Abstract
The protective immune response against Trypanosoma cruzi is improved by treatment with the natural killer (NK) T-cell glycolipid antigen α-galactosylceramide (α-GalCer). A single α-GalCer treatment of mice before T. cruzi infection decreases parasitaemia and prolongs survival. This protection is dependent on CD1d-restricted NKT cells and interferon-γ (IFN-γ) suggesting that α-GalCer-activated NKT cells produce IFN-γ, which stimulates the cells of the innate and adaptive immune responses to provide protection. To learn which cells provide protection we investigate here α-GalCer treatment of mice deficient in different immune cells. Surprisingly, although NK cells provide protection against T. cruzi, and are a major source of IFN-γ following α-GalCer treatment, NK cells are not required for the α-GalCer-induced protection. The α-GalCer treatment of NK-cell-depleted mice controlled parasitaemia and prevented death. In contrast, phagocytes, helper T cells and cytotoxic T cells are required. Furthermore, α-GalCer treatment of MHC II–/– or CD8α–/– mice exacerbated the infection, demonstrating that α-GalCer treatment induces some responses that favour the parasite. In summary α-GalCer protection against T. cruzi required multiple cellular responses, but not the response of NK cells. These results provide useful information because α-GalCer is being developed as therapy for infections, autoimmune diseases, allergy and cancers.
Keywords: cellular activation, natural killer cells, natural killer T cells, parasite: protozoan, T lymphocytes
Introduction
Trypanosoma cruzi is a protozoan parasite that chronically infects many mammalian species. During the acute infection T. cruzi disseminates in the mammalian host and a patent parasitaemia occurs.1 The natural acute infection is rarely lethal, and once infected individuals remain so for their lifetime. Thirty per cent of people infected develop Chagas’ disease, a chronic inflammatory disease that causes significant morbidity and mortality.1 The pathogenesis of Chagas’ disease remains unclear, but previous studies argue that the severity of the chronic disease correlates with both the parasite burden and the regulation of the anti-T. cruzi immune response.2,3 Safe and effective treatments for acute and chronic T. cruzi infection are lacking. Treatments that can lower parasite burden or improve regulation of the anti-parasite immune response are needed.
Natural killer T (NKT) cells are a distinct subset of T cells that rapidly secrete large amounts of cytokines when activated, and are known to regulate immune responses of infections, cancers and autoimmune diseases.4 In contrast with conventional T cells, NKT cells are stimulated by glycolipid antigens presented by the major histocompatibility complex (MHC) class I-like CD1d molecular complex.4 Most NKT cells express an invariant T-cell receptor α-chain and are referred to as invariant NKT (iNKT) cells. α-galactosylceramide (α-GalCer) is a glycolipid antigen of iNKT cells. Treatment of mice with α-GalCer causes iNKT cells to rapidly secrete large amounts of interferon-γ (IFN-γ) and interleukin-4 (IL-4), and results in the downstream activation of NK cells, macrophages and lymphocytes.5–10 Treatment of mice with NKT-cell-specific glycolipid antigens can improve the outcome of cancers, autoimmune diseases and infections. The safety of NKT-cell-specific glycolipid antigen treatment is currently being investigated in human clinical trials (reviewed in refs 11,12).
To investigate the pathogenesis of Chagas’ disease we used the T. cruzi CL strain, a strain that infects humans and mice and that permits the examination of both the acute and chronic stages of the infection. We have previously reported that treatment of mice before T. cruzi infection with α-GalCer provides protection that is dependent on iNKT cells and IFN-γ.13 Other investigators have also demonstrated that α-GalCer treatment provides protection against more virulent T. cruzi strains.14 To determine which cells contribute to α-GalCer-induced T. cruzi protection we have investigated α-GalCer treatment of mice deficient in cells known to be activated downstream of iNKT cells, i.e. NK cells, phagocytes and lymphocytes. These investigations demonstrate that α-GalCer protection requires phagocytes and lymphocytes but, surprisingly, not NK cells.
