Abstract
ClpB is a member of a multichaperone system in Escherichia coli (with DnaK, DnaJ, and GrpE) that reactivates aggregated proteins. The sequence of ClpB contains two ATP-binding regions that are enclosed between the N- and C-terminal extensions. Whereas it has been found that the N-terminal region of ClpB is essential for the chaperone activity, the structure of this region is not known, and its biochemical properties have not been studied. We expressed and purified the N-terminal fragment of ClpB (residues 1–147). Circular dichroism of the isolated N-terminal region showed a high content of α-helical structure. Differential scanning calorimetry showed that the N-terminal region of ClpB is thermodynamically stable and contains a single folding domain. The N-terminal domain is monomeric, as determined by gel-filtration chromatography, and the elution profile of the N-terminal domain does not change in the presence of the N-terminally truncated ClpB (ClpBΔN). This indicates that the N-terminal domain does not form strong contacts with ClpBΔN. Consistently, addition of the separated N-terminal domain does not reverse an inhibition of ATPase activity of ClpBΔN in the presence of casein. As shown by ELISA measurements, full-length ClpB and ClpBΔN bind protein substrates (casein, inactivated luciferase) with similar affinity. We also found that the isolated N-terminal domain of ClpB interacts with heat-inactivated luciferase. Taken together, our results indicate that the N-terminal fragment of ClpB forms a distinct domain that is not strongly associated with the ClpB core and is not required for ClpB interactions with other proteins, but may be involved in recognition of protein substrates.
Keywords: ClpB, molecular chaperone, folding domain, protein stability, protein, protein interactions, differential scanning calorimetry
ClpB belongs to a family of proteins known as Hsp100 or Clp ATPases (Schirmer et al. 1996). Several well-characterized members of this family (ClpA, ClpX, and ClpY) associate with peptidase subunits (ClpP and ClpQ) and regulate energy-dependent protease complexes (Gottesman et al. 1997; Wickner et al. 1999). The function of Clp ATPases in such complexes is recognition and unfolding of protein substrates and their subsequent delivery to the peptidase subunits (Weber-Ban et al. 1999; Kim et al. 2000; Singh et al. 2000). In contrast, ClpB from Escherichia coli and Thermus thermophilus, and its yeast homolog Hsp104, are not involved in protein degradation and instead cooperate with other molecular chaperones (DnaK, DnaJ, GrpE in E. coli, Ssa1 and Ydj1 in yeast) in efficient disaggregation and reactivation of misfolded and aggregated proteins (Glover and Lindquist 1998; Goloubinoff et al. 1999; Motohashi et al. 1999; Zolkiewski 1999; Tomoyasu et al. 2001). The mechanism of ClpB-assisted protein reactivation is currently unknown.
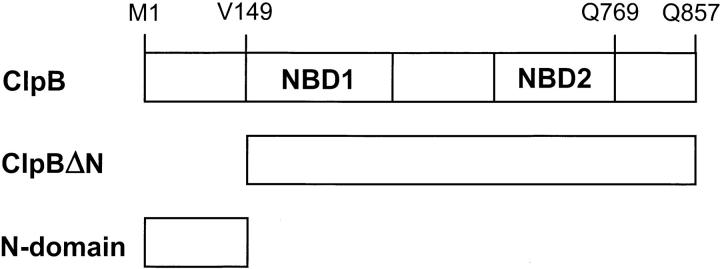
Clp ATPases contain one or two nucleotide binding domains (NBD) (Schirmer et al. 1996). In ClpA and ClpB, two highly conserved NBDs are enclosed between less-conserved N- and C-terminal regions and are separated by a middle sequence region (see Fig. 1 ▶). All Clp ATPases form ring-shaped oligomers in the presence of ATP or at high protein concentration (Kessel et al. 1995; Grimaud et al. 1998; Zolkiewski et al. 1999). Deletion analysis has shown that the C-terminal region of ClpB is involved in stabilization of intersubunit contacts within the ClpB oligomer (Barnett et al. 2000). Loss of intersubunit interactions in the C-terminally truncated ClpB is coupled with inhibition of the ATPase and chaperone activity.
Fig. 1.
Postulated domain structure of ClpB and protein variants produced in this study. The diagram shows two nucleotide binding domains in ClpB (NBD1, NBD2) enclosed between the N- and C-terminal regions. In ClpBΔN, 148 N-terminal amino acids have been removed and Val149 has been replaced with a methionine. The N-terminal domain characterized in this work contains 147 N-terminal residues of ClpB (Mr 15,600).
In vivo expression of the ClpB gene produces two polypeptide chains as follows: full-length 95-kD ClpB and the N-terminally truncated 80-kD ClpBΔN (Squires et al. 1991; Woo et al. 1992). The internal translation initiation site in ClpB corresponds to Val149 at the N terminus of NBD1 (see Fig. 1 ▶). Thus, the truncated form ClpBΔN does not contain the whole N-terminal domain of ClpB. In vivo, both forms of ClpB contribute to bacterial thermotolerance (Squires et al. 1991; Clarke and Eriksson 2000).
