Abstract
The role of neutrophils in the immune response has long been regarded as mainly phagocytic, but recent publications have indicated the production of several cytokines by polymorphonuclear leucocytes (PMN). The results of the individual reports, however, vary considerably. In this study, we established a cytokine profile of pure human neutrophils and demonstrated that minor contamination of peripheral blood mononuclear cells (PBMCs) in PMN preparations can lead to false-positive results. In our hands, peripheral blood PMN fail to produce the pro-inflammatory cytokines interleukin (IL)-1β, IL-6 and tumour necrosis factor-α (TNF-α). Instead, they secrete large amounts of the chemokine IL-8 and the anti-inflammatory IL-1 receptor antagonist (IL-1ra). Additionally, PMN preparations of a high purity show production of the chemokines macrophage inflammatory protein (MIP)-1α, MIP-1β and growth-related oncogene-α (GRO-α), as well as macrophage colony-stimulating factor (M-CSF). The neutrophil therefore represents a novelty by producing the antagonist of IL-1β (i.e. IL-1ra) in the absence of IL-1β itself. To support our results, we differentiated stem cells from human cord blood into PMN and monocytes, respectively. These in vitro-differentiated PMN showed the same cytokine profile as peripheral blood PMN lacking IL-1β, while differentiated monocytes produced the expected IL-1β in addition to IL-1ra. The clear anti-inflammatory nature of their cytokine profile enables PMN to antagonize pro-inflammatory signals in experimental conditions. It is therefore possible that PMN play a key role in immune regulation by counteracting a dysregulation of the inflammatory process. Clinical studies, in which administration of recombinant G-CSF had a favourable effect on the outcome of severe infections and even sepsis without worsening inflammation, could thus be explained by our results.
Keywords: granulocytes, IL-1ra, inflammation, neutrophils, PMN
Introduction
Of the leucocytes in peripheral blood, 40–75% are granulocytes, and with only low levels of eosinophils and basophils, neutrophils represent their major fraction. They are the first cells that migrate into infected tissue and provide innate immunity through phagocytosis and degranulation.1 The terminally differentiated, mature polymorphonuclear leucocytes (PMN) in circulation contain few ribosomes and a small amount of endoplasmic reticulum, and they are therefore traditionally thought to have only a limited capability for protein synthesis.
Nevertheless, recent studies have described cytokine production by PMN with results that are quite controversial. Several groups have detected production of pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6 and tumour necrosis factor-α (TNF-α).1,2 However, some studies reported no IL-6 production,3,4 and others only found production of the chemokine IL-85 or the anti-inflammatory cytokine, interleukin-1 receptor antagonist (IL-1ra), in addition to IL-1β, IL-8 and TNF-α.6 Secretion of other chemokines, such as macrophage inflammatory protein (MIP)-1α (CCL3),7–9 MIP-1β (CCL4)7,9 and growth-related oncogene-α (GRO-α) (CXCL1),10 has also been described. The production of granulocyte–macrophage colony-stimulating factor (GM-CSF),11 granulocyte colony-stimulating factor (G-CSF),1 IL-3,11 IL-12,7,12 interferon-α,13 and TGF-β114,15 and -2,16 have been reported by few or single groups, whereas IL-8 (CXCL8) has been detected by most groups.
However, the detection of cytokine mRNA by polymerase chain reaction (PCR) amplification must be interpreted carefully. It is possible that the presence of cytokine mRNA may be an effect of contaminating monocytes in neutrophil preparations,5 especially as these cells have a much higher capacity of RNA and protein synthesis.
G-CSF, a stimulant for the maturation and mobilization of neutrophils in bone marrow, increases the level of circulating PMN.17,18 Several studies have therefore shown beneficial therapeutic effects in applying recombinant G-CSF (rG-CSF) to patients with chemotherapy-associated leukopenia.17,19,20 In addition, rG-CSF has been used, along with antibiotics, to treat pneumonia in animal models,21 and it was found that leukopenic animals showed an increased survival under rG-CSF therapy. Other studies show a beneficial effect of rG-CSF, even in non-neutropenic animals.22,23 Recently, beneficial effects of G-CSF therapy or granulocyte transfusions have been reported in connection with serious infections in neutropenic human patients.24–26 However, a cytokine panel, as described above, would not explain successful results of a therapy that increases neutrophil counts. If neutrophils indeed produce pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α, they should rather worsen the situation by enhancing the inflammatory reaction. Interestingly, a human model for immunity impairment, achieved by ethanol treatment, demonstrated that after G-CSF application, anti-inflammatory cytokines, such as IL-1ra and soluble TNF-receptor p55, were increased, whereas the pro-inflammatory cytokine response was attenuated.27
We therefore questioned whether the pro-inflammatory cytokines mentioned above truly are produced by PMN, and we aimed to establish a revised cytokine profile of non-contaminated peripheral blood neutrophils. We have previously established an improved method to isolate neutrophils of a high purity and with no detectable prestimulation from human blood.5 This high purity should exclude false-positive results of cytokine production that may be caused by contaminating cells.
Materials and methods
Cell isolation
Neutrophils were isolated from buffy coats of healthy donors, as described previously.5 Briefly, the cells were separated by two density gradients of Percoll (Pharmacia, Uppsala, Sweden) after sedimentation through hydroxyethyl starch (Plasmasteril; Fresenius AG, Bad Homburg, Germany). Any remaining erythrocytes were removed by hypotonic lysis. The cell suspension was adjusted to 3 × 106/ml in RPMI-1640 (BioWhittaker, Verviers, Belgium) containing 10% low-endotoxin fetal calf serum (PAA Laboratories, Coelbe, Germany) and supplemented with 2 mm l-glutamine, 100 U/ml of penicillin and 100 µg/ml of streptomycin (all from Biochrom KG, Berlin, Germany). Peripheral blood mononuclear cells (PBMC) were prepared by Ficoll gradient centrifugation, as previously described.28 For experiments showing the effects of contamination, plastic-adherent PBMC were used and incubated for 1 hr in 10-ml Petri dishes (37°, 5% CO2), as previously described.29
Purity and flow cytometry analysis
Purity and degree of activation of the cells were measured by flow cytometry analysis (Coulter XL; Coulter Electronics, Krefeld, Germany, and FACScalibur, BD Biosciences, Heidelberg, Germany) using CD66b as a granulocyte marker and CD62L as a marker for activation. The cells were pelleted, resuspended in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA), and incubated with CD66b conjugated to fluorescein isothiocyanate (FITC) (formerly CD67; Immunotech, Hamburg, Germany) and with CD62L conjugated to phycoerythrin (PE) (alternative names: LECAM-1, l-selectin; Immunotech), for 15 min. As the control, immunoglobulin G1 (IgG1)–FITC/IgG1–PE mouse (Immunotech) was used. The cells were either washed again with 1% PBS/BSA and fixation was performed automatically in a Multi-Q-Prep (Coulter Electronics) or they were analysed immediately.
A total of 98 to > 99% of the cells were positive for CD66b as well as for CD62L.
May–Gruenwald–Giemsa staining (Merck, Darmstadt, Germany) showed that contaminating mononuclear cells amounted to < 0·5%.
