Abstract
Toll-like receptors (TLRs) expressed in mast cells play important roles in orchestrating host defence against bacterial pathogens. Previous studies demonstrated that TLR2 agonist tripalmitoyl-S-glycero-Cys-(Lys)4 (Pam3Cys) stimulates both degranulation and cytokine production in human mast cells but only induces cytokine production in murine mast cells. To determine the molecular basis for this difference, we utilized a human mast cell line LAD 2, murine lung and bone marrow-derived mast cells (MLMC and BMMC). We found that Pam3Cys caused a sustained Ca2+ mobilization and degranulation in LAD 2 mast cells but not in MLMC or BMMC. Despite these differences, Pam3Cys stimulated equivalent chemokine CCL2 generation in all mast cell types tested. Cyclosporin A (CsA), an inhibitor of Ca2+/calcineurin-mediated nuclear factor of activated T cells (NFAT) activation, blocked chemokine production in LAD 2 but not in MLMC or BMMC. In contrast, inhibitors of nuclear factor kappa B (NF-κB) completely blocked CCL2 production in MLMC and BMMC but not in LAD 2 mast cells. Pertussis toxin and U0126, which, respectively, inhibit Gαi, extracellular signal-regulated kinase (ERK) phosphorylation substantially inhibited Pam3Cys-induced CCL2 generation in LAD 2 mast cells but had little or no effect on chemokine generation in MLMC and BMMC. These findings suggest that TLR2 activation in human LAD 2 mast cells and MLMC/BMMC promotes the release of different classes of mediators via distinct signalling pathways that depend on Ca2+ mobilization and G protein usage.
Keywords: mast cells, TLR, signal transduction, chemokine, cytokine
Introduction
Mast cells are located throughout the body in close proximity of external environment, blood vessel and nerve endings.1 The role of mast cells in immediate hypersensitivity reactions and allergic diseases is well established.2–5 Mast cells also express Toll-like receptors (TLRs) that allow them to respond to diverse microbial, viral and fungal-derived products to mediate innate immunity.1,6 To date, 13 TLR family members have been identified in the human and murine genome and mast cells express TLR1, -2, -3, -4, -6, -7 and -9 but not -5.7–12 TLR2 is critical for the propagation of inflammatory response to components of Gram-positive, Gram-negative bacteria, mycobacteria, yeast and protozoa.13–19 TLR2 are predominantly expressed in cells involved in first line of host defence including mast cells, neutrophils, dendritic cells and macrophages. Mice deficient in TLR2 have been used to demonstrate an important role of this receptor in mast cell-dependent-innate immunity.20
One feature that distinguishes mast cells from other immune cells is the marked phenotypic and functional heterogeneity that exists between cell types obtained from different tissues and species.21–23 Thus, TLR2 agonists induce degranulation in human cord blood-derived mast cells (CMBC)7,8,10 but not in murine skin or bone-marrow derived mast cells (BMMCs).9,20,24 In contrast, TLR2 ligands induce chemokine/cytokine production in all mast cell types tested.7,8,10,24–26 The molecular basis for the differences in TLR2 function in humans and murine mast cells, however, remains unknown.
It is generally accepted that all TLRs activate a common signalling pathway which culminates in the activation of nuclear factor kappa B (NF-κB).27,28 Fan et al.29,30 recently showed that while TLR2-mediated tumour necrosis factor-α production in splenocytes does not require Gαi2, this G protein is essential for cytokine generation in macrophages. TLR activation also promote other signalling pathways such as mitogen activated protein kinases (extracellular signal-regulated kinase (ERK), p38 and c-Jun NH2-terminal kinase) and phosphatidylinositol-3 kinase (PI3K) to induce the expression of proinflammatory cytokines.31 Nuclear factor of activated T cells (NFAT) is a family of transcription factors expressed in a wide variety of cell types.32–35 Ca2+ mobilization in response to FcεRI cross-linking36,37 G-protein coupled receptor activation38 nerve growth factor activation39 in mast cells leads to NFAT activation and chemokine gene expression. Waters et al.40 recently showed that TLR2 agonist tripalmitoyl-S-glycero-Cys-(Lys)4 (Pam3Cys), which induces Ca2+ mobilization in human airway epithelial cells, requires NFAT but not NF-κB for cytokine production. The possibility that TLR could utilize different signalling pathways to induce chemokine generation in different mast cell types has not been tested.
This study was undertaken to better understand the signalling pathway via which TLR2 agonist tripalmitoyl-S-glycero-Cys-(Lys)4 (Pam3Cys) activates human and murine mast cells. For this study, we utilized a human mast cell line LAD 2, murine lung mast cell (MLMC) and BMMC. Here, we demonstrate the novel finding that Pam3Cys activates human LAD 2 and murine mast cells to promote the release of different classes of mediators via distinct signalling pathways that depend on Ca2+ mobilization and G protein usage.
Materials and methods
Materials
Pam3Cys was obtained from Invivogen (San Diego, CA). Recombinant human stem cell factor (rhSCF), recombinant murine stem cell factor (rmSCF) and interleukin-3 (rmIL-3) were purchased from Peprotech (Rocky Hill, NJ). DNP-BSA (2, 4-ninitrophenyl hapten conjugated to bovine serum albumin) was purchased from Biosearch Technologies (Novato, CA). Anti-DNP-immunoglobulin E (IgE) was a generous gift from Dr Juan Rivera (NIH). Reagents for enzyme-linked immunosorbent assay (ELISA) kits were purchased from R & D Systems (Minneapolis, MN). Phosphoplus p44/42 mitogen-activated protein kinase (ERK; Thr202/Tyr204) and Phosphoplus Akt (Ser 473) kits were from Cell Signalling (Beverly, MA). All tissue culture reagents and pertussis toxin (PTX) were purchased from Invitrogen (Gaithersburg, MD). Indo-1 and pluronic F-127 were from Molecular Probes (Eugene, OR). Supersignal Western blotting analysis kits were purchased from Pierce (Rockford, IL). LY294002, U0126, pyrrolidine dithiocarbamate (PDTC) (E)3-[(4-methylphenyl)sulphonyl]-2-propenenitrile (Bay 11-7082) and cyclosporin A (CsA) were from Calbiochem (La Jolla, CA).
