273(02)00690-6. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75:463–476. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Molnar Z, Tarabykin V, Stoykova A. Molecular mechanisms of cortical differentiation. Eur J Neurosci. 2006;23:857–868. doi: 10.1111/j.1460-9568.2006.04626.x. [DOI] [PubMed] [Google Scholar]
- Gutierrez R. The GABAergic phenotype of the ‘glutamatergic’ granule cells of the dentate gyrus. Prog Neurobiol. 2003;71:337–358. doi: 10.1016/j.pneurobio.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Hand R, Bortone D, Mattar P, Nguyen L, Heng JI, Guerrier S, et al. Phosphorylation of Neurogenin2 specifies the migration properties and the dendritic morphology of pyramidal neurons in the neocortex. Neuron. 2005;48:45–62. doi: 10.1016/j.neuron.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Helms AW, Battiste J, Henke RM, Nakada Y, Simplicio N, Guillemot F, Johnson JE. Sequential roles for Mash1 and Ngn2 in the generation of dorsal spinal cord interneurons. Development. 2005;132:2709–2719. doi: 10.1242/dev.01859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms AW, Johnson JE. Specification of dorsal spinal cord interneurons. Curr Op Neurobiol. 2003;13:42–49. doi: 10.1016/s0959-4388(03)00010-2. [DOI] [PubMed] [Google Scholar]
- Hendricks T, Francis N, Fyodorov D, Deneris ES. The ETS domain factor Pet-1 is an early and precise marker of central serotonin neurons and interacts with a conserved element in serotonergic genes. J Neurosci. 1999;19:10348–10356. doi: 10.1523/JNEUROSCI.19-23-10348.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, et al. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Hirsch M-R, Tiveron M-C, Guillemot F, Brunet J-F, Goridis C. Control of noradrenergic differentiation and Phox2a expression by MASH1 in the central and peripheral nervous system. Development. 1998;125:599–608. doi: 10.1242/dev.125.4.599. [DOI] [PubMed] [Google Scholar]
- Hoshino MM, Nakamura S, Mori K, Kawauchi T, Terao M, Nishimura Y, et al. Ptf1a, a bHLH transcriptional gene, defines GABAergic neuronal fates in cerebellum. Neuron. 2005;47:201–213. doi: 10.1016/j.neuron.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Howard MJ. Mechanisms and perspectives on differentiation of autonomic neurons. Dev Biol. 2005;277:271–286. doi: 10.1016/j.ydbio.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Hsieh J, Gage FH. Chromatin remodeling in neural development and plasticity. Curr Opin Cell Biol. 2005;17:664–671. doi: 10.1016/j.ceb.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Jan L, Jan YN. HLH proteins, fly neurogenesis, and vertebrate myogenesis. Cell. 1993;75:827–830. doi: 10.1016/0092-8674(93)90525-u. [DOI] [PubMed] [Google Scholar]
- Jeong JY, Einhorn Z, Mercurio S, Lee S, Lau B, Mione M, Wilson SW, Guo S. Neurogenin1 is a determinant of zebrafish basal forebrain dopaminergic neurons and is regulated by the conserved zinc finger protein Tof/Fezl. Proc Natl Acad Sci U S A. 2006;103:5143–5148. doi: 10.1073/pnas.0600337103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nat Rev Genet. 2000;1:20–29. doi: 10.1038/35049541. [DOI] [PubMed] [Google Scholar]
- Kaern M, Elston TC, Blake WJ, Collins JJ. Stochasticity in gene expression: from theories to phenotypes. Nat Rev Genet. 2005;6:451–464. doi: 10.1038/nrg1615. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. New York, U S A: McGraw-Hill; 2000. [Google Scholar]
- Kele J, Simplicio N, Ferri AL, Mira H, Guillemot F, Arenas E, Ang SL. Neurogenin 2 is required for the development of ventral midbrain dopaminergic neurons. Development. 2006;133:495–505. doi: 10.1242/dev.02223. [DOI] [PubMed] [Google Scholar]
- Kiecker C, Lumsden A. Compartments and their boundaries in vertebrate brain development. Nat Rev Neurosci. 2005;6:553–564. doi: 10.1038/nrn1702. [DOI] [PubMed] [Google Scholar]
- Kim JH, Auerbach JM, Rodriguez-Gomez JA, Velasco I, Gavin D, Lumelsky N, et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418:50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- Koshiba-Takeuchi K, Takeuchi JK, Matsumoto K, Momose T, Uno K, Hoepker V, et al. Tbx5 and the retinotectum projection. Science. 2000;287:134–137. doi: 10.1126/science.287.5450.134. [DOI] [PubMed] [Google Scholar]