Stimulation and measurement of cytokines
PMN were cultured in either 6- or 24-well plates with the following stimuli: zymosan (a mixture of magnesium-containing glycoproteins from yeast, 0·16 mg/ml; Serva, Heidelberg, Germany), GM-CSF (10 U/ml; Strathmann Biotec, Hamburg, Germany) and lipopolysaccharide (LPS) (1 µg/ml or 250 ng/ml; Escherichia coli serotype O111:B4; Sigma, Deisenhofen, Germany). Supernatants were collected after 24 hr and stored at −80° until measurement. Enzyme-linked immunosorbent assay (ELISA) kits were obtained from Bender MedSystems Diagnostics GmbH (Vienna, Austria) (IL-1β, IL-6, IL-8, IL-12, TNF-α); R & D Systems GmbH (Wiesbaden, Germany) [IL-1ra, IL-3, IL-6, IL-8, GROα, macrophage colony-stimulating factor (M-CSF), MIP-1α, MIP-1β, transforming growth factor (TGF)-β1, TGF-β2, TNF-α]; Amersham (Buckinghamshire, UK) (IL-1ra, G-CSF); Laboserve GmbH (Giessen, Germany) (GM-CSF); and BD Biosciences (IL-1β, IL-6, IL-8). ELISAs were quantified using an ELISA reader (Anthos by Labotec and Sunrise by Tecan Austria, both Salzburg, Austria). Only values equal to or higher than the lowest standard were regarded as positive.
Cell extracts and western blotting
A total of 107 cells, stimulated as described above (24 hr, 250 ng of LPS per ml) were lysed in ice-cold buffer (pH 8) containing 50 mm NaCl, 50 mm Tris, 1 mm EDTA, 1% Triton X-100, 0·1 mm Na-Vanadate, 0·5 mm dithiothreitol (DTT), 0·5 mm Pefabloc (Roche, Mannheim, Germany) and 10 µl/ml of Protease Inhibitor Cocktail (Sigma). Lysates were frozen for at least 1 hr at −80°, then cleared by centrifugation (22 000 g, 20 min, 4°). Supernatants were collected and assayed for protein concentration using the Bradford micro assay (Biorad, Munich, Germany). Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) was performed using a variant of the Laemmli method.30 Briefly, equivalent amounts of protein (usually between 10 and 20 µg) were supplemented with sample buffer (1·5 m Tris, pH 6·8) containing 56% Saccharose, 14% SDS, 1·4%β-mercaptoethanol and 0·02% Bromphenol blue, and separated using a 15% SDS polyacrylamide gel. Suitable recombinant controls (Strathmann Biotec) and biotinylated protein ladders (Cell Signaling Technology, Danvers, MA) were included. The protein was electrophoretically transferred onto a nitrocellulose membrane (Biorad), and equal amounts of protein per lane were verified by Ponceau staining (Fluka, Buchs, Switzerland). After blocking overnight with 5% dried milk in Tris-buffered saline, membranes were incubated for 2 hr with the primary antibodies, washed in Tris-buffered saline, incubated again for 1 hr with the secondary antibody and, if necessary, washed and incubated for 1 hr with a tertiary antibody [biotin-anti-IL-1β, R & D Systems; biotin-anti-IL-1ra, R & D Systems; anti-IL-8, BD Biosciences, Heidelberg, Germany; anti-biotin-horseradish peroxidase (HRP)-linked immunoglobulin, Cell Signaling Technology; biotin-anti-Ms IgG, Becton Dickinson]. Proteins were detected using the Phototope® HRP Westernblot detection system (Cell Signaling Technology). In the case of IL-6, the membrane was stripped and then reprobed as described above.
Isolation and in vitro differentiation of CD34+haematopoetic progenitor cells
Isolation of CD34+ cells from cord blood, and their expansion and differentiation, was performed as previously reported.31–34 In each case, one third of the undifferentiated cells from the same donor was incubated at 3 × 106 cells/ml, with and without LPS (250 ng/ml), for 3 hr. The cells were then lysed and the cytokines detected by western blotting, as described above. Another two thirds were differentiated into granulocytic and monocytic cells. For differentiation, G-CSF (10 ng/ml) or M-CSF (25 ng/ml), respectively, was added to RPMI-1640, otherwise supplemented as described above. Monocytic cells were differentiated for 7 days and granulocytic cells for 14 days. After the differentiation period, the cells were incubated with or without LPS for 3 hr and then lysed and analysed as described above.
Differentiation was examined by May–Gruenwald–Giemsa staining (Merck) and by flow cytometry with antibodies against CD14 (BD Pharmingen, Heidelberg Germany) and CD66b (BD Pharmingen).
Statistical analysis
Experimental data are expressed as means ± standard error. Where appropriate, significances of difference were analysed by the Student's t-test for paired samples, using the program spss. In a conservative manner, negative values were regarded as 0·1 pg less than the lowest standard value.
Results
Lack of pro-inflammatory cytokine production
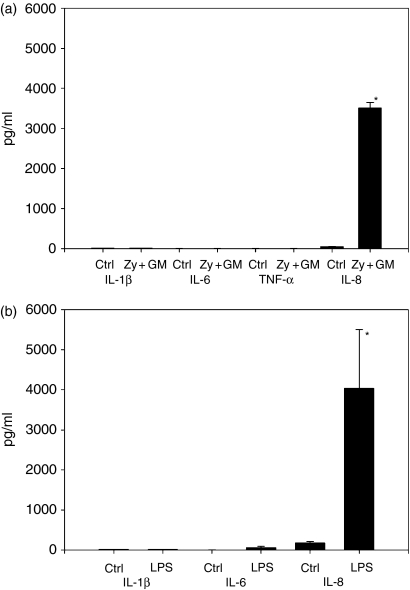
As previously shown, human neutrophils that are stimulated with zymosan do not produce the pro-inflammatory cytokines IL-1β, IL-6 and TNF-α, yet produce large amounts of the chemokine IL-8.5 These results differ from various reports in the literature, and to verify them, we extended the spectrum of stimuli used. By using GM-CSF alone (data not shown) or in combination with zymosan, we again were unable to detect IL-1β, IL-6 or TNF-α(Fig. 1a). However, there was significant production of IL-8 in response to the stimulants. We then stimulated PMN with LPS, and again the cells did not secrete any IL-1β. A minor secretion of IL-6 was observed in response to LPS, but the increase in IL-6 production compared with the negative-control conditions proved to be statistically insignificant. As further experiments using western blotting did not detect any IL-6, this may be false-positive noise owing to the sensitivity of the assay. As before, IL-8 was significantly induced in stimulated cells (Fig. 1b). G-CSF alone did not show any stimulative activity and, in combination with zymosan, it did not change the outcome compared to stimulation with zymosan alone (data not shown). By extending the range of our stimulants, we were thus able to show that our previous results cannot be explained with a limited response to zymosan.
Figure 1.
Lack of pro-inflammatory cytokine production by polymorphonuclear leucocytes (PMN). (a) PMN (3 × 106/ml) were incubated with zymosan plus granulocyte–macrophage colony-stimulating factor (GM-CSF) (Zy + GM; 0·16 mg/ml and 10 U/ml, respectively) for 24 hr, and control cultures (Ctrl) remained unstimulated. Cytokines in supernatants were measured by enzyme-linked immunosorbent assay (ELISA). Only interleukin (IL)-8 was detected. Zymosan and GM-CSF significantly increased cytokine production compared with control conditions (*P < 0·001 versus control). n = 4–7 experiments are shown. Neither zymosan stimulation in combination with granulocyte colony-stimulating factor (G-CSF) (data not shown), nor with GM-CSF alone (data not shown), stimulated PMN to produce IL-1, IL-6 or tumour necrosis factor-α (TNF-α). (b) PMN (3 × 106/ml) were incubated with lipopolysaccharide (LPS) (250 ng/ml) for 24 hr, whereas control cultures remained unstimulated. Cytokines in supernatants were measured by enzyme-linked immunosorbent assay (ELISA). Production of IL-8 was significantly increased upon stimulation with LPS (*P < 0·027 versus control). Again, there was no significant production of IL-1 or IL-6. n = 5 experiments are shown.
