Abstract
Serological analysis of expression cDNA libraries (SEREX) derived from two small cell lung cancer (SCLC) cell lines using pooled sera of SCLC patients led to the isolation of 14 genes, including 4 SOX group B genes (SOX1, SOX2, SOX3, and SOX21) and ZIC2. SOX group B genes and ZIC2 encode DNA-binding proteins; SOX group B proteins regulate transcription of target genes in the presence of cofactors, whereas ZIC2 is also suspected to be a transcriptional regulator. These genes are expressed at early developmental stages in the embryonic nervous system, but are down-regulated in the adult. Although SOX2 mRNA can be detected in some adult tissues, ZIC2 is expressed only in brain and testis, and SOX1, SOX3, and SOX21 transcripts are not detectable in normal adult tissues. Of SCLC cell lines tested, 80% expressed ZIC2 mRNA, and SOX1, SOX2, and SOX3 expression was detected in 40%, 50%, and 10%, respectively. SOX group B and ZIC2 antigens elicited serological responses in 30–40% of SCLC patients in this series, at titers up to 1:106. In sera from 23 normal adults, no antibody was detected against SOX group B or ZIC2 proteins except for one individual with low-titer anti-SOX2 antibody. Seroreactivity against SOX1 and 2 was consistently higher titered than SOX3 and 21 reactivity, suggesting SOX1 and/or SOX2 as the main antigens eliciting anti-SOX responses. Although paraneoplastic neurological syndromes have been associated with several SCLC antigens, neurological symptoms have not been observed in patients with anti-SOX or anti-ZIC2 antibodies.
Small cell lung cancer (SCLC) is a highly aggressive form of lung cancer that accounts for approximately 20% of all cases of lung cancer (1). Although initially responsive to chemotherapy, SCLC is almost invariably fatal. SCLC is a carcinoma of neuroendocrine origin, in contrast to non-SCLC (NSCLC), which is of bronchial epithelial origin. Dense-core neurosecretory granules and other neuroendocrine markers are characteristic features of SCLC (2, 3). Some of these markers, including synaptophysin, chromogranin A, and neuron-specific enolase, are expressed normally by neuroendocrine cells throughout development (4). In contrast, other SCLC gene products have been detected only in the embryonic nervous tissue and have not been found in normal adult tissues. The restricted expression of human acheate-scute homologue (hASH) is one such example. hASH1 has been shown to be essential for the development of neuroendocrine cells in the lung and for the maintenance of the neuroendocrine features of SCLC (5, 6).
The association of SCLC with paraneoplastic neurological syndromes is well known (7, 8). These syndromes, defined as neurological disorders that are related to cancer but cannot be accounted for by metastasis or treatment complications, are rare manifestations of a small subset of malignancies, including SCLC (8, 9). Current knowledge points to immune-mediated neuronal injury as the cause of these paraneoplastic syndromes, resulting from the amplified or aberrant expression of immunogenic neural antigens by the cancer. The elicited humoral and/or cell-mediated immune responses react with normal neuronal cells, leading to structural damages and clinical manifestations. The presence of high-titered antibodies in patients with paraneoplastic disorders has facilitated the identification of antigenic targets associated with these syndromes (9). A number of these antigens have been isolated by using antibodies from SCLC patients, and all of them are expressed normally by selected neuronal cell populations. The current list of SCLC-related antigens associated with paraneoplastic neurological syndromes includes HuD, a neuronal antigen homologous to Drosophila Elav and SxI genes (10); recoverin, a photoreceptor protein (11, 12); amphiphysin, a 128-kDa synaptic vesicular protein (13); Ri (Nova), an antigen with homology to RNA-binding proteins, expressed in the developing motor neurons (14); VGCC, located at the presynaptic region of the neuromuscular junction (15); and CRMP-5, a neuron-specific collapsin-response mediator protein (16). Clearly, SCLC is a highly immunogenic tumor likely the result of the expression of a wide array of normal neuroectodermal antigens to which the host is not tolerant.
To extend the search for SCLC antigens with immunogenicity in humans, we have analyzed the humoral immune response of SCLC patients by using serological analysis of expression cDNA libraries (SEREX), an approach that is being applied to a broad range of different human tumor types (17–19). SEREX analysis, which involves serological screening of cDNA expression libraries with sera from cancer patients, has shown that cancer patients mount a vigorous humoral immune response to a wide variety of cellular antigens, including differentiation antigens, mutational antigens, cancer-testis (CT) antigens, and amplified antigens (20). What has been clear from SEREX is that high-titered IgG response to tumor antigens is not a rare event and such responses are not limited to patients with paraneoplastic syndromes. In the present study, SEREX of SCLC cell lines has identified several genes with expression patterns predominantly restricted to the embryonic nervous system.
Materials and Methods
Cell Lines, Tissues, and Patient Sera.
Cell lines were obtained from the repository maintained at the Ludwig Institute for Cancer Research (LICR), New York Branch at the Memorial Sloan–Kettering Cancer Center, or from American Tissue Culture Collection. Eleven SCLC cell lines were used, including nine classical (SK-LC-13, NCI-H69, 128, 146, 187, 209, 378, 889, and 740) and two variant (NCI-H82 and 526) forms. The variant SCLC lines differ from the classical lines in lacking or having diminished neuroendocrine features, as well as in other biochemical, morphological, and growth properties (21, 22). Normal and tumor tissues were obtained from the departments of Pathology, New York Presbyterian Hospital (NYPH), and Memorial Sloan–Kettering Cancer Center (MSKCC). Patient sera were obtained from the Department of Medicine, NYPH, and from the LICR Melbourne Branch, Australia.