The role of the N-terminal region in ClpB is not clear. Previous biochemical studies identified a number of differences between purified full-length ClpB and ClpBΔN. Whereas ClpB as well as ClpBΔN form oligomers, the self-association of ClpBΔN is more pronounced at low protein concentration (Barnett et al. 2000). The increased population of oligomers manifests itself in an increased basal ATPase activity of ClpBΔN (Park et al. 1993; Barnett et al. 2000). However, whereas the ATPase of full-length ClpB is strongly activated by casein, the ATPase of ClpBΔN responds to casein to a much lower extent (Park et al. 1993; Barnett et al. 2000). Finally, the chaperone activity of ClpB in vitro is strongly inhibited upon deletion of the N-terminal region (Barnett et al. 2000). These results suggest that the N-terminal region of ClpB may either mediate interactions with protein substrates or may be involved in conformational rearrangements linking protein binding and ATP hydrolysis by ClpB.
No high-resolution structural information is currently available for ClpB. ClpY (HslU), the only Clp ATPase, for which the structure is available, does not contain a domain homologous to the N-terminal region of ClpB (Bochtler et al. 2000). In ClpA, the isolated N-terminal region has been shown to form a stable domain that was not essential for the ClpA activity (Lo et al. 2001; Singh et al. 2001).
In this study, we produced the isolated N-terminal region of ClpB (amino acids 1–147, 15.6 kD) and the N-terminally truncated ClpBΔN (see Fig. 1 ▶). We characterized biochemical properties of the N-terminal region of ClpB and tested its interactions with ClpBΔN as well as with potential protein substrates. Our results indicate that the N-terminal region forms a distinct stable domain that is linked to the remaining part of ClpB by a peptide bond, but does not form strong tertiary contacts with other domains of ClpB. The N-terminal domain of ClpB may also be involved in substrate recognition and/or substrate processing by ClpB.
Results
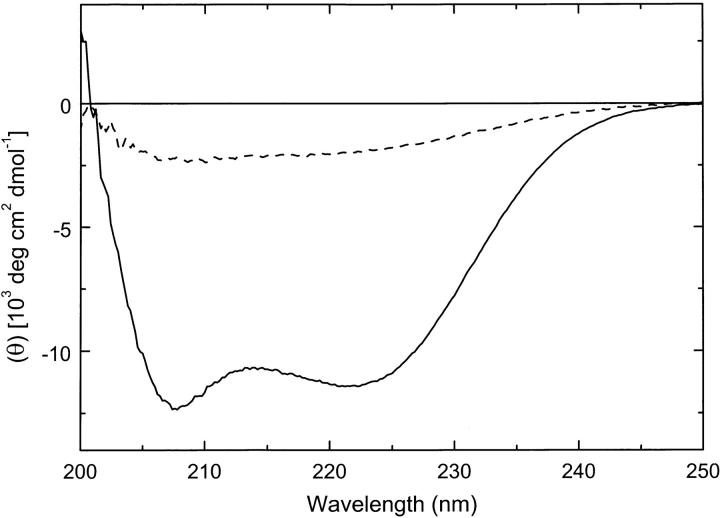
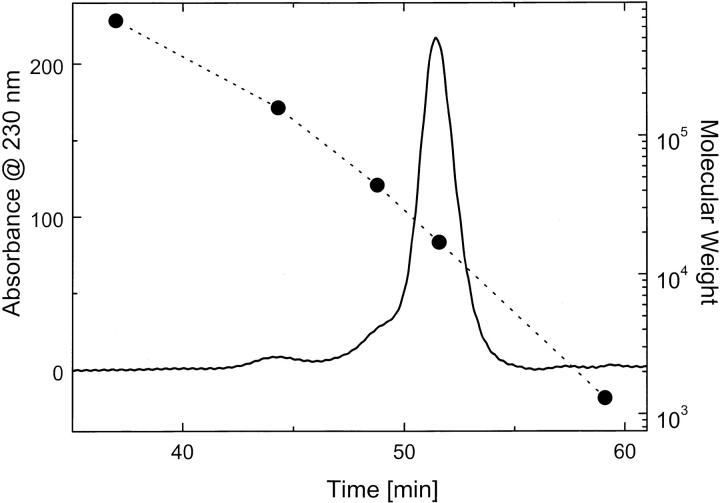
Because protein fragments may not always fold into stable conformations, we first tested the secondary structure of the isolated N-terminal region of ClpB. The circular dichroism (CD) spectrum of the N-terminal fragment at 25°C showed a negative double band with the minima at 208 and 222 nm (Fig. 2 ▶). The shape of the CD spectrum indicates that the N-terminal fragment of ClpB folds into a structure dominated by α-helices. The CD spectrum shown in Figure 2 ▶ is similar to that obtained previously for the N-terminal domain of ClpA (Lo et al. 2001). The elution time of the N-terminal fragment of ClpB on a gel-filtration column (Fig. 3 ▶) is very close to that of myoglobin (Mr 17,000). This indicates that the N-terminal fragment of ClpB is predominantly monomeric in solution.
Fig. 2.
Circular dichroism spectrum of the N-terminal fragment of ClpB (4.24 mg/mL in 50 mM Tris-HCl at pH 7.5, 0.2 M KCl, 20 mM MgCl2, 10% glycerol, 1 mM EDTA, 1 mM DTT) expressed as mean molar residue ellipticity (θ), measured at 25°C (solid line) and 85 °C (broken line).
Fig. 3.
Gel filtration chromatography of the N-terminal fragment of ClpB. An aliquot of the N-terminal fragment (20 μL, 1.0 mg/mL) was injected onto a Superose 6 column. Protein elution profile was obtained with a 0.05 mL/min flow-rate in 50 mM Tris-HCl (pH 7.5), 0.2 M KCl, 20 mM MgCl2, 1 mM EDTA, and 1 mM DTT. (•) Elution times of thyroglobulin (Mr 670,000`), γ-globulin (Mr 158,000), ovalbumin (Mr 44,000), myoglobin (Mr 17,000), and vitamin B-12 (Mr 1350).