Cytokine pattern of neutrophils
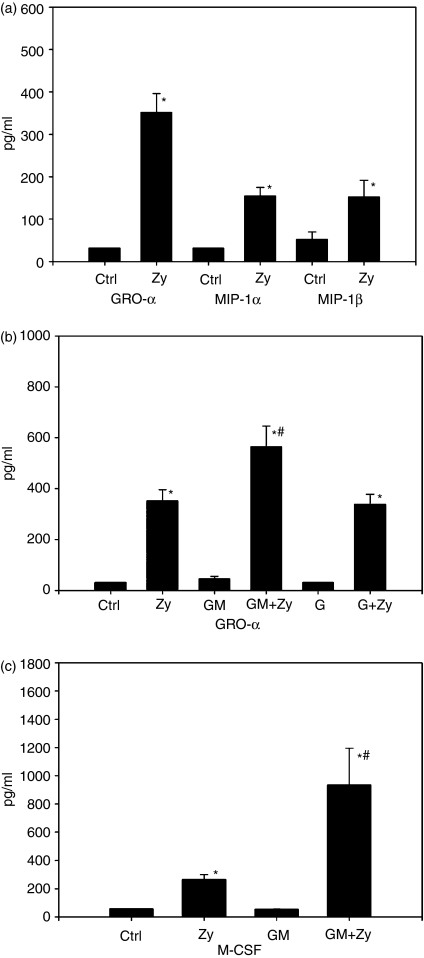
To examine the spectrum of cytokines produced by neutrophils, we tested our cell preparations for additional cytokines. The production of IL-8 implies that PMN might also produce other chemokines, and indeed, after stimulation with zymosan, we found significant production of GRO-α, MIP-1α and MIP-1β(Fig. 2a). Also, the capacity of different stimuli to induce the production of GRO-α was examined. As before, stimulation with zymosan results in a release of significant amounts of GRO-α. Stimulation with GM-CSF or G-CSF alone did not result in any significant production of GRO-α, and the combination of G-CSF and zymosan induced an amount of GRO-α that was similar to stimulation with zymosan only. However, the combined use of GM-CSF and zymosan induced an amount of secreted GRO-α that was significantly higher than stimulation with zymosan alone (Fig. 2b).
Figure 2.
Cytokine production by polymorphonuclear leucocytes (PMN). (a) PMN (3 × 106/ml) were stimulated with zymosan (Zy; 0·16 mg/ml) for 24 hr, whereas control cultures (Ctrl) remained untreated. Cytokines in supernatants were measured by enzyme-linked immunosorbent assay (ELISA). PMN showed significant production of the chemokines, growth-related oncogene-α (GRO-α), macrophage inflammatory protein (MIP)-1α and MIP-1β after stimulation with zymosan (*P < 0·001 for GRO-α, *P < 0001 for MIP-1α and *P < 0·002 for MIP-1β, all versus the control). n = 6 experiments are shown. (b) PMN were stimulated for 24 hr with zymosan (Zy; 0·16 mg/ml), granulocyte–macrophage colony-stimulating factor (GM-CSF) (GM; 10 U/ml), GM-CSF + zymosan (GM + Zy), granulocyte colony-stimulating factor (G-CSF) (G; 10 U/ml) and G-CSF + zymosan (G + Zy). Control cultures remained unstimulated. GRO-α was measured in supernatants by ELISA. A significant level of GRO-α was only detected after stimulation with zymosan alone or in combination with other stimulants (*P < 0·001 versus control). GM-CSF and G-CSF alone showed no significant effect. However, GM-CSF in combination with zymosan increased GRO-α secretion significantly compared to stimulation with zymosan alone (#P < 0·003 versus zymosan). G-CSF did not show this effect. n = 7 experiments are shown. (c) PMN preparations were tested for their capacity to produce the colony-stimulating factors G-CSF, macrophage colony-stimulating factor (M-CSF) and GM-CSF in response to zymosan and/or GM-CSF. Only M-CSF was detected after stimulation with zymosan (Zy; 0·16 mg/ml) and zymosan + GM-CSF (GM + Zy; 10 U/ml + 0·16 mg/ml), respectively (*P < 0·001 versus control). GM-CSF as a stimulus alone did not induce M-CSF production, yet in combination with zymosan, it increased the amount of secreted M-CSF significantly, even compared with zymosan alone (#P < 0·001 versus zymosan). n = 5 experiments are shown.
PMN were also tested for their capacity to produce the colony-stimulating factors G-CSF, M-CSF and GM-CSF in response to zymosan and/or GM-CSF. The only detectable factor was M-CSF, which was significantly up-regulated in response to zymosan. GM-CSF alone did not have any significant effect compared with control conditions, yet again, when the cells were stimulated with GM-CSF in combination with zymosan, the increase in M-CSF production was significantly higher than stimulation with zymosan alone (Fig. 2c). These results indicate an important role of GM-CSF as an accessory stimulant for neutrophils. However, as already shown in Fig. 1(a), even the combination of GM-CSF and zymosan did not induce detectable levels of the pro-inflammatory cytokines IL-1β, IL-6 and TNF-α.
In contrast to other reports, ELISAs for IL-3, IL-12, TGF-β1 and TGF-β2 were all negative for controls and for 24 hr of stimulation with zymosan (data not shown, summarized in Table 1). As previously shown, PMN do not produce type-I interferons in response to viral stimuli.35
Table 1.
Comparison of cytokine production of polymorphonuclear leucocytes (PMN) described in the literature and in highly purified preparations
| Cytokine | Detected/not detected in highly purified PMN1 | Reference |
|---|---|---|
| IL-1β | – | 1,2 |
| IL-1ra | + | 6, 35 |
| IL-3 | – | 11 |
| IL-6 | – | 1,2 |
| IL-8 | + | 1,2,4,5 |
| IL-12 | – | 12,7 |
| TNF-α | – | 1,2,12 |
| G-CSF | – | 1 |
| GM-CSF | – | 11 |
| M-CSF | + | 1 |
| IFN-α | – | 13 |
| GRO-α | + | 10 |
| MIP-1α | + | 7,8,9 |
| MIP-1β | + | 7,9 |
| TGF-β1 | – | 14,15 |
| TGF-β2 | – | 16 |
–, absent; +, present.
G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte–macrophage colony-stimulating factor; GRO-α, growth-related oncogene-α; IFN-α, interferon-α; IL, interleukin; M-CSF, macrophage colony-stimulating factor; MIP, macrophage inflammatory protein; TNF-α, tumour necrosis factor-α; TGF, transforming growth factor.
Anti-inflammatory cytokine pattern of neutrophils
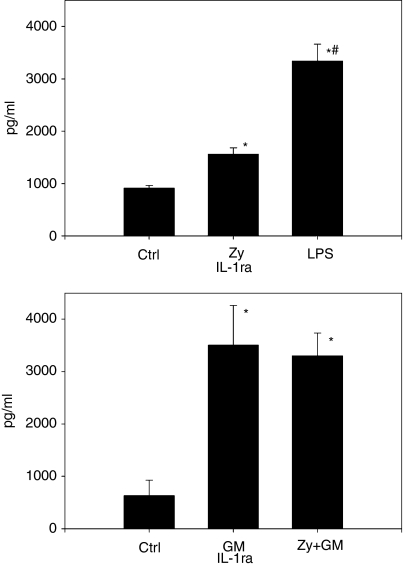
The cytokine profile of purified neutrophils determined so far shows a lack of the pro-inflammatory cytokines IL-β, IL-6 and TNF-α. We therefore questioned whether reports of IL-1ra detection could be inaccurate because there are no data on cells producing the natural antagonist of IL-1β, while IL-1β itself is not detectable. Surprisingly, neutrophils constitutively secreted low levels of IL-1ra, and stimulation with either zymosan or LPS increased the secreted level significantly (Fig. 3a). Interestingly, in contrast to the other cytokines examined, GM-CSF as a stimulant alone caused a significant increase of IL-1ra, similar to the use of GM-CSF and zymosan combined (Fig. 3b).
Figure 3.
