Abstract
Injection of the same antigen following primary immunization induces a classic secondary response characterized by a large quantity of high-affinity antibody of an immunoglobulin G class produced more rapidly than in the initial response – the products of memory B cells are qualitatively distinct from that of the original naive B lymphocytes. Very little is known of the help provided by the CD4 T cells that stimulate memory B cells. Using antigen-specific T-cell receptor transgenic CD4 T cells (DO11.10) as a source of help, we found that naive transgenic T cells stimulated memory B cells almost as well (in terms of quantity and speed) as transgenic T cells that had been recently primed. There was a direct correlation between serum antibody levels and the number of naive transgenic T cells transferred. Using T cells from transgenic interleukin-2-deficient mice we showed that interleukin-2 was not required for a secondary response, although it was necessary for a primary response. The results suggested that the signals delivered by CD4 T cells and required by memory B cells for their activation were common to both antigen-primed and naive CD4 T cells.
Keywords: adoptive transfer, CD4 T cells, CD45RB, memory, transgenic
Introduction
During the immune response to infection, activated T cells undergo numerous phenotypic as well as functional changes.1–3 These changes allow the T cell to orchestrate a protective immune response, part of which is the provision of help for B-cell antibody production.4–6 Following an initial expansion of the responding cells and clearance of the infection, the immune response contracts.7–10 This ensures that the immune repertoire does not become skewed following infection.11 However, despite this mass cull of activated lymphocytes immunological memory is usually established, which is defined as a faster and stronger response following re-infection. Evidence shows that the secondary humoral response is both qualitatively [higher affinity and immunoglobulin G (IgG) instead of IgM] and quantitatively different from the primary response.4,5,12,13 Memory B cells have been fundamentally altered and are substantially different from naive B cells.14 However, the type of CD4 T cell that provides help for memory B cells is poorly understood. Whereas antigen-primed CD4 T cells may be distinguished from naive cells on the basis of phenotype, a heightened recall response to antigen and committed cytokine production,2,3,15–17 there is no evidence that the ability of T cells to respond rapidly or that the T helper type 1 (Th1)/Th2 cytokines they produce are required to help memory B cells produce antibody.
Many of the cell surface markers used to distinguish memory CD4 T cells are indicative of recent antigen experience. One of these, CD45R (CD45RB in mice), may express high or low molecular weight isoforms depending upon its antigen experience.18 CD4 T cells that have not encountered antigen are CD45Rhi; upon antigen stimulation they express the low molecular weight isoform, CD45Rlo. However, under the right conditions the antigen-experienced T cell may re-express the high molecular weight isoform and return to a naive phenotype.19–21 Hence one may not always be able to distinguish truly naive T cells from those that have previously encountered antigen. To determine the T-cell requirements needed to stimulate memory B cells, we used a T-cell receptor (TCR) transgenic model where it was possible to obtain defined populations of antigen-specific naive or primed CD4 T cells.
During a primary response, previous studies showed that TCR transgenic CD4+ T cells specific for a peptide of ovalbumin (OVA) from the DO11.10 strain9 provided cognate help for naive B cells by direct physical contact, initially at the T-cell–B-cell junction and a day later within the follicles of draining lymph nodes.22 This model has not been used before in combination with memory B cells. In the present study we asked whether naive as well as primed T cells could provide the help needed to initiate antibody synthesis by OVA-specific memory B cells. We evaluated the relative importance of an increased frequency of antigen-specific CD4 T cells compared with the enhanced activity that T cells acquire following antigen priming. We also determined whether some of the changes induced in naive T cells following antigen stimulation [i.e. interleukin-2 (IL-2) production] were required for a secondary humoral response.
Materials and methods
Animals
BALB/c.Ighb mice (Balb/c) (from MK Jenkins, Minneapolis, MN) were bred in specific pathogen-free conditions and maintained after weaning under conventional husbandry in the Biological Services Unit of the University of Manchester. The DO11.10 and DO11.10-severe combined immunodeficient (SCID) mice on a BALB/c background (breeding colony from the University of Glasgow), DO11.10 IL-2−/− mice (kindly provided by T. Hunig, Wurtzburg) and SCID mice (University of Manchester colony) were housed in isolator air-filtered cages.
Antibodies
KJ1-26 clonotypic monoclonal antibody (mAb), specific for the OVA-peptide-specific TCR, was produced from a cell hybridoma line held by the Scottish Antibody Production Unit (Carluke, UK) and was purified using Prosep affinity chromatography. The following mAbs were used for flow cytometry analysis: fluorescein isothiocyanate (FITC)-CD45RB, phycoerythrin (PE)-CD4, PE-B220 (all BD-Pharmingen, Oxford, UK), streptavidin-PE (SA-PE) (Sigma, Poole, UK) and streptavidin Tri-colour (Caltag-Medsystems Ltd, Botolph Claydon, UK). The following biotinylated (bio) mAbs were used for cell purification: bio-CD4, bio-CD8, bio-Thy-1.2, bio-CD45RB (all Pharmingen) and bio-F4/80 (Caltag-Medsystems Ltd).
Antigen
For primary immunization of B cells 100 μg alum-precipitated chicken ovalbumin (ap-OVA) (Grade V, Sigma) was injected intraperitoneally (i.p.). For secondary challenge 10 μg or 100 μg soluble OVA (sol-OVA) was injected i.p. The OVA peptide 323–339 (synthesized by Sigma Genosys, Cambridge, UK) was used to prime T cells.
