Abstract
Primary immunization of infants with protein–polysaccharide conjugate vaccines induces antipolysaccharide antibody and is highly effective in preventing invasive disease caused by encapsulated bacteria. However, recent experience from the UK indicates that this immunity is not sustained in the absence of booster doses of vaccine. This study aimed to establish the kinetics and phenotype of B-cell subpopulations responding to booster immunization with a heptavalent pneumococcal conjugate vaccine (Pnc7), which is to be introduced into the primary immunization schedule in the UK during 2006. Six adult volunteers received a booster dose of Pnc7 12–18 months after primary immunization. CD27hi CD38hi CD20+/– IgG antibody-forming cells were detected in peripheral blood with maximum frequency at days 6–7 after immunization. This was accompanied by a more prolonged rise in memory B cells that required in vitro stimulation with Staphylococcus aureus Cowan strain and interleukin-2 to induce antibody secretion. These data provide evidence for at least two subsets of antibody-forming cells involved in the secondary humoral response to a glycoconjugate vaccine in primed individuals. A briefly circulating subset of B cells that spontaneously secrete immunoglobulin G may be responsible for early defence against re-encountered encapsulated bacteria. However, the kinetics of the appearance of these cells may indicate that the humoral immune response is too slow in defence against an organism that invades within days of acquisition. The more sustained presence of a memory population may provide persistence of antipolysaccharide antibody after a booster dose of vaccine and may also include re-circulatory populations responsible for further anamnestic responses.
Keywords: pneumococcal vaccine, memory B cells, B-cell subsets, antibody-forming cells, conjugate vaccine
Introduction
Streptococcus pneumoniae is a major cause of serious disease including pneumonia and meningitis at the extremes of age and is responsible for up to 1 million deaths per year globally.1 The polysaccharide capsules of the 90 pneumococcal serotypes are conventionally thought to be T-cell independent type-2 (TI-2) antigens that induce a short-lived rise in serum antibody following vaccination and do not elicit immune memory. In infants under the age of two, the TI-2 response is absent as the splenic marginal zone is immature resulting in an unsuitable environment for activation of marginal zone B cells.2 The need to provide protection in young infants has led to the development of protein–polysaccharide conjugate vaccines since polysaccharides can be made immunogenic in infants by conjugation to a protein carrier that recruits CD4+ T helper (Th) cells to provide signals for differentiation of naïve B cells into plasma cells and memory B cells.3 This T-cell dependent (TD) response4 involves germinal centre formation in infants. Using this approach, a heptavalent, pneumococcal glycoconjugate vaccine (Pnc7), which uses a mutant diphtheria toxoid (CRM197) as protein carrier, was introduced for routine use in infants in the USA in 2000 and has had a dramatic impact on rates of disease in early childhood.5 This vaccine was introduced with a three dose primary schedule at 2, 4 and 6 months of age and a booster dose administered in the second year of life and is to be included in the primary immunization schedule in the UK during 2006.
Immunological memory, as determined by an anamnestic antibody response, has been demonstrated years after priming with other conjugate vaccines and previously it was argued that booster doses of conjugate vaccines were not be required.6 However, recent experience in the UK with Haemophilus influenzae type b and serogroup C meningococcal conjugate vaccines has shown that their efficacy is not sustained after priming, in the absence of a booster.7,8
One explanation for the failure of immunological memory to provide sustained protection is that the memory response is not quick enough to produce protective antibody before invasive disease has occurred. We examined the kinetics of plasma and memory cells and antibody after a booster dose of the pneumococcal conjugate vaccine in adults.
Materials and methods
Subjects and clinical procedures
Ten healthy adult volunteers (aged 28–44 years, three male and seven female) received a primary dose of the Pnc7 (Wyeth Vaccines, Pearl River, MA) by intramuscular injection in the left deltoid. The 0·5 ml dose of the vaccine contained a concentration of polysaccharides of 2·0 µg/ml for each of serotypes 4, 9V, 4, 18C, 19F, 23F and 4 µg/ml of 6B. Each polysaccharide is conjugated to CRM197 (mutant diphtheria toxoid) and adsorbed on aluminium phosphate. Blood was drawn prior to vaccination and again 7 days later. Six of the 10 original volunteers received a booster dose of Pnc7, 12–18 months after the initial dose. Following booster immunization 20 ml of venous blood was collected in a heparin tube on days 0, 3, 5, 6, 7, 9, 11, 13, 15 from two adults. The remaining four volunteers provided blood samples on days 0, 6, 7, 15 and 4–6 weeks following vaccination.
Informed consent was obtained from the volunteers and the protocol was approved by the Oxfordshire's Research Ethics Committee (OxREC number C02.005).
Culture of peripheral blood mononuclear cells (PBMC) for memory B-cell activation and expansion
PBMC were isolated from peripheral blood using density-gradient centrifugation and cultured with (final well concentrations) Staphylococcus aureus Cowan strain (SAC) at 1 : 5000 dilution of the Pansorbin™ cell suspension (Calbiochem-Novabiochem, Nottingham, UK) and interleukin (IL)-2 (Roche Diagnostics, Mannheim, Germany) at 50 U/ml, for 5 days at 37 °C in 5% CO2 and 95% humidity.
Antibodies
Enzyme-linked immunospot assay (ELISpot)
The capture antibody was polyvalent goat-antihuman immunoglobulin (Caltag, H17000, Buckingham, UK).9 The detection antibodies were goat anti-human immunoglobulin G (IgG), γ-chain specific alkaline phosphatase conjugate (#401442), goat anti-human IgA, α-chain specific-alkaline phosphatase conjugate (#401132), goat anti-human IgM, µ-chain specific alkaline phosphatase conjugate (#401902; Calbiochem-Novabiochem).
