Abstract
Glucocorticoid-induced tumour necrosis factor receptor family related protein (GITR) is the 18th member of the tumour necrosis factor receptor superfamily (TNFRSF18) and is known to interact with its cognate ligand GITRL (TNFSF18). We investigated the potential role of GITR in the pro-inflammatory activation of macrophages. Immunohistochemistry and in situ hybridization analyses of human atherosclerotic plaques demonstrated that GITR and its ligand are expressed mainly in lipid-rich macrophages. We then investigated the role of GITR in human and mouse monocyte/macrophage functions. Stimulation of GITR caused nuclear factor (NF)-κB-dependent activation of matrix metalloproteinase-9 (MMP-9) and pro-inflammatory cytokine expression in both the human and mouse monocytic/macrophage cell lines, THP-1 and RAW264.7, respectively. These cellular responses were also observed when the THP-1 cells were treated with phorbol-12 myristate-13 acetate (PMA), which is known to induce macrophage differentiation. To demonstrate that these responses are not restricted to cultured cell lines, we tested primary macrophages. Both peritoneal and bone marrow-derived macrophages responded to GITR stimulation with induction of MMP-9 and tumour necrosis factor-α (TNF-α). Furthermore, the GITR staining pattern overlapped with those of MMP-9 and TNF-α in atherosclerotic plaques. These data indicate that GITR-mediated macrophage activation may promote atherogenesis via the induction of pro-atherogenic cytokines/chemokines, and destabilize the atherosclerotic plaques via the induction of the matrix-degrading enzyme, MMP-9.
Keywords: atherosclerosis, cytokines, glucocorticoid-induced tumour necrosis factor receptor family-related protein, inflammation, monocytes/macrophages, tumour necrosis factor receptor superfamily 18
Introduction
Members of the tumour necrosis factor superfamily (TNFSF) and the TNF receptor superfamily (TNFRSF) play pivotal roles in atherogenesis. TNF-α is expressed in lipid-rich macrophages, smooth muscle cells and mast cells1,2 in atherosclerotic plaques. CD40 ligand(CD40L/TNFSF5)/CD40(TNFRSF5) are also expressed in vascular endothelial cells, smooth muscle cells and macrophages.3 Recently, LIGHT (TNFSF14) and its receptor TR2 (TNFRSF14/HVEM) have been found to be expressed in the macrophages of human atherosclerotic plaques.4,5 The interaction between LIGHT and TR2 mediated expression of pro-inflammatory cytokines and matrix metalloproteinases (MMPs) in macrophages. Furthermore, TL1A (TNFSF15) and its receptor DR3 (TNFRSF12) have also been detected in the macrophage-rich regions of human atherosclerotic plaques and the stimulation of DR3 in THP-1 cells induced MMP-1, −9 and −13 expression.6,7 Collectively, interactions between the members of the TNFSF and their cognate receptors result in a variety of the biological responses associated with atherosclerosis, including the secretion of pro-inflammatory cytokines and MMPs, as well as the expression of adhesion molecules and tissue factor.8 These responses render the plaque unstable.
GITR (TNFRSF18/AITR) was initially identified in activated T lymphocytes, functioning as a regulator of T-cell receptor-mediated cell death.9 Subsequently, GITR expression was observed in CD25+ CD4+ regulatory T cells and GITR stimulation blocked suppressor activity of these cells.10–12 CD25– effector T cells also express GITR and the interaction of GITR with its ligand provides an early co-stimulatory signal, enhancing proliferation and cytokine production.13–15 Signalling through GITR is mediated by TNFR-associated factors (TRAFs) and activates the nuclear factor-κB (NF-κB).16 The ligand of GITR (GITRL) (TNFSF18/AITRL/TL6) is expressed in endothelial cells, dendritic cells, macrophages and B cells but not in T cells.13,16–18
Although there have been extensive studies on the role of GITR in various immune reactions, its role in atherogenesis and macrophage function has not been studied so far. To determine whether the interaction between GITR and GITRL plays a role in the atherogenic processes, we analysed the expression patterns of these molecules in human atherosclerotic plaque specimens, and macrophages were found to be the major cell type expressing both GITR and GITRL. To investigate the role of these molecules in macrophage function, we evaluated cellular responses after stimulation of GITR at the surface of human and mouse macrophages.
Materials and methods
Histological analysis
For the immunohistochemical analysis, carotid endoarterectomy specimens were obtained from patients, aged between 63 and 81, who had undergone surgery at the Samsung Seoul Hospital after obtaining informed consent. The current study was approved by the internal review board. Atherosclerotic plaque specimens were washed with saline and embedded in optimal cutting temperature medium to produce frozen sections. Standard 5 μm sections were stained using an LSAB kit (DAKO, Copenhagen, Denmark) according to the manual provided by the manufacturer. The sections were then counterstained with haematoxylin, which stains the nucleus blue. For the quantification of the macrophage expression of GITR and GITRL, two researchers analysed the slide separately and combined the scores to get the final value.