Materials and methods
Mice
Age-matched and weight-matched female mice were used in each experiment. Wild-type C57BL/6 mice were obtained from Charles River (Wilmington, MA). MHC II–/– (I-Aβ–/–), CD8α–/– (Cd8atm1Mak) and perforin–/– (Pfptm1Sdz) mice, all on the C57BL/6 background, were obtained from Jackson Laboratory (Bar Harbor, ME). The Institutional Animal Care and Use Committee approved all of the animal procedures.
Trypanosoma cruzi and parasitaemia determination
A recently derived clone of the CL strain subclone 3 was used.15 Trypomastigotes were obtained from culture supernatants of infected 3T3 cells grown in Dulbeccos’ modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated calf serum and 50 000 U penicillin/streptomycin (all from BioWhittaker, Walkersville, MD). Mice were infected by intraperitoneal (i.p.) injection with T. cruzi trypomastigotes at the stated inoculum. Parasitaemia was monitored by venesection of the tail; 2 μl blood was diluted in 1·66% ammonium chloride in phosphate-buffered saline (PBS) and the trypomastigotes were counted on a haemocytometer by an investigator unaware of experimental status.
α-GalCer administration and mouse monitoring
Mice were injected i.p. with 5 μg α-GalCer (supplied by Kirin Brewery Ltd, Gunma, Japan) 1 day before T. cruzi infection. α-GalCer was diluted in DMEM (BioWhittaker) immediately before administration. Mice were weighed just before α-GalCer or diluent treatment and again 1 day later, before T. cruzi infection. For analyses, the per cent weight change of each individual mouse was calculated, and from individual per cent changes, the mean and SE per cent weight change of each group of mice were calculated. Serum glutamic pyruvic transaminase (GPT) activity was determined in blood (collected from individual mice by venesection of the tail) using a colorimetric test according to the manufacturers’ instructions (GP-transaminase; Sigma, St Louis, MO).
Mouse treatments
NK cells were depleted by i.p. injection of 80 μg anti-asialo GM1 antibody [40 μl 2 mg/ml antibody in PBS (Wako Chemicals, Richmond, VA)]. Depletion of NK cells was confirmed by analyses of liver and spleen mononuclear cells stained with anti-NK1.1 phycoerythrin-conjugated monoclonal antobody (mAb) and anti-pan T-cell receptor β-chain Cy-Chrome-conjugated mAb (both from PharMingen, San Diego, CA) followed by flow cytometry as previously described.16 Phagocyte function was inhibited by i.p. injection of 20 mg silica [200 μl 100 mg/ml in PBS (Sigma)] 2 days and 1 day before T. cruzi infection, and again on day 7 of infection.
Statistics
The P-values for cumulative parasitaemia and weight change were determined using Student’s t-test (Microsoft Excel, Microsoft Corporation, Redmond, WA). The P-values for survival were calculated by determining the log rank statistic using Kaplan–Meier survival analysis (SPSS Inc., Chicago, IL).