Flow cytometry
Fluorescein isothiocyanate (FITC)-labelled anti-CD3, anti-CD27, anti-IgA; phycoerythrin (PE) labelled anti-CD27, anti-CD38, anti-IgG, anti-IgD; peridinin chlorophyll protein (PerCP) labelled anti-CD20 and BD-Cy-chrome™ (PE-Cy5) labelled anti-IgM. Antibody isotype controls used were BD SimulTest™-isotype control (anti-IgG1–FITC plus anti-IgG2a–PE) anti-IgG1–PerCP. All antibodies for flow cytometry were obtained from BD Biosciences Ltd (Oxford, UK), except for anti-IgA-FITC, which was obtained from Calbiochem-Novabiochem.
Enzyme-linked immunosorbent assay antibodies
Goat anti-human IgG (AHI0305), IgA (AHI0105) and IgM (AHI0605) all conjugated to alkaline phosphatase, were obtained from Biosource (Camarillo, CA).
The ELISpot assay for the detection of antigen-specific IgG, IgA and IgM antibody-forming cells (AFC)
Multiscreen™ hydrophobic polyvinylidene fluoride (PVDF) membrane plates (Millipore, Watford, UK) were coated with anti-IgG, tetanus toxoid, diphtheria toxoid (Statens Seruminstitute, Copenhagen, Denmark) or pneumococcal polysaccharides (4, 14, 23F, LGC Promochem, Teddington, UK) conjugated to methylated human serum albumin (mHSA, National Instititute for Standards and Controls, Potters Bar, UK)10 at 10 µg/ml in phosphate-buffered saline (PBS) except tetanus toxoid and serotype 23F which were coated at 5 and 20 µg/ml, respectively. Because availability of PBMC was limited only three of the seven vaccine serotypes were studied. Washed B cells cells were seeded at 200–2 × 105 cells per well on ELISpot plates, previously blocked with 10% newborn calf serum (NBCS), and incubated overnight at 37 °C in 5%CO2 and 95% humidity. The cells were washed with PBS-Tween and bound IgG antibody detected using an alkaline phosphatase conjugate and AP-conjugate substrate kit (nitroblue tetrazolium + 5-bromo-4-chloro-3-indolyl phosphate in dimethylformamide; Bio-Rad Laboratories Hemel Hempstead, UK) prior to counting using an AID ELISpot reader ELR02 and software version 3.2.3. (Cadama Medical Ltd, Stourbridge, UK).
Separation of PBMC for the detection of spontaneous, immunoglobulin-secreting cells by ELISpot
On day 7 after vaccination PBMC were separated using an AutoMACs™ cell separator (Miltenyi Biotec Ltd. Bisley, UK) on the basis of CD20, CD27 or CD38 expression using the microbeads as per the manufacturer's instructions.
Flow cytometric phenotyping of the unseparated and separated PBMC
Briefly, 5 × 105 cells for each assay were incubated with labelled antibodies for 15 min, in the dark, washed and fixed with 1% paraformaldehyde. Analysis was performed using a three colour BD FacSCAN™ and BD CellQuest™ software version 3.1.
Detection of antipolysaccharide immunoglobulins
The World Health Organization standard protocol was used for detection of human IgG, IgA and IgM antibodies against S. pneumoniae capsular polysaccharides11 (http://www.vaccine.uab.edu). In brief, plasma samples were preabsorbed with 10 µg/ml of cell wall polysaccharide (CPS; Statens Seruminstitut) and 10 µg/ml of a non-vaccine-related polysaccharide 22F (LGC; Promochem) The standard serum, 89SF (FDA) was used for the concentration curve and control sera were obtained from NIBSC. Antibodies specific for the capsular polysaccharides 4, 14 and 23F were detected using alkaline-phosphatase conjugates and antibody concentrations were calculated in µg/ml from a four point, sigmoidal plot using Revelation™ software (ThermoLabs Inc.).
Statistics
Comparison between individuals and time points was carried out using the Wilcoxon Signed Rank test and SPSS v. 12.
Results
The frequency of spontaneous IgG secretion by ex-vivo, antigen specific-AFC, isolated from adult donor PBMC during 15 days post booster immunization of adults with Pnc7
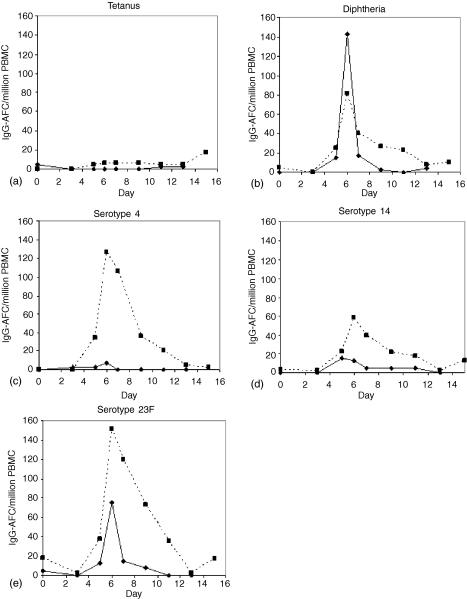
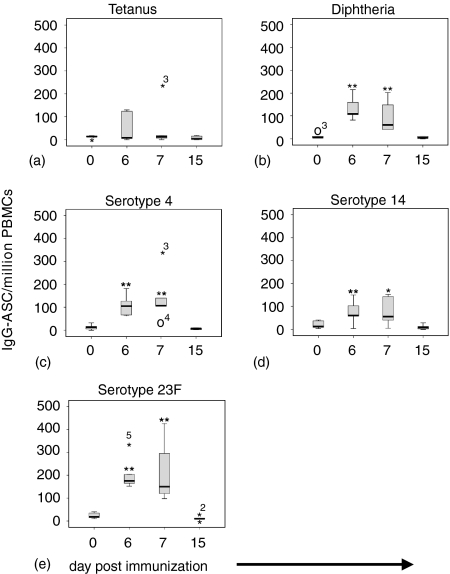
Spontaneously secreting IgG-AFC appeared in peripheral blood between days 5 and 15 (Fig. 1) after immunization. The magnitude and rate of decline in frequency was variable between individuals and for each antigen, while the peak response occurred consistently on days 6–7 (Fig. 2) (P < 0.05). There were no detectable alterations in the frequency of tetanus toxoid specific IgG–AFC, except for two individuals, in whom there was a rise at day 6–7 (Fig. 1a and Fig. 2a).