- Koyano-Nakagawa N, Wettstein D, Kintner C. Activation of Xenopus genes required for lateral inhibition and neuronal differentiation during primary neurogenesis. Mol Cell Neurosci. 1999;14:327–339. doi: 10.1006/mcne.1999.0783. [DOI] [PubMed] [Google Scholar]
- Kramer I, Sigrist M, de Nooij JC, Taniuchi I, Jessell TM, Arber S. A role for Runx transcription factor signaling in dorsal root ganglion sensory neuron diversification. Neuron. 2006;49:379–393. doi: 10.1016/j.neuron.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Lee JE. NeuroD and neurogenesis. Dev Neuroscience. 1997;19:27–32. doi: 10.1159/000111182. [DOI] [PubMed] [Google Scholar]
- Lee JE, Hollenberg SM, Snider L, Turner DL, Lipnick N, Weintraub H. Conversion of Xenopus ectoderm into neurons by NeuroD, a basic-helix-loop-helix protein. Science. 1995;268:836–844. doi: 10.1126/science.7754368. [DOI] [PubMed] [Google Scholar]
- Lee SK, Pfaff SL. Transcriptional networks regulating neuronal identity in the developing spinal cord. Nat Neurosci. 2001;4:1183–1191. doi: 10.1038/nn750. [DOI] [PubMed] [Google Scholar]
- Li Q, Harju S, Peterson KR. Locus control regions: coming of age at a decade plus. Trends Genet. 1999;15:403–408. doi: 10.1016/s0168-9525(99)01780-1. [DOI] [PubMed] [Google Scholar]
- Li MZ, Wang JS, Jiang DJ, Xiang CX, Wang FY, Zhang KH, Williams PR, Chen ZF. Molecular mapping of developing dorsal horn-enriched genes by microarray and dorsal/ventral subtractive screening. Dev Biol. 2006;292:555–564. doi: 10.1016/j.ydbio.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Livesey FJ, Cepko CL. Vertebrate neural cell-fate determination: lessons from the retina. Nat Rev Neurosci. 2001;2:109–118. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- Ma Q, Fode C, Guillemot F, Anderson DJ. Neurogenin1 and neurogenin2 control two distinct waves of neurogenesis in developing dorsal root ganglia. Genes Dev. 1999;13:1717–1728. doi: 10.1101/gad.13.13.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Kintner C, Anderson DJ. Identification of neurogenin, a vertebrate neuronal determination gene. Cell. 1996;87:43–52. doi: 10.1016/s0092-8674(00)81321-5. [DOI] [PubMed] [Google Scholar]
- Ma Q, Sommer L, Cserjesi P, Anderson DJ. Mash1 and neurogenin1 expression patterns define complementary domains of neuroepithelium in the developing CNS and are correlated with regions expressing notch ligands. J Neurosci. 1997;17:3644–3652. doi: 10.1523/JNEUROSCI.17-10-03644.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell SK. Constructing the cerebral cortex: neurogenesis and fate determination. Neuron. 1995;15:761–768. doi: 10.1016/0896-6273(95)90168-x. [DOI] [PubMed] [Google Scholar]
- McNay DE, Pelling M, Claxton S, Guillemot F, Ang SL. Mash1 is required for generic and subtype differentiation of hypothalamic neuroendocrine cells. Mol Endocrinol. 2006;20:1623–1632. doi: 10.1210/me.2005-0518. [DOI] [PubMed] [Google Scholar]
- Maier H, Ostraat R, Gao H, Fields S, Shinton SA, Medina KL, et al. Early B cell factor cooperates with Runx1 and mediates epigenetic changes associated with mb-1 transcription. Nature Immunol. 2004;5:1069–1077. doi: 10.1038/ni1119. [DOI] [PubMed] [Google Scholar]
- Mann RS, Carroll SB. Molecular mechanisms of selector gene function and evolution. Curr Opin Genet Dev. 2002;12:592–600. doi: 10.1016/s0959-437x(02)00344-1. [DOI] [PubMed] [Google Scholar]
- Marmigere F, Montelius A, Wegner M, Groner Y, Reichardt LF, Ernfors P. The Runx1/AML1 transcription factor selectively regulates development and survival of TrkA nociceptive sensory neurons. Nat Neurosci. 2006;9:180–187. doi: 10.1038/nn1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland RH. Neuronal cell types. Curr Biol. 2004;14:R497–R500. doi: 10.1016/j.cub.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Mattick JS, Makunin IV. Small regulatory RNAs in mammals. Hum Mol Genet. 2005;14:R121–R132. doi: 10.1093/hmg/ddi101. [DOI] [PubMed] [Google Scholar]