Cell culture
Human LAD 2 mast cells was isolated from the bone marrow of a patient with mast cell leukemia.41 These cells were cultured in serum-free media StemPro-34 (Invitrogen) supplemented with l-glutamine (2 mm), penicillin (100 IU/ml), streptomycin (100 µg/ml) and rhSCF (100 ng/ml). Hemidepletions were performed weekly with media containing rhSCF (100 ng/ml).41,42 MLMC were cultured as described by Zhong et al.43 Briefly, lung mast cells were cultured from the upper airways of C57BL6 mice by cutting the tissue into ∼3-mm3 pieces, which were then incubated in Dulbecco’s modified Eagle’s minimal essential medium containing 10% fetal bovine serum, glutamine (2 mm), HEPES (10 mm), penicillin (100 units/ml), streptomycin (100 µg/ml), rmSCF (10 ng/ml) and rmIL-3 (10 ng/ml). Culture medium was changed every other day for the first week. Non-adherent cells were collected and passaged in the same manner for additional 2–3 weeks. Cells that had been in culture for 4–8 weeks were used. Murine BMMC were obtained by flushing bone marrow cells from the femurs of C57BL6 mice, then culturing for 4–6 weeks in Iscove’s medium supplemented with 10% fetal calf serum, glutamine (2 mm), α-monothioglycerol (150 µm), penicillin (100 units/ml), streptomycin (100 µg/ml), and rmIL-3 (10 ng/ml) and rmSCF (10 ng/ml). The homogeneity of the mast cells was confirmed by acid toluidine blue staining.43 More than 95% pure MLMC and BMMC population was used for these studies.
Calcium measurement
Ca2+ mobilization was determined as described previously.38,44 Briefly, cells (0·25 × 106 for LAD 2 or 1 × 106 for MLMC and BMMC) were loaded with 1 µm indo-1 AM in the presence of 1 µm pluronic F-127 for 30 min at room temperature. Cells were washed and resuspended in 1·5 ml of HEPES-buffered saline. Ca2+ mobilization was measured in a Hitachi F-2500 spectrophotometer with an excitation wavelength of 355 nm and an emission wavelength of 410 nm.38
Assay of degranulation
Mast cells (5 × 103 for LAD 2 or 2 × 104 for MLMC and BMMC) were seeded into 96-well plates in a total volume of 50 µl of buffer containing 0·1% BSA and exposed to different concentrations of Pam3Cys or DNP-BSA for 30 min. For total β-hexosaminidase release, control cells were lysed in 50 µl of 0·1% Triton-X-100. Aliquots (15 µl) of supernatants or cell lysates were incubated with 15 µl of 1 mmp-nitrophenyl-N-acetyl-β-d-glucosamine for 1·5 hr at 37°. Reaction was stopped by adding 250 µl of a 0·1 m Na2CO3/0·1 m NaHCO3 buffer and absorbance was measured at 405 nm.44
Gel electrophoresis and immunoblotting
Mast cells (0·5 × 106) were stimulated with Pam3Cys (10 µg/ml) for 0, 1, 3, 5, 10 or 30 min and were lysed in sample buffer. Proteins were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and Western blotting was performed with anti-phospho-ERK and anti-phospho-Akt antibody. Blot was stripped and reprobed with anti-ERK and anti-Akt antibody. Immunoreactive bands were visualized by SuperSignal West Femto maximum sensitivity substrate (Pierce).
Assay of CCL2 and IL-6 production
LAD 2 mast cells, MLMC or BMMC (0·2 × 106 per well in 125 µl basal medium) were stimulated with Pam3Cys (30 µg/ml for LAD 2 cells, 1 µg/ml for MLMC and BMMC) for 6 hr. For inhibitor studies, cells were preincubated with PTX (100 ng/ml, 18 hr), U0126 (1 µm, 30 min), LY294002 (20 µm, 30 min), PDTC (100 µm; 1 hr), Bay 117082 (5 µm, 1 hr) or CsA (100 nm; 1 hr) and then stimulated with Pam3Cys for 6 hr. Supernatants were collected and stored frozen at −80°, until analysis. Human CCL2 or JE (murine chemokine equivalent to human CCL2) and interleukin-6 (IL-6) were quantified using a sandwich ELISA kit as described in the manufacturer’s protocols. The reaction was read at 450 nm in a micro plate reader (Molecular Devices).