Interleukin-1 receptor antagonist (IL-1ra) production of polymorphonuclear leucocytes (PMN). (a) PMN (3 × 106/ml) were incubated with zymosan (Zy; 0·16 mg/ml) and lipopolysaccharide (LPS) (250 ng/ml) for 24 hr, control cultures remained unstimulated. Cytokines in supernatants were measured by enzyme-linked immunosorbent assay (ELISA). IL-1ra was released constitutively; however, there was a significant increase upon stimulation with either zymosan or LPS compared with control conditions (Ctrl) (*P < 0·001 versus control). Interestingly, LPS induced a much higher production of IL-1ra than zymosan (#P < 0·001 versus zymosan). A minimum of n = 9 experiments are shown. (b) PMN were stimulated with granulocyte–macrophage colony-stimulating factor (GM-CSF) (GM; 10 U/ml) and GM-CSF + zymosan (Zy + GM; 0·16 mg/ml and 10 U/ml, respectively) for 24 hr. Control cultures remained unstimulated. IL-1ra in supernatants was measured by ELISA. Upon stimulation with GM-CSF, as well as the combination of zymosan and GM-CSF, the increase of IL-1ra production was significant (*P < 0·034 for GM-CSF, and *P < 0·001 for zymosan + GM-CSF, both versus the control). A total of n = 5 experiments are shown.
IL-1β in neutrophil preparations results from PBMC contamination
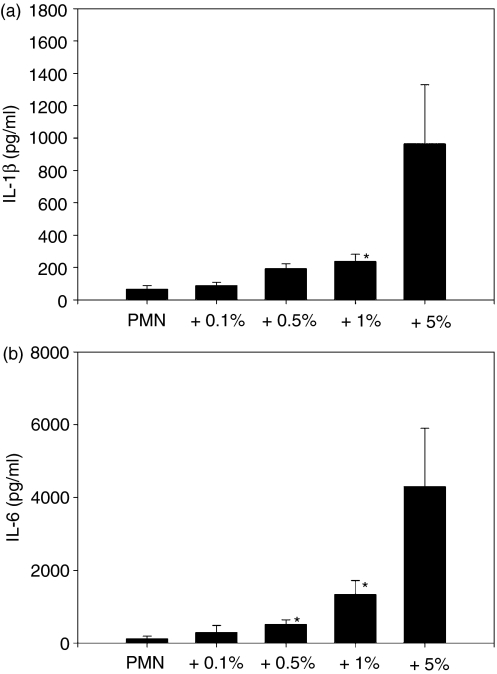
As a possible explanation for the presence of IL-1β and IL-6, reported in some publications, we deliberately contaminated our highly purified neutrophil preparations with plastic-adherent PBMCs (enriched monocytes) of the same donor at concentrations of 0·1, 0·5, 1 and 5%. The samples were stimulated with LPS, and the production of pro-inflammatory cytokines was measured by ELISA. Contamination with 1% PBMCs raised the IL-1β level significantly, as seen in Fig. 4(a), and IL-6 levels showed a significant elevation at a contamination of 0·5% (Fig. 4b).
Figure 4.
Production of interleukin (IL)-1β (a) and IL-6 (b) in a coculture of polymorphonuclear leucocytes (PMN) and peripheral blood mononuclear cells (PBMC). Pure PMN were contaminated by adherent PBMC of the same donor in concentrations from 0·1% up to 5% (3 × 106 cells/ml). Cocultures were incubated with lipopolysaccharide (LPS) (1 µg/ml) for 24 hr and cytokines in supernatants were measured by enzyme-linked immunosorbent assay (ELISA). The level of IL-1β was significantly increased at a contaminating level of 1% PBMC (*P < 0·04 versus pure PMN). For IL-6, a contamination of 0·5% was sufficient to cause a significant increase [*P < 0·02 (for 0·5% PBMC) and *P < 0·04 (for 1% PBMC) versus pure PMN].
Cytokine pattern determined in PMN lysates
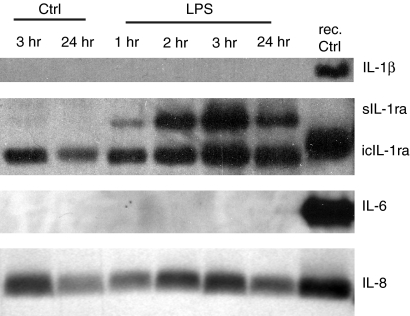
To exclude the possibility that none of the stimuli employed were able to induce a release of pro-inflammatory cytokines from potential intracellular storage, we detected cytokines in PMN cell extracts by means of western blotting. As shown in Fig. 5, no IL-1β or IL-6 was detected, whereas IL-1ra and IL-8 are clearly visible. In the case of IL-6, the ELISA values were not unequivocal, and so the membrane was stripped and reprobed with anti-IL-1ra as a positive control. One isoform of IL-1ra, the 16 000 molecular weight (MW) intracellular form, is produced constitutively, and another isoform, of 23 000 MW (the glycosylated 17 000 MW isoform36), is induced by stimulation with LPS.37 IL-8 is also produced constitutively. The weaker bands of the 24-hr control and LPS experiments indicate that owing to the short life span of PMN, longer incubation times are not appropriate for investigating intracellular cytokines.
Figure 5.
Detection of cytokines in polymorphonuclear leucocyte (PMN) lysate. PMN were stimulated with lipopolysaccharide (LPS; 250 ng/ml) for 1, 2, 3 and 24 hr. Control cultures (Ctrl) remained unstimulated for 3 and 24 hr. Lysates were separated on a 15% sodium dodecyl sulphate (SDS) polyacrylamide gel, blotted onto nitrocellulose membrane and detected with appropriate antibodies. Recombinant controls (rec.) were included. Production of the pro-inflammatory cytokines, interleukin (IL)-1β and IL-6, was not established. Instead, production of IL-8 and two different isoforms of IL-1ra were detected. Production of the 16 000 molecular weight (MW) isoform of intracellular IL-1 receptor antagonist (icIL-1ra) (identified by comparison with the 17 000 MW recombinant control) was constitutive. The glycosylated secreted form [soluble IL-1 receptor antagonist (sIL-1ra), c. 23 000 MW] was induced upon stimulation with lipopolysaccharide (LPS). In each case, one out of n = 3 independent experiments is shown.
Neutrophilic progenitors display the same anti-inflammatory cytokine profile as mature PMN
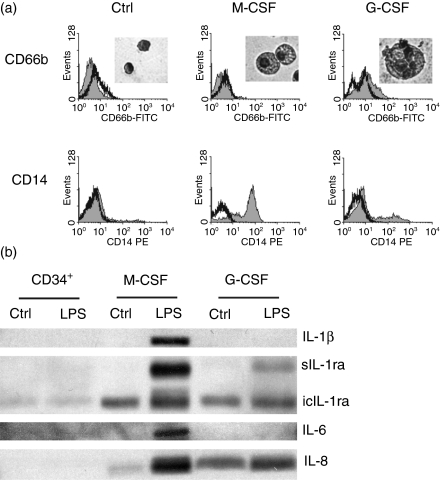
To exclude an enrichment of an anti-inflammatory PMN subpopulation by our purification method, CD34+ haematopoietic progenitor cells from human cord blood were isolated and differentiated in vitro into granulocytic cells and also into monocytic cells. As progenitor cells of the same donor were differentiated into both cell types, the detection of different cytokines must result from a switch in the cytokine profile during commitment to either monocytic or granulocytic lineage. Differentiation of the cells was examined by May–Gruenwald–Giemsa staining and by flow cytometric analysis of characteristic surface markers (Fig. 6a). Undifferentiated cells did not express CD14 or CD66b. Granulocytic cells, however, expressed low levels of CD14 and CD66b, and monocytic cells expressed high levels of CD14 only after 14/7 days of culture. The undifferentiated source cells, and both the granulocytic and the monocytic cells, were incubated with or without LPS for 3 hr. Again, the cytokines were detected from the lysates by western blotting (Fig. 6b). Production of IL-1β and IL-6 were seen only in the LPS-stimulated monocytic cells. No expression of IL-1β or IL-6 was observed in either the undifferentiated source cells or the granulocytic cells, not even after stimulation with LPS for 3 hr. Successful differentiation, however, could be assumed for the granulocytic cells, because, in contrast to the undifferentiated cells, expression was found of IL-1ra and IL-8. Down to the same two IL-1ra variants [intracellular IL-1 receptor antagonist (icIL-1ra) 16 000 MW and soluble IL-1 receptor antagonist (sIL-1ra)], the differentiated granulocytic cells showed the same cytokine profile as PMN isolated from peripheral blood (see Fig. 5). As described for monocytes,36 the differentiated monocytic cells additionally expressed the 18 000 MW icIL-1ra. The differentiation experiment therefore supports our previous results that PMN do not produce the pro-inflammatory cytokines IL-1β and IL-6, but instead produce IL-8 and two variants of IL-1ra.