Results
During acute T. cruzi infection α-GalCer treatment protects mice depleted of NK cells
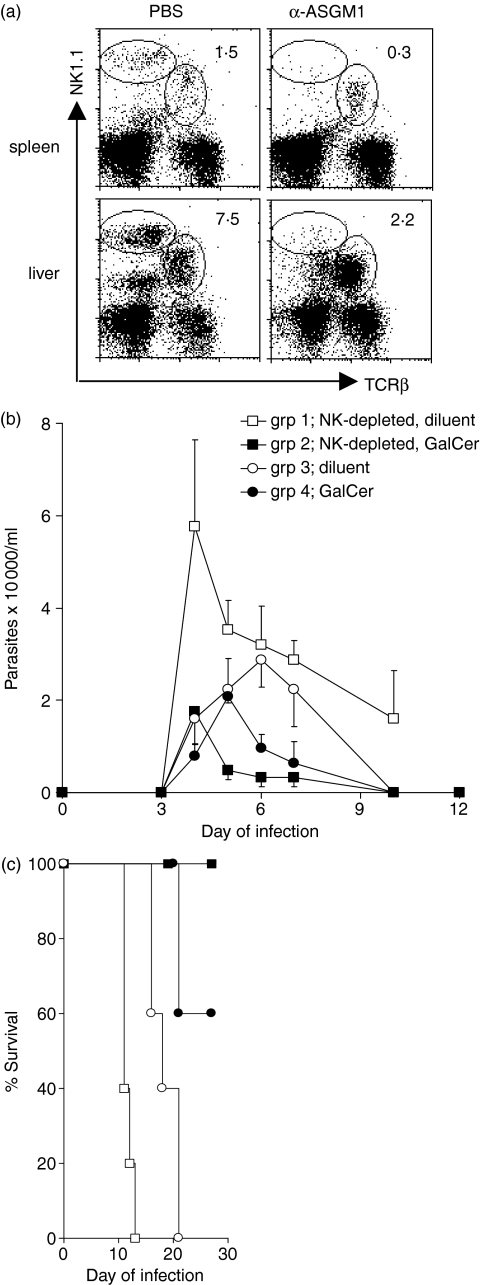
We have previously demonstrated that mice treated with α-GalCer 1 day before T. cruzi infection are protected and that this protection is dependent on IFN-γ.13 In this report we investigate what aspects of the immune response are activated by α-GalCer to provide protection. It is known that α-GalCer-activated iNKT cells stimulate NK cells to secrete IFN-γ.6,17,18 Furthermore, NK cells are critical for survival during acute T. cruzi infection.19–21 Together, these data strongly suggested to us that α-GalCer-induced protection against T. cruzi would require iNKT cell activation of NK cells. In previous studies we have demonstrated that anti-asialo GM1 (ASGM1) antibody treatment before T. cruzi infection selectively depletes the NK cells and results in increased parasitaemia and more rapid death.21 To investigate if α-GalCer-induced protection required NK cells, groups of mice were injected with PBS (control injection) or anti-asialo GM1 antibody and the spleen and liver NK cells were analysed by flow cytometry (Fig. 1a). In the spleen and liver of the mice injected with anti-asialo GM1 antibody the NK-cell populations appeared greatly diminished, whereas the NKT cell populations appeared unaffected (Fig. 1a). After confirming the selective depletion of NK cells, PBS-injected and anti-asialo GM1 antibody-injected groups of mice were treated with α-GalCer or diluent and 1 day later they were inoculated with 1 × 105 trypomastigotes. As expected, mice depleted of NK cells (group 1) experienced the highest parasitaemia and most rapid death (Fig. 1b,c). Furthermore, as we have previously shown, mice with normal NK-cell populations treated with α-GalCer (group 4), compared to mice with normal NK-cell populations treated with diluent (group 3), developed lower parasitaemia (P = 0·009) and improved survival (P = 0·017) confirming that α-GalCer treatment provides protection against T. cruzi (Fig. 1b,c).13 Surprisingly, NK-cell-depleted, α-GalCer-treated mice (group 2), compared to NK-cell-depleted, diluent-treated mice (group 1), exhibited much lower parasitaemia (P = 0·002) and strikingly improved survival (P = 0·002), indicating that a normal NK-cell population is not required for α-GalCer-induced protection (Fig. 1b,c). Remarkably, NK-cell-depleted, α-GalCer-treated mice (group 2) and NK-cell normal, α-GalCer-treated mice (group 4) exhibited similar parasitaemia (P = 0·166) and survival (P = 0·134), which is consistent with α-GalCer treatment being able to overcome the NK-cell deficiency (Fig. 1b,c). These experiments demonstrate that NK cells are not required for α-GalCer-mediated protection against T. cruzi infection and that a single α-GalCer treatment before T. cruzi infection can prevent the rapid death experienced by NK-cell-depleted mice.
Figure 1.
α-GalCer-induced Trypanosoma cruzi protection does not require NK cells. Mice were injected with 80 μg anti-asialo GM1 antibody or PBS, 1 day later they were treated with 5 μg α-GalCer or diluent and the next day they were inoculated with 1 × 105 trypomastigotes. (a) Spleen and liver mononuclear cells of mice treated with anti-asialo GM1 antibody or PBS were stained with anti-TCR mAb and anti-NK1.1 mAb and analysed by flow cytometry. In each flow cytometry plot the upper left ellipse indicates the NK cells and the central ellipse indicates the NKT cells. The number indicates the percentage of cells (NK cells) present in the left ellipse. (b) Mean parasitaemia and SE per group; (c) survival curves of five mice per group. Results are representative of three similar experiments.