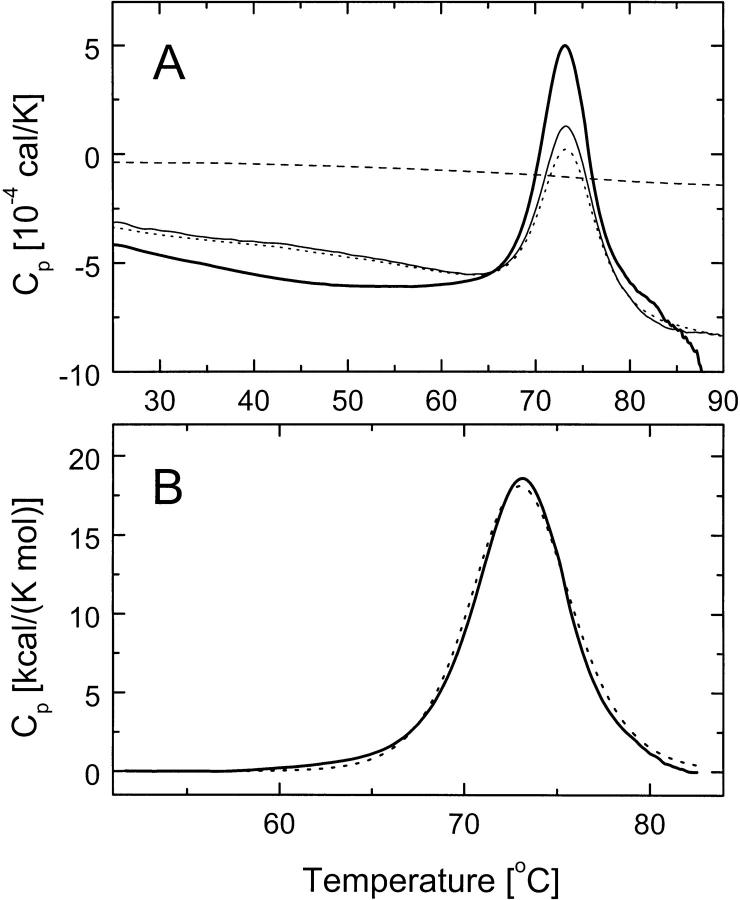
We investigated whether the conformation of the N-terminal fragment of ClpB is thermodynamically stable. Differential scanning calorimetry (DSC) of the N-terminal fragment showed a single unfolding transition with a maximum at 73°C (Fig. 4A ▶). The thermal unfolding is accompanied by a loss of the α-helical structure of the N-terminal fragment of ClpB (see Fig. 2 ▶, broken line). The temperature-induced unfolding of the N-terminal fragment was partially reversible, as shown by the heat capacity peaks observed during three consecutive scans. Partial irreversibility of unfolding is most likely due to protein aggregation at high temperature, as evidenced by exothermic shifts of the post-transition baseline above ∼85°C (Fig. 4A ▶, thick solid line). A cooperative thermal transition, as shown in Figure 4A ▶, indicates that the conformation of the N-terminal fragment of ClpB is thermodynamically stable at the temperature range of the biochemical assays (see below).
Fig. 4.
Differential scanning calorimetry (DSC) of the N-terminal fragment of ClpB. (A) Shown are the DSC data obtained with a 1 K/min scan-rate for the dialysate buffer (broken line) and for the N-terminal domain of ClpB (2.0 mg/mL in 50 mM Tris-HCl at pH 7.5, 0.2 M KCl, 20 mM MgCl2, 10% glycerol, 1 mM EDTA, 1 mM DTT) during the first scan (thick solid line), second scan (thin solid line), and third scan (dotted line). (B) DSC data from the first scan of the N-terminal domain of ClpB (solid line) after subtraction of the buffer scan and a linear baseline in the region of the thermal transition with a two-state model fit of the data (broken line).
We have tested whether thermal unfolding of the N-terminal fragment of ClpB occurs in a single step, from folded to unfolded conformation. We assumed that the DSC data within the cooperative transition are not affected to a high degree by irreversible aggregation. In such cases, the shape of the thermal transition can be described with equations of equilibrium thermodynamics (Freire et al. 1990). Figure 4B ▶ shows that the DSC data for the N-terminal fragment can be well approximated by a two-state conformational transition with the mid-point temperature, Tm = 73.1°C and the transition enthalpy, ΔH = 131 kcal/mole. Two-state cooperative unfolding indicates that the N-terminal fragment of ClpB is not only stable, but it contains a single folding domain.
We performed several experiments aimed at characterizing interactions between the N-terminal domain and the remaining part of ClpB (ClpBΔN). A mixture of ClpBΔN and the N-terminal domain has been subjected to gel-filtration chromatography (data not shown, experimental conditions as in Fig. 3 ▶). Fractions collected during the experiments were analyzed subsequently with SDS-PAGE to determine the elution profile of the N-terminal domain. We found that the N-terminal domain and ClpBΔN were separated on the column and the elution profile of the N-terminal domain in the presence of ClpBΔN was identical to that in the absence of ClpBΔN (see Fig. 3 ▶). Similar results have been obtained in the presence of ATP in the running buffer, which promotes self-association of ClpBΔN (Barnett et al. 2000), and with a doubly truncated ClpBΔNC, which does not self-associate (Barnett et al. 2000). These results indicate that the isolated N-terminal domain of ClpB does not form stable contacts with the remaining domains.