Results
TLR2 agonist Pam3Cys-induced degranulation in LAD 2 mast cells requires Ca2+ mobilization and heterotrimeric G protein activation
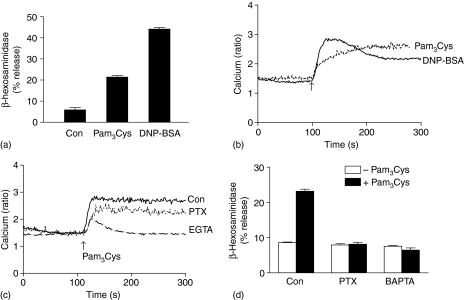
TLR2 is expressed in human CD34+-derived mast cells and cord blood derived mast cells (CBMC). A synthetic TLR2 agonist Pam3Cys induces degranulation in human CBMC.8 LAD 2 is a relatively new and highly differentiated human mast cell line, which we have recently used as a model to study G protein coupled receptor signalling.42,45 Because LAD 2 cells endogenously express TLR210 we first sought to determine whether Pam3Cys causes degranulation in this cell line. We found that, as in CBMCs8 Pam3Cys caused ∼15% degranulation in LAD 2 mast cells (Fig. 1a). This response was two- to threefold less than that induced by antigen (DNP-BSA) (Fig. 1a). Previously, it has been shown that another TLR2 agonist peptidoglycan (PGN) also induces approximately twofold degranulation in CBMCs.8 Similarly, we found that PGN (50 µg) induces ∼2·4-fold degranulation in LAD 2 mast cells (6·4 ± 1·0%β-hexasaminidase release in the presence of PGN compared to 2·6 ± 0·8% in its absence). Given that Ca2+ mobilization is required for mast cell degranulation46 we tested effects of Pam3Cys on the kinetics of Ca2+ response in mast cells. As shown in Fig. 1(b), Pam3Cys induced a slow but sustained Ca2+ mobilization. In contrast, antigen induced a less sustained Ca2+ response.
Figure 1.
Role of G protein signalling on Pam3Cys-induced degranulation and Ca2+ mobilization in human LAD 2 mast cells. (a) Cells were exposed to buffer (Con), Pam3Cys (30 µg/ml) or DNP-BSA (20 ng/ml) for 30 min β-hexosaminidase release was determined. (b) Cells were loaded with Indo-1AM and stimulated with Pam3Cys (1 µg/ml) or DNP-BSA (20 ng/ml) and intracellular Ca2+ was measured. (c) Cells cultured in the absence or presence of PTX (100 ng/ml, 16 hr), loaded with Indo-1AM and Pam3Cys (10 µg/ml)-induced intracellular calcium mobilization. For experiments in the absence of Ca2+, indo-1 loaded cells were exposed to EGTA (4 mm, 3 min) before stimulation with Pam3Cys. (d) Cells were pretreated with or without PTX (100 ng/ml, 16 hr) or BAPTA (100 µm, 30 min), exposed to with buffer (Con) or Pam3Cys (30 µg/ml) for 30 min and β-hexosaminidase release was determined. Data shown are representative of three similar experiments.
Fan et al.29,30 showed that TLR2 activates G-protein dependent signalling pathway in murine macrophages but not in splenocytes. To test the role of G protein signalling on Pam3Cys-induced responses, LAD 2 mast cells were pretreated with pertussis toxin (PTX, 100 ng/ml) and Ca2+ mobilization and degranulation were determined. PTX partially inhibited Pam3Cys-induced Ca2+ mobilization (Fig. 1c) but completely blocked degranulation (Fig. 1d). We found that in the absence of extracellular Ca2+, Pam3Cys-induced Ca2+ mobilization was substantially reduced. (Fig. 1c). Furthermore, chelation of intracellular Ca2+ with (1,2-bis 2-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid-acetoxymethyl ester (BAPTA) completely blocked Pam3Cys-induced degranulation (Fig. 1d). These findings suggest that Pam3Cys-induced degranulation in LAD 2 mast cells requires both Gαi-independent and Gαi-dependent signalling pathways.
Pam3Cys-induced chemokine production in human mast cells requires Ca2+-mediated NFAT and Gαi-mediated ERK and PI3 kinase activation
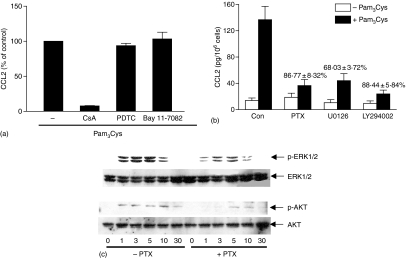
It is generally accepted that TLRs induce cytokine/chemokine gene expression via the activation of NF-κB. In contrast, Waters et al.40 recently demonstrated that Pam3Cys causes Ca2+ mobilization, NFAT activation and cytokine production in human airway epithelial cells. Furthermore, CsA, an inhibitor of NFAT activation blocked Pam3Cys-induced cytokine production but NF-κB inhibitor had no effect. Because Pam3Cys induces Ca2+ mobilization in LAD 2 cells, we hypothesized that chemokine production in this cell line may be mediated via NFAT activation. To test this possibility, we determined the effect of CsA on Pam3Cys-induced CCL2 generation in LAD 2. As shown in Fig. 2(a), CsA almost completely inhibited Pam3Cys-induced CCL2 generation. In contrast, PDTC or Bay 11-7082 (NF-κB inhibitors) had no effect on Pam3Cys response. These findings suggest that Pam3Cys causes chemokine production in LAD 2 mast cells via Ca2+-mediated NFAT but not NF-κB activation.
Figure 2.