Antibody assays
Anti-OVA antibody was detected by enzyme-linked immunosorbent assay (ELISA) from serum samples stored at − 20° before use, as described previously.23 The class of antibody produced was predominantly IgG1 with low levels of IgG2a and almost no IgG2b. Ninety-six-well flat-bottom microtitre flexible assay plates (Dynex, Ashford, UK) were coated for 1 hr with 50 μl/well 100 μg/ml OVA. Plates were washed five times with phosphate-buffered saline containing 0.02% Tween (PBST) and were incubated for 1 hr with 100 μl blocking buffer per well (Megablock from Bionostics, Devens, MA, 1/500 in PBS). Plates were washed and 50 μl blocking buffer plus 25 μl of either sample (experimental) or the standard, which had a known concentration of IgG-specific anti-OVA antibody (1 unit approximates 0.1 μg) or control normal serum (used as a background subtraction) was added to individual wells. All samples were diluted threefold starting from 1/90 and run in duplicate. After 1 hr the incubation plates were washed and incubated with 50 μl of alkaline phosphatase-conjugated rabbit anti-mouse IgG (Sigma) (1/1000 in PBST) per well for 1 hr. Plates were washed and incubated with 50 μl p-nitrophenyl phosphate substrate (5 mg tablet, from Sigma, dissolved in 5 ml diethanolamine buffer, 1 mg/ml, pH 9.8) per well in the dark for 15–20 min. The reaction was stopped with 100 μl 3 m NaOH per well, and the optical density (OD) was read at 405 nm using a Dynex MRX II ELISA reader (Dynex). The results were analysed using Dynex Revelation 4.21, which calculated the concentration of anti-OVA in units/ml using a standard curve included on each plate.
Cell separation
To obtain primed B cells, normal BALB/c mice were injected i.p. with 100 μg ap-OVA. Eight weeks later the mice were killed and spleen, mesenteric, inguinal, cervical and axillary lymph nodes were removed, teased apart with forceps and filtered through a nylon monofilament mesh filter. Red blood cells were lysed in Boyles solution (1 ml per spleen) at 37° for 5 min, the remaining cells were washed with PBS/fetal calf serum and pooled; any viable cells were counted using an electronic Scharfe system CASY1 counter. The total cell population was stained with bio-CD8 (12 pg per 108 cells), bio-CD4 (24 pg per 108 cells), bio-Thy-1.2 (96 pg per 108 cells), and bio-F4/80 (96 pg per 108 cells) for 30 min on ice. A sample was removed and stained with SA-PE for analysis before depletion. Biomag® Streptavidin beads (Metachem Diagnostics Ltd, Piddington, UK) were washed, mixed with the cells at a concentration of 50 μl beads per 108 cells and incubated with occasional resuspension for 30 min on ice. The cell/bead suspension was placed on a strong magnet, the ferrous beads with cells attached were removed by magnetic adhesion, the cells that remained in suspension recovered and were washed twice with PBS/fetal calf serum. A sample was stained with SA-PE to assess the purity of the separation. The cells were depleted a second time as before using 200 μl beads per 108. Finally, the cells were counted, a sample was stained with PE-B220 to determine the percentage of B cells and the concentration was adjusted for injection.
To obtain primed T cells a single cell suspension of lymph node cells from DO11.10-SCID mice was washed and stained with mAbs KJ1-26 and anti-CD4. KJ+ CD4+ T cells, either 1 × 106 or 5 × 106, were injected intravenously to SCID recipients which were then primed i.p. with 100 μg ap-OVA-peptide. Recipient mice were killed 7–14 days later, spleen and lymph nodes were removed, the cells were counted and a sample was stained with KJ1-26 and anti-CD4 to determine the number of donor transgenic T cells. The remaining cells were stained with rat anti-mouse CD45RB (0.5 μg per 108 cells) and depleted of CD45RBhi cells using two rounds of Biomag® goat anti-rat IgG beads (Metachem Diagnostics Ltd) as described above; 20 μl beads were used per 106 cells. The remaining population of cells was stained with PE-CD4, FITC-CD45RB, bKJ1-26 and Tri-SA and analysed by flow cytometry (FACScan®, Becton Dickson, Cowley, UK) to determine purity and number of cells for transfer.
Statistical analysis
Differences between means were evaluated using Student’s t-test.
Results
A model to evaluate the ability of transgenic T cells to provide help for memory B cells
Transgenic T cells from DO11.10 donors were adoptively transferred with B cells to immunodeficient SCID recipients and challenged with OVA. Similar to many proteins,24,25 OVA in soluble form (sol-OVA) induces tolerance in unprimed animals whereas OVA plus alum-precipitated adjuvant (ap-OVA) stimulates a primary response.23 However, once primed, a secondary response can be evoked by OVA in soluble form. To test this in our model, naive transgenic T cells, identified by mAb KJ1-26 (KJ+), were adoptively transferred with purified B cells from either naive or ap-OVA-primed BALB/c.Igh (BALB/c) donors to SCID recipients and challenged with sol-OVA. Primed B cells were also transferred on their own to assess the effectiveness of the CD4 T-cell depletion. On day 14 after transfer, recipients were killed and serum antibody responses were measured. The results demonstrated that naive transgenic T cells did not help naive B cells to produce antibody following sol-OVA challenge (Fig. 1a) as expected23 but clearly showed that they were competent to assist antibody synthesis by B cells that had previously been primed.
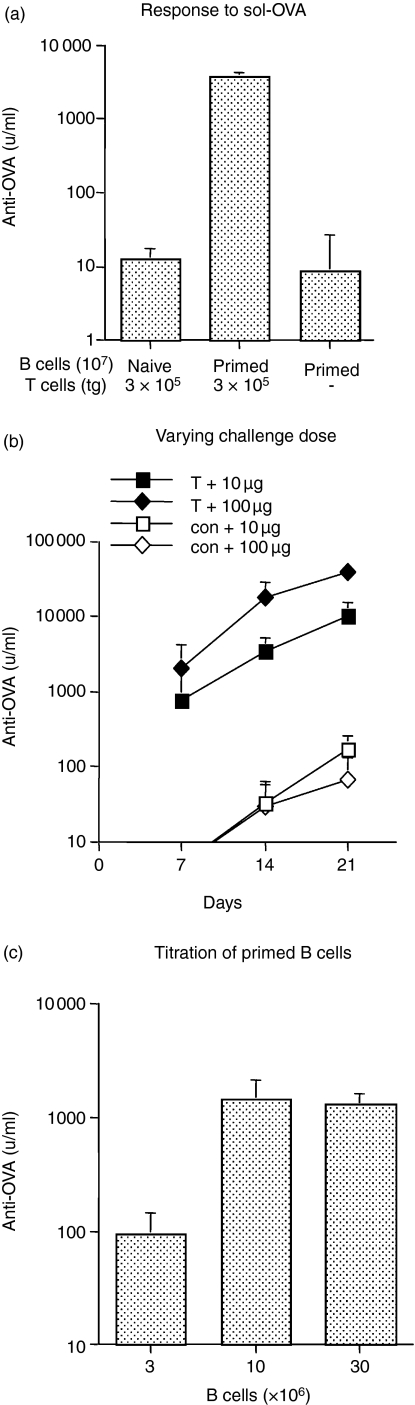
Figure 1.