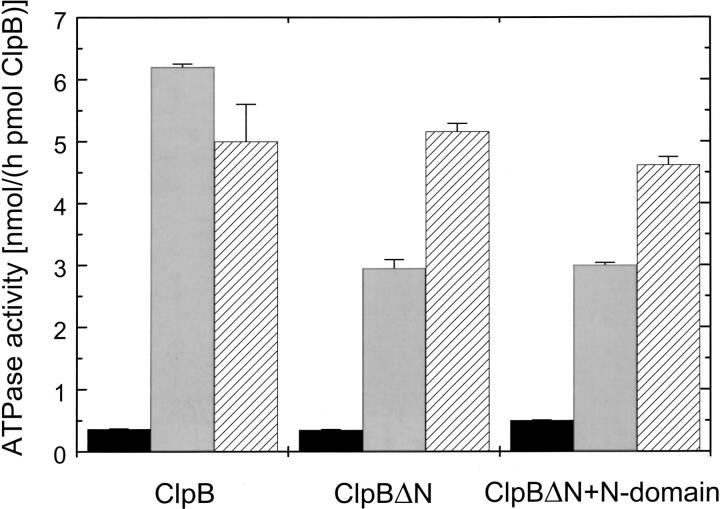
We then investigated whether the isolated N-terminal domain of ClpB can affect the activity of ClpBΔN in the presence of potential protein substrates. It has been found previously that the casein-induced activation of the ClpB ATPase is inhibited upon deletion of the N-terminal domain (Park et al. 1993; Barnett et al. 2000). Figure 5 ▶ shows the ATPase activity of different ClpB variants in the absence and presence of κ-casein or poly-L-lysine. Either casein or polylysine activates strongly the ATPase of ClpB. However, whereas the ATPase activity of ClpBΔN in the presence of casein is half that of the full-length ClpB, the ATPase of ClpBΔN is similar to that of the full-length ClpB in the presence of polylysine. Thus, the character of a protein that interacts with ClpB determines whether the N-terminal domain is essential for the protein-induced activation of the ClpB ATPase. Moreover, we found that even a large molar excess of the isolated N-terminal domain does not increase the ATPase activity of ClpBΔN in the presence of casein (Fig. 5 ▶). Similarly, addition of the N-terminal domain does not increase the chaperone activity of ClpBΔN in the luciferase reactivation assay (data not shown; Barnett et al. 2000). Taken together, the above results confirm that the N-terminal domain does not associate strongly with other domains of ClpB.
Fig. 5.
ATPase activity of ClpB, ClpBΔN, and ClpBΔN with the N-terminal fragment of ClpB. The rate of ATP hydrolysis has been measured by incubating the ClpB variants (5.6 pmole ClpB, 6.3 pmole ClpBΔN without and with 1.35 nmole N-terminal fragment) for 15 min at 37°C in 50 μL of the assay buffer (see Materials and Methods) in the absence of other proteins (black bars), with 0.2 mg/mL κ-casein (grey bars), or with 0.01 mg/mL poly-L-lysine (hatched bars).
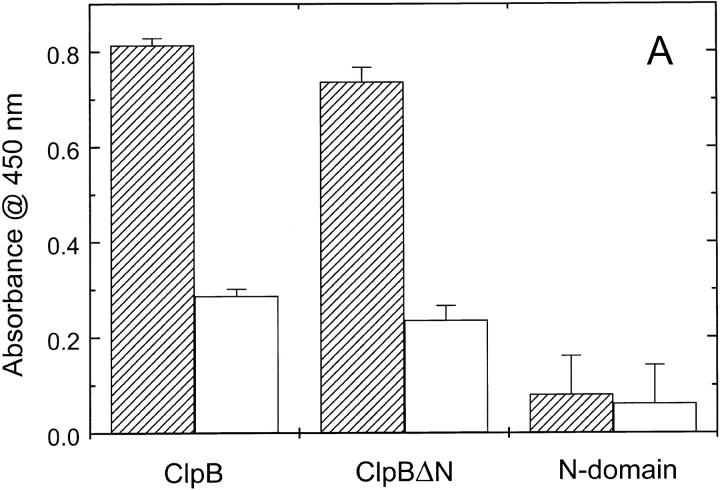
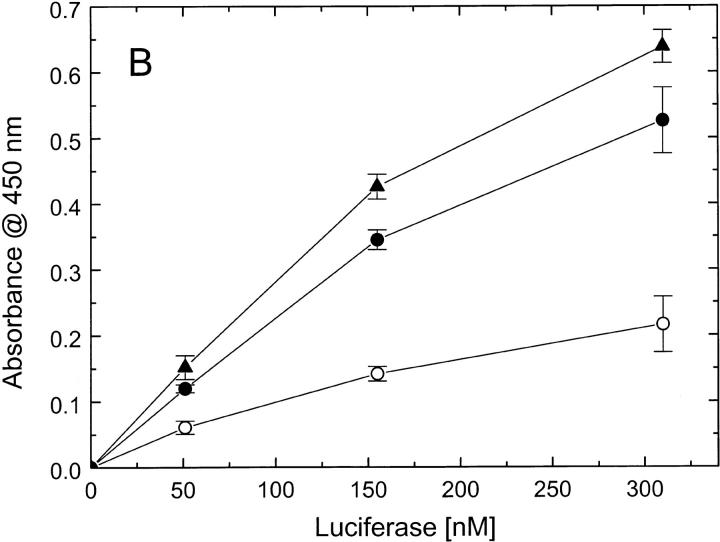
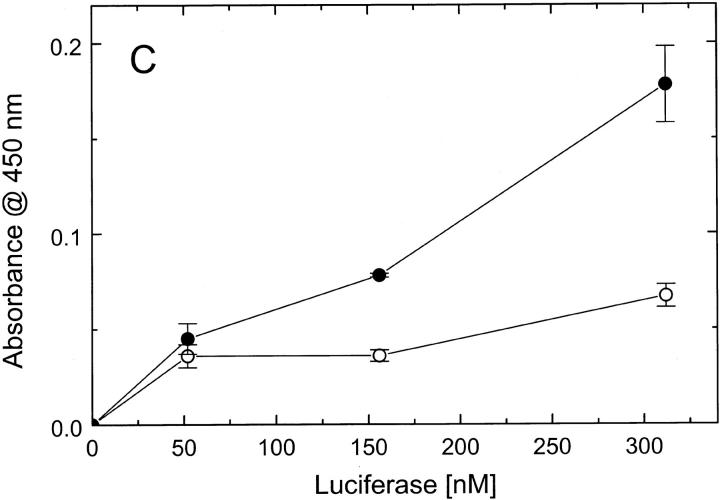
To determine whether the defect of ClpBΔN in responding to casein (see Fig. 5 ▶) is due to a loss of protein-binding capability of ClpBΔN, we performed a series of ELISA experiments (Fig. 6 ▶). First, we studied the binding of different ClpB variants to a plate covered with κ-casein (Fig. 6A ▶). We found that similar amounts of the full-length ClpB and ClpBΔN bound to the plate, which indicates that the N-terminal domain of ClpB is not essential for interaction with casein. We have also studied the interactions of ClpB with heat-inactivated luciferase, which had been used as a substrate in ClpB chaperone activity assays (Schlee et al. 2001). We found that the affinity of ClpBΔN for inactivated luciferase is not smaller than that of the full-length ClpB (Fig. 6B ▶). This indicates that the inhibition of the chaperone activity of ClpBΔN (Barnett et al. 2000) is not due to a decrease in binding affinity for a protein substrate. We have also found that inactivated luciferase binds to an ELISA plate covered with the isolated N-terminal domain of ClpB (Fig. 6C ▶). We conclude that, whereas the N-terminal domain is not essential for the luciferase interactions with ClpB, this domain may provide some molecular contacts in support of the binding.