Pam3Cys-induced CCL2 production in human LAD 2 mast cells requires Ca2+-mediated NFAT and Gαi-mediated ERK and PI3 kinase activation (a) Cells were pretreated with vehicle or cyclosporine A (CsA, 100 nm; 1 hr), PDTC (100 µm, 1 hr) or Bay 11-7082 (5 µm; 1 hr) and exposed to buffer or Pam3Cys (30 µg/ml) for 6 hr and chemokine/cytokine production was determined by ELISA. Data are expressed as percent stimulated with Pam3Cys in the absence of inhibitor (% of control). Data shown is mean ± SEM of three experiments performed in triplicate. (b) Cells were pretreated with vehicle or PTX (100 ng/ml, 16 hr), U0126 (1 µm, 30 min) or LY294002 (20 µm, 30 min) and exposed to buffer (Con) or Pam3Cys (30 µg/ml) for 6 hr and CCL2 production was determined by ELISA. Data shown are mean ± SEM of three experiments performed in triplicate. P < 0·05 in the absence or presence of inhibitors. (c) Cells pretreated with vehicle (–PTX) or PTX (+PTX; 100 ng/ml, 16 hr) were stimulated with Pam3Cys (10 µg/ml) for 0, 1, 3, 5, 10 and 30 min. Cell lysates were separated on SDS–PAGE and Western blotting was performed with anti-phospho-ERK1/2 and anti-phospho-Akt antibody. Blot was stripped and reprobed with anti-ERK and anti-Akt antibody followed by anti-rabbit-IgG-horseradish peroxidase. Immunoreactive bands were visualized by SuperSignal West Femto maximum sensitivity substrate. Data shown are representative of three similar experiments.
To determine whether Gαi-mediated signalling pathway interacts with Gαi independent Ca2+ mobilization, we tested the effect of PTX on Pam3Cys-induced CCL2 generation. As shown in Fig. 2(b), PTX caused substantial inhibition of Pam3Cys-induced CCL2 generation. Pam3Cys caused robust ERK and Akt phosphorylation in LAD 2 mast cells (Fig. 2c). Furthermore, PTX substantially inhibited both ERK and Akt phosphorylation (Fig. 2c). In addition, inhibitors of ERK (U0126) and PI3 kinase (LY294002) blocked Pam3Cys-induced CCL2 production by (68·03 ± 3%) and LY294002 (88·4 ± 5%), respectively (Fig. 2b). These findings demonstrate that Pam3Cys-induced CCL2 production in LAD 2 mast cells requires both Ca2+ mobilization and Gαi-mediated ERK and PI3 kinase activation.
Pam3Cys does not induce Ca2+ mobilization or degranulation but induces chemokine and cytokine production in murine mast cells via NF-κB activation
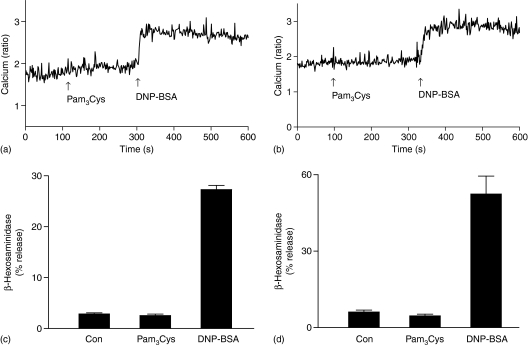
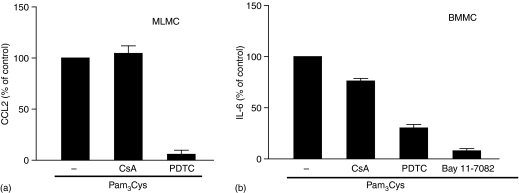
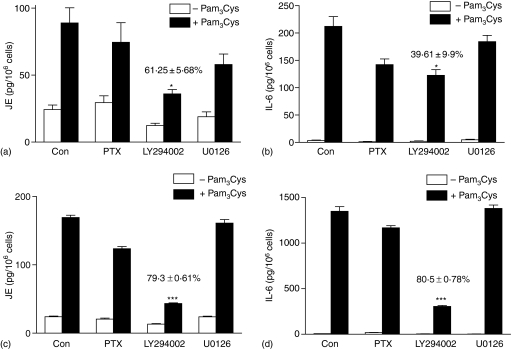
While TLR2 activation leads to degranulation in human CBMC8 and LAD 2 mast cells (Fig. 1a), it has no effect on degranulation in murine BMMC and fetal-skin derived mast cells.9,24 All murine mast cells are, however, highly responsive to TLR2 agonists for cytokine generation. MLMC display some properties that are distinct from BMMC. Thus, activation of MLMC via adenosine A3 receptors leads to degranulation whereas the same receptor expressed in BMMC does not induce degranulation.43,47 We therefore sought to determine the effects of TLR2 activation on mediator release and signalling pathways in MLMC and BMMC. As shown in Fig. 3, Pam3Cys did not cause Ca2+ mobilization or degranulation in MLMC and BMMC. However, these responses were induced by Ag in both MLMC and BMMC. In contrast, Pam3Cys induced chemokine, JE as well as IL-6 in murine mast cells (Figs 4 and 5). There were, however, important differences in the mechanism by which Pam3Cys induced chemokine/cytokine production in MLMC/BMMC when compared to human LAD 2 mast cells that reflects differences in Ca2+ mobilization and G protein coupling. Thus, in contrast to LAD 2 cells (Fig. 2a), CsA did not inhibit either JE (Fig. 4a) or IL-6 (Fig. 4b) in murine mast cells. Furthermore, inhibitors of NF-κB (PDTC and Bay 11-7082), which had no effect on chemokine generation in LAD 2 cells, almost completely inhibited both JE and IL-6 in murine mast cells (Fig. 4). Unlike the situation in LAD 2 cells, PTX and U0126 had no effect on JE or IL-6 induction in murine mast cells (Fig. 5). Although LY294002 blocked Pam3Cys-induced responses, there were differences in the magnitude of inhibition between MLMC and BMMC (Fig. 5).
Figure 3.