Figure 6.
Cytokine profile of cells differentiated from hematopoietic progenitors. (a) CD34+ cells were isolated from cord blood and differentiated in vitro into granulocytic (G-CSF) and monocytic (M-CSF) cells. Successful differentiation compared with undifferentiated cells (Ctrl) was analysed by May–Gruenwald–Giemsa staining and flow cytometric analysis. (b) The differentiated cells and the undifferentiated source cells were stimulated with lipopolysaccharide (LPS) (250 ng/ml) for 3 hr. Control cultures remained unstimulated. Lysates were separated on a 15% sodium dodecyl sulphate (SDS) polyacrylamide gel, blotted onto nitrocellulose membrane and detected with appropriate antibodies. Production of interleukin-1 receptor antagonist (IL-1ra) and interleukin (IL)-8 by granulocytic and monocytic cells was established. Production of the pro-inflammatory cytokines IL-1β and IL-6 was detectable in stimulated monocytic cells only. One representative out of n = 3 independent experiments is shown. FITC, fluorescein isothiocyanate; icIL-1ra, intracellular IL-1 receptor antagonist; sIL-1ra, soluble IL-1 receptor antagonist; PE, phycoerythrin.
Anti-inflammatory influence of PMN on PBMC
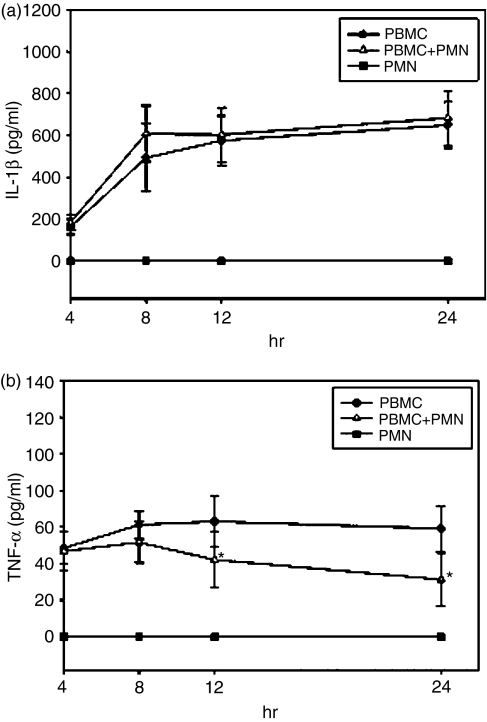
Isolated PMN do not show any production of IL-1β after stimulation in vitro, but in coculture with other blood cells they might react differently. Consequently, the IL-1β production in the previous experiment could as well be attributed to the PMN fraction of the coculture. To determine the origin of the detected IL-1β, we measured IL-1β in the supernatant of pure PMN, in pure PBMC and in a coculture of both, at a ratio that resembles in vivo conditions (2 × 106 PMN and 1 × 106 PBMC per ml). As expected, pure PMN showed no production of IL-1β after stimulation with LPS for 4, 8, 12 or 24 hr. The level of IL-1β production of PMN and PBMC in coculture was not elevated compared with IL-1β production of pure PBMC, indicating that the total amount of IL-1β is indeed secreted by PBMC exclusively, and not by PMN (Fig. 7a).
Figure 7.
Interleukin (IL)-1β production from peripheral blood mononuclear cells (PBMC) is not directly influenced by polymorphonuclear leucocytes (PMN). (a) PMN (2 × 106 cells/ml) alone show no production of IL-1β after stimulation with lipopolysaccharide (LPS). The level of IL-1β produced by PBMC (106 cells/ml) after stimulation with LPS is constant with or without PMN. The mean values of n = 5 experiments are shown. (b) Tumour necrosis factor-α (TNF-α) production of PBMC is reduced in coculture with PMN. Adding PMN to PBMC (both 106 cells/ml) decreases the TNF-α level produced by PBMC after stimulation with LPS. A significant difference is reached after 12 and 24 hr of incubation (*P < 0·05). Mean values of n = 5 experiments are shown. As described before, PMN alone fail to produce TNF-α.
Because PMN produce IL-1ra in the absence of IL-1β, the capability of neutrophils to inhibit the pro-inflammatory effect of IL-1β was investigated. In a similar experiment, we measured the production of the pro-inflammatory TNF-α in a coculture of PMN and PBMC. The TNF-α level was significantly reduced in the coculture compared with PBMC alone. As shown previously, pure PMN did not secrete any TNF-α (Fig. 7b).
Discussion
In our hands, peripheral blood PMN produced IL-8, along with other chemokines such as GRO-α, MIP-1α and MIP-1β. Additionally, PMN produced the anti-inflammatory antagonist of IL-1β, IL-1ra. In contrast to earlier publications, we were not able to detect the pro-inflammatory cytokines IL-1β and IL-6. Table 1 summarizes all the cytokines measured by our group from the highly purified PMN of seven to 21 individuals, regarding each cytokine in different experimental set ups as opposed to reported cytokines in the literature.
While it is relatively easy to detect low levels of cytokines, it is rather difficult to determine the real cytokine profile of a purified population, excluding any influence of contaminating cells. In highly purified PMN, we found no production of the pro-inflammatory IL-1β or IL-6, yet by adding increasing amounts of PBMC, we were able to detect significant levels of IL-1β and IL-6, which occurred at a very low percentage of PBMC contamination (Fig. 4). Varying results observed in different studies, and difficulties in measuring the actual cytokine pattern of neutrophils, may therefore be explained by low levels of contaminating cells. These cells may be responsible for pro-inflammatory cytokine production, especially in response to LPS. Recently, the ability of neutrophils to internalize IL-1β rapidly via the IL-1 decoy receptor has been reported.38 This ‘scavenging’ endocytosis of IL-1β in a pro-inflammatory milieu may present another explanation for the presence of IL-1β in neutrophils.
The production of IL-8, which acts as a chemoattractant on T cells and also on PMN,39,40 provides an autocrine mechanism to recruit more neutrophils to the site of an infection. GRO-α, a member of the CXC subfamily of chemokines, like IL-8, is a neutrophil-activating factor that attracts neutrophils and causes degranulation and enzyme release.40 MIP-1α and MIP-1β, members of the C-C subfamily of chemokines, are also secreted by neutrophils. They act as a chemoattractant on monocytes and natural killer cells, leading additional cells to the site of an infection. With this wide spectrum of chemoattractant cytokines, neutrophils are therefore well equipped, and being the first cells that migrate into infected tissue, they are thus able to induce and regulate the following events of an immune response. This is in concordance with Hayashi et al., who implied that neutrophils participate in the recruitment of innate immune cells to sites of infection.41
Just as important as the fast induction of an immune response, however, is its tight control. A dysregulated inflammation results not only in damage to the surrounding tissue, but septic patients die of the effects of overproduced pro-inflammatory cytokines.42,43 An imbalance in the IL-1β/IL-1ra system, resulting in an elevated IL1β/IL-1ra ratio, assumingly contributes to tissue damage.44 In a coculture of PBMC and different concentrations of PMN, Kolling et al. found a dose-dependent increase of anti-inflammatory cytokines.45 A study by Rupp et al. indicated that cells of healthy controls have a greater capacity to compensate for the activity of IL-1β by increased production of IL-1ra than those of patients suffering from chronic obstructive pulmonary disease (COPD).46 This, and our findings, that PMN produce IL-1ra and do not secrete pro-inflammatory cytokines, such as IL-1β and TNF-α, indicate an anti-inflammatory capacity of neutrophils.