In situ hybridization
Riboprobes containing 402-base and 328-base fragments of GITR and GITRL coding sequences, respectively, were generated using a DIG RNA labelling kit according to the manufacturer’s instructions (Roche Molecular Biochemicals, Mannheim, Germany). The RNA probes were ethanol-precipitated and stored at − 70° until use. For in situ hybridization, tissue sections were rehydrated and sequentially treated with 0·2 m HCl for 15 min followed by rinse buffer [0·3% Triton X-100 in Diethylpyrocarbonate-treated-phosphate-buffered saline (DEPC-PBS)]; 2 μg/ml proteinase K for 30 min followed by rinsing (0·3% Triton X-100 in DEPC-PBS and DEPC-PBS twice); 4% paraformaldehyde for 30 min followed by rinsing (DEPC-PBS); 0·1 m triethanolamine (pH 8·0); and 0·1 m triethanolamine (pH 8·0) in 0·25% acetic anhydride while agitating the slides. Prehybridization was performed in 100 μl hybridization buffer [4 × saline sodium citrate (SSC), 50% formamide, 10% dextran sulphate, 1 × Denhardt’s solution, 2 mm ethylenediaminetetraacetic acid, and 125 μg/ml boiled yeast tRNA] at 42° for 2–3 hr. Thirty nanograms of probes, preheated at 80° for 3 min in 100 μl hybridization solution, was added and hybridization continued at 42° overnight in a humidified chamber. The slides were then rinsed sequentially with 2 × SSC in 50% formamide; 2 × SSC; and 0·2 × SSC. The slides were then processed bon a Dig Wash and Block Buffer Set (Roche) following the manufacturer’s instructions before incubation in anti-digoxygenin-AP (1 : 500) for 2 hr at 37°. The colour reaction was performed with 5-bromo-4-chloro-3-indolylphosphate p-toluidine salt/nitroblue tetrazolium dye (BCIP/NBT) (purple colour) and the slides were mounted on aqueous mounting media.
Monoclonal antibodies
Monoclonal antibody (mAb) to CD68 (KP1) was purchased from DAKO (Glostrup, Denmark); mAbs for human GITR (clone 621), human GITRL (clone EB11), and mouse GITR were purchased from Immunomics (Ulsan, Korea); mAb for mouse GITRL were from eBioscience (San Diego, CA, USA); rabbit polyclonal antibody to IκB from Cell Signalling (Danvers, MA, USA); goat polyclonal antibody to actin from Santa Cruz (Santa Cruz, CA, USA); mouse immunoglobulin G1 (IgG1) from Becton-Dickinson (Mountain View, CA); and human IgG1 from Calbiochem International, Inc. (Darmstadt, Germany). Endotoxin levels in the anti-GITR or GITRL stock solution (2 mg/ml) were below 20 pg/ml (tested with QCL-1000 Chromogenic Limulus Amoebocyte lysate test method, Bio-Whittaker, Walkersville, MD). Recombinant human GITRL (rhGITRL), mouse GITRL (rmGITRL), rat IgG1, and rat IgG2 were purchased from R & D Systems, Inc. (Minneapolis, MN, USA). N-tosyl-l-phenylalanine chloromethyl ketone (TPCK), ethyl pyruvate and sulphasalazine were purchased from Sigma (St Louis, MO, USA).
Cell culture
RAW264.7 and THP-1 cells were cultured as described by the American Type Culture Collection (Rockville, MD). For macrophage differentiation, THP-1 cells were treated with 100 nm 12-phorbol 13-myristate acetate (PMA) for 2 days and further incubated with normal medium for 3 days before stimulation. To isolate the peritoneal macrophages, 0·5% thioglycollate medium was injected into the peritoneum of 2-month-old ICR mice. Four days after the injection, peritoneal cells were collected and counted. Non-adherent cells were washed away after 3 hr of incubation and the remaining cells were used for analysis. For the differentiation of bone marrow cells into macrophages, bone marrow cells were collected and non-adherent cells were washed out. The remaining adherent cells were incubated for more than 10 days in a culture medium supplemented with 10% L929 culture supernatant as a source of macrophage colony-stimulating factor. Macrophage differentiation was confirmed by staining the cells with MOMA-2, a macrophage marker, which stained more than 95% of the cells in both cases.
Flow cytometric analysis
Flow cytometric analysis was performed on the FACS-calibur system (Becton-Dickinson, Mountain View, CA). Cells (5 × 105) were pelleted and incubated with 0·3 μg antibodies in 30 μl FACS solution (PBS containing 0·5% bovine serum albumin and 0·1% sodium azide) for 20 min on ice. Cells were washed twice and incubated with 0·3 μg of fluorescein isothiocyanate-labelled secondary antibody in a 30 μl FACS solution. For the background fluorescence, cells were stained with isotype-matching control antibody. The fluorescence profiles of 1 × 104 cells were collected and analysed.