Fig. 6.
Binding of protein substrates to ClpB, ClpBΔN, and the N-terminal fragment of ClpB. (A) Wells in an ELISA plate have been covered with κ-casein (hatched bars), blocked with BSA, and subsequently incubated with 1.0 μM ClpB, ClpBΔN, or the N-terminal fragment. As a control, a group of wells has not been covered with casein (open bars). After washing, the amounts of ClpB variants remaining on the plate have been determined with anti-ClpB antibody (see Materials and Methods). The data points represent average results from three independent determinations with standard deviations shown. (B) Wells in an ELISA plate have been covered with ClpB (•) or ClpBΔN (▴), blocked, and incubated with heat-inactivated luciferase at the indicated concentrations. As a control, a group of wells has not been covered with ClpB (○). The amounts of luciferase bound to the plate have been determined with anti-luciferase antibody. (C) Wells in an ELISA plate have been covered with the N-terminal fragment of ClpB (•) or only blocked with BSA (○), and incubated with inactivated luciferase. The amounts of luciferase remaining on the plate after washing have been determined with anti-luciferase antibody.
Discussion
In the absence of published high-resolution structural information for ClpB and other similar Clp ATPases, it is essential to characterize the role and properties of distinct protein domains. In this work, we have purified the N-terminal fragment of ClpB. We found that the N-terminal fragment is monomeric and folds into a stable structure, which contains a single cooperative domain. Previously, two apparent sequence repeats have been identified within the N-terminal sequences of Clp ATPases (Lo et al. 2001). The two sequence repeats correspond in ClpB to residues 1–77 and 78–145, respectively. Our results indicate that the two sequence repeats are not equivalent to two folding domains within the N-terminal region of ClpB (Fig. 4B ▶). This conclusion is in agreement with those of Lo et al. (2001) who studied the properties of the N-terminal domain of ClpA.
It is evident from a number of our observations that the N-terminal domain does not associate with the remaining domains of ClpB. The N-terminal domain easily separates from ClpBΔN in gel-filtration chromatography and it does not affect the properties of ClpBΔN in activity assays (see Fig. 5 ▶). These results are consistent with a model in which the N-terminal domain is linked to the NBD1 region of ClpB with a peptide bond, but does not form extensive tertiary contacts with the other domains in ClpB. This conclusion is consistent with those of Maurizi and coworkers, who studied the N-terminal fragments of ClpA and ClpX (Singh et al. 2001). It appears that the N-terminal extensions of many Clp ATPases form distinct domains that are structurally independent from the ATPase cores.