Figure 1.
The kinetics of the appearance of spontaneously secreting, IgG-AFC in peripheral blood of two adult volunteers after a booster immunization with a pneumococcal conjugate vaccine (Pnc7), 1 year after a primary dose. The IgG-AFC specific for tetanus toxoid (a) diphtheria toxoid (b) serotype-4 (c) serotype-14 (d) and serotype 23F (e) enumerated by ELISpot are shown. The data are expressed as the number of antigen specific IgG-AFC per million PBMC.
Figure 2.
The kinetics of IgG secreting plasma cells isolated from the peripheral blood of six adults following a booster dose of a pneumococcal conjugate vaccine (Pnc7), one year after a primary dose. IgG-AFC were enumerated by ELISPOT specific for tetanus toxoid (a) diphtheria toxoid (b) serotype 4 (c) serotype 14 (d) and serotype 23F (e) were enumerated by ELISpot. The box represents the 25th and 75th quartiles and the whiskers are the outliers. Extreme outliers are represented by (⋆, higher or ○, lower with numbers representing the individual subject in the data set. i.e. 3 = individual 3). (**P < 0·05, *P = 0·08).
The phenotype of B cells involved in the IgG-AFC response after a booster Pnc7 immunization
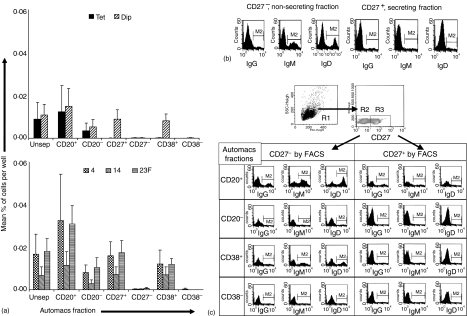
On the peak day of the AFC response (Fig. 3a), IgG–AFC specific for the capsular polysaccharides were present in both the CD20+ and CD20– fractions of the PBMC population, although they were more frequent in the CD20+ fraction. There was almost a complete absence of IgG–AFC within the CD27– and CD38– fractions with all AFC activity appearing within the CD27+ and CD38+ fractions. Tetanus toxoid-specific IgG–AFC were present in the unseparated fraction, but became undetectable in this assay following sorting. Diphtheria-specific IgG–AFC showed the same distribution of phenotype as polysaccharide specific cells (CD20+/–, CD27+ and CD38+).
Figure 3.
The phenotype of the IgG-AFC isolated on day 7 after a booster dose of pneumococcal conjugate vaccine (Pnc7), 1 year after a primary dose. (a) PBMCs were separated, using magnetic beads, on the basis of CD20, CD27 or CD38 expression. The purity of the sorted fractions were as follows: CD27+ = 89% (77–97%); CD27– = 97% (94–98%); CD38+ = 79% (70–88%); CD38– = 65% (61–70%); CD20+ = 72% (71–74%); CD20– = 93% (92–94%). Anti-diphtheria toxoid, pneumococcal polysaccharide (serotypes 4, 14 and 23F) and tetanus toxoid IgG-AFC were detected by ELISpot from the positive and negative fractions. The data represents the mean and standard error from three individuals. The expression of immunoglobulin and CD27 on B cells within each fraction was determined by flow cytometry (b, c, representative data from one donor of three tested.) The lymphocytes were gated on the basis of side scatter versus forward scatter (R1). The presence or absence of CD27 was then assessed (R2 and R3) The expression of immunoglobulin within R2 and R3 was then determined for each fraction.
The percentage of IgG, IgM and IgD positive cells was higher in the non-secreting, CD27– fraction than in the CD27+ fraction (Fig. 3b). The CD27– B cells in all of the fractions expressed far more immunoglobulin on their surface than did the memory, CD27+ B cells (Fig. 3c). The CD20+ CD27+ B cells had higher expression of immunoglobulin than any of the other CD27+ populations. The fractions that had AFC activity (CD27+, CD20+/– and CD38+) had lower surface expression of immunoglobulin than the non-secreting fractions.
Frequency of SAC + IL-2-induced IgG–AFC during the 4–6 weeks post booster immunization of adults with Pnc7
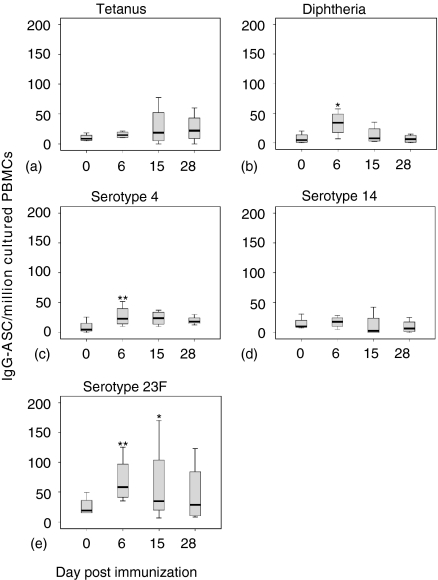
The frequency of SAC + IL-2-induced tetanus-specific IgG–AFC rose in all individuals except one, following Pnc7 immunization and remained elevated at days 28–42 post vaccination, although the peak in frequency varied (Fig. 4a) Diphtheria-specific IgG–AFC induced by SAC + IL-2 were at the limit of detection of this assay at day 0 but rose to a peak at day 6, for all but one individual, where the rise in the frequency was delayed until day 15. Diphtheria-specific IgG–AFC declined after day 15 but remained detectable even at days 28–42, in all but one volunteer (Fig. 4b) The IgG-AFC response to the pneumococcal capsular polysaccharides also demonstrated more of a plateau than seen in the ex-vivo response. At day 0 (12–18 months post primary immunization) cultured IgG–AFC were detectable for all serotypes with serotype 23F > 14 > 4 (Fig. 4d–e) At day 6, post immunization there were equal numbers of responders and non-responders to serotype-14 following booster Pnc7 administration but there was a significant rise in both serotype 4 and 23F memory cells. This response had fallen below baseline by day 15 for all vaccinees except two (Fig. 4d) Most individuals responded with an increase in frequency of serotype-4 and 23F specific IgG-AFC (Fig. 4c and e).