Cell activation and analysis of cytokine/MMP-9 expression
For the activation utilizing immobilized mAbs, 50 μl/well PBS containing 1–200 μg/ml antibody was incubated overnight on a 96-well plate. The wells were washed twice with PBS, after which THP-1 cells (1 × 105/well) in 100 μl serum-free RPMI-1640 medium were added. Supernatants were collected 24–48 hr after activation. The levels of interleukin-8 (IL-8), monocyte chemoattractant protein-1 (MCP-1), and TNF-α in the supernatants were measured by sandwich enzyme-linked immunosorbent assay (ELISA; R & D Systems, Inc., Minneapolis, MN, USA). Detection limits were < 10 pg/ml for all cytokines. The MMP activity in the culture supernatant was determined via substrate gel electrophoresis, as previously described.4,5
Results and discusssion
Expression of GITR and GITRL was detected in the macrophages of human atherosclerotic plaques
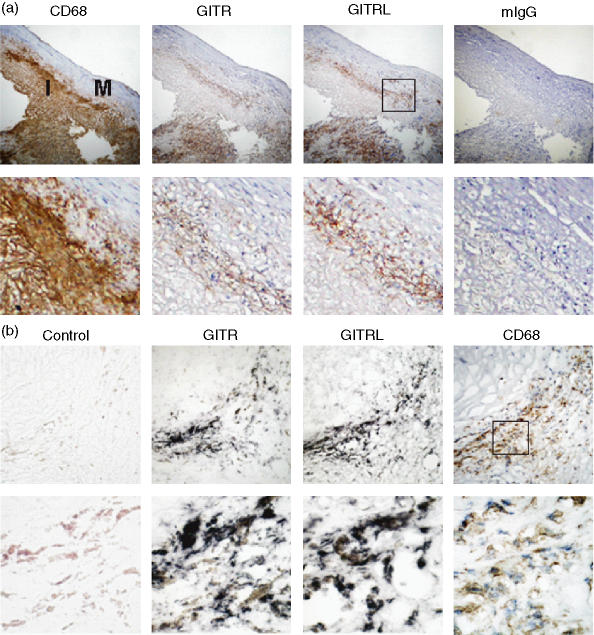
Immunohistochemical analysis was performed on human atherosclerotic plaques using mAbs specific to GITR, GITRL and CD68 (a macrophage marker). GITR and GITRL expression was observed in regions that were rich in foamy macrophages (Fig. 1a). The expression of GITR and GITRL was detected in macrophage-rich areas in all 11 plaque specimens tested. GITR and GITRL expression was especially high in macrophages present at the intima-media junctions and in areas around the necrotic core (Table 1). We then analysed GITR and GITRL expression using in situ hybridization. The GITR- and GITRL-specific riboprobes, but not the non-specific control probe, were hybridized in the macrophage-rich areas (Fig. 1b). These data indicate that macrophages are the major cell type expressing GITR and GITRL in the atherosclerotic plaque specimens we tested.
Figure 1.
Macrophages express GITR and GITRL in the atherosclerotic plaques. (a) Plaque sections were stained with antibodies against CD68, GITR, or GITRL. Isotype-matching control antibody (mouse IgG1) was used to demonstrate the specificity of the staining. Low magnification (× 100) pictures of consecutive sections of atherosclerotic plaque are shown in the upper panel and high magnification (× 400) pictures are shown in the lower panel. I, intima; M, media. (b) Serial sections of an atherosclerotic plaque were used for in situ hybridization using GITR- or GITRL-specific probes or a non-specific control probe as described in the Materials and methods section. The hybridization was visualized as dark purple. For comparison, an adjacent section was immunostained using anti-CD68 mAb (brown colour). Magnifications are × 400 for the upper panel and × 1000 for the lower panel.
Table 1.
Expression patterns of GITR and GITRL in macrophages in different parts of atherosclerotic plaques
| GITR | GITRL | |||||||
|---|---|---|---|---|---|---|---|---|
| Tissues | Whole | M-I1 | Core2 | Shoulder3 | Whole | M-I1 | Core2 | Shoulder3 |
| #1 | + + + 4 | + + + | + + | + + + | + + | + + | – | + |
| #2 | + + + | + + + | + + + | + + + | + + + | + + + | + + + | + + |
| #3 | + + + | + + | + + + | na | + + + | + + + | + + + | na |
| #4 | + + | + + + | na5 | na | + + + | + + | na | na |
| #5 | + + + | + + + | + + + | + + | + + | + + | + + | + |
| #6 | + + | + | na | na | + + + | + + + | na | na |
| #7 | + + | + | + + + | na | + + + | + + | + | na |
| #8 | + + + | + + | + + | na | + + | + + + | + + + | na |
| #9 | + + + | + + | + + | na | + + | + + + | + + + | na |
| #10 | + + + | + + + | na | + + + | + + | + + + | na | + |
| #11 | + + + | + + | + + + | na | + + + | + + + | + + + | na |
Boundary regions between media and intima
areas around necrotic core
shoulder regions of the plaque
–, no expression; +, <30%; + +, 30–70%; + + +, >70% of macrophages express GTIR or GITRL
na, not available because this area was not present in the tissue specimen.
We then tested whether GITR and GITRL are expressed in human monocyte/macrophage cell lines. Flow cytometric analysis detected both GITR and GITRL expression on the cell surfaces of THP-1 (Fig. 2a) and U937 cells (data not shown). Expression of GITRL by monocytes/macrophages has also been reported in peripheral blood monocytes after stimulation with staphylococcal enterotoxin B.19 Our immunohistochemistry and flow cytometry data, in combination with the previous reports, clearly demonstrate that macrophages can express both GITR and GITRL.