Also, gene expression profiling of PMN from patients with x-linked chronic granulomatous disease (XCGD) showed an up-regulation of pro-inflammatory capacity compared with healthy PMN.47 Studies have demonstrated that the cell redox status regulates the expression of chemokines and receptors for inflammatory mediators,48,49 and PMN from XCGD-patients are defective in their ability to produce reactive oxygen species. These patients aquire life-threatening bacterial and fungal infections and develop granulomas, which are indicative of a chronic inflammatory response.50–53 Interestingly, Conti et al. were able to decrease significantly the size and weight of induced granulomas in mice by treatment with rIL-1ra.54 Together, these findings underline the importance of anti-inflammatory mediators in the course of an inflammatory response. In concordance with our results, the secretion of IL-1ra by healthy PMN seems to be an essential factor to prevent dysregulation of the immune response.
Currently, PMN seem to be the only cells with this rather unusual constellation of producing the antagonist of IL-1β in the absence of IL-1β itself. While IL-1ra competes with IL-1β for the same receptor, it does not activate the cells,55 and the secreted isoform (sIL-1ra) released by PMN can therefore influence the pro-inflammatory signals triggered by the IL-1β secretion of other cells. In combination with the ‘scavenging’ of IL-1β by the type II IL-1 receptor on PMN,38 this indicates a new, anti-inflammatory role in immune regulation for the phagocyte.
Our experiments clearly demonstrate an anti-inflammatory effect that neutrophils exert on TNF-α production (Fig. 7b). TNF-α has been reported to induce endothelial cell dysfunction in the lung and to inhibit gene transcription of surfactant protein C.56 By decreasing the TNF-α level, PMN might even play a role in preventing or limiting tissue damage.
An anti-inflammatory effect has also been discussed in conjunction with the application of recombinant human G-CSF, which induces the maturation and mobilization of PMN.57–59
Our anti-inflammatory cytokine profile of PMN has been established using peripheral blood neutrophils. A reasonable point of criticism may therefore be that these circulating PMN might be resting cells and that only fully activated PMN still produce pro-inflammatory cytokines. However, we have applied several stimulants, none of which were able to induce pro-inflammatory cytokines in PMN, yet which resulted in the production of IL-1ra, IL-8 and other chemokines. Additionally, considering the short half-life and low capacity of neutrophils to synthesize proteins, it seems unlikely that the observed reactions to the stimulants represent only a status of pre-activation. The cytokine profile of granulocytic cells that were differentiated from CD34+ haematopoietic progenitors is similar to that of peripheral blood neutrophils because these cells produce IL-1ra and IL-8, but not IL-1β or IL-6 (Fig. 6b). In contrast, monocytic cells differentiated from the same donor were found to produce the pro-inflammatory IL-1β and IL-6, in addition to IL-1ra and IL-6. Commitment to the granulocytic lineage therefore implies an anti-inflammatory cytokine profile, which is in line with the observations described recently.60
In summary, we hypothesize a central regulatory role for the neutrophil in the early stages of infection: extravasating PMN recruit more neutrophils and activate themselves using the autocrine mechanisms of IL-8 and GRO-α secretion. Production of MIP-1α and MIP-1β then attracts other cells, such as monocytes, to the site of infection. GM-CSF secreted by activated monocytes inhibits neutrophil apoptosis.61 Additionally, it stimulates neutrophils to release large amounts of IL-1ra (Fig. 3b). IL-1ra regulates the cytokine environment by interfering with the pro-inflammatory IL-1 system. The secretion of IL-1ra by PMN in tissue and also in the circulation therefore keeps inflammation under control, prevents chronic progression and assists in turning the inflammatory response off after clearance of an infection. Interestingly, IL-1ra is the only cytokine examined that is significantly up-regulated upon stimulation with GM-CSF alone (Fig. 3b). Yet, PMN themselves do not produce GM-CSF (Table 1), and it is therefore possible that the secretion of GM-CSF by activated monocytes thus serves as a regulatory mechanism. The secreted GM-CSF induces IL-1ra production of neutrophils which, in turn, down-regulates the production of pro-inflammatory cytokines in the inflamed area.
Beyond this, it is quite possible that neutrophils – as the first cells at the site of an infection – might be able to clear a minor infection before monocytes arrive. We therefore suggest the clearance of an infection by neutrophils without the classical symptoms of inflammation. Symptoms like reddening, swelling, pain and potential tissue damage are all induced by pro-inflammatory cytokines that are secreted by the later arriving monocytes.
Acknowledgments
This work was supported, in part, by the START program of the medical faculty, RWTH Aachen University.
We thank Professor Stefan Rose-John (Christian Albrechts University, Kiel, Germany) for kindly providing hyper-IL-6, and Romney Haylett for critical reading of the manuscript.
Glossary
Abbreviations
- BSA
bovine serum albumin
- ELISA
enzyme-linked immunosorbent assay
- FITC
fluorescein isothiocyanate
- G-CSF
granulocyte colony-stimulating factor
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- GRO-α
growth-related oncogene-α
- IFN-α
interferon-α
- IgG1
immunoglobulin G1
- IL
interleukin
- IL-1ra
interleukin-1 receptor antagonist
- LPS
lipopolysaccharide
- M-CSF
macrophage colony-stimulating factor
- MIP
macrophage inflammatory protein
- PBMC
peripheral blood mononuclear cells
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PE
phycoerythrin
- PMN
polymorphonuclear leucocytes
- SDS–PAGE
sodium dodecyl sulphate–polyacrylamide gel electrophoresis
- TNF-α
tumour necrosis factor-α
- TGF
transforming growth factor
References
- 1.Lloyd AR, Oppenheim JJ. Poly's lament: the neglected role of the polymorphonuclear neutrophil in the afferent limb of the immune response. Immunol Today. 1992;13:169–72. doi: 10.1016/0167-5699(92)90121-M. [DOI] [PubMed] [Google Scholar]
- 2.Cassatella MA. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–6. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 3.Bazzoni F, Cassatella MA, Rossi F, Ceska M, Dewald B, Baggiolini M. Phagocytosing neutrophils produce and release high amounts of the neutrophil-activating peptide 1/interleukin 8. J Exp Med. 1991;173:771–4. doi: 10.1084/jem.173.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang P, Wu P, Anthes JC, Siegel MI, Egan RW, Billah MM. Interleukin-10 inhibits interleukin-8 production in human neutrophils. Blood. 1994;83:2678–83. [PubMed] [Google Scholar]