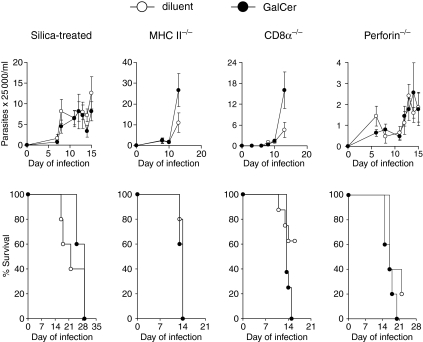
The protective effect of α-GalCer during T. cruzi infection requires silica-sensitive cells, and helper and cytotoxic T cells. As the α-GalCer protection against T. cruzi is IFN-γ-dependent, we hypothesized that IFN-γ-activated phagocytes contributed.13 If this were correct, then inhibition of phagocytic cell activity would reduce or eliminate the α-GalCer protection. To investigate this possibility, mice were injected with silica and then treated with α-GalCer or diluent before subsequent T. cruzi infection.22–24α-GalCer treatment failed to provide protection because it did not reduce parasitaemia (P = 0·343) or improve survival (P = 0·284) (Fig. 2). These data indicate that α-GalCer-induced protection against T. cruzi requires phagocytic cell functions.
Figure 2.
α-GalCer-mediated protection against Trypanosoma cruzi is not observed in MHC II–/–, CD8α–/–, perforin–/– or silica-treated mice. Mice were treated with 5 μg α-GalCer or diluent and the next day were inoculated with 2 × 105 trypomastigotes. Five mice per group were monitored for parasitaemia (mean and SE) and five or more mice per group were monitored for survival. The results are representative of two or more experiments for each mouse strain.
We next analysed the role of adaptive T cells in the α-GalCer-induced protection. First, MHC II–/– mice were treated with α-GalCer or diluent and 1 day later were infected with T. cruzi.α-GalCer treatment failed to reduce parasitaemia (P = 0·417) or to prolong survival (P = 0·513) (Fig. 2). In fact, the α-GalCer-treated mice appeared to develop a higher parasitaemia than the diluent-treated mice shortly before dying (Fig. 2).
Next, we investigated the role of cytotoxic T cells. β2-microglobulin–/– mice were not investigated because these mice do not express CD1d, and therefore do not develop NKT cells or respond to α-GalCer.25 Instead, perforin–/– and CD8α–/– mice were analysed. As with the MHC II–/– mice, α-GalCer treatment of perforin–/– mice, compared to diluent treatment, did not reduce parasitaemia (P = 0·993) or prolong survival (P = 0·303) arguing that perforin-expressing, cytotoxic cells are required for the α-GalCer-induced protection (Fig. 2). Since NK cells are not required for the α-GalCer-induced protection (Fig. 1), but perforin is (Fig. 2), these results argue that cytotoxic cells other than NK cells are required. To further examine this possibility we treated CD8α–/– mice with α-GalCer or diluent. Interestingly the α-GalCer-treated CD8α–/– mice exhibited greater parasitaemia (P = 0·061) and earlier death (P = 0·046) (Fig. 2). These results argue that CD8α is required for α-GalCer-induced protection against T. cruzi, further suggesting that CD8 T cells are required. In addition, the data argue that α-GalCer treatment stimulates aspects of the immune response that favour the parasite.