Immunoscreening of SCLC Cell Line Libraries and Characterization of Immunoreactive Clones.
Construction of cDNA expression libraries from NCI-H740 and SK-LC-13 SCLC cell lines in the λ-ZAP vector (Stratagene) and immunoscreening of the libraries were done as described previously (20, 23), with the following modifications. Sera from five SCLC patients (Lu94, Lu100, Lu101, Lu104, and Lu113) were pooled and absorbed (19). The pooled serum was diluted 1:200 (final dilution, 1:1,000 for each serum) in Tris-buffered saline (TBS) containing 1% BSA and .02% NaN3 and used to screen the NCI-H740 library [5.6 × 105 plaque-forming units (pfu)] and the SK-LC-13 library (2.2 × 105 pfu). Immunoreactive clones were isolated and sequence was analyzed as described previously (23). Selected immunoreactive clones were tested for reactivity against 10-fold serially diluted sera (1:103 to 1:106) from individual lung cancer patients and normal individuals by using the same plaque assay. A λ-ZAP clone without an insert was coplated and included in each assay as a negative control.
Reverse Transcription–PCR (RT-PCR) Analysis.
RT was performed with total RNA isolated from tissue or cell lines by the guanidium thiocyanate/CsCl method (24). Primers used to amplify ZIC2 were designed based on the published sequence (AF104902): ZIC2A1, 5′-CATGAATATGAACATGGGTATGAACATGG; and ZIC2B1, 5′-TCGCAGCCCTCAAACTCACACTG. PCR was performed with initial denaturation and AmpliTaq Gold (Perkin–Elmer) activation at 94°C for 10 min, followed by 35 cycles of amplification (denaturation at 94°C for 1 min, annealing at 60°C for 1 min, and primer extension for 1 min at 72°C), followed by 6-min extension at 72°C. Amplification products were analyzed by agarose gel electrophoresis and visualized by ethidium bromide staining.
Northern Blot Analysis.
Adult normal tissue mRNA blots were obtained from CLONTECH and contained 2 μg of poly(A)+ RNA per lane. SCLC cell line total RNA was isolated as described above, and poly(A)+ mRNA was prepared by using the Microfast Track kit (Invitrogen). Two micrograms of mRNA was transferred to nylon membranes (Schleicher & Schuell) after denaturing gel electrophoresis. Hybridization was carried out in the ExpressHyb buffer (CLONTECH); hybridization and washing conditions were as described by the manufacturer. The probes used for Northern blot analysis were 450-bp SOX2 fragment (nucleotides 630-1,080, GenBank accession no. Z31560), 751-bp SOX1 fragment (nucleotides 1,520–2,271, Y13436), 330-bp SOX3 fragment (nucleotides 442–772, X71135), 680-bp SOX21 fragment (nucleotides 2,720–3,400, AF104902), and full-length ID4 cDNA (1,322 bp, U28368).
Results
Isolation of Immunoreactive Clones from SCLC Cell Lines by SEREX.
SEREX of the SCLC cell line NCI-H740 with a pool of five sera from SCLC patients resulted in the isolation of 37 clones coding for 8 known gene products (Table 1). These eight genes were given SEREX gene designations of NY-SCLC-1 to NY-SCLC-8. The most frequently isolated genes were SOX2 and ZIC2, comprising 51% and 24% of all clones. A single clone corresponding to SOX1 was also isolated from this library. SOX- and ZIC2-encoding clones showed very strong immunoreactivity with the SCLC patient sera. Other genes isolated included ID4, MPP11, MAZ, eIF2B, and RBP-1. ID4 protein is a member of the dominant negative helix–loop–helix (HLH) protein family (25). This protein can interact with other HLH proteins (such as Achaete-scute), and, by virtue of not containing a DNA-binding domain, it acts as a repressor (25). ID4 mRNA expression in normal tissues was found to be universal by Northern blot analysis (data not shown). Seroreactivity against ID4 was of moderate intensity. MPP11 (MPM2-reactive phosphoprotein 11), another HLH protein-binding factor, originally was identified in HeLa cells by M-phase protein-recognizing antibodies (26). Seroreactivity against MPP11 was strong. MPP11 has been identified previously by SEREX of gastric and breast cancer (SEREX database: http://www.licr.org/SEREX.html; clones NGO-St-58 and LONY-BR-7), and MPP11 mRNA is universally expressed. The other genes isolated from the NCI-H740 library—myc-associated Zinc-finger protein MAZ, the eukaryotic translation initiation factor eIF2B, and the J-κ recombination signal-binding protein (RBP-1)—also have been identified by SEREX. MAZ, eIF2B, and RBP-1 mRNA are broadly expressed in normal adult tissues.
Table 1.