- Miyoshi G, Bessho Y, Yamada S, Kageyama R. Identification of a novel basic helix-loop-helix gene, Heslike, and its role in GABAergic neurogenesis. J Neurosci. 2004;24:3672–3682. doi: 10.1523/JNEUROSCI.5327-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi R, Kriks S, Cordes R, Gossler A, Ma Q, Goulding M. Ascl1 and Gsh1/2 control inhibitory and excitatory cell fate in spinal sensory interneurons. Nat Neurosci. 2006;9:770–778. doi: 10.1038/nn1706. [DOI] [PubMed] [Google Scholar]
- Molyneaux BJ, Arlotta P, Hirata T, Hibi M, Macklis JD. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron. 2005;47:817–831. doi: 10.1016/j.neuron.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Mui SH, Hindges R, O'Leary DD, Lemke G, Bertuzzi S. The homeodomain protein Vax2 patterns the dorsoventral and nasotemporal axes of the eye. Development. 2002;129:797–804. doi: 10.1242/dev.129.3.797. [DOI] [PubMed] [Google Scholar]
- Muller T, Anlag K, Wildner H, Britsch S, Treier M, Birchmeier C. The bHLH factor Olig3 coordinates the specification of dorsal neurons in the spinal cord. Genes Dev. 2005;19:733–743. doi: 10.1101/gad.326105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T, Brohmann H, Pierani A, Heppenstall PA, Lewin GR, Jessell TM, Birchmeier C. The homeodomain factor Lbx1 distinguishes two major programs of neuronal differentiation in the dorsal spinal cord. Neuron. 2002;34:551–562. doi: 10.1016/s0896-6273(02)00689-x. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y, O'Leary DD. Combinatorial expression patterns of LIM-homeodomain and other regulatory genes parcellate developing thalamus. J Neurosci. 2001;21:2711–2725. doi: 10.1523/JNEUROSCI.21-08-02711.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Gull K. A pol I transcriptional body associated with VSG mono-allelic expression in Trypanosoma brucei. Nature. 2001;414:759–763. doi: 10.1038/414759a. [DOI] [PubMed] [Google Scholar]
- Pak W, Hindges R, Lim YS, Pfaff SL, O'Leary DD. Magnitude of binocular vision controlled by islet-2 repression of a genetic program that specifies laterality of retinal axon pathfinding. Cell. 2004;119:567–578. doi: 10.1016/j.cell.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Parras CM, Schuurmans C, Scardigli R, Kim J, Anderson DJ, Guillemot F. Divergent functions of the proneural genes Mash1 and Ngn2 in the specification of neuronal subtype identity. Genes Dev. 2002;16:324–338. doi: 10.1101/gad.940902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish JZ, Kim MD, Jan LY, Jan YN. Genome-wide analyses identify transcription factors required for proper morphogenesis of Drosophila sensory neuron dendrites. Genes Dev. 2006;20:820–835. doi: 10.1101/gad.1391006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattyn A, Simplicio N, van Doorninck JH, Goridis C, Guillemot F, Brunet JF. Ascl1/Mash1 is required for the development of central serotonergic neurons. Nat Neurosci. 2004;7:589–595. doi: 10.1038/nn1247. [DOI] [PubMed] [Google Scholar]
- Perez SE, Rebelo S, Anderson DJ. Early specification of sensory neuron fate revealed by expression and function of neurogenins in the chick embryo. Development. 1999;126:1715–1728. doi: 10.1242/dev.126.8.1715. [DOI] [PubMed] [Google Scholar]
- Ramirez M, Gutierrez R. Activity-dependent expression of GAD67 in the granule cells of the rat hippocampus. Brain Res. 2001;917:139–146. doi: 10.1016/s0006-8993(01)02794-9. [DOI] [PubMed] [Google Scholar]
- Raser JM, O'Shea EK. Noise in gene expression: origins, consequences, and control. Science. 2005;309:2010–2013. doi: 10.1126/science.1105891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Represa A, Ben-Ari Y. Trophic actions of GABA on neuronal development. Trends Neurosci. 2005;28:278–283. doi: 10.1016/j.tins.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Sandberg M, Kallstrom M, Muhr J. Sox21 promotes the progression of vertebrate neurogenesis. Nat Neurosci. 2005;8:995–1001. doi: 10.1038/nn1493. [DOI] [PubMed] [Google Scholar]
- Schulte D, Furukawa T, Peters MA, Kozak CA, Cepko CL. Misexpression of the Emx-related homeobox genes cVax and mVax2 ventralizes the retina and perturbs the retinotectal map. Neuron. 1999;24:541–553. doi: 10.1016/s0896-6273(00)81111-3. [DOI] [PubMed] [Google Scholar]