α-GalCer treatment induces weight loss
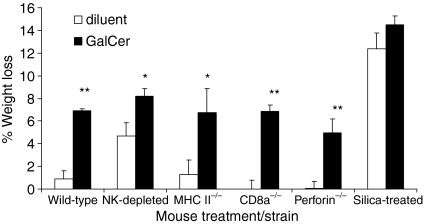
The results argue that α-GalCer-mediated protection against T. cruzi requires phagocytic cells, T helper cells and cytotoxic T lymphocytes. Although protection was not observed in these experiments, other effects of the treatment were. First, surprisingly, the MHC II–/– mice and CD8α–/– mice treated with α-GalCer displayed increased parasitaemia and decreased survival (Fig. 2). In addition, α-GalCer treatment is known to cause a hepatitis that is detected by measuring increased serum GPT, and significantly increased serum GPT levels were detected 24 hr after α-GalCer treatment of the MHC II–/– mice and perforin–/– mice (data not shown).26 Furthermore, 1 day after treatment all the mice treated with α-GalCer (except for silica-treated mice) experienced significant weight loss (Fig. 3). Thus α-GalCer treatment (and silica treatment) appeared to cause weight loss (Fig. 3). This is the first report that α-GalCer treatment of mice causes weight loss. These results indicate that all the mice treated with α-GalCer, except for the silica-treated mice, were affected by the α-GalCer treatment even if protective α-GalCer effects were not observed.
Figure 3.
α-GalCer-treated mice exhibit weight loss. Wild-type, NK-cell-depleted, MHC II–/–, CD8α–/–, perforin–/– and silica-treated mice were treated with 5 μg α-GalCer or diluent. Five mice per group were monitored for weight just before α-GalCer treatment and 24 hr later (just before T. cruzi infection) and weight change is presented as the mean and SE of the per cent weight change., The results are representative of two or more experiments for each mouse strain; *P < 0·05 and **P < 0·01, versus same strain, diluent-treated.
Discussion
α-GalCer treatment rapidly activates iNKT cells to release large amounts of cytokines that stimulate aspects of the innate and adaptive immune responses.27 We have previously demonstrated that α-GalCer treatment augments protection against T. cruzi through an IFN-γ-dependent mechanism.13 To investigate what aspects of the innate and adaptive immune responses contribute to that protection, we examined cells known to become activated following α-GalCer treatment.4,28 This study demonstrates that α-GalCer treatment can protect against T. cruzi despite depletion of NK cells, but not if functional phagocytes, helper T cells or cytotoxic T cells are deficient. These results suggest that phagocytes, helper T cells and cytotoxic T cells, but surprisingly not NK cells, are required for α-GalCer-induced protection against T. cruzi.
The α-GalCer-activated iNKT cells secrete IFN-γ, which stimulates NK cells to produce abundant IFN-γ.6,9,17,18,29 Furthermore, NK cells and NK-cell-derived IFN-γ are critical to α-GalCer-mediated protection against many tumours.17,30 Moreover, because IFN-γ is essential for the α-GalCer-induced protection against T. cruzi, and because NK cells are normally required for survival after acute T. cruzi infection, we speculated that NK-cell-produced IFN-γ would be required for the α-GalCer-induced protection against T. cruzi.13,19,21 Surprisingly, while T. cruzi-infected mice depleted of NK cells and treated with diluent developed high parasitaemia and died rapidly, those treated with α-GalCer controlled parasitaemia well and survived. These results are in agreement with previous studies investigating α-GalCer protection against Plasmodium yoelii and indicate that α-GalCer treatment is protective against T. cruzi infection in mice depleted of normal numbers of NK cells.31 Moreover, the α-GalCer protection of NK-cell-depleted mice was comparable to the α-GalCer protection of normal wild-type mice, demonstrating not only that NK cells are not required, but also that α-GalCer treatment is extremely potent because it protected NK-cell-depleted mice that died rapidly during the acute infection. This protection might be facilitated by secretion of large amounts of IFN-γ by α-GalCer-activated iNKT cells, which more than compensates for the deficiency in NK cell-produced IFN-γ.