Model to evaluate the ability of transgenic KJ+ CD4+ T cells to provide help for memory B cells. (a) Naive KJ+ CD4+ T cells help primed B cells but not naive B cells following sol-OVA challenge. SCID recipients were injected with 3 × 105 naive KJ+ CD4+ T cells together with 107 primed B cells or 107 naive B cells or with 107 primed B cells alone as a control. Mice were challenged i.p. with 10 μg sol-OVA immediately after transfer and anti-OVA antibody levels were measured on day 14. The values represent the geometric means + SD of six recipients per group. (b) The effect of antigen dose on antibody production by primed B cells. SCID recipients received 107 primed B cells alone (con), or together with 3 × 105 naive KJ+ CD4+ T cells, and were challenged i.p. with 10 μg or 100 μg sol-OVA. The values shown represent the geometric means + SD of five or two (control) recipients per group. (c) Titration of primed B cells. SCID recipients were injected with 3 × 106, 107 or 3 × 107 primed B cells together with 3 × 106 KJ+ CD4+ T cells; mice were challenged i.p. with 10 μg sol-OVA immediately after transfer and bled 14 days later. The values represent the geometric means + SD of six recipients per group.
Based on similar experiments in the rat,23 10 μg sol-OVA was chosen to stimulate a secondary response. The effect of increasing the dose to 100 μg was examined in direct comparison with 10 μg (Fig. 1b). The larger dose evoked antibody levels nearly three times higher on day 7 than those which received 10 μg sol-OVA (Fig. 1b), a difference that increased to five- and four-fold on days 14 and 21, respectively. The lower dose of antigen was selected as a standard because this produced a substantial response and provided sufficient scope for detecting a difference, should there be one, between naive and primed T cells.
To measure T-cell help for B cells, it was important to optimize the number of primed B cells required to elicit a response. B cells were purified from ap-OVA-primed BALB/c donors. Because a majority of the population would contain B cells with other specificities, relatively large numbers of ‘primed’ cells (3 × 106, 107, and 3 × 107) were transferred to SCID recipients with a high dose of transgenic T cells (3 × 106). In comparison with 107 B cells, the response of 3 × 106 B cells measured on day 14, was considered too low (Fig. 1c). Since the response of 3 × 107 B cells was not significantly different, we opted to transfer 107 B cells in subsequent experiments. Furthermore, to exclude transgenic T cells that may have been primed because they coexpress an endogenous TCR,26 donor T cells were obtained hereafter from DO11.10-SCID mice which contain only naive CD4 T cells that express the DO11.10 TcR.
Comparison of help from naive and primed transgenic T cells for primed B cells
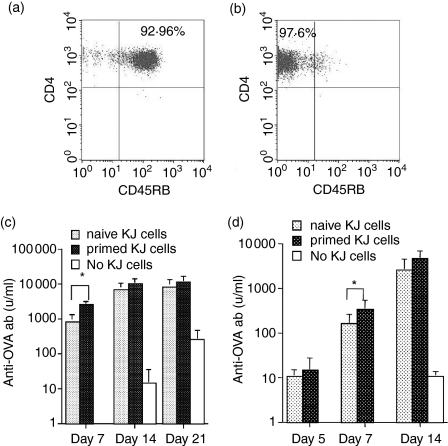
It is generally held that CD4 T cells activated during the primary response are essential in providing the quality of T-cell help needed to stimulate memory B cells. However, the experiments in Fig. 1 clearly indicated that naive transgenic T cells could also provide help for memory B cells. We wanted to compare, on a cell-for-cell basis, the ability of naive versus primed transgenic CD4 T cells to stimulate antibody synthesis by primed B cells in vivo. It was not possible to recover transgenic T cells from directly primed DO11.10 mice. We confirmed what others have reported,27 that transgenic T cells that will be primed by injecting DO11.10 mice directly with OVA could not be identified by phenotype as a source for transfer. Nor was it feasible to recover large numbers of primed transgenic T cells following transfer to wild-type BALB/c hosts. Although KJ+ CD4+ T cells could be readily identified in BALB/c recipients, they represented less than 3% of the lymphocyte population at the peak of the response (day 7) and numbers declined exponentially thereafter.9,28–31 Hence, transgenic T cells from DO11.10-SCID donors were transferred to intermediate SCID recipients where donor T cells could survive longer because they did not have to compete with host lymphocytes for limited resources.32,33 SCID recipients were challenged with 100 μg ap-OVA and primed transgenic T cells recovered from lymph nodes 10 days later. Before transfer, about 90–95% of the KJ+ CD4+ T cells were CD45RBhi (e.g. Fig. 2a); 10 days following transfer and challenge, the KJ+ CD4+ donor T cells were approximately 70% CD45RBlo, CD62Llo and CD44hi. Lymph node cells were depleted of CD45RBhi cells and a population that was 97.6% CD45RBlo was obtained (Fig. 2b). CD45RBlo KJ+ CD4+ T cells (3 × 105) were transferred with 107 OVA-primed B cells to SCID recipients and challenged with sol-OVA. For a direct comparison, 3 × 105 CD45RBhi KJ+ CD4+ T cells (Fig. 2a) from naive DO11.10-SCID donors were transferred with 107 OVA-primed B cells and challenged with sol-OVA. As a control, 107 B cells were also transferred on their own. Apart from a small difference at day 7 the help provided by naive transgenic T cells was nearly equal to that of primed transgenic T cells (Fig. 2c). To confirm this unexpected result, the experiment was repeated with a lower dose of KJ+ CD4+ T cells (1 × 105) and the response was measured at day 5, a point where low levels of antibody were just within the limits of detection (Fig. 2d). The results were similar to the above experiment. Apart from a small transient difference on day 7, naive transgenic T cells were as capable as primed transgenic T cells on a cell-for-cell basis in helping memory B cells produce antibody.