Figure 4.
The frequency of IgG-producing B cells (presumed memory population) isolated from the peripheral blood of six adults following a booster dose of pneumococcal conjugate vaccine (Pnc7), 1 year after a primary dose and stimulated in vitro. IgG-AFC specific for tetanus toxoid (a) diphtheria toxoid (b) pneumococcal serotype 4 (c) serotype 14 (d) and serotype 23F (e) were enumerated by ELISpot. (**P = < 0·05, *P = 0·08). Box represents the 25th and 75th quartiles and the whiskers represent the outliers.
Determination of plasma IgG, IgA and IgM concentrations following a primary dose of Pnc7 immunization
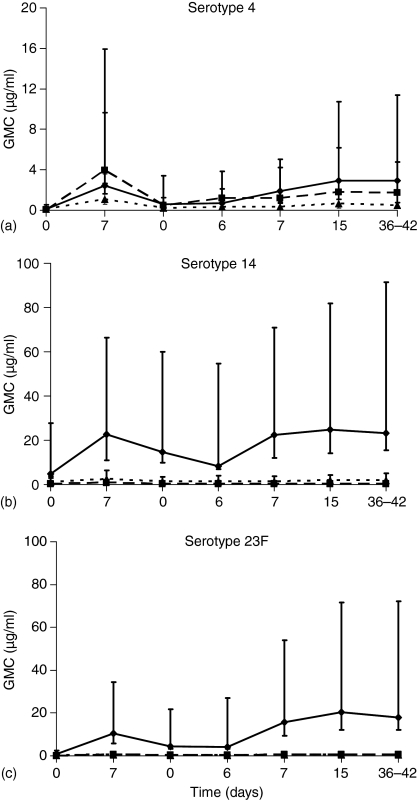
There were significant increases in the geometric mean concentration (GMC) of IgG, IgA and IgM in response to serotype 4 by day 7 following a single dose of Pnc7 (P = 0.0004, 0.0001 and 0.0022, respectively, Fig. 5a) This was also the case for serotype 23F (P = 0.0004, 0.0002 and 0.0039, Fig. 5c) Serotype-14 induced significant increases in IgG and IgA (P = 0.03 and 0.015, Fig. 5b) but the prevaccination antibody levels against this serotype were already higher than those for serotype 23F and serotype 4 (IgG GMC: 4.80, 0.81 and 0.14 µg/ml, respectively, and IgA GMC: 0.23, 0.07, 0.07 µg/ml, respectively.) The magnitude of the IgG response to serotype 4 was much lower than that for 14 and 23F (Fig. 5).
Figure 5.
The anticapsular antibody concentrations in plasma from adults following both a primary and booster dose of Pnc7 (1 year after a primary dose). Ten adult volunteers received a dose of Pnc7 and blood was drawn prior to and 7 days after immunization. Six of the adults received a booster dose 12–18 months later. Antipolysaccharide IgG (solid line) IgA (dashed line) and IgM (dotted line) were measured for serotypes 4 (a) 14 (b) and 23F (c). Data represent the GMC with 95% confidence intervals.
Determination of plasma IgG, IgA and IgM concentrations following a booster dose of Pnc7, 12–18 months post primary immunization (Fig. 5a–c)
Prior to administration of the booster dose, IgG, IgA and IgM concentrations for serotype 4 and 23F had declined almost to prevaccination levels. However, the IgG GMCs were still above 0.20 µg/ml for all three serotypes. The booster response peaked by day 15 after immunization and was maintained to 28–42 days for IgG, IgA and IgM to all three serotypes (Fig. 5a–c). Serotype 4 induced slightly higher levels of IgG following Pnc7 boost (GMC: 2.91 µg/ml versus 2.43 µg/ml) while levels of IgA and IgM were lower than after primary immunization (IgA GMC: 1.79 µg/ml versus 3.97 µg/ml and IgM GMC: 0.63 µg/ml versus 1.10 µg/ml) There was no significant IgA or IgM response to boosting of the serotype 14 response, while 23F induced a small, but significant increase in IgA and IgM, no higher than seen following the first dose.
Discussion
This study provides the first description of the peripheral blood kinetics and phenotype of populations of B cells appearing after immunization of adults with a booster dose of a pneumococcal glycoconjugate vaccine. B cells that spontaneously secrete antibody ex vivo appear briefly in peripheral blood, peaking on day 7 following immunization with the rise above baseline occurring at only 4–5 days after immunization, highlighting the slow response time of the secondary immune response. These cells are of two phenotypes (CD20+ CD38+ CD27+ or CD20– CD38+ CD27+) and both of these subsets have low surface immunoglobulin expression, with IgM+ and IgD+ cells being most prominent in the secreting cell fractions and are phenotypically plasma cells. Furthermore, the appearance of these antibody secreting cells is accompanied by a more sustained, though lower magnitude, rise in appearance of a non-immunoglobulin secreting B-cell subset in the peripheral blood that have the in vitro characteristics of memory B cells.