Genes isolated by SEREX of the SCLC cell line NCI-H740
| Gene designation | Gene/sequence identity (GenBank accession no.) | No. of clones (% of total) |
|---|---|---|
| NY-SCLC-1 | SOX2 (Z31560) | 19 (51) |
| NY-SCLC-2 | SOX1 (Y13436) | 1 (3) |
| NY-SCLC-3 | ZIC2 (AF104902) | 9 (24) |
| NY-SCLC-4 | ID4 (U28368) | 2 (5) |
| NY-SCLC-5 | MAZ (M94046) | 1 (3) |
| NY-SCLC-6 | MPP11 (X98260) | 3 (8) |
| NY-SCLC-7 | eIF2B (U23028) | 1 (3) |
| NY-SCLC-8 | RBP-1 (L07872) | 1 (3) |
| Total: 37 |
SEREX of the second SCLC line SK-LC-13 with the same pooled sera from SCLC patients resulted in the identification of 14 clones corresponding to 10 genes (Table 2). Four genes were identical to those isolated from NCI-H740, and six were distinct (NY-SCLC-9 to NY-SCLC-14). SOX2 was isolated twice, and SOX3 and SOX21 each were represented by a single clone. ZIC2 was isolated twice. Other genes isolated in both SK-LC-13 and NCI-H740 libraries were ID4, isolated once, and MPP11, which was represented by three immunoreactive clones. NY-SCLC-11 (KIAA0963) is an unknown gene with identical expressed sequence tag sequences derived from many tissues. Two novel genes (NY-SCLC-13 and NY-SCLC-14) showed no sequence identity to current GenBank entries. These two genes are intriguing because their DNA sequences contain homopolymers of 24- and 6-bp repeats that would encode tandem octapeptides and dipeptides. This repeat structure has been found in other immunogenic antigens associated with cancer, such as CT7 (23) and CDR34 (27). NY-SCLC-12, lymphocyte activation gene-3 (LAG-3), is related to CD4 and has a restricted tissue expression pattern, which is characteristic of differentiation antigens of lymphoid origin (28).
Table 2.
Genes isolated by SEREX of the SCLC cell line SK-LC-13
| Gene designation | Gene/sequence identity (GenBank accession no.) | No. of clones (% of total) |
|---|---|---|
| NY-SCLC-1 | SOX2 (Z31560) | 2 (14) |
| NY-SCLC-9 | SOX3 (X71135) | 1 (7) |
| NY-SCLC-10 | SOX21 (AF107044) | 1 (7) |
| NY-SCLC-3 | ZIC2 (AF104902) | 2 (14) |
| NY-SCLC-4 | ID4 (U28368) | 1 (7) |
| NY-SCLC-6 | MPP11 (X98260) | 3 (21) |
| NY-SCLC-11 | KIAA0963 (AB023180.1) | 1 (7) |
| NY-SCLC-12 | LAG-3 (X51985) | 1 (7) |
| NY-SCLC-13 | Novel-1 | 1 (7) |
| NY-SCLC-14 | Novel-2 | 1 (7) |
| Total: 14 |
Immunogenic Epitopes of ZIC2 and SOX Proteins.
Of 11 ZIC2 clones isolated, 7 clones were sequenced and 4 were evaluated by restriction mapping. The longest ZIC2 clone was ≈2.6 kb, with a sequence that extends beyond both 5′ and 3′ ends of the ZIC2 cDNA entry in the GenBank database (AF104902). The shortest clone is of ≈1 kb in size, and its 5′ end corresponds to nucleotide position 692 (amino acid residue 231) of AF104902. Because the seroreactivity of this shorter clone was comparable to that of larger ZIC2 clones, it is likely that the antigenic epitope(s) of ZIC2 resides between amino acid residue 231 and the C-terminal end (amino acid residue 533).
Of 24 SOX clones isolated, 8 SOX2 clones and the SOX1, SOX3, and SOX21 clones were sequenced. The remaining 13 SOX2 clones were analyzed by restriction mapping. All SOX2 clones contained full-length cDNA (1,085 bp), and the longest clone had 54 additional nucleotides at its 5′ untranslated region as compared with the SOX2 GenBank entry (accession no. Z31560). The two SOX1 and SOX3 clones contained truncated cDNA inserts lacking sequences 5′ to those encoding the high-mobility group (HMG) box, whereas the SOX21 clone encoded the full-length SOX21 protein, which has only five residues N-terminal to its HMG box (Fig. 1). The most conserved region in SOX cDNA clones is the HMG box-encoding region, which is 88–96% identical in SOX group B family members. Sera that reacted with SOX1 also reacted with SOX2, SOX3, and SOX21 (see below), suggesting that the immunoreactivity of SCLC patient sera is directed primarily against the conserved HMG box of SOX proteins.
Figure 1.
Alignment of predicted protein sequences of SOX1, 2, 3, and 21 (GenBank accession nos. O00570, P48431, P41225, and AAC95381.1). Sequences encoded within the SEREX-isolated clones are in boldface type, and sequences absent in these clones are in italics. The DNA-binding HMG domain is boxed. Amino acids shared by three or four SOX proteins are lightly and darkly shaded.
Exclusive Expression of ZIC2 in Brain, Testis, and Tumors.
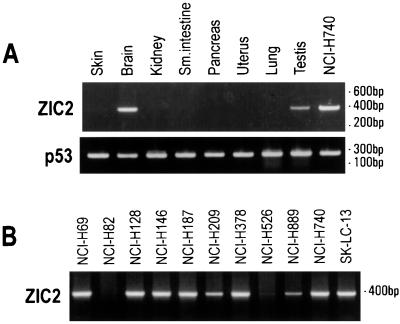
ZIC2 gene expression was analyzed by RT-PCR. Among normal tissues, ZIC2 mRNA was detectable in brain and in testis, but not in skin, kidney, small intestine, pancreas, uterus, or lung (Fig. 2A). Of 11 SCLC cell lines analyzed, all 9 classical SCLC lines had detectable ZIC2 mRNA, whereas the two variant SCLC cell lines (NCI-H82 and NCI-H526) showed no or minimal expression. Among other cell lines, ZIC2 mRNA could be amplified in 100% (7 of 7) of lines derived from non-small cell lung tumor and 83% (10 of 12) of melanoma cell lines (Table 3). Among tumor tissues, ZIC2 mRNA was detectable in 50% or more of a wide range of malignancies, including melanoma, colon cancer, breast cancer, head and neck cancer, lung cancer, bladder cancer, leiomyosarcoma, and synovial sarcoma (Table 3).