Figure 2.
Comparison of help provided by naive and primed KJ+ CD4+ T cells for primed B cells. (a) A representative analysis of naive KJ+ CD4+ T cells from DO11.10-SCID donors expressing a CD45RBhi phenotype. (b) An analysis of primed KJ+ CD4+ T cells used for injection recovered from intermediate SCID mice injected 10 days earlier with 100 μg ap-OVA-pep and depleted of CD45RBhi T cells. SCID recipients were injected with 3 × 105 (c) or 1 × 105 (d) naive or primed KJ+ CD4+ T cells together with 107 primed B cells, challenged immediately with 10 μg sol-OVA, and antibody levels measured on selected days. Control groups received 107 B cells alone. Values represent the geometric means + SD of six recipients per group (naive vs primed, *P < 0·05).
Number of transgenic T cells determines levels of antibody response
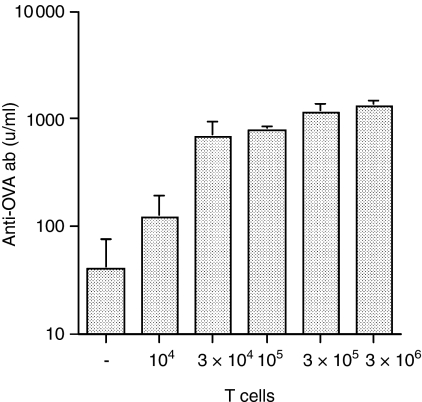
Following proliferation of specific T and B cells during the primary response, some members of the clonally expanded population remain and are responsible for long-term memory. It was of interest to determine whether the size of the secondary response was controlled by the number of CD4 T cells. The experiment in Fig. 1(c) showed that when CD4 T cells were in excess, the size of the antibody response was a function of the number of primed B cells available. To determine the effect of CD4 T-cell numbers, the response by 107 primed B cells, cotransferred to SCID recipients with increasing numbers of naive KJ+ CD4+ T cells (104, 3 × 104, 105, 3 × 105 or 3 × 106) was measured following challenge with 10 μg sol-OVA. The antibody response obtained with 104 transgenic T cells was just above background (Fig. 3) and 3 × 105 transgenic T cells induced a near maximum response; 10-fold more transgenic T cells did not significantly increase antibody levels further. The titration of naive transgenic T cells showed that the amount of antibody produced by the same cohort of memory B cells correlated directly with the dose of CD4 T cells transferred.
Figure 3.
The response by primed B cells is limited by the number of transgenic T cells transferred. SCID recipients were injected with 107 primed B cells together with graded doses of KJ+ CD4+ T cells, challenged i.p. with 10 μg sol-OVA immediately after cell transfer and serum was collected on day 14. The values represent the geometric means + SD of four to six recipients per group.
IL-2 was required for the primary but not the secondary antibody response
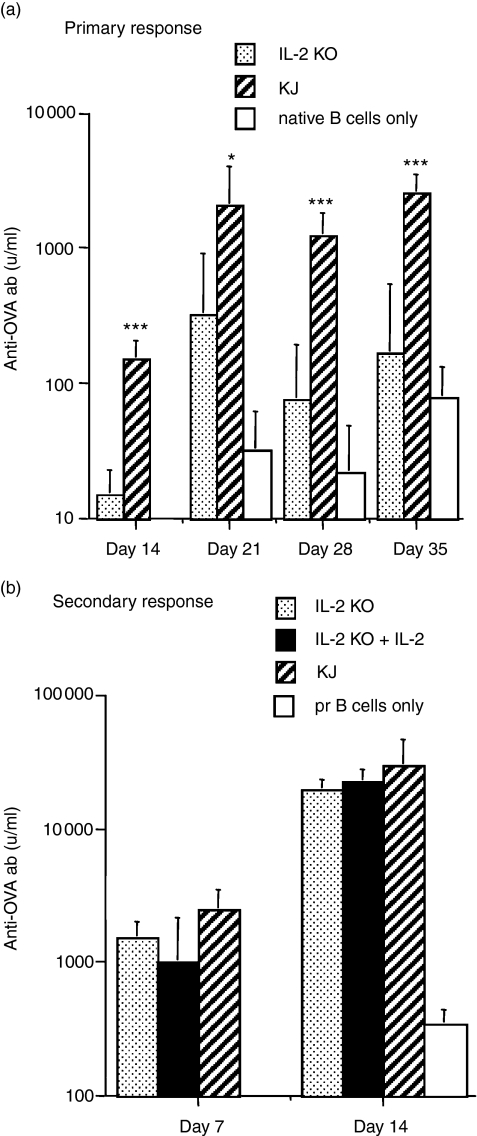
The role of cytokines has been extensively studied in the primary antibody response but very little is known of the cytokine requirements for stimulating memory B cells. The fact that naive transgenic T cells provided help for memory B cells suggested that the requirements for stimulating memory B cells were less stringent and that cytokines produced by naive T cells, e.g. IL-2,34 may be involved. Our first examination of the role of IL-2 was to transfer primed B cells alone to SCID mice in the absence of T cells, challenge with sol-OVA and treat the recipients with human recombinant IL-2 (rIL-2). Human rIL-2 has been used successfully in numerous murine studies.35,36 SCID recipients received 107 memory B cells and were injected twice daily with 3 × 105 IU of rIL-2 i.p. on days 1, 2, 3 and 4 after cell transfer. No antibody synthesis was detected (data not shown). This experiment would not exclude the possibility that primed B cells required T-cell-derived signals in addition to IL-2. To test this possibility we made use of IL-2–/– knockout (KO) mice37 that had been back-crossed onto the DO11.10 strain (transgenic IL-2–/–)38. Studies showed that naive CD4 T cells from IL-2–/– mice were unable to induce IgM antibody synthesis by naive B cells in vitro37 although these mice had normal IgM and elevated IgG1 serum levels and produced a normal IgM response to a viral challenge.39 To assess their function in vivo, naive KJ+ IL-2–/– CD4+ T cells were transferred with naive B cells to SCID recipients and injected with the adjuvant form of OVA (ap-OVA) to induce a primary antibody response. In comparison with KJ+ CD4+ T cells from the wild-type strain, the transgenic-IL-2 KO T cells were markedly deficient in inducing a primary response (Fig. 4a). To test their ability to provide help for a secondary response, naive transgenic IL-2 KO T cells were transferred with primed B cells to SCID recipients and challenged with sol-OVA. In an initial experiment the antibody response at day 7 in the transgenic IL-2–/– group was reduced compared with the wild-type transgenic T cells (P < 0.05) but there was no difference at day 14 (data not shown). Since this small difference could have arisen by normal variation, the experiment was repeated and included a group receiving rIL-2. In contrast with the primary response, naive transgenic IL-2–/– T cells provided help that was equivalent to that of conventional KJ+ CD4+ T cells (Fig. 4b); the addition of rIL-2 did not enhance the response. There was no evidence that IL-2 was required to stimulate memory B cells.