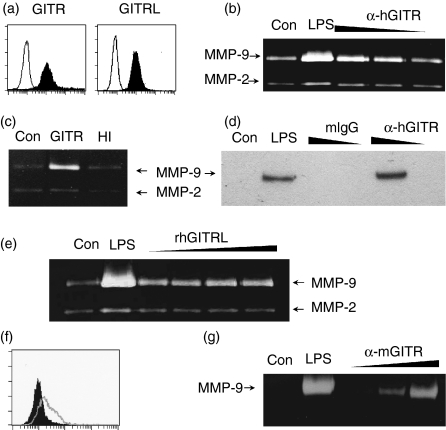
Figure 2.
Stimulation of GITR induces MMP-9 expression in monocyte/macrophage cell lines. (a) THP-1 cells were stained with anti-GITR or anti-GITRL mAbs as indicated. Fluorescence profiles obtained from specific staining (filled area) and background staining (open area, stained with isotype-matching control antibody) are compared. (b) THP-1 cells were stimulated with anti-hGITR mAb immobilized at 20, 6 and 2 μg/ml concentrations. Culture supernatants were collected over a 24 hr period to measure MMP-9 activity using gelatin zymogram. (c) THP-1 cells were stimulated with immobilized anti-GITR mAb (10 μg/ml) that had been pretreated with (HI) or without (GITR) heat (95° for 30 min). (d) THP-1 cells were stimulated with anti-GITR mAb or mIgG immobilized at 20 and 2 μg/ml concentrations. Culture supernatants were collected after 24 hr and condensed approximately 10-fold before Western blot analysis with anti-MMP-9 mAb. (e) THP-1 cells were stimulated with 0·1, 0·3, 1 and 3 μg/ml of rhGITRL for 24 hr before gelatin zymogram. (f) RAW 264.7 cells were stained with anti-GITR mAb. Histograms from specific staining (open area) and background staining (filled area, stained with isotype-matching control antibody) are compared. (g) RAW264.7 cells were stimulated with anti-mGITR mAb which was added to culture medium at 1, 10 and 30 μg/ml concentrations. Culture supernatants were collected after 48 hr for gelatin zymogram. As a positive control, the cells were treated with 1 μg/ml lipopolysaccharide (LPS). Con, no treatment control.
Macrophages play a pivotal role in the pathogenesis of various inflammatory diseases. Rheumatoid arthritis is the best-known autoimmune disease in which inflammatory destruction of cartilage and bone is mediated by mediators produced by inflammatory cells including macrophages. To test whether the expression of GITR by macrophages is a general phenomenon in inflammatory diseases, we performed a similar analysis in rheumatoid arthritis synovial tissue sections. Both GITR and GITRL were expressed in synovial macrophages (our unpublished observation), raising the possibility that macrophages express GITR and GITRL in most inflammatory processes.
Activation of GITR induces MMP-9 expression in THP-1 and RAW264.7 cells
Macrophages play a major role in atherogenesis by mediating various inflammatory reactions. To investigate the role of GITR in the inflammatory activation of monocytes/macrophages, we stimulated THP-1 cells with immobilized mAbs against GITR. The culture supernatants were collected and MMP-9 expression levels were detected using a gelatin zymogram. As shown in Fig. 2(b), immobilized anti-GITR mAb induced MMP-9 expression in a dose-dependent manner. Heat inactivation of the mAb before THP-1 cell stimulation resulted in the abolition of MMP-9 induction, thereby refuting the possibility that this response had been induced by contaminating heat-resistant endotoxins (Fig. 2c). We also confirmed the GITR mediated induction of MMP-9 through Western blot analysis of the culture supernatants with MMP-9-specific mAb. Stimulation of the THP-1 cells with isotype-matching mouse antibody failed to induce MMP-9 expression (Fig. 2d). Since the mAb is not the natural molecule interacting with GITR, we stimulated the cell with rhGITRL. Treatment with rhGITRL induced MMP-9 expression at concentrations as low as 100 ng/ml (Fig. 2e).
We then tested the mouse monocyte/macrophage cell line RAW264.7 for the expression of GITR and GITRL. RAW264.7 cells expressed low levels of GITR (Fig. 2F). GITRL expression was not detected in RAW264.7 cells (data not shown), but Tone et al. previously reported that GITRL expression is induced in RAW264.7 cells after stimulation with lipopolysaccharide.13 RAW264.7 cells also expressed MMP-9 after stimulation with anti-mGITR mAb that had been added to the culture medium (Fig. 2g).
Stimulation of GITR induced cytokine expression in THP-1 and RAW264.7 cells
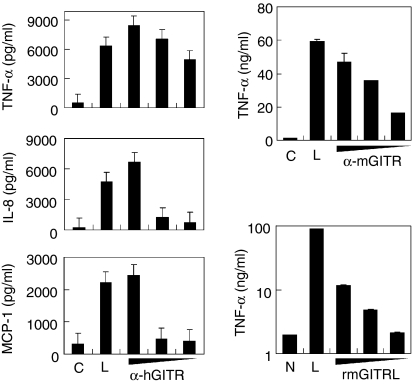
Cytokine/chemokines, such as TNF-α, IL-8 and MCP-1, are involved in atherogenesis by mediating a variety of inflammatory processes which render the plaque unstable.8 Stimulation of THP-1 cells with immobilized anti-GITR mAb induced TNF-α in a dose-dependent manner. The antibody also induced IL-8 and MCP-1 expression at the highest dose (Fig. 3a). Stimulation of cytokine expression tended to require a much higher dose of antibody treatment than MMP-9 induction. When RAW264.7 cells were stimulated with anti-mGITR mAb, TNF-α induction was detected dose-dependently (Fig. 3b). Recombinant mouse GITRL also induced TNF-α expression in RAW264.7 cells, albeit at lower levels (Fig. 3c).