Figure 2.
RT-PCR analysis of ZIC2 gene expression in normal tissues (A) and SCLC cell lines (B). RNA quality was assessed by amplification of p53 exons 5 and 6.
Table 3.
ZIC2 gene expression in cancer
| Tumor specimen or cell line | ZIC2 mRNA expression |
|---|---|
| Melanoma cell line | 10/12 (83%) |
| SCLC cell line | 9/11 (82%) |
| NSCLC cell line | 7/7 (100%) |
| Melanoma | 5/10 (50%) |
| Colon cancer | 2/4 (50%) |
| Breast cancer | 3/4 (75%) |
| Head and neck cancer | 12/14 (86%) |
| Lung cancer | 6/9 (66%) |
| Bladder cancer | 7/14 (50%) |
| Leiomyosarcoma | 1/2 (50%) |
| Synovial sarcoma | 2/2 (100%) |
Expression of SOX Group B Genes.
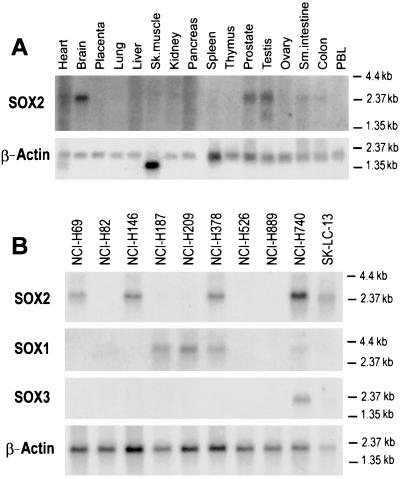
Because SOX group B genes are intronless (29–32), their gene expression was evaluated by Northern blot analysis. Among normal tissues, SOX2 mRNA could be detected in brain, testis, and prostate and at lower levels in small intestine and colon, but not in heart, placenta, lung, liver, skeletal muscle, kidney, pancreas, spleen, thymus, ovary, and peripheral blood leukocytes (Fig. 3A). SOX1, SOX3, and SOX21 mRNA was not detected in normal adult tissues, which is consistent with the current literature (33, 34). In tumor cell lines, SOX2 was expressed in 5 of 10 SCLC cell lines, including NCI-H740 and SK-LC-13 (Fig. 3B). SOX2 message was not detected in three NSCLC lines (SK-LC-7, 8, and 17) or in eight melanoma cell lines (SK-MEL-10, 12, 14, 24, 26, 28, and 37 and Mz19) (data not shown). SOX1 mRNA was detected in 4 of 10 SCLC cell lines, SOX3 mRNA was detected in 2 of 10 lines, and SOX21 was not detected even after prolonged exposure (1 week) (Fig. 3B). SOX1 and SOX3 required longer exposure times than SOX2, indicating lower expression levels. The two variant SCLC cell lines, NCI-H82 and NCI-H526, had no detectable SOX group B gene expression.
Figure 3.
Northern blot analysis of SOX group B gene expression. SOX2 expression in normal tissues (A) and SOX1, 2, and 3 expression in SCLC cell lines (B). A β-actin probe was used to assess RNA quality and quantity. SCLC blots were exposed for 24 hr (SOX2), 72 hr (SOX1), or 1 week (SOX3). Normal tissue blot for SOX2 was exposed for 1 week.
High-Titer Antibodies to SOX and ZIC2 Proteins in SCLC Patients.
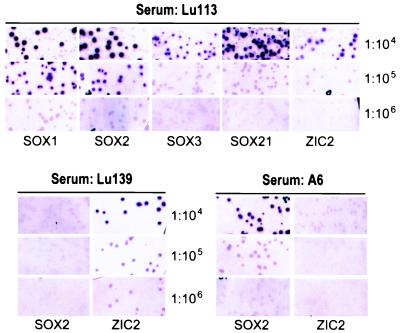
Sera from 17 SCLC patients and 23 normal adults were titered against phage clones coding for SOX1, 2, 3, or 21 or ZIC2. Only 1 of the 23 normal sera showed weak reactivity against SOX2 (titer, 1:1,000). In contrast, 7 of 17 SCLC patients (41%) had antibodies reactive with SOX1- and SOX2-containing phagemids, whereas 29% (5 of 17) and 35% (6 of 17) had antibodies to SOX3 and SOX21, respectively. Five of 17 (29%) patients had detectable anti-ZIC2 antibodies. Antibody titers measured up to 1:106 (Table 4 and Fig. 4). The five patients with antibodies against ZIC2 also had reactivity against SOX proteins; one (Lu113) showed high-titered reactivity (1:106), whereas another (Lu139) was reactive at a 1:103 dilution. Two patients (Lu100 and A6) with strong SOX1 and SOX2 reactivity showed no reactivity with ZIC2 (Table 4 and Fig. 4). All patients with antibodies against SOX3 or SOX21 had higher reactivity against SOX1 and SOX2. The presence of consistently higher-titer antibodies against SOX1 and 2 suggests SOX1 and/or 2 as the main immunogenic tumor antigen in these patients; seroreactivity to SOX3 and SOX21 might be secondary to the shared antigenic epitopes located within the highly conserved HMG box of SOX proteins.
Table 4.