- 5.Altstaedt J, Kirchner H, Rink L. Cytokine production of neutrophils is limited to interleukin-8. Immunology. 1996;89:563–8. doi: 10.1046/j.1365-2567.1996.d01-784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassatella MA, Meda L, Gasperini S, Calzetti F, Bonora S. Interleukin 10 (IL-10) upregulates IL-1 receptor antagonist production from lipopolysaccharide-stimulated human polymorphonuclear leukocytes by delaying mRNA degradation. J Exp Med. 1994;179:1695–9. doi: 10.1084/jem.179.5.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bliss SK, Marshall AJ, Zhang Y, Denkers EY. Human polymorphonuclear leukocytes produce IL-12, TNF-alpha, and the chemokines macrophage-inflammatory protein-1 alpha and -1 beta in response to Toxoplasma gondii antigens. J Immunol. 1999;162:7369–75. [PubMed] [Google Scholar]
- 8.Kasama T, Strieter RM, Standiford TJ, Burdick MD, Kunkel SL. Expression and regulation of human neutrophil-derived macrophage inflammatory protein 1 alpha. J Exp Med. 1993;178:63–72. doi: 10.1084/jem.178.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasama T, Strieter RM, Lukacs NW, Burdick MD, Kunkel SL. Regulation of neutrophil-derived chemokine expression by IL-10. J Immunol. 1994;152:3559–69. [PubMed] [Google Scholar]
- 10.Rudack C, Jorg S, Sachse F. Biologically active neutrophil chemokine pattern in tonsillitis. Clin Exp Immunol. 2004;135:511–8. doi: 10.1111/j.1365-2249.2003.02390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kita H, Ohnishi T, Okubo Y, Weiler D, Abrams JS, Gleich GJ. Granulocyte/macrophage colony-stimulating factor and interleukin 3 release from human peripheral blood eosinophils and neutrophils. J Exp Med. 1991;174:745–8. doi: 10.1084/jem.174.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cassatella MA, Meda L, Gasperini S, D'Andrea A, Ma X, Trinchieri G. Interleukin-12 production by human polymorphonuclear leukocytes. Eur J Immunol. 1995;25:1–5. doi: 10.1002/eji.1830250102. [DOI] [PubMed] [Google Scholar]
- 13.Shirafuji N, Matsuda S, Ogura H, et al. Granulocyte colony-stimulating factor stimulates human mature neutrophilic granulocytes to produce interferon-alpha. Blood. 1990;75:17–9. [PubMed] [Google Scholar]
- 14.Fava RA, Olsen NJ, Postlethwaite AE, Broadley KN, Davidson JM, Nanney LB, Lucas C, Townes AS. Transforming growth factor beta 1 (TGF-beta 1) induced neutrophil recruitment to synovial tissues: implications for TGF-beta-driven synovial inflammation and hyperplasia. J Exp Med. 1991;173:1121–32. doi: 10.1084/jem.173.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grotendorst GR, Smale G, Pencev D. Production of transforming growth factor beta by human peripheral blood monocytes and neutrophils. J Cell Physiol. 1989;140:396–402. doi: 10.1002/jcp.1041400226. [DOI] [PubMed] [Google Scholar]
- 16.Szymkowiak CH, Csernok E, Reinhold D, Bank U, Gross WL, Kekow J. Neutrophils synthesize and activate TGF beta 2. Cytokine. 2000;12:397–400. doi: 10.1006/cyto.1999.0572. [DOI] [PubMed] [Google Scholar]
- 17.Bronchud MH, Scarffe JH, Thatcher N, Crowther D, Souza LM, Alton NK, Testa NG, Dexter TM. Phase I/II study of recombinant human granulocyte colony-stimulating factor in patients receiving intensive chemotherapy for small cell lung cancer. Br J Cancer. 1987;56:809–13. doi: 10.1038/bjc.1987.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caspar CB, Seger RA, Burger J, Gmur J. Effective stimulation of donors for granulocyte transfusions with recombinant methionyl granulocyte colony-stimulating factor. Blood. 1993;81:2866–71. [PubMed] [Google Scholar]
- 19.Yoshida T, Nakamura S, Ohtake S, et al. Effect of granulocyte colony-stimulating factor on neutropenia due to chemotherapy for non-Hodgkin's lymphoma. Cancer. 1990;66:1904–9. doi: 10.1002/1097-0142(19901101)66:9<1904::aid-cncr2820660908>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 20.Crawford J, Ozer H, Stoller R, et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991;325:164–70. doi: 10.1056/NEJM199107183250305. [DOI] [PubMed] [Google Scholar]
- 21.Smith WS, Sumnicht GE, Sharpe RW, Samuelson D, Millard FE. Granulocyte colony-stimulating factor versus placebo in addition to penicillin G in a randomized blinded study of gram-negative pneumonia sepsis: analysis of survival and multisystem organ failure. Blood. 1995;86:1301–9. [PubMed] [Google Scholar]
- 22.Kullberg BJ, Netea MG, Curfs JH, Keuter M, Meis JF, van der Meer JW. Recombinant murine granulocyte colony-stimulating factor protects against acute disseminated Candida albicans infection in nonneutropenic mice. J Infect Dis. 1998;177:175–81. doi: 10.1086/513812. [DOI] [PubMed] [Google Scholar]
- 23.Dunne JR, Dunkin BJ, Nelson S, White JC. Effects of granulocyte colony stimulating factor in a nonneutropenic rodent model of Escherichia coli peritonitis. J Surg Res. 1996;61:348–54. doi: 10.1006/jsre.1996.0128. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka H, Nishino M, Nakamori Y, Ogura H, Ishikawa K, Shimazu T, Sugimoto H. Granulocyte colony-stimulating factor (G-CSF) stiffens leukocytes but attenuates inflammatory response without lung injury in septic patients. J Trauma. 2001;51:1110–6. doi: 10.1097/00005373-200112000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Grigull L, Schrauder A, Schmitt-Thomssen A, Sykora K, Welte K. Efficacy and safety of G-CSF mobilized granulocyte transfusions in four neutropenic children with sepsis and invasive fungal infection. Infection. 2002;30:267–71. doi: 10.1007/s15010-002-2133-y. [DOI] [PubMed] [Google Scholar]
- 26.Cheng AC, Stephens DP, Anstey NM, Currie BJ. Adjunctive granulocyte colony-stimulating factor for treatment of septic shock due to melioidosis. Clin Infect Dis. 2004;38:32–7. doi: 10.1086/380456. [DOI] [PubMed] [Google Scholar]
- 27.Dalhoff K, Hansen F, Dromann D, Schaaf B, Aries SP, Braun J. Inhibition of neutrophil apoptosis and modulation of the inflammatory response by granulocyte colony-stimulating factor in healthy and ethanol-treated human volunteers. J Infect Dis. 1998;178:891–5. doi: 10.1086/515350. [DOI] [PubMed] [Google Scholar]
- 28.Schober I, Braun R, Reiser H, Munk K, Leroux M, Kirchner H. la-positive T lymphocytes are the producer cells of interferon gamma. Exp Cell Res. 1984;152:348–56. doi: 10.1016/0014-4827(84)90636-0. [DOI] [PubMed] [Google Scholar]
- 29.Rink L, Nicklas W, Alvarez OL, Fagin U, Kirchner H. Microbial superantigens stimulate T cells by the superantigen bridge and independently by a cytokine pathway. J Interferon Cytokine Res. 1997;17:489–99. doi: 10.1089/jir.1997.17.489. [DOI] [PubMed] [Google Scholar]
- 30.Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage-T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 31.Ju XS, Zenke M. Differentiation of human antigen-presenting dendritic cells from CD34+ hematopoietic stem cells in vitro. Methods Mol Biol. 2003;215:399–407. doi: 10.1385/1-59259-345-3:399. [DOI] [PubMed] [Google Scholar]
- 32.Hacker C, Kirsch RD, Ju XS, et al. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat Immunol. 2003;4:380–6. doi: 10.1038/ni903. [DOI] [PubMed] [Google Scholar]