Macrophages help to control T. cruzi through their own anti-trypanocidal actions and indirectly by activating other cells.22 In addition, α-GalCer stimulation of iNKT cells results in macrophage activation.8,9 These data argue that α-GalCer treatment will stimulate macrophages and other phagocytic cells to provide protection against T. cruzi. To test this possibility we inhibited phagocytic cells in vivo by injection of silica before α-GalCer treatment.22–24 Since silica treatment induced weight loss and increased serum GPT levels, these parameters could not be used as evidence that the α-GalCer treatment had activated iNKT cells. Recent studies, however, indicate that in the absence of functional macrophages α-GalCer is still effectively presented by CD1d of dendritic cells to activate NKT cells.32 Together these data argue that following silica treatment α-GalCer is presented to NKT cells by dendritic cells, and that the failure of α-GalCer to provide protection is caused by the loss of phagocytic cell functions other than α-GalCer CD1d presentation.
Our previous study indicated that α-GalCer protects against T. cruzi through IFN-γ, and strongly suggested that CD4+ or CD8+ T cells were involved.13 In this report we analysed the role of T cells in α-GalCer treatment of T. cruzi infection using gene-deficient mice. MHC II–/– mice were not protected by α-GalCer treatment, which suggested that during T. cruzi infection CD4+ T helper cells are necessary for the α-GalCer-induced protection. Having demonstrated the involvement of CD4+ T cells and phagocytes, we next wanted to consider MHC class I-restricted T cells. β2-microglobulin–/– mice could not be investigated, as these mice do not develop CD1d-restricted NKT cells. Rather, perforin–/– and CD8α–/– mice were used. As expected, α-GalCer-induced treatment did not protect these mouse strains. It was surprising, however, that α-GalCer treatment of CD8α–/– mice exacerbated parasitaemia and death similar to the effect we observed following α-GalCer treatment of IFN-γ–/– mice.13 These results argue that in the absence of CD8α, α-GalCer-induced immune responses favour the parasite.
Our result that intraperitoneal α-GalCer treatment augments protection against T. cruzi infection agrees with another study.14 That study also determined that α-GalCer treatment did not enhance protection by a T. cruzi DNA vaccine.14 It remains possible that NKT-cell glycolipid antigens can be used as adjuvants for other types of vaccines against T. cruzi.
In human clinical trials α-GalCer treatment appears safe.26,33 It is known that α-GalCer treatment of mice causes a transient hepatitis, and here we report that mice, following α-GalCer treatment, also suffer a transient weight loss. This weight loss appears to occur in the absence of MHC II-restricted helper T cells and MHC class I-restricted cytotoxic T cells. Although we do not know the mechanism of the weight loss, it might be caused by activated liver iNKT cells, which rapidly induce a robust inflammatory response.
The results presented here indicate that following α-GalCer injection, iNKT-cell activation triggers a complex cellular response that provides protection during T. cruzi infection. Surprisingly, despite a requirement of NK cells and IFN-γ to control acute T. cruzi infection, NK cells were not required for the α-GalCer-induced protection, arguing that α-GalCer activated other cells to overcome the absence of NK cells. These results further our understanding of NKT cells as a potential therapeutic target during infections and other diseases.
Acknowledgments
We thank Kirin Brewery Co., Ltd. (Gunma, Japan) for providing α-GalCer. This work was supported by the National Institutes of Health Grant AI49455. The authors thank Maria Kahn for critical discussion of the manuscript.