SOX and ZIC2 reactivity of sera from SCLC patients
| Patient | Serum/protein | SOX1 | SOX2 | SOX3 | SOX21 | ZIC2 |
|---|---|---|---|---|---|---|
| 1 | Lu 94* | 1:105 | 1:105 | 1:104 | 1:104 | 1:105 |
| 2 | Lu 100* | 1:105 | 1:105 | 1:104 | 1:104 | — |
| 3 | Lu 101* | — | — | — | — | — |
| 4 | Lu 104* | — | — | — | — | — |
| 5 | Lu 113* | 1:106 | 1:106 | 1:105 | 1:105 | 1:105 |
| 6 | Lu 139 | 1:103 | 1:103 | — | — | 1:106 |
| 7 | Lu 159 | — | — | — | — | — |
| 8 | A1 | 1:105 | 1:106 | 1:105 | 1:105 | 1:104 |
| 9 | A2 | — | — | — | — | — |
| 10 | A3 | — | — | — | — | — |
| 11 | A4 | — | — | — | — | — |
| 12 | A5 | — | — | — | — | — |
| 13 | A6 | 1:105 | 1:105 | 1:105 | 1:104 | — |
| 14 | A7 | — | — | — | — | — |
| 15 | A8 | — | — | — | — | — |
| 16 | A9 | — | — | — | — | — |
| 17 | A10 | 1:104 | 1:104 | — | 1:105 | 1:104 |
| 7/17 (41%) | 7/17 (41%) | 5/17 (29%) | 6/17 (35%) | 5/17 (29%) |
*Sera contributing to serum pool used in SEREX of the SCLC cell lines.
Figure 4.
Seroreactivity of SCLC patients (Lu113, Lu139, and A6) for SOX1, 2, 3, and 21 and ZIC2. Serum dilutions and cloned gene products are indicated. λZAP phages without insert are mixed with test clones and serve as internal negative controls; these are visible as a background to the positive clones (see 1:104 dilution of Lu113 serum). Assays are scored positive only if test clones are clearly distinguishable from control phages. Lu113 seroreactivity can be observed up to 1:106 for SOX1 and SOX2 and 1:105 for SOX3, SOX21, and ZIC2. Lu139 serum showed strong reactivity with ZIC2 but not SOX2, and A6 serum is reactive with SOX2 but not ZIC2.
Discussion
SOX family proteins (Sry-like HMG box), currently with more than 30 members in vertebrates, belong to the HMG box superfamily of DNA-binding proteins (33, 34). They originally were identified based on their homology within the HMG box to the prototype protein SRY, encoded by the testis-determining gene on chromosome Y (35). Within the DNA-binding 79-aa HMG box domain, SOX proteins share more than 50% identity. They are classified into groups A–G, with SRY being the sole member of group A (33, 34). Within each group, the HMG sequences are more than 90% identical and the homology extends into N- and C-terminal regions. Group B SOX proteins (SOX1, 2, 3, 14, and 21) bind similar DNA sequences (32, 36). SOX1, 2, and 3 have been shown to be transcriptional activators (37), and SOX3 recently has been shown to regulate Wnt signaling in Xenopus by binding to β-catenin (38). Whereas the mouse Sox2 knockout is embryonic lethal (39), Sox1 knockout mice are viable at birth but develop seizures and are defective in eye and lens development (40).
The expression of human SOX 1, 2, 3, and 21 gene counterparts in other vertebrates has been studied in detail (33, 34). They are generally expressed within the developing nervous system of the vertebrate embryo, and their expression either ceases or is reduced at later stages. The genes have spatial and temporal patterns of expression that are distinct but mostly overlapping. Chick Sox3 (cSox3) is detectable throughout the epiblast (the layer from which the embryonic tissues will develop) during Hamburger–Hamilton (HH) stage 1 before gastrulation (41), and cSox2 is first expressed in the presumptive neural ectoderm at HH stage 3–4 (41, 42). In both chicken and mouse, Sox1, 2, 3, and 21 are expressed predominantly in the developing central nervous system and are down-regulated as neural differentiation progresses (36, 37, 39, 42–46). Outside the nervous system, low-level mouse Sox group B gene expression has been reported in gut epithelium, developing limb buds, and genital ridges of the embryo (36, 47). Although this embryonic expression pattern generally holds true for all species examined, some interspecies variations in temporal expression of Sox group B genes exist. For example, in contrast to chick Sox2, mouse Sox2 is expressed before gastrulation. Also, whereas Sox3 is expressed in the mouse gonads, chick and Xenopus Sox3 are not (36, 43–45, 47).
The ZIC genes (Zinc-finger gene of the cerebellum), currently with four members, also encode DNA-binding proteins (48–50). ZIC proteins are structurally similar to each other, particularly within a 150-aa domain that encodes five Zinc-finger motifs (51). Mouse Zic genes, although unrelated to Sox group B genes, have similar temporal expression patterns, and Sox2 and Zic1 are subject to common developmentally regulatory elements (52). All Zic genes are expressed from the gastrula stage on and show different temporal expression patterns (51, 53). Xenopus Zic2 expression commences earlier than XZic1 and 3 (51). In the adult mouse, Zic2 and 3 expression is restricted to the granule cells of the cerebellum, whereas Zic1 expression is detectable in the olfactory bulb and cerebrum in addition to the cerebellum (48, 50). Mutations in human ZIC2 and ZIC3 are related to holoproensephaly and situs abnormalities, respectively, whereas mZic1 knockout mice develop severe ataxia because of a hypoplastic cerebellum (54–56).