- 33.Ju XS, Hacker C, Madruga J, Kurz SM, Knespel S, Blendinger G, Rose-John S, Zenke M. Towards determining the differentiation program of antigen-presenting dendritic cells by transcriptional profiling. Eur J Cell Biol. 2003;82:75–86. doi: 10.1078/0171-9335-00294. [DOI] [PubMed] [Google Scholar]
- 34.Schreiber A, Otto B, Ju X, Zenke M, Goebel U, Luft FC, Kettritz R. Membrane proteinase 3 expression in patients with Wegener's granulomatosis and in human hematopoietic stem cell-derived neutrophils. J Am Soc Nephrol. 2005;16:2216–24. doi: 10.1681/ASN.2004070609. [DOI] [PubMed] [Google Scholar]
- 35.von der Ohe M, Altstaedt J, Gross U, Rink L. Human neutrophils produce macrophage inhibitory protein-1beta but not type i interferons in response to viral stimulation. J Interferon Cytokine Res. 2001;21:241–7. doi: 10.1089/107999001750169899. [DOI] [PubMed] [Google Scholar]
- 36.Malyak M, Smith MF, Abel AA, Hance KR, Arend WP. The differential production of three forms of IL-1 receptor antagonist by human neutrophils and monocytes. J Immunol. 1998;161:2004–10. [PubMed] [Google Scholar]
- 37.Schröder AK, von der Ohe M, Fleischer D, Rink L, Uciechowski P. Differential synthesis of two interleukin-1 receptor antagonist variants and interleukin-8 by peripheral blood neutrophils. Cytokine. 2005;32:246–53. doi: 10.1016/j.cyto.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Bourke E, Cassetti A, Villa A, Fadlon E, Colotta F, Mantovani A. IL-1 beta scavenging by the type II IL-1 decoy receptor in human neutrophils. J Immunol. 2003;170:5999–6005. doi: 10.4049/jimmunol.170.12.5999. [DOI] [PubMed] [Google Scholar]
- 39.Leonard EJ. NAP-1 (IL-8) Immunol Today. 1990;11:223–4. doi: 10.1016/0167-5699(90)90087-p. [DOI] [PubMed] [Google Scholar]
- 40.Zwahlen R, Walz A, Rot A. In vitro and in vivo activity and pathophysiology of human interleukin-8 and related peptides. Int Rev Exp Pathol. 1993;34(Part B):27–42. doi: 10.1016/b978-0-12-364935-5.50008-0. [DOI] [PubMed] [Google Scholar]
- 41.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–9. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 42.Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119:771–8. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- 43.Glauser MP, Zanetti G, Baumgartner JD, Cohen J. Septic shock: pathogenesis. Lancet. 1991;338:732–6. doi: 10.1016/0140-6736(91)91452-z. [DOI] [PubMed] [Google Scholar]
- 44.Tsao TCY, Hong J, Li LF, Hsieh MJ, Liao SK, Chang KSS. Imbalances between tumor necrosis factor-alpha and its soluble receptor forms, and interleukin-1 beta and interleukin-1 receptor antagonist in BAL fluid of cavitary pulmonary tuberculosis. Chest. 2000;117:103–9. doi: 10.1378/chest.117.1.103. [DOI] [PubMed] [Google Scholar]
- 45.Krolling UK, Hansen F, Braun J, Rink L, Katus HA, Dalhoff K. Leucocyte response and anti-inflammatory cytokines in community acquired pneumonia. Thorax. 2001;56:121–5. doi: 10.1136/thorax.56.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rupp J, Kothe H, Mueller A, Maass M, Dalhoff K. Imbalanced secretion of IL-1beta and IL-1RA in Chlamydia pneumoniae-infected mononuclear cells from COPD patients. Eur Respir J. 2003;22:274–9. doi: 10.1183/09031936.03.00007303. [DOI] [PubMed] [Google Scholar]
- 47.Kobayashi SD, Voyich JM, Braughton KR, Whitney AR, Nauseef WM, Malech HL, Deleo FR. Gene expression profiling provides insight into the pathophysiology of chronic granulomatous disease. J Immunol. 2004;172:636–43. doi: 10.4049/jimmunol.172.1.636. [DOI] [PubMed] [Google Scholar]
- 48.Saccani A, Saccani S, Orlando S, Sironi M, Bernasconi S, Ghezzi P, Mantovani A, Sica A. Redox regulation of chemokine receptor expression. Proc Natl Acad Sci USA. 2000;97:2761–6. doi: 10.1073/pnas.97.6.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Michalec L, Choudhury BK, Postlethwait E, Wild JS, Alam R, Lett-Brown M, Sur S. CCL7 and CXCL10 orchestrate oxidative stress-induced neutrophilic lung inflammation. J Immunol. 2002;168:846–52. doi: 10.4049/jimmunol.168.2.846. [DOI] [PubMed] [Google Scholar]
- 50.Malech HL, Nauseef WM. Primary inherited defects in neutrophil function: etiology and treatment. Semin Hematol. 1997;34:279–90. [PubMed] [Google Scholar]
- 51.Lekstrom-Himes JA, Gallin JI. Immunodeficiency diseases caused by defects in phagocytes. N Engl J Med. 2000;343:1703–14. doi: 10.1056/NEJM200012073432307. [DOI] [PubMed] [Google Scholar]
- 52.Halamish A, Klar A, Shoseyov D, Blinder G, Hurvitz H. Corticosteroid therapy reversed progressive chronic granulomatous lung disease following deterioration on interferon-gamma treatment. Pediatr Pulmonol. 2001;32:257–60. doi: 10.1002/ppul.1116. [DOI] [PubMed] [Google Scholar]
- 53.Chin TW, Stiehm ER, Falloon J, Gallin JI. Corticosteroids in treatment of obstructive lesions of chronic granulomatous disease. J Pediatr. 1987;111:349–52. doi: 10.1016/s0022-3476(87)80452-3. [DOI] [PubMed] [Google Scholar]
- 54.Conti P, Panara MR, Fridas S, Barbacane RC, Grilli A, Placido FC, Reale M, Fiore S. Inhibition of granuloma formation induced by potassium permanganate in the mouse by a specific human recombinant receptor antagonist for interleukin-1 (hrIL-1ra) Cell Immunol. 1993;147:446–57. doi: 10.1006/cimm.1993.1083. [DOI] [PubMed] [Google Scholar]
- 55.Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- 56.Rink L, Kirchner H. Recent progress in the tumor necrosis factor-alpha field. Int Arch Allergy Immunol. 1996;111:199–209. doi: 10.1159/000237369. [DOI] [PubMed] [Google Scholar]
- 57.Wiedermann FJ, Mayr AJ, Hobisch-Hagen P, Fuchs D, Schobersberger W. Association of endogenous G-CSF with anti-inflammatory mediators in patients with acute respiratory distress syndrome. J Interferon Cytokine Res. 2003;23:729–36. doi: 10.1089/107999003772084842. [DOI] [PubMed] [Google Scholar]
- 58.Stephens DP, Fisher DA, Currie BJ. An audit of the use of granulocyte colony-stimulating factor in septic shock. Intern Med J. 2002;32:143–8. doi: 10.1046/j.1445-5994.2002.00195.x. [DOI] [PubMed] [Google Scholar]
- 59.Schneider C, von Aulock S, Zedler S, Schinkel C, Hartung T, Faist E. Perioperative recombinant human granulocyte colony-stimulating factor (Filgrastim) treatment prevents immunoinflammatory dysfunction associated with major surgery. Ann Surg. 2004;239:75–81. doi: 10.1097/01.sla.0000103062.21049.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xing LY, Remick DG. Neutrophils as firemen, production of anti-inflammatory mediators by neutrophils in a mixed cell environment. Cell Immunol. 2004;231:126–32. doi: 10.1016/j.cellimm.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 61.Klein JB, Rane MJ, Scherzer JA, Coxon PY, Kettritz R, Mathiesen JM, Buridi A, McLeish KR. Granulocyte-macrophage colony-stimulating factor delays neutrophil constitutive apoptosis through phosphoinositide 3-kinase and extracellular signal-regulated kinase pathways. J Immunol. 2000;164:4286–91. doi: 10.4049/jimmunol.164.8.4286. [DOI] [PubMed] [Google Scholar]