Plasma cells appeared by day 5 and peaked at days 6–7 following booster immunization, but had fallen to undetectable levels by day 15. Both polysaccharide-specific and diphtheria (a conventional T-dependent antigen)-specific B cells showed the same kinetics. There was a simultaneous rise in serum anticapsular IgG, IgA and IgM antibodies from day 6 following the administration of the booster dose with a peak by day 15. We did not study the plasma cell response following the primary immunization of our volunteers; however, similar kinetics have been described following primary immunization of toddlers and adults with Pnc7 or 23-valent pneumococcal polysaccharide vaccine12 and mice.13 A single dose of a 14-valent pneumococcal polysaccharide vaccine led to a peak in cell supernatant antipolysaccharide (Ps) antibody of all isotypes by day 8. This was followed by a peak in serum anti-Ps antibody by day 14.14 The reproducibility of the kinetics in circulating antigen-specific AFC and serum antibody between different individuals in this and other studies suggests that these responses are a programmed physiological response to immunization. Because organisms like pneumococci are carried in the nasopharynx, it is not clear whether primary immunization of adults with the vaccine results in stimulation of naïve B cells or cells that had been previously primed through nasopharyngeal exposure to the organism. Other published data indicate that these responses are not limited to Pnc7 as the appearance of antigen specific, spontaneously secreting B cells in the peripheral blood, at days 5–8 after immunization, have been described after immunization with tetanus, influenza and pneumococcal vaccines.12,15–18 The time from immunization to the production of these effector B cells indicates that, in the absence of a sustained protective antibody concentration, there is a window of several days in which invasive bacteria could cause disease even in a primed individual.
In our study the spontaneously secreting, antigen-specific AFC seen in peripheral blood are probably derived from memory cells residing in secondary lymphoid tissues after primary immunizations. Booster immunization induces activation and rapid proliferation of vaccine specific, memory B cells19 in a germinal centre and T-cell independent fashion. By 6–7 days after immunization there is a rapid efflux of antigen-specific AFC into the peripheral blood20 the spontaneously secreting B cells being identified in our ex-vivo assay. It is also possible that, at least some, of these cells are derived from naïve antigen-specific B cells.21
After administration of a booster dose of Pnc7 serotype 14 and 23F specific AFCs could be detected in an ELISpot assay after in vitro culture but the frequencies of the antigen specific AFC had returned to almost prebooster levels by 1 month. In-vitro stimulation through the B-cell receptor (BCR) with SAC (which cross-links the BCR) and activation of costimulatory molecules (IL-2 in our assay which stimulates proliferation of activated B cells and T cells present in PBMC cultures) provides the signals necessary for the expansion of these antigen specific memory B-cell populations, facilitating their detection by ELISpot.22 Our observations suggest that memory B cells induced by the primary immunization 12–18 months earlier were mobilized to circulate in peripheral blood following the booster dose of Pnc7. Alternatively, these cells may represent newly formed memory B cells stimulated by exposure of naïve B cells to the conjugate vaccine. In this study we could detect these cells at a rate of 0–0.01/1000 PBMC at day 0 and 0.02–0.05/1000 PBMC at day 6.
The hypothesis that these antibody secretion-inducible cells are memory B cells is supported by previous studies showing that it is necessary to stimulate PBMC in vitro to detect pre-existing, re-circulating memory cells.23 ELISpot analysis of AFC ex-vivo only allowed detection of fully differentiated plasma cells already secreting antigen-specific antibody.24 Pre-existing, mature plasma cells only survive in culture for up to 48 hr and after 5 days stimulation with SAC + IL-2, only those B cells entering culture as memory B cells or preplasma cells remain.25 Analysis of B-cell division by flow cytometry and carboxy-fluorescein diacetate, succinimidyl ester (CFSE) labelling has shown that five days of in-vitro stimulation is long enough for the cells to undergo at least four division cycles and that this number of division cycles is necessary for antibody secretion.19 This system preferentially activates memory B cells,26,27 and induces IgM memory B cells (CD27+ IgM+ IgD+) to secrete IgM and IgG, while class switched memory B cells (CD27+ IgM– IgD–) will secrete IgG and IgA.28 The SAC + IL-2 system probably induces a mixture of pre-existing memory B cells and newly generated precursor plasma cells to secrete antibody. Migration of the precursor plasma cells occurs over a limited period before they become resident in the bone marrow, and this may explain the observed peaks in SAC + IL-2 induced cells at days 6–7 and the return to prebooster levels of detectable AFC at 4–6 weeks post immunization.
To provide sustained protection through antibody it may be necessary for these cells to ‘turn over’ and differentiate into plasma cells. We included tetanus toxoid (TT) in our memory B-cell system as a non-vaccine-related control and found that Pnc7 immunization appeared to induce increased frequencies of TT-specific IgG-AFC in addition to the vaccine specific B-cell responses. The appearance of AFC of unrelated specificities in the peripheral blood may be caused by the displacement of long-lived plasma cells from the bone marrow or bystander activation of B-cells during an immune response.25,29–31
In our study the antipneumococcal polysaccharide serum antibody concentrations rose after primary immunization and remained above baseline before rising again after secondary immunization. The kinetics to day 7 were similar for both the primary and booster immunizations, perhaps supporting our view that these adults may have been primed by prior nasopharyngeal carriage. We cannot be sure of the kinetics of the response beyond day 7 following primary immunization but the antibody levels had declined somewhat by the time of the booster dose. The process behind maintenance of serum antibody is poorly understood. Three mechanisms have been suggested for: the long-term maintenance of protective antibody levels;32 presence of long-lived plasma cells;33,34 bystander or polyclonal activation of B cells;29 and re-stimulation of memory B cells by antigen persisting on follicular dendritic cells.35
An alternative explanation in the case of pneumococcal antibody may be exposure to the organism through nasopharyngeal colonization. Nasopharyngeal carriage of pneumococci was not investigated during this study but previous epidemiological studies, including one carried out in Oxford children, showed that 23F was among the most commonly detected serotype among nasopharyngeal isolates, while serotype 4 was the rarest and 14 was detected at an intermediate rate.36–38 Similar observations were made in a multinational epidemiological study of pneumococcal disease and carriage rates.39 It is possible, therefore, that frequent exposure to serotypes 14 and 23F, via nasopharyngeal carriage has helped to boost the AFC and antibody responses seen for 14 and 23F polysaccharides, leading to a predominantly IgG mediated response caused by memory B-cell formation in exposed individuals. Conversely, little or no exposure to the infrequently colonizing serotype 4 means that a more naïve response is generated following immunization. This view is supported by our study in which the serotype 4 response did not improve in magnitude following the booster dose, although there was a switch to a more predominantly IgG response. In addition, IgA and IgM were more apparent in the type 4 response than in the immune response to type 14 or 23F polysaccharides. These differences in the level of antibody and response to immunization are also reflected in higher rates of invasive pneumococcal disease caused by this serotype.