Figure 3.
Stimulation of GITR induces cytokine/chemokine expression in monocyte/macrophage cell lines. (a) THP-1 cells were stimulated with immobilized anti-hGITR mAb or mIgG. Culture supernatants were collected 24 hr after activation and cytokine concentrations were measured using ELISA. Concentrations used for the immobilization were 200, 20 and 2 μg/ml for the anti-GITR mAb and 200 μg/ml for mIgG. (b,c) RAW264.7 cells were stimulated with anti-mGITR mAb added to the culture medium at 30, 10 and 1 μg/ml concentrations or 30 μg/ml of rat IgG (b) or rmGITRL added at 10, 3 and 1 μg/ml concentrations (c). Culture supernatants were collected 48 hr after activation and TNF-α concentrations were measured using ELISA. C, treatment with isotype-matching control antibody; N, no treatment control; L, treatment with 1 μg/ml lipopolysaccharide as a positive control. Mean values of triplicate measurements ± SD are shown.
GITR-mediated activation of MMP-9 requires NF-κB activation
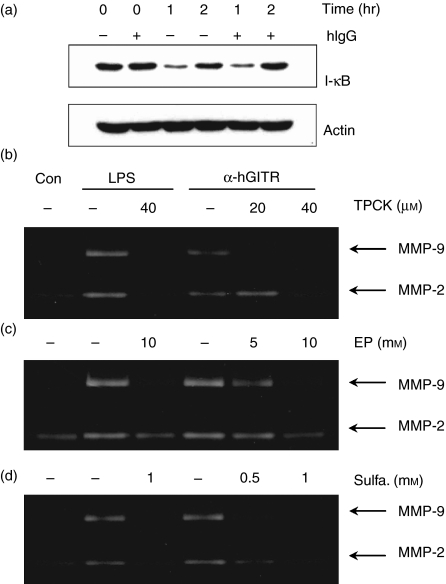
NF-κB is the key transcription factor involved in activation of cytokines/chemokines and MMPs.20,21 In resting cells, NF-κB/IκB complexes are present in the cytoplasm. Activation of the cells under appropriate conditions leads to the phosphorylation and subsequent degradation of IκB. The free NF-κB then translocates into the nucleus to activate genes with NF-κB binding sites. Therefore, we attempted to determine whether GITR stimulation at the surface of THP-1 cells induces degradation of IκB. When cells were stimulated with immobilized anti-GITR mAb, IκB degradation was detected after 1 hr and the original level was restored after 2 hr (Fig. 4a).
Figure 4.
Stimulation of GITR induces the NF-κB activation, which is required for MMP-9 induction. (a) THP-1 cells were preincubated with or without human IgG (20 μg/ml) for 30 min to block FcRs. These cells were then stimulated with immobilized anti-GITR mAb (20 μg/ml). Whole cell lysates were then collected 0, 1 or 2 hr after the activation and IκB levels were measured by Western blot analysis. Actin levels were measured as a protein loading control. (b–d), THP-1 cells were activated with immobilized anti-GITR mAb in the presence or absence of NF-κB inhibitors TPCK (b), ethyl pyruvate (c), and sulphasalazine (d). Culture supernatants were collected after 24 hr for the measurement of MMP-9 activity using gelatin zymogram. Con, no treatment control; LPS, treatment with 1 μg/ml lipopolysaccharide as a positive control.
We used immobilized antibodies to activate THP-1 cells. Although it is likely that the antibodies interact with the corresponding antigens, it is possible that some of the antibodies interact with FcR on the surface of THP-1 cells. In such a case, stimulation through GITR and FcR may work synergistically to activate the cells. On the other hand, some of the liberated antibodies may cause adhesion between cells and subsequent activation by bidirectional interaction: binding to the corresponding antigen on one cell and to the FcR on another cell. To prevent interaction between the antibody Fc portion and the FcR on the THP-1 cell surface, we pretreated the THP-1 cells with human IgG before stimulation with the anti-GITR mAb. As shown in Fig. 4(a), pretreatment with human IgG failed to block the IκB degradation caused by stimulation with immobilized anti-GITR, indicating that THP-1 cell activation is induced by specific interaction between the anti-GITR mAb and its corresponding antigen.
To confirm that activated NF-κB mediates GITR-induced MMP-9 expression, we pretreated THP-1 cells with known NF-κB inhibitors, such as TPCK,22 ethyl pyruvate23 and sulphasalazine.24 All of these compounds inhibited the GITR-induced activation of MMP-9 in a dose-dependent manner (Fig. 4b), indicating that GITR-mediated induction of MMP-9 expression depends on NF-κB activation.