Pam3Cys does not induce Ca2+ mobilization or degranulation in MLMC and BMMC. (a) MLMC or (b) BMMC were loaded with Indo-1AM, stimulated with Pam3Cys (10 µg/ml) or DNP-BSA (20 ng/ml) and intracellular calcium was measured. (c) MLMC and (d) BMMC were stimulated with Pam3Cys (10 µg/ml) or DNP-BSA (20 ng/ml) for 30 min and β-hexosaminidase release was determined. Data shown are representative of three similar experiments.
Figure 4.
Pam3Cys induces JE and IL-6 production in murine mast cells via NF-κB activation. (a) MLMC or (b) BMMC were pretreated with vehicle or CsA (100 nm; 1 hr), PDTC (100 µm; 1 hr) or Bay 11-7082 (5 µm; 1 hr) and exposed to buffer or Pam3Cys (1 µg/ml) for 6 h and chemokine/cytokine production was determined by ELISA. Data are expressed as percent stimulated with Pam3Cys in the absence of inhibitor (% of control). Data shown is mean ± SEM of three experiments performed in triplicate.
Figure 5.
Pam3Cys-induced JE and IL-6 production in MLMC and BMMC require PI3 kinase but not G protein, ERK activation. (a and b) MLMC and (c and d) BMMC were pretreated with vehicle (Con) or PTX (100 ng/ml, 16 hr), LY294002 (20 µm, 30 min) or U0126 (1 µm, 30 min) and exposed to buffer (Con) or Pam3Cys (1 µg/ml) for 6 h and (a and c) JE as well as (b and d) IL-6 production was determined by ELISA. Data shown are mean ± SEM of two experiments performed in triplicate. *P < 0·05 or ***P < 0·0001 in the absence or presence of inhibitors.
Discussion
Mast cells are multifunctional immune cells that play important roles in host defence1,6 but also mediate allergic and inflammatory diseases.2–5 One feature that distinguishes mast cells from other immune cells is the marked phenotypic and functional heterogeneity that exists between cell types obtained from different tissues and species.21–23 Recent studies demonstrated important differences in the ability of TLR2 ligands to induce the release of early and late mediators in human and murine mast cells.8–10,24 Thus, TLR2 ligands induce degranulation in human CBMCs8 but not in murine BMMCs, fetal skin mast cells or a murine mast cell line, MC/9.9,24,48 In contrast, TLR2 activation promotes cytokine generation in all mast cell types tested. The molecular basis for the differences in TLR2 function in human and murine mast cells, however, remains unknown. In the present study, we utilized a human mast cell line LAD 2, murine lung as well as BMMC and demonstrated the novel findings that Pam3Cys induces the release of different classes of mediators with a specificity that depends on Ca2+ mobilization and G protein usage.
An important finding of the present study was that Pam3Cys induced degranulation in LAD 2 mast cells equal in magnitude to that previously reported for human CBMCs.8 We also showed that Pam3Cys caused a sustained Ca2+ mobilization in LAD 2 mast cells and that this response was dependent on the presence of extracellular Ca2+. Furthermore, chelation of Ca2+ with BAPTA completely inhibited Pam3Cys-induced degranulation. Consistent with previous reports9,24 we failed to detect any degranulation by Pam3Cys in murine lung or BMMC. Furthermore, unlike the situation in LAD 2 mast cells, Pam3Cys did not induce Ca2+ mobilization in murine lung or BMMCs. These findings suggest that susceptibility of human CBMC8 and LAD 2 mast cells to undergo degranulation in response to Pam3Cys and the resistance of murine mast cells reflect differences in Ca2+ mobilization.
Interestingly, despite the differences in Pam3Cys-induced Ca2+ mobilization and degranulation in human LAD 2 and murine mast cells, it caused chemokine CCL2 production in all mast cell types tested. While NF-κB is the major transcription factor that mediates TLR2-induced cytokine production in a variety of cell types, a recent report indicated that Ca2+-induced phosphatase calcineurin activates NFAT, but not NF-κB, to induce cytokine production in human lung epithelial cells.40 Qiao et al.24 have also shown that FcεRI-mediated cytokine production in BMMC and MC/9 cells requires Ca2+ mobilization and NFAT activation. These findings raise the interesting possibility that Pam3Cys induces chemokine in different mast cell populations via the utilization of NFAT or NF-κB depending on its ability to induce Ca2+ mobilization. Indeed, we found that CsA, an inhibitor of calcineurin-mediated NFAT activation, blocked Pam3Cys-induced CCL2 production in human LAD 2 mast cells but had no effect on murine mast cells. In contrast, inhibitors of NF-κB completely inhibited CCL2 as well as IL-6 production in murine but not in LAD 2 mast cells. Another important difference was that while Pam3Cys-induced chemokine generation in LAD 2 mast cells required Gαi-mediated ERK phosphorylation, these signalling pathways were not required for chemokine/cytokine generation in MLMC or BMMC. These findings suggest that Pam3Cys causes chemokine production in human LAD 2 and MLMC/BMMC via distinct signalling pathways that depend on Ca2+ mobilization and G protein usage.
The reason for the differences in Pam3Cys-induced chemokine production in human LAD 2 and murine mast cells is not clear. LAD 2 cells are highly differentiated connective tissue type mast cells whereas murine mast cells tested in this study are immature mucosal mast cells. It is therefore possible that this phenotypic variation could explain the observed differences in mechanism of TLR2 signalling. Matsushima et al.9 recently compared the properties of murine skin (connective tissue) and BMMC (mucosal) with respect to TLR function. They found no difference in TLR2 signalling in skin mast cells and BMMCs. However, skin mast cells express TLR3, TLR7 and TLR9 that are not present in BMMCs. These findings suggest that phenotypic variation in mast cells regulate expression profile of different TLRs but may not explain the differences in TLR2 signalling observed in the present study. In general, it is extremely difficult to extrapolate data from one mast cell type to another. Therefore, the further experiments are required to delineate the relevance of the studies described herein with mast cell line.