Figure 4.
Transgenic T cells from DO11.10-IL-2–/– mice help memory but not naive B cells produce antibody. (a) Primary response: SCID mice received 107 naive B cells from non-immunized mice together with 3 × 105 T cells from naive DO11.10-IL-2–/– mice (IL-2 KO), or from naive conventional DO11.10 mice (KJ) or received no transgenic T cells. All recipients were challenged with 100 μg ap-OVA on the day of transfer. Values represent the geometric means + SD of six recipients per group (IL-2 KO versus KJ: *P < 0·05; ***P < 0·001). (b) Secondary response: SCID mice were injected with 107 B cells from OVA-primed mice together with 3 × 105 naive transgenic T cells from DO11.10-IL-2–/– (IL-2 KO), or DO11.10 donors (KJ), or 3 × 105 T cells from DO11.10-IL-2–/– mice plus twice daily injections of 3 × 105 IU rIL-2 per day for 6 days (IL-2 KO + IL-2), or no T cells (pr B cells only). All recipients were challenged with 10 μg sol-OVA on the day of transfer. Values represent the geometric means + SD of six recipients per group.
Discussion
The present investigation explored the role of CD4 T cells, not in generating memory B cells, but in stimulating them into antibody synthesis once they had been produced. Although responsible for the classic secondary response, very little is known of the signals required from CD4 T cells to transform resting memory B cells into antibody production. A detailed analysis of the memory response has been constrained by the low frequency of antigen-specific CD4 T cells in wild-type animals and the paucity of reagents that recognize the TCR for major histocompatibility complex class II-presented peptides.
The DO11.10 strain provided a source of TCR-specific CD4 T cells that could be used quantitatively to provide help for OVA-specific memory B cells. Using this model it was possible to explore the effects of both naive and antigen-primed CD4 T cells. In agreement with in vivo28,30,40 and in vitro41 studies, sol-OVA challenge induced a majority of the KJ+ CD4+ T cells to express the typical CD45RBlo CD62Llo CD44hi phenotype associated with primed T cells. Hence, antigen-primed CD45RBlo transgenic T cells were used in the present study to provide help for memory B cells. At an early stage in the investigation it became apparent that naive transgenic T cells were quite capable of helping memory B cells produce antibody; no pre-encounter with antigen was necessary. Although others have shown the effects of a high frequency of transgenic T cells in generating an enhanced primary antibody response,42 to our knowledge the present study is the first report to show that the size of the memory B-cell response was limited by the frequency of antigen-specific T cells. Transgenic T cells that had been deliberately stimulated in vivo with OVA and expressing the CD45RBlo isoform increased antibody production significantly 7 days after challenge (P < 0.05) but not at earlier (day 5) or later (days 14–21) times. The modestly enhanced, although transient, antibody synthesis could be used as evidence to support current concepts as to the value of a primed T-cell population. Alternatively, the same evidence could be used to argue that primed T cells offered very little advantage over the naive transgenic T cells either in the speed of onset or quantity of antibody produced by memory B cells. The latter view appears to be at odds with traditional ideas of memory. Perhaps the results were a product of the experimental model.
It is important to consider the T-cell-deficient environment of the SCID recipients where unprovoked proliferation may occur. Perhaps naive transgenic T cells were activated in the lymphocyte-deficient tissues as a consequence of homeostatic proliferation.43–45 We do not think this was likely because other studies have shown that KJ+ CD4+ T cells failed to proliferate in the absence of antigen in either irradiated46 or SCID recipients (D. Duffy, E.B. Bell, unpublished observations) 1 or 2 weeks after transfer. Even after 3 weeks, only a fraction of the population transferred to RAG–/– mice proliferated homeostatically and those that did underwent at most one or two cycles of division.44,47 In contrast, the effect of antigen challenge on the transferred transgenic T cells would be immediate and long before any influence could be exerted by the T-cell-deficient host.
Could the results have been a function of the high frequency of antigen-specific T cells transferred, for example, enabling memory B cells to accept help from an inappropriate source. This explanation implies that a naive T lymphocyte, not yet programmed by antigen contact, could provide the precise requirements for memory B cells simply by force of numbers. Such a hypothesis would be difficult to defend. The memory B-cell response was also very sensitive to T-cell dose. Large numbers of transgenic T cells were not required and the injection of as few as 30 000 naive transgenic T cells was able to evoke a sizable response.
Given that the model was optimized for naive transgenic T cells, could this have obscured the benefits of a primed population? Perhaps the premium effects of primed transgenic T cells would have been observed had lower numbers of transgenic T cells been transferred or a lower dose of sol-OVA used for challenge. In fact, in vitro studies showed that primed transgenic CD4 T cells were more sensitive to antigen-induced proliferation.9,17,30 Although the present evidence does not include a full titration of primed transgenic T cells or the use of smaller antigen doses, the experiments were deliberately designed to allow detection of an enhanced response by primed transgenic T cells. The antigen dose used (10 μg sol-OVA) was exceptionally low, 10-fold below the usual dose and insufficient to generate a maximum response from the same cohort of primed B cells (Fig. 1b). We showed elsewhere that the effect of reducing the antigen dose in vivo was to reduce the number of transgenic T cells that were recruited into the response.28 The two doses of transgenic T cells transferred (3 × 105 and 105) were both suboptimal for the number of memory B cells transferred. Note also that the amount of antibody produced by the low dose of primed T cells was proportionally lower (Fig. 2c,d). Further experimentation will help to clarify these issues. However, on a cell-for-cell basis, the present results suggested that naive transgenic T cells were nearly as effective as those that had been preprimed.