Stimulation of GITR induces MMP-9 and TNF-α expression in primary macrophages
To demonstrate that the role of GITR-mediated cellular activation is not restricted to cultured cell lines, THP-1 cells were differentiated into macrophages with PMA treatment.25,26 PMA treatment resulted in increased basal expression levels of both MMP-9 and cytokines. Stimulation of THP-1 macrophages with anti-GITR mAb further enhanced MMP-9 expression in a dose-dependent manner (Fig. 5a). Anti-GITR mAb treatment also up-regulated TNF-α secretion in a dose-dependent manner (Fig. 5b). Stimulation with rhGITRL, however, failed to induce MMP-9 (Fig. 5a) and TNF-α (data not shown).
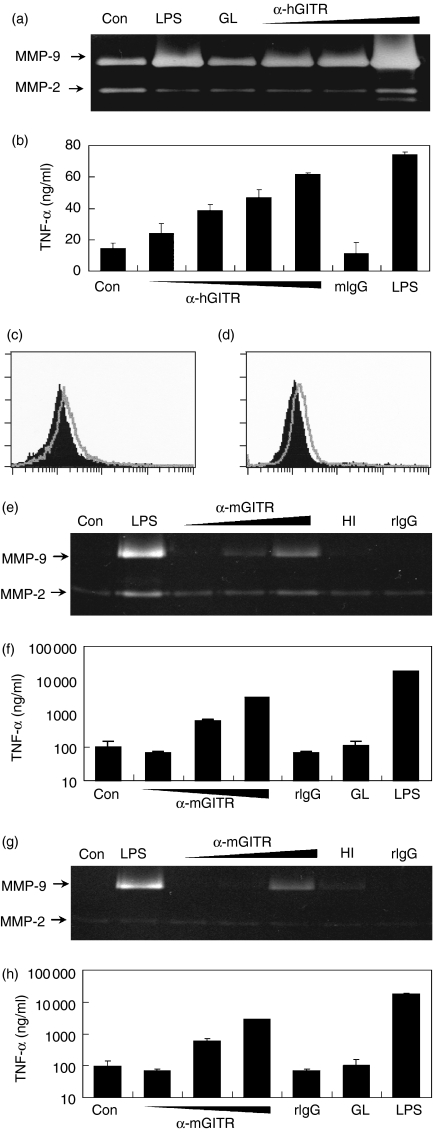
Figure 5.
GITR stimulation activates macrophages to express MMP-9 and TNF-α. THP-1 cells were treated with PMA to differentiate into macrophages, as described in the Materials and methods section. Cells were then stimulated with 3 μg/ml rhGITRL (GL) or 3, 10 and 30 μg/ml anti-hGITR mAb, which was added to the culture medium. As a positive control, cells were stimulated with 1 μg/ml LPS. Culture supernatants were collected after 24 hr and MMP-9 levels were measured using gelatin zymogram (a) and TNF-α levels were measured using ELISA (b). Peritoneal macrophages (c, e, f) and bone marrow-derived macrophages (d, g, h) were analysed. Fluorescence profiles of GITR specific staining (open area) were compared with that obtained using isotype-matching control antibody (filled area) (c, d). Cells were stimulated with anti-mGITR mAb added to the culture medium at 3, 10 and 30 μg/ml concentrations or 3 μg/ml of rmGITRL (GL). As negative controls, cells were also stimulated with heat-inactivated (HI) anti-GITR mAb or rat IgG (rIgG) at 30 μg/ml. Culture supernatants were collected after 48 hr and MMP-9 levels were measured using gelatin zymogram (e, g). TNF-α levels were measured using ELISA (f, h).
We then tested primary macrophages derived from mice. Both peritoneal and bone marrow-derived macrophages expressed low levels of GITR at their cell surface (Fig. 5c,d). Although no expression of GITRL was detected in the current study, these cells have been reported previously to express GITRL transiently after stimulation.13 When these cells were stimulated with anti-mGITR mAb, MMP-9 expression was induced dose-dependently (Fig. 5e,g) and TNF-α expression levels were up-regulated (Fig. 5f,h). However, recombinant mouse GITRL failed to induce MMP-9 (data not shown) and TNF-α (Fig. 5f,h).
The expression of GITR and GITRL was also detected in human primary macrophages derived from peripheral blood and in synovial macrophages in RA patients. Furthermore, stimulation of synovial macrophages with anti-GITR mAb resulted in a dose-dependent induction of MMP-9 and pro-inflammatory cytokines (our unpublished observations). This indicates that GITR-mediated inflammatory activation of macrophages occurs in both macrophage cell lines and primary macrophages. Our observation that it occurs in both mouse and human macrophages further indicates that the role of GITR in macrophage function is an evolutionarily conserved process.