What could be the role of the N-terminal domain in ClpB? In ClpAP and ClpXP complexes, the N-terminal regions of ClpA and ClpX are located at the distal surfaces, facing away from the peptidase subunits (Singh et al. 2001). Such location of the N-terminal domains suggests that they may be involved in interaction with protein substrates. A similar function may be performed by the N-terminal domain of ClpB. Previously observed defects in activity of ClpBΔN suggested that the N-terminal domain may contain a protein-binding site (Park et al. 1993; Barnett et al. 2000). However, our results show that substrates, such as casein or inactivated luciferase, bind to ClpBΔN as well as to the full-length ClpB (see Fig. 6A,B ▶). This indicates that inhibition of activity of ClpBΔN is related to not-yet-characterized conformational rearrangements in ClpB and/or in a protein substrate, which are mediated by the N-terminal domain.
Taking into account the results of this work, we propose that the N-terminal domain of ClpB is separated from the other domains with an unstructured, possibly flexible region. In oligomeric ClpB, the N-terminal domains may form a ring of structures that are accessible to potential protein substrates. The N-terminal domain is not essential but may support binding of a protein substrate to ClpB (see Fig. 6C ▶). Conformational flexibility of the link between ClpBΔN and the N-terminal domain may provide a mechanism for inducing structural changes in a substrate, which may be essential for the protein-disaggregating activity of ClpB (Goloubinoff et al. 1999; Zolkiewski 1999).
Materials and methods
Protein purification
DNA fragment encoding the N-terminal domain of ClpB (residues 1–147) was produced by PCR and subcloned between the NdeI and BamHI sites of pET-14b (Novagen). The plasmid pET-20b containing the sequence of ClpB (Barnett et al. 2000) was used as a PCR template.
The N-terminal domain of ClpB with an N-terminal extension containing a 6-His-tag and a thrombin-specific site was overexpressed in E. coli strain BL21(DE3)LysS (Novagen). E. coli cells were grown at 37°C to A600 nm ∼0.6 in LB broth containing 0.1 mg/mL ampicillin. Protein expression was induced with 0.4 mM isopropyl-β-D-thiogalactoside. Cells were grown at 37°C for 2 h after induction and collected by centrifugation. Protein purification steps were performed on ice or at 4°C. Bacterial pellet (∼10 g) was suspended in 90 mL of buffer A (50 mM Tris-HCl at pH 8.0, 300 mM NaCl) and disrupted by sonication. Cell extract was centrifuged at 20,000g for 30 min. Supernatant was incubated for 4 h at 4°C with 6 mL of Ni-NTA agarose gel (QIAGEN), poured into a column, and then washed with buffer A with 20 mM imidazole. The N-terminal domain of ClpB was eluted from the column with buffer A with 250 mM imidazole. The N-terminal protein extension was cleaved with biotinylated thrombin (Novagen). Thrombin was subsequently removed with streptavidin-agarose (Novagen). The final protein sample showed a single band on SDS-PAGE (>95% purity) with an apparent molecular weight of ∼15 kD. For further use, the N-terminal domain of ClpB was extensively dialyzed against 50 mM Tris-HCl (pH 7.5), 0.2 M KCl, 20 mM MgCl2, 10% glycerol, 1 mM EDTA, and 1 mM DTT. Protein concentration was measured with the Bradford dye-binding method (Bio-Rad) using bovine gamma globulin as a standard.
Full-length ClpB and the N-terminally truncated ClpBΔN were purified as described before (Barnett et al. 2000).
Circular dichroism spectroscopy
Circular dichroism spectra were measured with a Jasco J-720 spectrometer using a 0.02-cm water-jacketed cylindrical cell. The temperature of the cell was controlled with an external water bath (Fisher Isotemp 1016P).
Gel filtration chromatography
Gel filtration experiments were performed using a Superose 6 PC 3.2/30 column (Amersham Pharmacia Biotech) with a Shimadzu HPLC system containing a LC-10ATvp solvent delivery unit and a SPD-M10Avp photodiode-array detector. Gel filtration protein standards were obtained from Bio-Rad.
Differential scanning calorimetry
Differential scanning calorimetry (DSC) experiments were performed using a VP-DSC calorimeter (MicroCal Inc.) at the scan-rate of 1 K/min. DSC data were corrected for the buffer baseline and protein concentration. Data analysis was performed with Origin software (MicroCal).
ATPase activity assays
ClpB samples were incubated for 15 min at 37°C in the assay buffer (100 mM Tris-HCl at pH 8.0, 10 mM MgCl2, 5 mM ATP, 1 mM EDTA, 1 mM DTT) without other proteins, with 0.2 mg/mL κ-casein (Sigma), or with 0.01 mg/mL poly-L-lysine (Sigma). Concentration of inorganic phosphate produced from ATP was measured using the malachite green colorimetric method (Hess and Derr 1975; Lanzetta et al. 1979).
Protein–protein interaction assays
Interactions between ClpB variants and protein substrates were investigated using an ELISA procedure. Rabbit polyclonal anti-ClpB antibodies were produced by Cocalico Biologicals using purified ClpB as an immunogen. Anti-luciferase antibody was from Sigma and anti-casein antibody was from Maine Biotechnology Services.