The effectiveness of naive T cells23,48,49 suggested that help for memory B cells may need to be viewed anew. Primed and naive T cells appeared to share a common property crucial for memory B-cell stimulation. Surprisingly the literature revealed very little information on cytokine requirements for activating memory B cells in vivo. One in vitro study showed that induction of IgG1 synthesis by primed B cells could occur via two separate pathways, one involving IL-2, and the other involving IL-4 and IL-5.50 Since the major cytokine produced by naive transgenic T cells was IL-2,34 we examined whether it was required by memory B cells. Injections of rIL-2 plus antigen in the absence of T cells failed to stimulate memory B cells. DO11.10 T cells lacking IL-2 (transgenic IL-2–/–)38 stimulated primed B cells to produce a strong secondary antibody response which was not enhanced by the addition of rIL-2 injections. Although IL-2 was not required to help memory B cells it was required for a primary antibody response in agreement with the work of others.37,51,52 DO11.10-IL2–/– T cells were unable to help naive B cells produce antibody. It remains to be seen whether cytokines represent the shared element between naive and primed T cells.
The present study is not the only investigation to report an effect of unprimed T cells in a memory response. Leclerc et al.53 found that memory B cells responded to defined B-cell and T-cell viral epitopes in the absence of primed T helper cells. In addition, using CD4 T cells from wild-type rather than transgenic mice, CD45Rhi T cells from naive donors readily stimulated OVA-specific memory B cells into antibody synthesis following adoptive transfer.23 However, not all investigators have found naive CD4 T cells to be as effective as primed T cells. In a model assessing protection against viral infection, Maloy et al.54 reported that naive transgenic CD4 T cells (tg7) specific for a vesicular stomatitis virus peptide, failed to protect wild-type mice, whereas 10-fold fewer tg7 T cells, primed in vitro to produce interferon-γ, were protective. The latter study highlights a major distinction between T cells designed to provide help for memory B cells and those, for example, engaged in attacking a viral infection where rapid production of cytokines is imperative. It has recently become clear that for CD4 T cells to make the transition from naive to the effector stage requires two encounters with antigen:55 the first to instruct a Th1 or Th2 programme (antigen presented in lymph nodes/spleen) and the second to initiate cytokine production, e.g. at the site of infection. T cells that have been primed and then driven to an end-stage effector cell would not represent a long-lived memory T cell. Memory B cells represent fully committed, class-switched, surviving members of an antigen-driven event.14 There was no evidence that CD4 T cells, which provide memory B cells with help, needed to have seen antigen previously or needed to be programmed to differentiate down a Th1 or Th2 pathway; clearly virgin CD4 T cells sufficed.
It may be relevant to note that only CD45Rhi CD4 T cells are known to have long life-spans.56–58 A recent study measuring the turn-over of human CD4 T cells in vivo58 observed that naive T cells were very long-lived (dividing on average less than once a year) whereas both so-called ‘effector/memory’ (CD45RO+ CD62Llo CCR7–) and ‘central memory’ (CD45RO+ CD62Lhi CCR7+) subsets were relatively short-lived (half-lives of 6 and 17 days, respectively). The observation that antigen-primed CD45Rlo CD4 T cells revert to a CD45Rhi naive phenotype in the absence of antigen20,23,31 suggests that a higher frequency of previously primed, but with time quiescent, CD45Rhi T helper cells could be responsible for ensuring that immunological memory is long-lived.
Acknowledgments
This work was funded by grants from the Arthritis Research Council (E.B.B.) and the UK Biotechnology and Biological Sciences Research Council (E.B.B. and P.G.).
Abbreviations
- ap
alum-precipitated
- bio
biotinylated
- ELISA
enzyme-linked immunosorbent assay
- IgG
immunoglobulin G
- IL-2
interleukin-2
- i.p.