It is an unexpected observation that rGITRL does not induce macrophage activation while anti-GITR mAb induces expression of inflammatory mediators such as MMP-9 and cytokines. Stimulation using rGITR tended to generate a weaker response than anti-GITR mAbs in both THP-1 and RAW264.7 cells (Figs 2 and 3). Since THP-1 macrophages and primary macrophages are in activated states because of macrophage differentiation (thus, the high basal levels of MMP-9 and cytokine expression), the stimulatory effects of rGITRL may have been masked. It is possible that cells exposed to GITRL require additional secondary stimulation to respond. Accordingly, we tested various stimulants including interferon-γ, various ligands of Toll-like receptors, and lysophosphatidylcholine, which were added in suboptimal levels in combination with rGITRL. However, none of these stimulants augmented the response to rGITRL. It is also possible that there could be an as yet unknown ligand of GITR which, through GITR, could induce inflammatory activation of the macrophages. We cannot exclude the possibility that stimulation of GITR on macrophages occurs through a direct cell-to-cell contact. GITRL and other co-stimulatory molecules present on the surface of contacting cells may work in concert to induce inflammatory activation of macrophages.
Staining patterns of TNF-α and MMP-9 overlap with that of GITR in atherosclerotic plaques
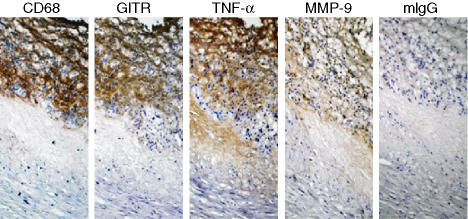
Our in vitro data indicate that GITR activation induces MMP-9 and pro-inflammatory cytokine expression in macrophages. To determine whether the presence of GITR correlates with the expression of MMP-9 and TNF-α in the pathological specimens, we compared the staining patterns of these molecules in serial sections of human carotid atherosclerotic plaques. As shown in Fig. 6, the GITR-staining pattern in the macrophage-rich area overlapped with MMP-9 and TNF-α staining patterns. Since macrophages in the atherosclerotic lesions express receptors for other inflammatory cytokines as well,1–7 stimulation through GITR and other inflammatory receptors can be expected to work in concert to induce expression of these inflammatory mediators.
Figure 6.
Expression of TNF-α and MMP-9 co-localized with that of GITR in human atherosclerotic plaques. Consecutive sections of a human carotid artherosclerotic plaque were stained for the expression of CD68, GITR, TNF-α and MMP-9. Pictures were taken at × 400 magnification. As a negative control, mouse IgG1 was used for staining.
Our data provided the first direct evidence that GITR and GITRL are expressed on the lipid-rich macrophages of human atherosclerotic plaques, and that they may play a role in the destabilization of atherosclerotic plaques, through inducing the expression of cytokines/chemokines, which exhibit multiple pro-inflammatory activities, and MMP-9, which can destabilize the integrity of atherosclerotic plaques via the degradation of the fibrous cap. On the basis of the present data, further analyses on GITR-mediated macrophage activation are warranted with regard to various atherogenic processes, such as induction of transmigration and expression of adhesion molecules and tissue factor.
Acknowledgments
This work was supported by an SRC fund to I.R.C., University of Ulsan, by Korea Science and Engineering Foundation.
Abbreviations
- FcR
Fc receptor
- GITR
glucocorticoid-induced tumour necrosis factor receptor family-related protein
- IL
interleukin
- mAb
monoclonal antibody
- MCP
monocyte chemoattractant protein
- MMP
matrix metalloproteinase
- NF-κB
nuclear factor-κB
- PMA
phorbol-12 myristate-13 acetate
- RA
rheumatoid arthritis
- TNF
tumour necrosis factor
- TNFSF
tumour necrosis factor superfamily
- TNFRSF
tumour necrosis factor receptor superfamily
- TRAF
tumour necrosis factor receptor-associated factors
References
- 1.Rus HG, Niculescu F, Vlaicu R. Tumor necrosis factor-alpha in human arterial wall with atherosclerosis. Atherosclerosis. 1991;89:247–54. doi: 10.1016/0021-9150(91)90066-c. [DOI] [PubMed] [Google Scholar]
- 2.Kaartinen M, Penttila A, Kovanen PT. Mast cells in rupture-prone areas of human coronary atheromas produce and store TNF-α. Circulation. 1996;94:2787–92. doi: 10.1161/01.cir.94.11.2787. [DOI] [PubMed] [Google Scholar]
- 3.Mach F, Schonbeck U, Sukhova GK, Bourcier T, Bonnefoy JY, Pober JS, Libby P. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40–CD40 ligand signaling in atherosclerosis. Proc Natl Acad Sci USA. 1997;94:1931–6. doi: 10.1073/pnas.94.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee WH, Kim SH, Lee Y, Lee BB, Kwon B, Song H, Kwon BS, Park JE. Tumor necrosis factor receptor superfamily 14 is involved in atherogenesis by inducing proinflammatory cytokines and matrix metalloproteinases. Arterioscler Thromb Vasc Biol. 2001;21:2004–10. doi: 10.1161/hq1201.098945. [DOI] [PubMed] [Google Scholar]
- 5.Kim WJ, Lee WH. LIGHT is expressed in foam cells and involved in destabilization of atherosclerotic plaques through induction of matrix metalloproteinase-9 and IL-8. Immune Network. 2004;4:116–22. [Google Scholar]