Two possibilities could account for the expression of SOX and ZIC2 in SCLC. One is that the normal progenitor cell of SCLC, generally presumed to be the neuroendocrine Kulchitsky cell (57), expresses ZIC2 and SOX group B genes; thus, the expression of these antigens in SCLC represents the persistence of these differentiation characteristics during neoplastic clonal expansion. Although we have not detected ZIC2 and SOX group B expression in normal lung, this cannot be taken as evidence for absence of expression in normal Kulchitsky cells, because this is a rare cell population poorly represented in normal adult lung (in contrast to a greater abundance of Kulchitsky cells in fetal and neonatal lung). The other possibility is that SOX group B and ZIC2 genes are not expressed in normal adult Kulchitsky cells or bronchial epithelium and that the expression of these genes in SCLC represents a reactivation of lineage-specific embryonic markers, reflecting the developmental stage at which ZIC2 and SOX2 are coexpressed. Neither of the two explanations, however, can account for the finding that the ZIC2 gene is expressed in many cancer types unrelated to neuroendocrine lineage. This suggests that various gene activation or derepression mechanisms are involved in the tumor expression of ZIC2 and maybe SOX genes.
From an immunological standpoint, the high frequency and high titers of anti-SOX and anti-ZIC2 antibodies in these SCLC patients are striking. At present, we can only speculate as to why these antigens elicit such a strong humoral immune response. A likely reason is that SCLC expresses a range of neuroectodermal antigens, including SOX group B and ZIC2, primarily restricted to the nervous system and that immune tolerance has not been established against these antigens. Growing outside the brain–blood barrier, SCLC would provide a potent immunogenic stimulus to the host. In our series, anti-ZIC2 antibody was observed in 29% and anti-SOX antibody was observed in 41% of the SCLC sera. These sera were collected from a heterogeneous group of SCLC patients who were at different stages of their diseases, receiving various treatments, and having variable responses; indeed, one of the antibody-positive patients (Lu113) had no clinical evidence of residual disease when serum was collected, but had a subsequent recurrence of tumor. A large-scale survey of anti-SOX and anti-ZIC2 antibodies in SCLC patients, correlating the seroreactivity with tumor stage, treatment, and survival, is needed to explore the clinical significance and possible utility of these antibodies as tumor markers.
In addition to the immunodiagnostic potential of SOX group B and ZIC2 gene products, the possibility that these antigens could serve as cancer vaccine targets should be considered. The expression of these genes in brain is a major concern, particularly given the paraneoplastic syndromes associated with SCLC (8, 9). However, despite the presence of high-titer anti-SOX and anti-ZIC2 antibodies, none of the seven antibody-positive patients in this study had neurological manifestations related to the disease. In fact, the only patient in this series with a paraneoplastic syndrome involving the cerebellum (patient A9) had no detectable anti-SOX group B or ZIC2 antibodies. Thus, immune responses against SOX group B and ZIC2 may not lead to autoimmune neurological disorders, but this must be explored in a much larger series of patients. Because SOX and ZIC2 genes are conserved in mice, SOX and/or ZIC2 vaccination can be assessed in an experimental model. Indeed, HuD antigen, one of the neuronal antigens associated with paraneoplastic syndromes (8, 9), recently has been used as a vaccine target in a murine model of SCLC, and antitumor activity was observed without neurological disease (58, 59). In considering immunological approaches to the therapy of SCLC, a major obstacle is obtaining tumor tissue for antigen analysis. SCLC usually is diagnosed by endoscopic biopsies rather than surgical resection, and an adequate specimen for RNA extraction and RT-PCR typing cannot be obtained in most cases. A further complication is the fact that SOX Group B genes are intronless and RT-PCR typing is often unreliable. Immunohistochemical typing with specific antibody reagents should circumvent these problems, and the generation of mAbs against individual SOX and ZIC2 gene products is underway.
Acknowledgments
We thank Dr. Jonathan Cebon of LICR, Austin and Repatriation Medical Center, Australia, for providing sera from SCLC patients (A1–A10).
Abbreviations
- SEREX
serological analysis of recombinant cDNA expression libraries
- SCLC
small cell lung cancer
- HMG
high-mobility group
- RT-PCR
reverse transcription–PCR
References