Glossary
Abbreviations
- α-GalCer
α-galactosylceramide
- ASGM1
asialo GM1
- DMEM
Dulbecco’s modified Eagles’s medium
- GPT
glutamic pyruvic transaminase
- IFN-γ
interferon-γ
- IL-4
interleukin-4
- iNKT
invariant NKT
- mAb
monoclonal antibody
- MHC
major histocompatibility complex
- NKT
natural killer T
- PBS
phosphate-buffered saline
References
- 1.World Health Organization. Control of Chagas Disease. WHO Technical Report Series, 905. Geneva: World Health Organization; 2002. pp. 1–109. [PubMed] [Google Scholar]
- 2.Marinho CR, D’Imperio Lima MR, Grisotto MG, Alvarez JM. Influence of acute-phase parasite load on pathology, parasitism, and activation of the immune system at the late chronic phase of Chagas’ disease. Infect Immun. 1999;67:308–18. doi: 10.1128/iai.67.1.308-318.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duthie MS, Kahn M, White M, Kapur RP, Kahn SJ. Critical proinflammatory and anti-inflammatory functions of different subsets of CD1d-restricted natural killer T cells during Trypanosoma cruzi infection. Infect Immun. 2005;73:181–92. doi: 10.1128/IAI.73.1.181-192.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 5.Kawano T, Cui J, Koezuka Y, et al. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 6.Carnaud C, Lee D, Donnars O, Park SH, Beavis A, Koezuka Y, Bendelac A. Cutting edge: cross-talk between cells of the innate immune system: NKT cells rapidly activate NK cells. J Immunol. 1999;163:4647–50. [PubMed] [Google Scholar]
- 7.Eberl G, MacDonald HR. Selective induction of NK cell proliferation and cytotoxicity by activated NKT cells. Eur J Immunol. 2000;30:985–92. doi: 10.1002/(SICI)1521-4141(200004)30:4<985::AID-IMMU985>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 8.Nieuwenhuis EE, Matsumoto T, Exley M, et al. CD1d-dependent macrophage-mediated clearance of Pseudomonas aeruginosa from lung. Nat Med. 2002;8:588–93. doi: 10.1038/nm0602-588. [DOI] [PubMed] [Google Scholar]
- 9.Wesley JD, Robbins SH, Sidobre S, Kronenberg M, Terrizzi S, Brossay L. Cutting edge: IFN-γ signaling to macrophages is required for optimal Vα14i NK T/NK cell cross-talk. J Immunol. 2005;174:3864–8. doi: 10.4049/jimmunol.174.7.3864. [DOI] [PubMed] [Google Scholar]
- 10.Eberl G, Brawand P, MacDonald HR. Selective bystander proliferation of memory CD4+ and CD8+ T cells upon NK T or T cell activation. J Immunol. 2000;165:4305–11. doi: 10.4049/jimmunol.165.8.4305. [DOI] [PubMed] [Google Scholar]
- 11.Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2:557–68. doi: 10.1038/nri854. [DOI] [PubMed] [Google Scholar]
- 12.Wilson MT, Singh AK, Van Kaer L. Immunotherapy with ligands of natural killer T cells. Trends Mol Med. 2002;8:225–31. doi: 10.1016/s1471-4914(02)02325-0. [DOI] [PubMed] [Google Scholar]
- 13.Duthie MS, Kahn SJ. Treatment with alpha-galactosylceramide before Trypanosoma cruzi infection provides protection or induces failure to thrive. J Immunol. 2002;168:5778–85. doi: 10.4049/jimmunol.168.11.5778. [DOI] [PubMed] [Google Scholar]
- 14.Miyahira Y, Katae M, Takeda K, et al. Activation of natural killer T cells by alpha-galactosylceramide impairs DNA vaccine-induced protective immunity against Trypanosoma cruzi. Infect Immun. 2003;71:1234–41. doi: 10.1128/IAI.71.3.1234-1241.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plata F, Garcia Pons F, Eisen H. Antigenic polymorphism of Trypanosoma cruzi: clonal analysis of trypomastigote surface antigens. Eur J Immunol. 1984;14:392–9. doi: 10.1002/eji.1830140503. [DOI] [PubMed] [Google Scholar]
- 16.Duthie MS, Wleklinski-Lee M, Smith S, Nakayama T, Taniguchi M, Kahn SJ. During Trypanosoma cruzi infection CD1d-restricted NK T cells limit parasitemia and augment the antibody response to a glycophosphoinositol-modified surface protein. Infect Immun. 2002;70:36–48. doi: 10.1128/IAI.70.1.36-48.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smyth MJ, Crowe NY, Pellicci DG, Kyparissoudis K, Kelly JM, Takeda K, Yagita H, Godfrey DI. Sequential production of interferon-gamma by NK1.1(+) T cells and natural killer cells is essential for the antimetastatic effect of alpha-galactosylceramide. Blood. 2002;99:1259–66. doi: 10.1182/blood.v99.4.1259. [DOI] [PubMed] [Google Scholar]