The plasma cell population in the peripheral blood on day 7 following immunization included two subpopulations, CD20+ CD27+ CD38+ Iglo and CD20– CD27+CD38+ Iglo. The CD20– cells are probably terminally differentiated plasma cells expressing high (hi) levels of CD27 and CD38 with concomitant down regulation of CD20 and surface immunoglobulin.34,40,41 The CD20+ subset represents intermediate plasmablasts with a more germinal centre phenotype, that are present in the peripheral blood and able to secrete antibody.21,42 The identification of the CD20+ subset as a plasmablast population was further confirmed by the low level, surface expression of immunoglobulin.
A memory B-cell compartment identified by CD27+/–IgM+ IgD+ expression has been identified as circulating marginal zone B cells (MZB).43 These cells express a similar phenotype of IgMhi IgDlo CD21hi CD23lo, in the spleen and peripheral blood of humans and mice43,44 and may be particularly important in B-cell responses to polysaccharide antigens. We have identified a population of B cells in our day 7 spontaneously secreting AFC fractions that express CD27+ IgM+ IgD+ that may be derived from such an MZB subset (Fig. 3c). These cells may represent an important component of innate defence against polysaccharide encapsulated bacteria, by secreting large amounts of IgM antibody followed by rapid class-switching to IgG.45
The importance of the splenic marginal zone for the formation of polysaccharide reactive plasma cells may explain the high risk of invasive pneumococcal disease in infants, who have immature splenic marginal zones.43 Conversely, the superior efficacy of glycoconjugates over purified polysaccharide vaccines in this age group arises from the formation of germinal centers as a result of T cells that are activated by processing of the carrier protein without the need for involvement of MZB cells. However, it is claimed that the MZB cells are involved in the immune response to both conjugates and polysaccharide vaccines once the splenic marginal zone is formed (over 2 years of age), an idea which gains some support from our observation that plasma cells detected in these studies had some characteristics of MZB.44,46
There was considerable variability in the magnitude of the response against each antigen in a given individual and between subjects. This was true for plasma cells, memory B cells and serum antibody concentrations. In our study, the immunogenicity of serotype 23F, as measured by ex-vivo AFC frequency, was greater than that of serotype 4, and similar observations have been made for antibody concentrations.47–49 This variation may result from induction of differing cytokine profiles within the germinal centres,50 genetic factors, or biochemical differences in the polysaccharides,51 or previous exposure to the organism through nasopharyngeal colonization.
The response to a booster dose of Pnc7 is marked by the generation of plasma cells that peaks at days 6–7 after immunization and is accompanied by an increase in the circulation of memory B cells. The responding AFC population includes a switched memory B-cell derived subset and a CD27+ IgD+ IgM+ subset that resembles the MZB phenotype. These cells are critical in protection against polysaccharide-encapsulated pathogens such as S. pneumoniae. It is anticipated that primary immunization of babies in the UK, following introduction of the vaccine during 2006, will induce the same B-cell responses as seen here in adults. If responses are similar in this age group, the planned introduction of a booster dose in the second year of life will maintain a pool of differentiated antibody secreting cells that may be the key to sustained protection.
Acknowledgments
This project was supported by a grant awarded by the Edward Jenner Institute for Vaccine Research, Compton, UK. The authors would like to thank the volunteers who participated in the study and the research nurses at the Oxford Vaccine Group, University of Oxford, who undertook the clinical procedures.
Glossary
Abbreviations
- AFC
antibody-forming cell
- SAC
Staphylococcus aureus Cowan strain
- TI
T-cell independent
- TD
T-cell dependent
- MZB
marginal zone B-cell
- Pnc7
heptavalent pneumococcal-CRM197 conjugate vaccine
- GMC
geometric mean concentration
References
- 1.Fedson DS, Scott JA. The burden of pneumococcal disease among adults in developed and developing countries. what is and is not known. Vaccine. 1999;17(Suppl. 1):S11–8. doi: 10.1016/s0264-410x(99)00122-x. [DOI] [PubMed] [Google Scholar]
- 2.Weller S, Braun MC, Tan BK, et al. Human blood IgM ‘memory’ B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–54. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan AQ, Lees A, Snapper CM. Differential regulation of IgG anti-capsular polysaccharide and antiprotein responses to intact Streptococcus pneumoniae in the presence of cognate CD4+ T cell help. J Immunol. 2004;172:532–9. doi: 10.4049/jimmunol.172.1.532. [DOI] [PubMed] [Google Scholar]
- 4.Adderson EE. Antibody repertoires in infants and adults: effects of T-independent and T-dependent immunizations. Springer Semin Immunopathol. 2001;23:387–403. doi: 10.1007/s281-001-8166-x. [DOI] [PubMed] [Google Scholar]
- 5.Black S, Shinefield H, Baxter R, Austrian R, Bracken L, Hansen J, Lewis E, Fireman B. Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal conjugate vaccine in Northern California Kaiser Permanente. Pediatr Infect Dis J. 2004;23:485–9. doi: 10.1097/01.inf.0000129685.04847.94. [DOI] [PubMed] [Google Scholar]