- 1.Boring C C, Squires T S, Tong T, Montgomery S. CA Cancer J Clin. 1994;44:7–26. doi: 10.3322/canjclin.44.1.7. [DOI] [PubMed] [Google Scholar]
- 2.Guinee D G, Jr, Fishback N F, Koss M N, Abbondanzo S L, Travis W D. Am J Clin Pathol. 1994;102:406–414. doi: 10.1093/ajcp/102.4.406. [DOI] [PubMed] [Google Scholar]
- 3.Travis W D, Linnoila R I, Tsokos M G, Hitchcock C L, Cutler G B, Jr, Nieman L, Chrousos G, Pass H, Doppman J. Am J Surg Pathol. 1991;15:529–553. doi: 10.1097/00000478-199106000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Linnoila R I. J Cell Biochem Suppl. 1996;24:92–106. doi: 10.1002/jcb.240630506. [DOI] [PubMed] [Google Scholar]
- 5.Ball D W, Azzoli C G, Baylin S B, Chi D, Dou S, Donis-Keller H, Cumaraswamy A, Borges M, Nelkin B D. Proc Natl Acad Sci USA. 1993;90:5648–5652. doi: 10.1073/pnas.90.12.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borges M, Linnoila R I, van de Velde H J, Chen H, Nelkin B D, Mabry M, Baylin S B, Ball D W. Nature (London) 1997;386:852–855. doi: 10.1038/386852a0. [DOI] [PubMed] [Google Scholar]
- 7.Darnell R B. Proc Natl Acad Sci USA. 1996;93:4529–4536. doi: 10.1073/pnas.93.10.4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Posner J B, Dalmau J. Curr Opin Immunol. 1997;9:723–729. doi: 10.1016/s0952-7915(97)80055-6. [DOI] [PubMed] [Google Scholar]
- 9.Dalmau J O, Posner J B. Arch Neurol. 1999;56:405–408. doi: 10.1001/archneur.56.4.405. [DOI] [PubMed] [Google Scholar]
- 10.Szabo A, Dalmau J, Manley G, Rosenfeld M, Wong E, Henson J, Posner J B, Furneaux H M. Cell. 1991;67:325–333. doi: 10.1016/0092-8674(91)90184-z. [DOI] [PubMed] [Google Scholar]
- 11.Thirkill C E, Keltner J L, Tyler N K, Roth A M. Arch Ophthalmol. 1993;111:931–937. doi: 10.1001/archopht.1993.01090070049018. [DOI] [PubMed] [Google Scholar]
- 12.Polans A S, Witkowska D, Haley T L, Amundson D, Baizer L, Adamus G. Proc Natl Acad Sci USA. 1995;92:9176–9180. doi: 10.1073/pnas.92.20.9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.David C, McPherson P S, Mundigl O, de Camilli P. Proc Natl Acad Sci USA. 1996;93:331–335. doi: 10.1073/pnas.93.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckanovich R J, Posner J B, Darnell R B. Neuron. 1993;11:657–672. doi: 10.1016/0896-6273(93)90077-5. [DOI] [PubMed] [Google Scholar]
- 15.Lennon V A, Kryzer T J, Griesmann G E, O'Suilleabhain P E, Windebank A J, Woppmann A, Miljanich G P, Lambert E H. N Engl J Med. 1995;332:1467–1474. doi: 10.1056/NEJM199506013322203. [DOI] [PubMed] [Google Scholar]
- 16.Yu Z, Kryzer T J, Griesmann G E, Kwiatkowski T J, Jr, Kim K, Lennon V A. Cancer Immunosurveillance 1999. New York: Cancer Research Institute; 1999. pp. A1–A66. [Google Scholar]
- 17.Sahin U, Tureci O, Schmitt H, Cochlovius B, Johannes T, Schmits R, Stenner F, Luo G, Schobert I, Pfreundschuh M. Proc Natl Acad Sci USA. 1995;92:11810–11813. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y T, Scanlan M J, Sahin U, Tureci O, Güre A O, Tsang S, Williamson B, Stockert E, Pfreundschuh M, Old L J. Proc Natl Acad Sci USA. 1997;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scanlan M J, Chen Y T, Williamson B, Güre A O, Stockert E, Gordan J D, Tureci O, Sahin U, Pfreundschuh M, Old L J. Int J Cancer. 1998;76:652–658. doi: 10.1002/(sici)1097-0215(19980529)76:5<652::aid-ijc7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 20.Old L J, Chen Y T. J Exp Med. 1998;187:1163–1167. doi: 10.1084/jem.187.8.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carney D N, Gazdar A F, Bepler G, Guccion J G, Marangos P J, Moody T W, Zweig M H, Minna J D. Cancer Res. 1985;45:2913–2923. [PubMed] [Google Scholar]
- 22.Park J G, Oie H K, Sugarbaker P H, Henslee J G, Chen T R, Johnson B E, Gazdar A. Cancer Res. 1987;47:6710–6718. [PubMed] [Google Scholar]
- 23.Chen Y T, Güre A O, Tsang S, Stockert E, Jager E, Knuth A, Old L J. Proc Natl Acad Sci USA. 1998;95:6919–6923. doi: 10.1073/pnas.95.12.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. In: Current Protocols in Molecular Biology. Chanda V B, editor. New York: Wiley; 1999. [Google Scholar]
- 25.Riechmann V, van Cruchten I, Sablitzky F. Nucleic Acids Res. 1994;22:749–755. doi: 10.1093/nar/22.5.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsumoto-Taniura N, Pirollet F, Monroe R, Gerace L, Westendorf J M. Mol Biol Cell. 1996;7:1455–1469. doi: 10.1091/mbc.7.9.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y T, Rettig W J, Yenamandra A K, Kozak C A, Chaganti R S, Posner J B, Old L J. Proc Natl Acad Sci USA. 1990;87:3077–3081. doi: 10.1073/pnas.87.8.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, Hercend T. J Exp Med. 1990;171:1393–1405. doi: 10.1084/jem.171.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malas S, Duthie S M, Mohri F, Lovell-Badge R, Episkopou V. Mamm Genome. 1997;8:866–868. doi: 10.1007/s003359900597. [DOI] [PubMed] [Google Scholar]