- 6.Kang YJ, Kim WJ, Bae HU, Kim DI, Park YB, Park JE, Kwon BS, Lee WH. Involvement of TL1A and DR3 in induction of pro-inflammatory cytokines and matrix metalloproteinase-9 in atherogenesis. Cytokine. 2005;29:229–35. doi: 10.1016/j.cyto.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Kim SH, Lee WH, Kwon BS, Oh GT, Choi YH, Park JE. Tumor necrosis factor receptor superfamily 12 may destabilize atherosclerotic plaques by inducing matrix metalloproteinases. Jpn Circ J. 2001;65:136–8. doi: 10.1253/jcj.65.136. [DOI] [PubMed] [Google Scholar]
- 8.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 9.Nocentini G, Giunchi L, Ronchetti S, Krausz LT, Bartoli A, Moraca R, Migliorati G, Riccardi C. A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:6216–21. doi: 10.1073/pnas.94.12.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4(+) CD25(+) immunoregulatory T cells. Gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–23. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+) CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–42. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 12.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4(+) CD25(+) suppressor T cells in vivo. Nat Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 13.Tone M, Tone Y, Adams E, Yates SF, Frewin MR, Cobbold SP, Waldmann H. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proc Natl Acad Sci USA. 2003;100:15059–64. doi: 10.1073/pnas.2334901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ronchetti S, Zollo O, Bruscoli S, Agostini M, Bianchini R, Nocentini G, Ayroldi E, Riccardi C. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol. 2004;34:613–22. doi: 10.1002/eji.200324804. [DOI] [PubMed] [Google Scholar]
- 15.Kanamaru F, Youngnak P, Hashiguchi M, Nishioka T, Takahashi T, Sakaguchi S, Ishikawa I, Azuma M. Costimulation via glucocorticoid-induced TNF receptor in both conventional and CD25+ regulatory CD4+ T cells. J Immunol. 2004;172:7306–14. doi: 10.4049/jimmunol.172.12.7306. [DOI] [PubMed] [Google Scholar]
- 16.Kwon BYuKY, Ni J, et al. Identification of a novel activation-inducible protein of the tumor necrosis factor receptor superfamily and its ligand. J Biol Chem. 1999;274:6056–61. doi: 10.1074/jbc.274.10.6056. [DOI] [PubMed] [Google Scholar]
- 17.Kim JD, Choi BK, Bae JS, et al. Cloning and characterization of GITR ligand. Genes Immun. 2003;4:564–9. doi: 10.1038/sj.gene.6364026. [DOI] [PubMed] [Google Scholar]
- 18.Yu KY, Kim HS, Song SY, Min SS, Jeong JJ, Youn BS. Identification of a ligand for glucocorticoid-induced tumor necrosis factor receptor constitutively expressed in dendritic cells. Biochem Biophys Res Commun. 2003;310:433–8. doi: 10.1016/j.bbrc.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 19.Cardona ID, Goleva E, Ou LS, Leung DY. Staphylococcal enterotoxin B inhibits regulatory T cells by inducing glucocorticoid-induced TNF receptor-related protein ligand on monocytes. J Allergy Clin Immunol. 2006;117:688–95. doi: 10.1016/j.jaci.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 20.Moon SK, Cha BY, Kim CH. ERK1/2 mediates TNF-alpha-induced matrix metalloproteinase-9 expression in human vascular smooth muscle cells via the regulation of NF-kappaB and AP-1: involvement of the ras dependent pathway. J Cell Physiol. 2004;198:417–27. doi: 10.1002/jcp.10435. [DOI] [PubMed] [Google Scholar]
- 21.Bond M, Fabunmi RP, Baker AH, Newby AC. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF-kappa B. FEBS Lett. 1998;435:29–34. doi: 10.1016/s0014-5793(98)01034-5. [DOI] [PubMed] [Google Scholar]
- 22.Ballif BA, Shimamura A, Pae E, Blenis J. Disruption of 3-phosphoinositide-dependent kinase 1 (PDK1) signaling by the anti-tumorigenic and anti-proliferative agent n-alpha-tosyl-l-phenylalanyl chloromethyl ketone. J Biol Chem. 2001;276:12466–75. doi: 10.1074/jbc.M009939200. [DOI] [PubMed] [Google Scholar]
- 23.Han Y, Englert JA, Yang R, Delude RL, Fink MP. Ethyl pyruvate inhibits nuclear factor-κB-dependent signaling by directly targeting p65. J Pharmacol Exp Ther. 2005;312:1097–105. doi: 10.1124/jpet.104.079707. [DOI] [PubMed] [Google Scholar]
- 24.Wahl C, Liptay S, Adler G, Schmid RM. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J Clin Invest. 1998;101:1163–74. doi: 10.1172/JCI992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Auwerx JH, Deeb S, Brunzell JD, Peng R, Chait A. Transcriptional activation of the lipoprotein lipase and apolipoprotein E genes accompanies differentiation in some human macrophage-like cell lines. Biochemistry. 1988;27:2651–5. doi: 10.1021/bi00408a003. [DOI] [PubMed] [Google Scholar]
- 26.Akuzawa N, Kurabayashi M, Ohyama Y, Arai M, Nagai R. Zinc finger transcription factor Egr-1 activates Flt-1 gene expression in THP-1 cells on induction for macrophage differentiation. Arterioscler Thromb Vasc Biol. 2000;20:377–84. doi: 10.1161/01.atv.20.2.377. [DOI] [PubMed] [Google Scholar]