intraperitoneally
- KJ+
monoclonal antibody KJ1-26 positive
- OD
optical density
- OVA
ovalbumin
- pep
peptide
- SCID
severe combined immune deficiency
- sol
soluble
- TCR
T-cell receptor
- Th
T helper
References
- 1.Sprent J, Surh CD. T cell memory. Annu Rev Immunol. 2002;20:551–79. doi: 10.1146/annurev.immunol.20.100101.151926. [DOI] [PubMed] [Google Scholar]
- 2.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol. 1998;16:201–23. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 4.MacLennan IC, Gulbranson-Judge A, Toellner KM, Casamayor-Palleja M, Chan E, Sze DM, Luther SA, Orbea HA. The changing preference of T and B cells for partners as T-dependent antibody responses develop. Immunol Rev. 1997;156:53–66. doi: 10.1111/j.1600-065x.1997.tb00958.x. [DOI] [PubMed] [Google Scholar]
- 5.Jacob J, Kassir R, Kelsoe G. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl) acetyl. I. The architecture and dynamics of responding cell populations. J Exp Med. 1991;173:1165–75. doi: 10.1084/jem.173.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toellner KM, Luther SA, Sze DM, Choy RK, Taylor DR, MacLennan IC, Acha-Orbea H. T helper 1 (Th1) and Th2 characteristics start to develop during T cell priming and are associated with an immediate ability to induce immunoglobulin class switching. J Exp Med. 1998;187:1193–204. doi: 10.1084/jem.187.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sprent J. Fate of H2-activated T lymphocytes in syngeneic hosts. I. Fate in lymphoid tissues and intestines traced with 3H-thymidine, 125I-deoxyuridine and 51chromium. Cell Immunol. 1976;21:278–302. doi: 10.1016/0008-8749(76)90057-5. [DOI] [PubMed] [Google Scholar]
- 8.Sprent J, Miller JF. Fate of H2-activated T lymphocytes in syngeneic hosts. III. Differentiation into long-lived recirculating memory cells. Cell Immunol. 1976;21:314–26. doi: 10.1016/0008-8749(76)90059-9. [DOI] [PubMed] [Google Scholar]
- 9.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–39. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 10.Rathmell JC, Thompson CB. The central effectors of cell death in the immune system. Annu Rev Immunol. 1999;17:781–828. doi: 10.1146/annurev.immunol.17.1.781. [DOI] [PubMed] [Google Scholar]
- 11.Goldrath AW, Bevan MJ. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402:255–62. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 12.Siekevitz M, Kocks C, Rajewsky K, Dildrop R. Analysis of somatic mutation and class switching in naive and memory B cells generating adoptive primary and secondary responses. Cell. 1987;48:757–70. doi: 10.1016/0092-8674(87)90073-0. [DOI] [PubMed] [Google Scholar]
- 13.Okumura K, Julius MH, Tsu T, Herzenberg LA. Demonstration that IgG memory is carried by IgG-bearing cells. Eur J Immunol. 1976;6:467–72. doi: 10.1002/eji.1830060704. [DOI] [PubMed] [Google Scholar]
- 14.McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. doi: 10.1146/annurev.immunol.23.021704.115732. [DOI] [PubMed] [Google Scholar]
- 15.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–12. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 16.Zinkernagel RM, Bachmann MF, Kundig TM, Oehen S, Pirchet H, Hengartner H. On immunological memory. Annu Rev Immunol. 1996;14:333–67. doi: 10.1146/annurev.immunol.14.1.333. [DOI] [PubMed] [Google Scholar]
- 17.Rogers PR, Dubey C, Swain SL. Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen. J Immunol. 2000;164:2338–46. doi: 10.4049/jimmunol.164.5.2338. [DOI] [PubMed] [Google Scholar]
- 18.Bell EB. Function of CD4 T cell subsets in vivo: expression of CD45R isoforms. Semin Immunol. 1992;4:43–50. [PubMed] [Google Scholar]
- 19.Bell EB, Sparshott SM. Interconversion of CD45R subsets of CD4 T cells in vivo. Nature. 1990;348:163–6. doi: 10.1038/348163a0. [DOI] [PubMed] [Google Scholar]
- 20.Bunce C, Bell EB. CD45RC isoforms define two types of CD4 memory T cells, one of which depends on persisting antigen. J Exp Med. 1997;185:767–76. doi: 10.1084/jem.185.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersen P, Smedegaard B. CD4(+) T-cell subsets that mediate immunological memory to Mycobacterium tuberculosis infection in mice. Infect Immun. 2000;68:621–9. doi: 10.1128/iai.68.2.621-629.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–9. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 23.Bell EB, Hayes S, McDonagh M, Bunce C, Yang C, Sparshott SM. Both CD45R(low) and CD45R(high) ‘revertant’ CD4 memory T cells provide help for memory B cells. Eur J Immunol. 2001;31:1685–95. doi: 10.1002/1521-4141(200106)31:6<1685::aid-immu1685>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 24.Mitchison NA. The dosage requirements for immunological paralysis by soluble proteins. Immunology. 1968;15:509–30. [PMC free article] [PubMed] [Google Scholar]
- 25.Bell EB, Shand FL. Cellular events in protein tolerant inbred rats. I. The fate of thoracic duct lymphocytes and memory cells during tolerance induction to human serum albumin. Eur J Immunol. 1973;3:259–67. doi: 10.1002/eji.1830030503. [DOI] [PubMed] [Google Scholar]
- 26.Lee WT, Cole-Calkins J, Street NE. Memory T cell development in the absence of specific antigen priming. J Immunol. 1996;157:5300–7. [PubMed] [Google Scholar]
- 27.Pape KA, Merica R, Mondino A, Khoruts A, Jenkins MK. Direct evidence that functionally impaired CD4+ T cells persist in vivo following induction of peripheral tolerance. J Immunol. 1998;160:4719–29. [PubMed] [Google Scholar]
- 28.Yang CP, Sparshott SM, Duffy D, Garside P, Bell EB. The phenotype and survival of antigen-stimulated transgenic CD4 T cells in vivo: the influence of persisting antigen. Int Immunol. 2006;18:515–23. doi: 10.1093/intimm/dxh392. [DOI] [PubMed] [Google Scholar]
- 29.Rogers WO, Weaver CT, Kraus LA, Li J, Li L, Bucy RP. Visualization of antigen-specific T cell activation and cytokine expression in vivo. J Immunol. 1997;158:649–57. [PubMed] [Google Scholar]
- 30.London CA, Perez VL, Abbas AK. Functional characteristics and survival requirements of memory CD4+ T lymphocytes in vivo. J Immunol. 1999;162:766–73. [PubMed] [Google Scholar]
- 31.Merica R, Khoruts A, Pape KA, Reinhardt RL, Jenkins MK. Antigen-experienced CD4 T cells display a reduced capacity for clonal expansion in vivo that is imposed by factors present in the immune host. J Immunol. 2000;164:4551–7. doi: 10.4049/jimmunol.164.9.4551. [DOI] [PubMed] [Google Scholar]