TLR2 is not the only TLR that displays differences between human and murine mast cells. Thus, human CBMCs do not response to TLR4 ligand unless the cells are primed with interleukin-4.7 In contrast, murine BMMC are fully responsive to the endotoxins in the absence of priming.9,49,50 We confirmed that while lipopolysaccharide stimulates the cytokine production in murine mast cells but not in LAD 2 mast cells (data not shown). This raises the interesting possibility that the observed differences in human LAD 2 and murine mast cells reflect species-specific differences in TLR2 signalling. Future studies with human mast cells from different locations are needed to confirm the validity of this contention.
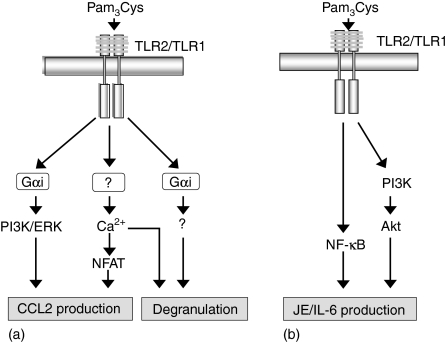
In summary, we have used a human mast cell line LAD 2, MLMC and BMMC to delineate the molecular basis for the previously described differences in TLR2 signalling in human and murine mast cells.8,9,24 We propose that the ability of Pam3Cys to induce degranulation in LAD 2 mast cells, MLMC and BMMC reflects difference in Ca2+ mobilization and Gαi activation. We have shown that Pam3Cys-induced Ca2+ mobilization synergizes with Gαi-mediated ERK and Akt phosphorylation to induce chemokine generation in LAD 2 mast cells. In contrast, Pam3Cys, which does not induce Ca2+ mobilization in MLMC or BMMC, causes chemokine production via a different mechanism involving NF-κB and Akt phosphorylation (see Model Fig. 6).
Figure 6.
Schematic representation of the signalling pathways involved in Pam3Cys-induced degranulation and cytokine/chemokine production in human LAD 2, MLMC and BMMC. We propose that the ability of Pam3Cys to induce degranulation in (a) LAD 2 mast cells, (b) MLMC and BMMC reflects differences in Ca2+ mobilization and Gαi activation. Pam3Cys-induced CCL2 production in (a) human LAD 2 mast cells requires Ca2+-mediated NFAT and Gαi-mediated ERK and PI3 kinase activation. However, in (b) MLMC and BMMC Pam3Cys does not induce Ca2+ mobilization therefore JE production is mediated via NF-κB activation. PI3 kinase pathway is also required for JE production in MLMC and BMMC. (→ stimulation).
Acknowledgments
We are grateful to Drs Arnold Kirshenbaum and Dean Metcalfe (NIAID/NIH) for human LAD 2 mast cell line. We also thank Dr Juan Rivera (NIAMS/NIH) for generous gift of Anti-DNP-IgE. This work was supported by National Institutes of Health Grant; 1RO1-HL63372 and a grant from the University of Pennsylvania Research Foundation.
Abbreviations
- BMMC
murine bone marrow derived mast cells
- CCL2
chemokine CC ligand 2
- ERK
extracellular-regulated kinase
- GPCR
G protein coupled receptor
- LAD 2
Laboratory of allergic diseases 2
- MLMC
murine lung mast cells
- NFAT
nuclear factor of activated T cells
- PTX
pertussis toxin
- TLR
Toll-like receptor
References
- 1.Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4:787–99. doi: 10.1038/nri1460. [DOI] [PubMed] [Google Scholar]
- 2.Rottem M, Mekori YA. Mast cells and autoimmunity. Autoimmun Rev. 2005;4:21–7. doi: 10.1016/j.autrev.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Puxeddu I, Piliponsky AM, Bachelet I, Levi-Schaffer F. Mast cells in allergy and beyond. Int J Biochem Cell Biol. 2003;35:1601–7. doi: 10.1016/s1357-2725(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 4.Tsai M, Grimbaldeston MA, Yu M, Tam SY, Galli SJ. Using mast cell knock-in mice to analyze the roles of mast cells in allergic responses in vivo. Chem Immunol Allergy. 2005;87:179–97. doi: 10.1159/000087644. [DOI] [PubMed] [Google Scholar]
- 5.Moller A, Grabbe J, Czarnetzki BM. Mast cells and their mediators in immediate and delayed immune reactions. Skin Pharmacol. 1991;4(Suppl. 1):56–63. doi: 10.1159/000210983. [DOI] [PubMed] [Google Scholar]
- 6.Marshall JS, Jawdat DM. Mast cells in innate immunity. J Allergy Clin Immunol. 2004;114:21–7. doi: 10.1016/j.jaci.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 7.Varadaradjalou S, Feger F, Thieblemont N, Hamouda NB, Pleau JM, Dy M, Arock M. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human mast cells. Eur J Immunol. 2003;33:899–906. doi: 10.1002/eji.200323830. [DOI] [PubMed] [Google Scholar]
- 8.McCurdy JD, Olynych TJ, Maher LH, Marshall JS. Cutting edge: distinct Toll-like receptor 2 activators selectively induce different classes of mediator production from human mast cells. J Immunol. 2003;170:1625–9. doi: 10.4049/jimmunol.170.4.1625. [DOI] [PubMed] [Google Scholar]
- 9.Matsushima H, Yamada N, Matsue H, Shimada S. TLR3-, TLR7-, and TLR9-mediated production of proinflammatory cytokines and chemokines from murine connective tissue type skin-derived mast cells but not from bone marrow-derived mast cells. J Immunol. 2004;173:531–41. doi: 10.4049/jimmunol.173.1.531. [DOI] [PubMed] [Google Scholar]