- 6.Heath PT, Booy R, Azzopardi HJ, et al. Antibody concentration and clinical protection after Hib conjugate vaccination in the United Kingdom. JAMA. 2000;284:2334–40. doi: 10.1001/jama.284.18.2334. [DOI] [PubMed] [Google Scholar]
- 7.Ramsay ME, McVernon J, Andrews NJ, Heath PT, Slack MP. Estimating Haemophilus influenzae type b vaccine effectiveness in England and Wales by use of the screening method. J Infect Dis. 2003;188:481–5. doi: 10.1086/376997. [DOI] [PubMed] [Google Scholar]
- 8.Trotter CL, Andrews NJ, Kaczmarski EB, Miller E, Ramsay ME. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet. 2004;364:365–7. doi: 10.1016/S0140-6736(04)16725-1. [DOI] [PubMed] [Google Scholar]
- 9.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells. a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286:111–22. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Concepcion N, Frasch CE. Evaluation of previously assigned antibody concentrations in pneumococcal polysaccharide reference serum; 89SF:by the method of cross-standardization. Clin Diagn Lab Immunol. 1998;5:199–204. doi: 10.1128/cdli.5.2.199-204.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wernette CM, Frasch CE, Madore D, et al. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin Diagn Laboratory Immunol. 2003;10:514–9. doi: 10.1128/CDLI.10.4.514-519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heilmann C, Henrichsen J, Pedersen FK. Vaccination-induced circulation of human B cells secreting type-specific antibodies against pneumococcal polysaccharides. Scand J Immunol. 1987;25:61–7. doi: 10.1111/j.1365-3083.1987.tb01047.x. [DOI] [PubMed] [Google Scholar]
- 13.Milligan GN, Fairchild RL, Sterner KE, Braley-Mullen H. Type 6 and 19 pneumococcal polysaccharides coupled to erythrocytes elicit pneumococcal cell wall-specific primary IgM responses and capsular polysaccharide-specific secondary IgG responses. Eur J Immunol. 1990;20:595–603. doi: 10.1002/eji.1830200320. [DOI] [PubMed] [Google Scholar]
- 14.Kehrl JH, Fauci AS. Characterization of the cellular immune response following in vivo immunization with pneumococcal polysaccharides in man. Bull Eur Physiopathol Respir. 1983;19:227–34. [PubMed] [Google Scholar]
- 15.Brokstad KA, Cox RJ, Olofsson J, Jonsson R, Haaheim LR. Parenteral influenza vaccination induces a rapid systemic and local immune response. J Infect Dis. 1995;171:198–203. doi: 10.1093/infdis/171.1.198. [DOI] [PubMed] [Google Scholar]
- 16.Falkoff RJ, Zhu LP, Fauci AS. The relationship between immunization and circulating antigen-specific plaque-forming cells. Cell Immunol. 1983;78:392–9. doi: 10.1016/0008-8749(83)90295-2. [DOI] [PubMed] [Google Scholar]
- 17.Nieminen T, Kayhty H, Virolainen A, Eskola J. Circulating antibody secreting cell response to parenteral pneumococcal vaccines as an indicator of a salivary IgA antibody response. Vaccine. 1998;16:313–9. doi: 10.1016/s0264-410x(97)00162-x. [DOI] [PubMed] [Google Scholar]
- 18.Odendahl M, Mei H, Hoyer BF, et al. Generation of migratory antigen-specific plasma blasts and mobilisation of resident plasma cells in a secondary immune response. Blood. 2005;105:1614–21. doi: 10.1182/blood-2004-07-2507. [DOI] [PubMed] [Google Scholar]
- 19.Hasbold J, Corcoran LM, Tarlinton DM, Tangye SG, Hodgkin PD. Evidence from the generation of immunoglobulin G-secreting cells that stochastic mechanisms regulate lymphocyte differentiation. Nat Immunol. 2004;5:55–63. doi: 10.1038/ni1016. [DOI] [PubMed] [Google Scholar]
- 20.Dilosa RM, Maeda K, Masuda A, Szakal AK, Tew JG. Germinal center B cells and antibody production in the bone marrow. J Immunol. 1991;146:4071–7. [PubMed] [Google Scholar]
- 21.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki N, Ueda Y, Sakane T. Differential mechanism for differentiation into immunoglobulin-secreting cells in human resting B lymphocyte subsets isolated on the basis of cell density. J Clin Invest. 1988;81:261–9. doi: 10.1172/JCI113304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nanan R, Heinrich D, Frosch M, Kreth HW. Acute and long-term effects of booster immunisation on frequencies of antigen-specific memory B-lymphocytes. Vaccine. 2001;20:498–504. doi: 10.1016/s0264-410x(01)00328-0. [DOI] [PubMed] [Google Scholar]
- 24.Soenawan E, Srivastava I, Gupta S, et al. Maintenance of long-term immunological memory by low avidity IgM-secreting cells in bone marrow after mucosal immunizations with cholera toxin adjuvant. Vaccine. 2004;22(11–12):1553–63. doi: 10.1016/j.vaccine.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Cassese G, Arce S, Hauser AE, et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol. 2003;171:1684–90. doi: 10.4049/jimmunol.171.4.1684. [DOI] [PubMed] [Google Scholar]
- 26.Maurer D, Fischer GF, Fae I, Majdic O, Stuhlmeier K, Von Jeney N, Holter W, Knapp W. IgM and IgG but not cytokine secretion is restricted to the CD27+ B lymphocyte subset. J Immunol. 1992;148:3700–5. [PubMed] [Google Scholar]
- 27.Nagumo H, Agematsu K, Kobayashi N, et al. The different process of class switching and somatic hypermutation; a novel analysis by CD27 (–) naive B cells. Blood. 2002;99:567–75. doi: 10.1182/blood.v99.2.567. [DOI] [PubMed] [Google Scholar]
- 28.Saiki O, Ralph P. IgM- and IgD-bearing peripheral blood lymphocytes differentiate to IgM but not IgG or IgA immunoglobulin-secreting cells. Eur J Immunol. 1982;12:506–10. doi: 10.1002/eji.1830120611. [DOI] [PubMed] [Google Scholar]