- 18.Matsuda JL, Gapin L, Baron JL, Sidobre S, Stetson DB, Mohrs M, Locksley RM, Kronenberg M. Mouse V alpha 14i natural killer T cells are resistant to cytokine polarization in vivo. Proc Natl Acad Sci USA. 2003;100:8395–400. doi: 10.1073/pnas.1332805100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rottenberg M, Cardoni RL, Andersson R, Segura EL, Orn A. Role of T helper/inducer cells as well as natural killer cells in resistance to Trypanosoma cruzi infection. Scand J Immunol. 1988;28:573–82. doi: 10.1111/j.1365-3083.1988.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 20.Cardillo F, Voltarelli JC, Reed SG, Silva JS. Regulation of Trypanosoma cruzi infection in mice by gamma interferon and interleukin 10: role of NK cells. Infect Immun. 1996;64:128–34. doi: 10.1128/iai.64.1.128-134.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duthie MS, Kahn SJ. NK cell activation and protection occur independently of natural killer T cells during Trypanosoma cruzi infection. Int Immunol. 2005;17:607–13. doi: 10.1093/intimm/dxh239. [DOI] [PubMed] [Google Scholar]
- 22.Kierszenbaum F, Knecht E, Budzko DB, Pizzimenti MC. Phagocytosis: a defense mechanism against infection with Trypanosoma cruzi. J Immunol. 1974;112:1839–44. [PubMed] [Google Scholar]
- 23.Melo RC. Depletion of immune effector cells induces myocardial damage in the acute experimental Trypanosoma cruzi infection: ultrastructural study in rats. Tissue Cell. 1999;31:281–90. doi: 10.1054/tice.1999.0040. [DOI] [PubMed] [Google Scholar]
- 24.Melo RC, Machado CR. Trypanosoma cruzi: peripheral blood monocytes and heart macrophages in the resistance to acute experimental infection in rats. Exp Parasitol. 2001;97:15–23. doi: 10.1006/expr.2000.4576. [DOI] [PubMed] [Google Scholar]
- 25.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells. Development, specificity, and function. Annu Rev Immunol. 1997;15:535–62. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 26.Osman Y, Kawamura T, Naito T, Takeda K, Van Kaer L, Okumura K, Abo T. Activation of hepatic NKT cells and subsequent liver injury following administration of alpha-galactosylceramide. Eur J Immunol. 2000;30:1919–28. doi: 10.1002/1521-4141(200007)30:7<1919::AID-IMMU1919>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, Koezuka Y, Kronenberg M. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192:741–54. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Kaer L. Regulation of immune responses by CD1d-restricted natural killer T cells. Immunol Res. 2004;30:139–53. doi: 10.1385/IR:30:2:139. [DOI] [PubMed] [Google Scholar]
- 29.Hayakawa Y, Takeda K, Yagita H, et al. Critical contribution of IFN-γ and NK cells, but not perforin-mediated cytotoxicity, to anti-metastatic effect of α-galactosylceramide. Eur J Immunol. 2001;31:1720–7. [PubMed] [Google Scholar]
- 30.Hayakawa Y, Takeda K, Yagita H, Smyth MJ, Van Kaer L, Okumura K, Saiki I. IFN-γ-mediated inhibition of tumor angiogenesis by natural killer T-cell ligand, alpha-galactosylceramide. Blood. 2002;100:1728–33. [PubMed] [Google Scholar]
- 31.Gonzalez-Aseguinolaza G, de Oliveira C, Tomaska M, et al. α-galactosylceramide-activated Vα14 natural killer T cells mediate protection against murine malaria. Proc Natl Acad Sci USA. 2000;97:8461–6. doi: 10.1073/pnas.97.15.8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmieg J, Yang G, Franck RW, Van Rooijen N, Tsuji M. Glycolipid presentation to natural killer T cells differs in an organ-dependent fashion. Proc Natl Acad Sci USA. 2005;102:1127–32. doi: 10.1073/pnas.0408288102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giaccone G, Punt CJ, Ando Y, et al. A phase I study of the natural killer T-cell ligand α-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. 2002;8:3702–9. [PubMed] [Google Scholar]