- 10.Kulka M, Alexopoulou L, Flavell RA, Metcalfe DD. Activation of mast cells by double-stranded RNA. evidence for activation through Toll-like receptor 3. J Allergy Clin Immunol. 2004;114:174–82. doi: 10.1016/j.jaci.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 11.Akira S, Yamamoto M, Takeda K. Role of adapters in Toll-like receptor signalling. Biochem Soc Trans. 2003;31:637–42. doi: 10.1042/bst0310637. [DOI] [PubMed] [Google Scholar]
- 12.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–63. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 13.Underhill DM, Ozinsky A, Hajjar AM, Stevens A, Wilson CB, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–5. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 14.Underhill DM, Ozinsky A, Smith KD, Aderem A. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci USA. 1999;96:14459–63. doi: 10.1073/pnas.96.25.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–51. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 16.Meier A, Kirschning CJ, Nikolaus T, Wagner H, Heesemann J, Ebel F. Toll-like receptor (TLR) 2 and TLR4 are essential for Aspergillus-induced activation of murine macrophages. Cell Microbiol. 2003;5:561–70. doi: 10.1046/j.1462-5822.2003.00301.x. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda Y, Adachi Y, Ishibashi K, Miura N, Ohno N. Activation of toll-like receptor-mediated NF-kappa beta by zymosan-derived water-soluble fraction. possible contribution of endotoxin-like substances. Immunopharmacol Immunotoxicol. 2005;27:285–98. doi: 10.1081/iph-200067943. [DOI] [PubMed] [Google Scholar]
- 18.Villamon E, Roig P, Gil ML, Gozalbo D. Toll-like receptor 2 mediates prostaglandin E (2) production in murine peritoneal macrophages and splenocytes in response to Candida albicans. Res Microbiol. 2005;156:115–8. doi: 10.1016/j.resmic.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Maldonado-Bernal C, Kirschning CJ, Rosenstein Y, et al. The innate immune response to Entamoeba histolytica lipopeptidophosphoglycan is mediated by toll-like receptors 2 and 4. Parasite Immunol. 2005;27:127–37. doi: 10.1111/j.1365-3024.2005.00754.x. [DOI] [PubMed] [Google Scholar]
- 20.Supajatura V, Ushio H, Nakao A, Akira S, Okumura K, Ra C, Ogawa H. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J Clin Invest. 2002;109:1351–9. doi: 10.1172/JCI14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oskeritzian CA, Zhao W, Min HK, Xia HZ, Pozez A, Kiev J, Schwartz LB. Surface CD88 functionally distinguishes the MCTC from the MCT type of human lung mast cell. J Allergy Clin Immunol. 2005;115:1162–8. doi: 10.1016/j.jaci.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as ‘tunable’ effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–86. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 23.Zaidi AK, Amrani Y, Panettieri RA, Ali H. Response to C3a, mast cells, and asthma. FASEB J. 2006;20:199. doi: 10.1096/fj.06-0204ufm. [DOI] [PubMed] [Google Scholar]
- 24.Qiao H, Andrade MV, Lisboa FA, Morgan K, Beaven MA. Fc{epsilon}RI and Toll-like receptors mediate synergistic signals to markedly augment production of inflammatory cytokines in murine mast cells. Blood. 2005;107:610–8. doi: 10.1182/blood-2005-06-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marshall JS, King CA, McCurdy JD. Mast cell cytokine and chemokine responses to bacterial and viral infection. Curr Pharm Des. 2003;9:11–24. doi: 10.2174/1381612033392413. [DOI] [PubMed] [Google Scholar]
- 26.Marshall JS, McCurdy JD, Olynych T. Toll-like receptor-mediated activation of mast cells: implications for allergic disease? Int Arch Allergy Immunol. 2003;132:87–97. doi: 10.1159/000073709. [DOI] [PubMed] [Google Scholar]
- 27.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 28.O’Neill LA. Signal transduction pathways activated by the IL-1 receptor/toll-like receptor superfamily. Curr Top Microbiol Immunol. 2002;270:47–61. [PubMed] [Google Scholar]
- 29.Fan H, Cook JA. Molecular mechanisms of endotoxin tolerance. J Endotoxin Res. 2004;10:71–84. doi: 10.1179/096805104225003997. [DOI] [PubMed] [Google Scholar]
- 30.Fan H, Zingarelli B, Peck OM, Teti G, Tempel GE, Halushka PV, Cook JA. Lipopolysaccharide and Gram-positive bacteria induced cellular inflammatory responses: role of heterotrimeric G{alpha}i proteins. Am J Physiol Cell Physiol. 2005;289:C293–301. doi: 10.1152/ajpcell.00394.2004. [DOI] [PubMed] [Google Scholar]