- 29.Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells. Science. 2002;298:2199–202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- 30.Dorner T, Radbruch A. Selecting B cells and plasma cells to memory. J Exp Med. 2005;201:497–9. doi: 10.1084/jem.20050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunkel EJ, Butcher EC. Plasma-cell homing. Nat Rev Immunol. 2003;3:822–9. doi: 10.1038/nri1203. [DOI] [PubMed] [Google Scholar]
- 32.Gourley TS, Wherry EJ, Masopust D, Ahmed R. Generation and maintenance of immunological memory. Semin Immunol. 2004;16:323–33. doi: 10.1016/j.smim.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 33.Manz RA, Hauser AE, Hiepe F, Radbruch A. Maintenance of serum antibody levels. Annu Rev Immunol. 2005;23:367–86. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- 34.Slifka MK, Ahmed R. Long-lived plasma cells: a mechanism for maintaining persistent antibody production. Curr Opin Immunol. 1998;10:252–8. doi: 10.1016/s0952-7915(98)80162-3. [DOI] [PubMed] [Google Scholar]
- 35.Zinkernagel RM, Hengartner H. On immunity against infections and vaccines: credo 2004. Scand J Immunol. 2004;60:9–13. doi: 10.1111/j.0300-9475.2004.01460.x. [DOI] [PubMed] [Google Scholar]
- 36.Brueggemann AB, Griffiths DT, Meats E, Peto T, Crook DW, Spratt BG. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J Infect Dis. 2003;187:1424–32. doi: 10.1086/374624. [DOI] [PubMed] [Google Scholar]
- 37.Brueggemann AB, Peto TE, Crook DW, Butler JC, Kristinsson KG, Spratt BG. Temporal and geographic stability of the serogroup-specific invasive disease potential of Streptococcus pneumoniae in children. J Infect Dis. 2004;190:1203–11. doi: 10.1086/423820. [DOI] [PubMed] [Google Scholar]
- 38.Hausdorff WP, Feikin DR, Klugman KP. Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis. 2005;5:83–93. doi: 10.1016/S1473-3099(05)01280-6. [DOI] [PubMed] [Google Scholar]
- 39.Hausdorff WP, Yothers G, Dagan R, et al. Multinational study of pneumococcal serotypes causing acute otitis media in children. Pediatr Infect Dis J. 2002;21:1008–16. doi: 10.1097/00006454-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Jacquot S, Kobata T, Iwata S, Morimoto C, Schlossman SF. CD154/CD40 and CD70/CD27 interactions have different and sequential functions in T cell-dependent B cell responses: enhancement of plasma cell differentiation by CD27 signaling. J Immunol. 1997;159:2652–7. [PubMed] [Google Scholar]
- 41.Joshua D, Pope B, Brown R, Brown L, Murray A, Gibson J. Phenotyping primitive plasma cells. Br J Haematol. 2002;117:252–3. doi: 10.1046/j.1365-2141.2002.3406_5.x. [DOI] [PubMed] [Google Scholar]
- 42.O'Connor BP, Gleeson MW, Noelle RJ, Erickson LD. The rise and fall of long-lived humoral immunity: terminal differentiation of plasma cells in health and disease. Immunol Rev. 2003;194:61–76. doi: 10.1034/j.1600-065x.2003.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weller S, Faili A, Aoufouchi S, Gueranger Q, Braun M, Reynaud CA, Weill JC. Hypermutation in human B cells in vivo and in vitro. Ann N Y Acad Sci. 2003;987:158–65. doi: 10.1111/j.1749-6632.2003.tb06044.x. [DOI] [PubMed] [Google Scholar]
- 44.Oliver AM, Martin F, Kearney JF. IgMhigh CD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J Immunol. 1999;162:7198–207. [PubMed] [Google Scholar]
- 45.Werner-Favre C, Bovia F, Schneider P, Holler N, Barnet M, Kindler V, Tschopp J, Zubler RH. IgG subclass switch capacity is low in switched and in IgM-only, but high in IgD+ IgM+, post-germinal center (CD27+) human B cells. Eur J Immunol. 2001;31:243–9. doi: 10.1002/1521-4141(200101)31:1<243::AID-IMMU243>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 46.Weller S, Reynaud CA, Weill JC. Vaccination against encapsulated bacteria in humans: paradoxes. Trends Immunol. 2005;26:85–9. doi: 10.1016/j.it.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Ekstrom N, Ahman H, Verho J, Jokinen J, Vakevainen M, Kilpi T, Kayhty H. Kinetics and avidity of antibodies evoked by heptavalent pneumococcal conjugate vaccines PncCRM and PncOMPC in the Finnish Otitis Media Vaccine Trial. Infect Immun. 2005;73:369–77. doi: 10.1128/IAI.73.1.369-377.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huebner RE, Mbelle N, Forrest B, Madore DV, Klugman KP. Long-term antibody levels and booster responses in South African children immunized with nonavalent pneumococcal conjugate vaccine. Vaccine. 2004;22:2696–700. doi: 10.1016/j.vaccine.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 49.Shinefield HR, Black S, Ray P, et al. Safety and immunogenicity of heptavalent pneumococcal CRM197 conjugate vaccine in infants and toddlers. Pediatr Infect Dis J. 1999;18:757–63. doi: 10.1097/00006454-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Mawas F, Feavers IM, Corbel MJ. Serotype of Streptococcus pneumoniae capsular polysaccharide can modify the Th1/Th2 cytokine profile and IgG subclass response to pneumococal-CRM (197) conjugate vaccines in a murine model. Vaccine. 2000;19:1159–66. doi: 10.1016/s0264-410x(00)00314-5. [DOI] [PubMed] [Google Scholar]
- 51.Kalka-Moll WM, Tzianabos AO, Bryant PW, Niemeyer M, Ploegh HL, Kasper DL. Zwitterionic polysaccharides stimulate T cells by MHC class II-dependent interactions. J Immunol. 2002;169:6149–53. doi: 10.4049/jimmunol.169.11.6149. [DOI] [PubMed] [Google Scholar]