- 32.Fry TJ, Mackall CL. Interleukin-7: master regulator of peripheral T-cell homeostasis? Trends Immunol. 2001;22:564–71. doi: 10.1016/s1471-4906(01)02028-2. [DOI] [PubMed] [Google Scholar]
- 33.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4:680–6. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 34.Khoruts A, Mondino A, Pape KA, Reiner SL, Jenkins MK. A natural immunological adjuvant enhances T cell clonal expansion through a CD28-dependent, interleukin (IL)-2-independent mechanism. J Exp Med. 1998;187:225–36. doi: 10.1084/jem.187.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aarts WM, Schlom J, Hodge JW. Vector-based vaccine/cytokine combination therapy to enhance induction of immune responses to a self-antigen and antitumor activity. Cancer Res. 2002;62:5770–7. [PubMed] [Google Scholar]
- 36.Thornton S, Boivin GP, Kim KN, Finkelman FD, Hirsch R. Heterogeneous effects of IL-2 on collagen-induced arthritis. J Immunol. 2000;165:1557–63. doi: 10.4049/jimmunol.165.3.1557. [DOI] [PubMed] [Google Scholar]
- 37.Schorle H, Holtschke T, Hunig T, Schimpl A, Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352:621–4. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- 38.Santner B, Wagner S, Wolf M, Kneitz B, Hunig T, Schimpl A. In vitro skewing of TCR-transgenic T cells from IL-2 deficient mice towards TH1 and TH2 in the absence of exogenous IL-2. Eur Cytok Network. 1998;9:17–25. [PubMed] [Google Scholar]
- 39.Kundig TM, Schorle H, Bachmann MF, Hengartner H, Zinkernagel RM, Horak I. Immune responses in interleukin-2-deficient mice. Science. 1993;262:1059–61. doi: 10.1126/science.8235625. [DOI] [PubMed] [Google Scholar]
- 40.Pape KA, Kearney ER, Khoruts A, et al. Use of adoptive transfer of T-cell-antigen-receptor-transgenic T cell for the study of T-cell activation in vivo. Immunol Rev. 1997;156:67–78. doi: 10.1111/j.1600-065x.1997.tb00959.x. [DOI] [PubMed] [Google Scholar]
- 41.Lee WT, Pelletier WJ. Visualizing memory phenotype development after in vitro stimulation of CD4(+) T cells. Cell Immunol. 1998;188:1–11. doi: 10.1006/cimm.1998.1341. [DOI] [PubMed] [Google Scholar]
- 42.Freer G, Burkhart C, Rulicke T, Ghelardi E, Rohrer UH, Pircher H, Zinkernagel RM, Hengartner H. Role of T helper cell precursor frequency on vesicular stomatitis virus neutralizing antibody responses in a T cell receptor beta chain transgenic mouse. Eur J Immunol. 1995;25:1410–16. doi: 10.1002/eji.1830250541. [DOI] [PubMed] [Google Scholar]
- 43.Bell EB, Sparshott SM. The peripheral T-cell pool: regulation by non-antigen induced proliferation? Semin Immunol. 1997;9:347–53. doi: 10.1006/smim.1997.0092. [DOI] [PubMed] [Google Scholar]
- 44.Gudmundsdottir H, Turka LA. A closer look at homeostatic proliferation of CD4+ T cells: costimulatory requirements and role in memory formation. J Immunol. 2001;167:3699–707. doi: 10.4049/jimmunol.167.7.3699. [DOI] [PubMed] [Google Scholar]
- 45.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–81. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 46.Bender J, Mitchell T, Kappler J, Marrack P. CD4+ T cell division in irradiated mice requires peptides distinct from those responsible for thymic selection. J Exp Med. 1999;190:367–74. doi: 10.1084/jem.190.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kassiotis G, Zamoyska R, Stockinger B. Involvement of avidity for major histocompatibility complex in homeostasis of naive and memory T cells. J Exp Med. 2003;197:1007–16. doi: 10.1084/jem.20021812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller JF, Sprent J. Cell-to-cell interaction in the immune response. VI. Contribution of thymus-derived cells and antibody-forming cell precursors to immunological memory. J Exp Med. 1971;134:66–82. doi: 10.1084/jem.134.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitchell GF, Chan EL, Noble MS, Weissman IL, Mishell RI, Herzenberg LA. Immunological memory in mice. 3. Memory to heterologous erythrocytes in both T cell and B cell populations and requirement for T cells in expression of B cell memory. Evidence using immunoglobulin allotype and mouse alloantigen theta markers with congenic mice. J Exp Med. 1972;135:165–84. doi: 10.1084/jem.135.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee WT, Vitetta ES. Virgin T cells do not provide help for antigen-specific B cells in the absence of IL-4, IL-5, and IL-6. Int Immunol. 1991;3:907–16. doi: 10.1093/intimm/3.9.907. [DOI] [PubMed] [Google Scholar]
- 51.Johnson-Leger C, Christenson JR, Holman M, Klaus GG. Evidence for a critical role for IL-2 in CD40-mediated activation of naive B cells by primary CD4 T cells. J Immunol. 1998;161:4618–26. [PubMed] [Google Scholar]
- 52.Grabstein KH, Maliszewski CR, Shanebeck K, Sato TA, Spriggs MK, Fanslow WC, Armitage RJ. The regulation of T cell-dependent antibody formation in vitro by CD40 ligand and IL-2. J Immunol. 1993;150:3141–7. [PubMed] [Google Scholar]
- 53.Leclerc C, Sedlik C, Lo-Man R, Charlot B, Rojas M, Deriaud E. Stimulation of a memory B cell response does not require primed helper T cells. Eur J Immunol. 1995;25:2533–8. doi: 10.1002/eji.1830250919. [DOI] [PubMed] [Google Scholar]
- 54.Maloy KJ, Burkhart C, Freer G, et al. Qualitative and quantitative requirements for CD4+ T cell-mediated antiviral protection. J Immunol. 1999;162:2867–74. [PubMed] [Google Scholar]
- 55.Mohrs K, Wakil AE, Killeen N, Locksley RM, Mohrs M. A two-step process for cytokine production revealed by IL-4 dual-reporter mice. Immunity. 2005;23:419–29. doi: 10.1016/j.immuni.2005.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sparshott SM, Bell EB. Membrane CD45R isoform exchange on CD4 T cells is rapid, frequent and dynamic in vivo. Eur J Immunol. 1994;24:2573–8. doi: 10.1002/eji.1830241102. [DOI] [PubMed] [Google Scholar]
- 57.Tough DF, Sprent J. Turnover of naive- and memory-phenotype T cells. J Exp Med. 1994;179:1127–35. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Macallan DC, Wallace D, Zhang Y, et al. Rapid turnover of effector-memory CD4(+) T cells in healthy humans. J Exp Med. 2004;200:255–60. doi: 10.1084/jem.20040341. [DOI] [PMC free article] [PubMed] [Google Scholar]