- 31.Jones BW, Heldwein KA, Means TK, Saukkonen JJ, Fenton MJ. Differential roles of Toll-like receptors in the elicitation of proinflammatory responses by macrophages. Ann Rheum Dis. 2001;60(Suppl. 3):iii6–12. doi: 10.1136/ard.60.90003.iii6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crabtree GR, Olson ENNFAT. signaling. choreographing the social lives of cells. Cell. 2002;109(Suppl.):S67–79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 33.Graef IA, Wang F, Charron F, Chen L, Neilson J, Tessier-Lavigne M, Crabtree GR. Neurotrophins and netrins require calcineurin/NFAT signaling to stimulate outgrowth of embryonic axons. Cell. 2003;113:657–70. doi: 10.1016/s0092-8674(03)00390-8. [DOI] [PubMed] [Google Scholar]
- 34.Hill-Eubanks DC, Gomez MF, Stevenson AS, Nelson MT. NFAT regulation in smooth muscle. Trends Cardiovasc Med. 2003;13:56–62. doi: 10.1016/s1050-1738(02)00212-8. [DOI] [PubMed] [Google Scholar]
- 35.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–47. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 36.Hutchinson LE, McCloskey MA. Fc epsilon RI-mediated induction of nuclear factor of activated T-cells. J Biol Chem. 1995;270:16333–8. doi: 10.1074/jbc.270.27.16333. [DOI] [PubMed] [Google Scholar]
- 37.Pandey V, Mihara S, Fensome-Green A, Bolsover S, Cockcroft S. Monomeric IgE stimulates NFAT translocation into the nucleus, a rise in cytosol Ca2+, degranulation, and membrane ruffling in the cultured rat basophilic leukemia-2H3 mast cell line. J Immunol. 2004;172:4048–58. doi: 10.4049/jimmunol.172.7.4048. [DOI] [PubMed] [Google Scholar]
- 38.Ali H, Ahamed J, Hernandez-Munain C, Baron JL, Krangel MS, Patel DD. Chemokine production by G protein-coupled receptor activation in a human mast cell line. Roles of extracellular signal-regulated kinase and NFAT. J Immunol. 2000;165:7215–23. doi: 10.4049/jimmunol.165.12.7215. [DOI] [PubMed] [Google Scholar]
- 39.Ahamed J, Venkatesha RT, Thangam EB, Ali H. C3a enhances nerve growth factor-induced NFAT activation and chemokine production in a human mast cell line, HMC-1. J Immunol. 2004;172:6961–8. doi: 10.4049/jimmunol.172.11.6961. [DOI] [PubMed] [Google Scholar]
- 40.Waters V, Sokol S, Reddy B, Soong G, Chun J, Prince A. The effect of cyclosporin A on airway cell proinflammatory signaling and pneumonia. Am J Respir Cell Mol Biol. 2005;33:138–44. doi: 10.1165/rcmb.2005-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirshenbaum AS, Akin C, Wu Y, Rottem M, Goff JP, Beaven MA, Rao VK, Metcalfe DD. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcepsilonRI or FcgammaRI. Leuk Res. 2003;27:677–82. doi: 10.1016/s0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 42.Thangam EB, Venkatesha RT, Zaidi AK, et al. Airway smooth muscle cells enhance C3a-induced mast cell degranulation following cell–cell contact. Faseb J. 2005;19:798–800. doi: 10.1096/fj.04-2797fje. [DOI] [PubMed] [Google Scholar]
- 43.Zhong H, Shlykov SG, Molina JG, Sanborn BM, Jacobson MA, Tilley SL, Blackburn MR. Activation of murine lung mast cells by the adenosine A3 receptor. J Immunol. 2003;171:338–45. doi: 10.4049/jimmunol.171.1.338. [DOI] [PubMed] [Google Scholar]
- 44.Ali H, Richardson RM, Tomhave ED, Didsbury JR, Snyderman R. Differences in phosphorylation of formylpeptide and C5a chemoattractant receptors correlate with differences in desensitization. J Biol Chem. 1993;268:24247–54. [PubMed] [Google Scholar]
- 45.Venkatesha RT, Ahamed J, Nuesch C, Zaidi AK, Ali H. Platelet-activating factor-induced chemokine gene expression requires NF-kappaB activation and Ca2+/calcineurin signaling pathways. Inhibition by receptor phosphorylation and beta-arrestin recruitment. J Biol Chem. 2004;279:44606–12. doi: 10.1074/jbc.M408035200. [DOI] [PubMed] [Google Scholar]
- 46.Maeyama K, Hohman RJ, Metzger H, Beaven MA. Quantitative relationships between aggregation of IgE receptors, generation of intracellular signals, and histamine secretion in rat basophilic leukemia (2H3) cells. Enhanced responses with heavy water. J Biol Chem. 1986;261:2583–92. [PubMed] [Google Scholar]
- 47.Marquardt DL, Walker LL. Alteration of mast cell responsiveness to adenosine by pertussis toxin. Biochem Pharmacol. 1988;37:4019–25. doi: 10.1016/0006-2952(88)90088-3. [DOI] [PubMed] [Google Scholar]
- 48.Ikeda T, Funaba M. Altered function of murine mast cells in response to lipopolysaccharide and peptidoglycan. Immunol Lett. 2003;88:21–6. doi: 10.1016/s0165-2478(03)00031-2. [DOI] [PubMed] [Google Scholar]
- 49.McCurdy JD, Lin TJ, Marshall JS. Toll-like receptor 4-mediated activation of murine mast cells. J Leukoc Biol. 2001;70:977–84. [PubMed] [Google Scholar]
- 50.Supajatura V, Ushio H, Nakao A, Okumura K, Ra C, Ogawa H. Protective roles of mast cells against enterobacterial infection are mediated by Toll-like receptor 4. J Immunol. 2001;167:2250–6. doi: 10.4049/jimmunol.167.4.2250. [DOI] [PubMed] [Google Scholar]