To study interactions between ClpB and casein (see Fig. 6A ▶), Reacti-Bind maleic anhydride-activated 96-well plates (Pierce) were covered with κ-casein (100 μL/well, 10 mg/mL κ-casein in 50 mM Na2CO3 at pH 9.4) and incubated for 2.5 h at room temperature. The plates were washed three times with 0.2% BSA in PBS and blocked by incubating with 200 μL/well of the same solution for 1.5 h at room temperature. After blocking and washing twice with 200 μL/well of buffer B (100 mM Tris-HCl at pH 8.0, 10 mM MgCl2, 2 mM ATP, 1 mM EDTA, 1 mM DTT), 100 μL/well of 1.0 μM ClpB in buffer B were added to the wells and incubated for 1 h at 37°C. Following the incubation with ClpB, the wells were washed twice with 200 μL/well of 0.2% BSA in PBST (PBS + 0.05% Tween-20) and twice with buffer B. Next, 100 μL/well of anti-ClpB serum in PBS with 0.2% BSA was added to the plate and incubated for 2 h at room temperature. After incubation with the antibody, the wells were washed six times with 200 μL of PBST and then incubated with 100 μL/well of goat anti-rabbit IgM + IgG conjugated to horseradish peroxidase (Southern Biotechnology Associates) for 45 min at room temperature. The wells were then washed 10 times with 200 μL of PBST and the amount of secondary antibody was measured using the TMB peroxidase substrate kit (Pierce). Absorbance at 450 nm was measured with a UV micro-plate reader (Molecular Devices). In a control experiment, the binding of κ-casein to the plate has been verified with direct ELISA using anti-casein antibodies.
To study interactions between ClpB and inactivated luciferase (see Fig. 6B C ▶), full-length ClpB, ClpBΔN, or the N-terminal domain of ClpB (100 μL of 0.1 mg/mL protein in buffer B) was incubated in the wells of MaxiSorp 96-well plates (Nalge Nunc International) for 3 h at room temperature. First, the amounts of ClpB and ClpBΔN bound in the wells were compared using direct ELISA with anti-ClpB antibodies. Wells were washed three times with 200 μL of PBST and three times with 200 μL of 0.2% BSA in PBS. For blocking, 200 μL of 0.2% BSA in PBS were incubated in wells for 2 h at room temperature. After washing four times with PBST, 100 μL of anti-ClpB serum diluted in PBS was added to the wells and incubated overnight at 4°C. After incubation with the antibody, the wells were washed six times with 200 μL of PBST, 100 μL of the secondary antibody was added to each well, and the ELISA signal was measured as described above. We found that similar amounts (± 5%) of the full-length ClpB and ClpBΔN bound to the plate.
In the binding assay, the wells were first covered with ClpB, ClpBΔN, or the N-terminal domain of ClpB, blocked with BSA (see the procedure above), and then washed twice with 200 μL of buffer C (25 mM Hepes-KOH at pH 7.6, 150 mM KCl, 25 mM NaCl, 1 mM DTT, 0.1 mM EDTA, 10 mM MgCl2, 2 mM ATP, 2.5% glycerol, 0.05% Triton-X-100, 0.2% BSA). Firefly luciferase (Promega) was inactivated by incubation at 45°C for 12 min at the concentration of 0.04 mg/mL in PBS. After diluting the heat-treated luciferase to a desired concentration with buffer C, the luciferase solutions were added to the wells covered with ClpB, ClpBΔN, the N-terminal domain, or only blocked with BSA (control). Wells were washed twice with 200 μL of buffer C, three times with 200 μL of 0.2% BSA in PBS, and once with 200 μL of buffer B. The plates were incubated with 100 μL/well of anti-luciferase serum (in PBS) for 2 h at room temperature. After washing three times with 200 μL of 0.2% BSA in PBST and twice with 0.2% BSA in PBS, 100 μL/well of the secondary antibody was added to the plate and the ELISA signal was measured as described above.
Acknowledgments
This work was supported by the American Heart Association, Heartland Affiliate (Grant no. 0060445Z) and by the National Institutes of Health (Grant no. GM58626). This is contribution 02–217-J from the Kansas Agricultural Experiment Station.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.4860102.