- Schuurmans C, Armant O, Nieto M, Stenman JM, Britz O, Klenin N, et al. Sequential phases of cortical specification involve Neurogenin-dependent and -independent pathways. EMBO J. 2004;23:2892–2902. doi: 10.1038/sj.emboj.7600278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurmans C, Guillemot F. Molecular mechanisms underlying cell fate specification in the developing telencephalon. Curr Opin Neurobiol. 2002;12:26–34. doi: 10.1016/s0959-4388(02)00286-6. [DOI] [PubMed] [Google Scholar]
- Schwarzer C, Sperk G. Hippocampal granule cells express glutamic acid decarboxylase-67 after limbic seizures in the rat. Neuroscience. 1995;69:705–709. doi: 10.1016/0306-4522(95)00348-m. [DOI] [PubMed] [Google Scholar]
- Serizawa S, Miyamichi K, Nakatani H, Suzuki M, Saito M, Yoshihara Y, Sakano H. Negative feedback regulation ensures the one receptor-one olfactory neuron rule in mouse. Science. 2003;302:2078–2079. doi: 10.1126/science.1089122. [DOI] [PubMed] [Google Scholar]
- Shykind BM. Regulation of odorant receptors: one allele at a time. Hum Mol Genet. 2005;14:R33–R39. doi: 10.1093/hmg/ddi105. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Dichter MA, Rachinsky TL, Dean E, Goodman JH, Sollas AL, Martin DL. Basal expression and induction of glutamate decarboxylase and GABA in excitatory granule cells of the rat and monkey hippocampal dentate gyrus. J Comp Neurol. 1996;373:593–618. doi: 10.1002/(SICI)1096-9861(19960930)373:4<593::AID-CNE8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Smallwood PM, Wang Y, Nathans J. Role of a locus control region in the mutually exclusive expression of human red and green cone pigment genes. Proc Natl Acad Sci U S A. 2002;99:1008–1011. doi: 10.1073/pnas.022629799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer L, Ma Q, Anderson DJ. Neurogenins, a novel family of atonal-related bHLH transcription factors, are putative mammalian neuronal determination genes that reveal progenitor cell heterogenity in the developing CNS and PNS. Mol Cell Neurosci. 1996;8:221–241. doi: 10.1006/mcne.1996.0060. [DOI] [PubMed] [Google Scholar]
- Spitzer NC, Root CM, Borodinsky LN. Orchestrating neuronal differentiation: patterns of Ca2+ spikes specify transmitter choice. Trends Neurosci. 2004;27:415–421. doi: 10.1016/j.tins.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Sun Y, Nadal-Vicens M, Misono S, Lin MZ, Zubiaga A, Hua X, Fan G, Greenberg ME. Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell. 2001;104:365–376. doi: 10.1016/s0092-8674(01)00224-0. [DOI] [PubMed] [Google Scholar]
- Sur M, Rubenstein JL. Patterning and plasticity of the cerebral cortex. Science. 2005;310:805–810. doi: 10.1126/science.1112070. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, William C, Jessell TM. Specification of motor neuron identity by the MNR2 homeodomain protein. Cell. 1998;95:67–80. doi: 10.1016/s0092-8674(00)81783-3. [DOI] [PubMed] [Google Scholar]
- Tapscott SJ. The circuitry of a master switch: Myod and the regulation of skeletal muscle gene transcription. Development. 2005;132:2685–2695. doi: 10.1242/dev.01874. [DOI] [PubMed] [Google Scholar]
- Thiel G. Transcription Factors in the Nervous System. Wiley-VCH-Verlag GmbH Weinheim; 2006. [Google Scholar]
- Vitalis T, Parnavelas JG. The role of serotonin in early cortical development. Dev Neurosci. 2003;25:245–256. doi: 10.1159/000072272. [DOI] [PubMed] [Google Scholar]
- Weimann JM, Zhang YA, Levin ME, Devine WP, Brulet P, McConnell SK. Cortical neurons require Otx1 for the refinement of exuberant axonal projections to subcortical targets. Neuron. 1999;24:819–831. doi: 10.1016/s0896-6273(00)81030-2. [DOI] [PubMed] [Google Scholar]
- Weintraub H. The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell. 1993;75:1241–1244. doi: 10.1016/0092-8674(93)90610-3. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Wildner H, Muller T, Cho SH, Brohl D, Cepko CL, Guillemot F, Birchmeier C. dILA neurons in the dorsal spinal cord are the product of terminal and non-terminal asymmetric progenitor cell divisions, and require Mash1 for their development. Development. 2006;133:2105–2113. doi: 10.1242/dev.02345. [DOI] [PubMed] [Google Scholar]
- Zhu X, Lin CR, Prefontaine GG, Tollkuhn J, Rosenfeld MG. Genetic control of pituitary development and hypopituitarism. Curr Opin Genet Dev. 2005;15:332–340. doi: 10.1016/j.gde.2005.04.011. [DOI] [PubMed] [Google Scholar]