- 30.Stevanovic M, Zuffardi O, Collignon J, Lovell-Badge R, Goodfellow P. Mamm Genome. 1994;5:640–642. doi: 10.1007/BF00411460. [DOI] [PubMed] [Google Scholar]
- 31.Stevanovic M, Lovell-Badge R, Collignon J, Goodfellow P N. Hum Mol Genet. 1993;2:2013–2018. doi: 10.1093/hmg/2.12.2013. [DOI] [PubMed] [Google Scholar]
- 32.Malas S, Duthie S, Deloukas P, Episkopou V. Mamm Genome. 1999;10:934–937. doi: 10.1007/s003359901118. [DOI] [PubMed] [Google Scholar]
- 33.Pevny L H, Lovell-Badge R. Curr Opin Genet Dev. 1997;7:338–344. doi: 10.1016/s0959-437x(97)80147-5. [DOI] [PubMed] [Google Scholar]
- 34.Wegner M. Nucleic Acids Res. 1999;27:1409–1420. doi: 10.1093/nar/27.6.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Munsterberg A, Vivian N, Goodfellow P, Lovell-Badge R. Nature (London) 1990;346:245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- 36.Collignon J, Sockanathan S, Hacker A, Cohen-Tannoudji M, Norris D, Rastan S, Stevanovic M, Goodfellow P N, Lovell-Badge R. Development. 1996;122:509–520. doi: 10.1242/dev.122.2.509. [DOI] [PubMed] [Google Scholar]
- 37.Kamachi Y, Uchikawa M, Collignon J, Lovell-Badge R, Kondoh H. Development. 1998;125:2521–2532. doi: 10.1242/dev.125.13.2521. [DOI] [PubMed] [Google Scholar]
- 38.Zorn A M, Barish G D, Williams B O, Lavender P, Klymkowsky M W, Varmus H E. Mol Cell. 1999;4:487–498. doi: 10.1016/s1097-2765(00)80200-2. [DOI] [PubMed] [Google Scholar]
- 39.Pevny L H, Sockanathan S, Placzek M, Lovell-Badge R. Development. 1998;125:1967–1978. doi: 10.1242/dev.125.10.1967. [DOI] [PubMed] [Google Scholar]
- 40.Nishiguchi S, Wood H, Kondoh H, Lovell-Badge R, Episkopou V. Genes Dev. 1998;12:776–781. doi: 10.1101/gad.12.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rex M, Orme A, Uwanogho D, Tointon K, Wigmore P M, Sharpe P T, Scotting P J. Dev Dyn. 1997;209:323–332. doi: 10.1002/(SICI)1097-0177(199707)209:3<323::AID-AJA7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 42.Streit A, Sockanathan S, Perez L, Rex M, Scotting P J, Sharpe P T, Lovell-Badge R, Stern C D. Development. 1997;124:1191–1202. doi: 10.1242/dev.124.6.1191. [DOI] [PubMed] [Google Scholar]
- 43.Wood H B, Episkopou V. Mech Dev. 1999;86:197–201. doi: 10.1016/s0925-4773(99)00116-1. [DOI] [PubMed] [Google Scholar]
- 44.Uchikawa M, Kamachi Y, Kondok H. Mech Dev. 1999;84:103–120. doi: 10.1016/s0925-4773(99)00083-0. [DOI] [PubMed] [Google Scholar]
- 45.Koyano S, Ito M, Takamatsu N, Takiguchi S, Shiba T. Gene. 1997;188:101–107. doi: 10.1016/s0378-1119(96)00790-1. [DOI] [PubMed] [Google Scholar]
- 46.Furuta Y, Hogan B L M. Genes Dev. 1998;12:3764–3775. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uwanogho D, Rex M, Cartwright E J, Pearl G, Healy C, Scotting P J, Sharpe P T. Mech Dev. 1995;49:23–36. doi: 10.1016/0925-4773(94)00299-3. [DOI] [PubMed] [Google Scholar]
- 48.Aruga J, Nagai T, Tokuyama T, Hayashizaki Y, Okazaki Y, Chapman V M, Mikoshiba K. J Biol Chem. 1996;271:1043–1047. doi: 10.1074/jbc.271.2.1043. [DOI] [PubMed] [Google Scholar]
- 49.Aruga J, Yozu A, Hayashizaki Y, Okazaki Y, Chapman V M, Mikoshiba K. Gene. 1996;172:291–294. doi: 10.1016/0378-1119(96)00111-4. [DOI] [PubMed] [Google Scholar]
- 50.Aruga J, Yokota N, Hashimoto M, Furuichi T, Fukuda M, Mikoshiba K. J Neurochem. 1994;63:1880–1890. doi: 10.1046/j.1471-4159.1994.63051880.x. [DOI] [PubMed] [Google Scholar]
- 51.Nakata K, Nagai T, Aruga J, Mikoshiba K. Mech Dev. 1998;75:43–51. doi: 10.1016/s0925-4773(98)00073-2. [DOI] [PubMed] [Google Scholar]
- 52.Mizuseki K, Kishi M, Matsui M, Nakanishi S, Sasai Y. Development. 1998;125:579–587. doi: 10.1242/dev.125.4.579. [DOI] [PubMed] [Google Scholar]
- 53.Nagai T, Aruga J, Takada S, Gunther T, Sporle R, Schughart K, Mikoshiba K. Dev Biol. 1997;182:299–313. doi: 10.1006/dbio.1996.8449. [DOI] [PubMed] [Google Scholar]
- 54.Brown S A, Warburton D, Brown L Y, Yu C Y, Roeder E R, Stengel-Rutkowski S, Hennekam R C, Muenke M. Nat Genet. 1998;20:180–183. doi: 10.1038/2484. [DOI] [PubMed] [Google Scholar]
- 55.Gebbia M, Ferrero G B, Pilia G, Bassi M T, Aylsworth A, Penman-Splitt M, Bird L M, Bamforth J S, Burn J, Schlessinger D, et al. Nat Genet. 1997;17:305–308. doi: 10.1038/ng1197-305. [DOI] [PubMed] [Google Scholar]
- 56.Aruga J, Minowa O, Yaginuma H, Kuno J, Nagai T, Noda T, Mikoshiba K. J Neurosci. 1998;18:284–293. doi: 10.1523/JNEUROSCI.18-01-00284.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kobzik L. In: Pathologic Basis of Disease. Cotran R S, Kumar V, Collins T, editors. Philadelphia: Saunders; 1999. pp. 697–755. [Google Scholar]
- 58.Carpentier A F, Rosenfeld M R, Delattre J Y, Whalen R G, Posner J B, Dalmau J. Clin Cancer Res. 1998;4:2819–2824. [PubMed] [Google Scholar]
- 59.Ohwada A, Nagaoka I, Takahashi F, Tominaga S, Fukuchi Y. Am J Respir Cell Mol Biol. 1999;21:37–43. doi: 10.1165/ajrcmb.21.1.3625. [DOI] [PubMed] [Google Scholar]