References
- Barnett, M.E., Zolkiewska, A., and Zolkiewski, M. 2000. Structure and activity of ClpB from Escherichia coli. Role of the amino- and -carboxyl-terminal domains. J. Biol. Chem. 275 37565–37571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochtler, M., Hartmann, C., Song, H.K., Bourenkov, G.P., Bartunik, H.D., and Huber, R. 2000. The structures of HsIU and the ATP-dependent protease HsIU-HsIV. Nature 403 800–805. [DOI] [PubMed] [Google Scholar]
- Clarke, A.K. and Eriksson, M.J. 2000. The truncated form of the bacterial heat shock protein ClpB/HSP100 contributes to development of thermotolerance in the cyanobacterium Synechococcus sp. strain PCC 7942. J. Bacteriol. 182 7092–7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire, E., van Osdol, W.W., Mayorga, O.L., and Sanchez-Ruiz, J.M. 1990. Calorimetrically determined dynamics of complex unfolding transitions in proteins. Annu. Rev. Biophys. Biophys. Chem. 19 159–188. [DOI] [PubMed] [Google Scholar]
- Glover, J.R. and Lindquist, S. 1998. Hsp104, Hsp70, and Hsp40: A novel chaperone system that rescues previously aggregated proteins. Cell 94 73–82. [DOI] [PubMed] [Google Scholar]
- Goloubinoff, P., Mogk, A., Zvi, A.P., Tomoyasu, T., and Bukau, B. 1999. Sequential mechanism of solubilization and refolding of stable protein aggregates by a bichaperone network. Proc. Natl. Acad. Sci. 96 13732–13737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman, S., Maurizi, M.R., and Wickner, S. 1997. Regulatory subunits of energy-dependent proteases. Cell 91 435–438. [DOI] [PubMed] [Google Scholar]
- Grimaud, R., Kessel, M., Beuron, F., Steven, A.C., and Maurizi, M.R. 1998. Enzymatic and structural similarities between the Escherichia coli ATP-dependent proteases, ClpXP and ClpAP. J. Biol. Chem. 273 12476–12481. [DOI] [PubMed] [Google Scholar]
- Hess, H.H. and Derr, J.E. 1975. Assay of inorganic and organic phosphorus in the 0.1–5 nanomole range. Anal. Biochem. 63 607–613. [DOI] [PubMed] [Google Scholar]
- Kessel, M., Maurizi, M.R., Kim, B., Kocsis, E., Trus, B.L., Singh, S.K., and Steven, A.C. 1995. Homology in structural organization between E. coli ClpAP protease and the eukaryotic 26 S proteasome. J. Mol. Biol. 250 587–594. [DOI] [PubMed] [Google Scholar]
- Kim, Y.I., Burton, R.E., Burton, B.M., Sauer, R.T., and Baker, T.A. 2000. Dynamics of substrate denaturation and translocation by the ClpXP degradation machine. Mol. Cell 5 639–648. [DOI] [PubMed] [Google Scholar]
- Lanzetta, P.A., Alvarez, L.J., Reinach, P.S., and Candia, O.A. 1979. An improved assay for nanomole amounts of inorganic phosphate. Anal. Biochem. 100 95–97. [DOI] [PubMed] [Google Scholar]
- Lo, J.H., Baker, T.A., and Sauer, R.T. 2001. Characterization of the N-terminal repeat domain of Escherichia coli ClpA-A class I Clp/HSP100 ATPase. Protein Sci. 10 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motohashi, K., Watanabe, Y., Yohda, M., and Yoshida, M. 1999. Heat-inactivated proteins are rescued by the DnaK.J-GrpE set and ClpB chaperones. Proc. Natl. Acad. Sci. 96 7184–7189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S.K., Kim, K.I., Woo, K.M., Seol, J.H., Tanaka, K., Ichihara, A., Ha, D.B., and Chung, C.H. 1993. Site-directed mutagenesis of the dual translational initiation sites of the clpB gene of Escherichia coli and characterization of its gene products. J. Biol. Chem. 268 20170–20174. [PubMed] [Google Scholar]
- Schirmer, E.C., Glover, J.R., Singer, M.A., and Lindquist, S. 1996. HSP100/Clp proteins: A common mechanism explains diverse functions. Trends Biochem. Sci. 21 289–296. [PubMed] [Google Scholar]
- Schlee, S., Groemping, Y., Herde, P., Seidel, R., and Reinstein, J. 2001. The chaperone function of ClpB from Thermus thermophilus depends on allosteric interactions of its two ATP-binding sites. J. Mol. Biol. 306 889–899. [DOI] [PubMed] [Google Scholar]
- Singh, S.K., Grimaud, R., Hoskins, J.R., Wickner, S., and Maurizi, M.R. 2000. Unfolding and internalization of proteins by the ATP-dependent proteases ClpXP and ClpAP. Proc. Natl. Acad. Sci. 97 8898–8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, S.K., Rozycki, J., Ortega, J., Ishikawa, T., Lo, J., Steven, A.C., and Maurizi, M.R. 2001. Functional domains of the ClpA and ClpX molecular chaperones identified by limited proteolysis and deletion analysis. J. Biol. Chem. 276 29420–29429. [DOI] [PubMed] [Google Scholar]
- Squires, C.L., Pedersen, S., Ross, B.M., and Squires, C. 1991. ClpB is the Escherichia coli heat shock protein F84.1. J. Bacteriol. 173 4254–4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoyasu, T., Mogk, A., Langen, H., Goloubinoff, P., and Bukau, B. 2001. Genetic dissection of the roles of chaperones and proteases in protein folding and degradation in the Escherichia coli cytosol. Mol. Microbiol. 40 397–413. [DOI] [PubMed] [Google Scholar]
- Weber-Ban, E.U., Reid, B.G., Miranker, A.D., and Horwich, A.L. 1999. Global unfolding of a substrate protein by the Hsp100 chaperone ClpA. Nature 401 90–93. [DOI] [PubMed] [Google Scholar]
- Wickner, S., Maurizi, M.R., and Gottesman, S. 1999. Posttranslational quality control: Folding, refolding, and degrading proteins. Science 286 1888–1893. [DOI] [PubMed] [Google Scholar]
- Woo, K.M., Kim, K.I., Goldberg, A.L., Ha, D.B., and Chung, C.H. 1992. The heat-shock protein ClpB in Escherichia coli is a protein-activated ATPase. J. Biol. Chem. 267 20429–20434. [PubMed] [Google Scholar]
- Zolkiewski, M. 1999. ClpB cooperates with DnaK, DnaJ, and GrpE in suppressing protein aggregation. A novel multi-chaperone system from Escherichia coli. J. Biol. Chem. 274 28083–28086. [DOI] [PubMed] [Google Scholar]
- Zolkiewski, M., Kessel, M., Ginsburg, A., and Maurizi, M.R. 1999. Nucleotide-dependent oligomerization of ClpB from Escherichia coli. Protein Sci. 8 1899–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]