Abstract
Systemic lupus erythematosus (SLE) is an autoimmune disease resulting from dysregulation of the immune system. Interleukin-6 (IL-6) is a multifunctional cytokine produced by macrophages, monocytes and T and B cells. It stimulates B-cell differentiation/maturation, immunoglobulin secretion, and T-cell functions. Elevated levels of IL-6 in serum, urine and renal glomeruli were detected in patients with active SLE and in murine models of SLE. Our study investigated the role of IL-6 in an SLE-like disease in New Zealand Black/White (NZB/W) F1 mice by administration of an anti-murine IL-6 monoclonal antibody (mAb). Intraperitoneal administration of the anti-IL-6 mAb suppressed the production of anti-dsDNA autoantibody. B-cell proliferation induced by anti-IgM and anti-CD40 was lower in the anti-IL-6 mAb-treated mice, ex vivo studies demonstrated that anti-IL-6 mAb treatment inhibited anti-dsDNA production. Anti-CD3-induced T-cell proliferation and mixed lymphocyte reactions were inhibited by anti-IL-6 mAb treatment, indicating a partial down-regulation of T cells. Histological analysis showed that treatment with anti-IL-6 mAb prevented the development of severe kidney disease. These results suggest that treatment with anti-IL-6 mAb has a beneficial effect on autoimmunity in murine SLE and that autoreactive B cells may be the primary target for anti-IL-6 mAb treatment; its effect on autoreactive T cells is also indicated.
Keywords: antibody, autoimmunity, interleukin-6, systemic lupus erythematosus
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune disorder characterized by the involvement of multiple organ systems with alternating clinical exacerbations and remissions. Circulating immune complexes and autoantibodies cause tissue damage and organ dysfunction with manifestations involving the skin, serosal surfaces, central nervous system and kidneys. These manifestations are believed to be the result of interactions among autoreactive lymphocytes that arise from both hereditary immunoregulatory defects and environmental factors, including chemicals and ultraviolet radiation. It is believed that autoreactive T and B cells drive the production of autoantibodies and the formation of immune complexes, which ultimately lead to tissue damage and organ failure in SLE.1
Interleukin-6 (IL-6) is a multifunctional cytokine that is critical for B-cell differentiation and maturation, immunoglobulin secretion, cytotoxic T-cell differentiation, acute-phase protein production, bone marrow progenitor stimulation, renal mesangial cell proliferation, and macrophage/monocyte functions.2 IL-6 mediates its biological activity through binding to a receptor complex consisting of two glycoproteins; an 80 000 molecular weight (MW) cognate receptor subunit IL-6 receptor α (IL-6Rα) and a 130 000 MW signal-transducing element (gp130).2 IL-6 binding to the IL-6Rα triggers the dimerization of gp130, which results in the activation of gp130-associated Janus kinase 1 (JAK1) and subsequently of two major signal transduction pathways: SHP2/GAB-mediated extracellular signal-related kinase (ERK), mitogen-activated protein kinase (MAPK), and signal transducers and activators of transcription 3 (stat3) mediated pathways.3,4 These two pathways are not only involved in immune activation and regulation, but are also associated with other physiological events and biological systems. Stat3 is tyrosine phosphorylated in the synovial tissue of rheumatoid arthritis (RA) patients, but not that of patients with osteoarthritis (OA).5 In the murine collagen induced arthritis model, stat3 phosphorylation in the ankle joints was observed around day 40, when disease was established.5 Thus, IL-6-dependent stat3 signalling may be critical for autoimmune responses.
As a result of its broad range of biological activities on various target cells, IL-6 plays a critical role in immune responses and inflammation. Overproduction of IL-6 has been associated with various autoimmune diseases, such as SLE, RA, Castleman’s disease, juvenile idiopathic arthritis and Crohn’s disease.6 Furthermore, IL-6 is also important for experimentally induced autoimmune diseases, such as type II collagen and antigen-induced arthritis,7,8 myelin oligodendrocyte protein induced experimental autoimmune encephalomyelitis9 and pristine induced autoantibody production.10 It has long been suggested that IL-6 plays a role in SLE pathogenesis and it is elevated in active SLE patients.11 B cells from SLE patients are hypersensitive to IL-6, which in turn enhances in vitro anti-dsDNA autoantibody production by these B cells, and anti-IL-6 monoclonal antibody (mAb) treatment blocks the enhancement of autoantibody production.11 The most compelling evidence supporting a critical role for IL-6 in the pathogenesis of SLE was demonstrated by the beneficial effects of IL-6 receptor blockade and the exacerbating effect of IL-6 in NZB/W F1 mice.12,13 However, the mechanisms underlying the IL-6-mediated pathogenesis are complex and remain to be further elucidated. Our anti-IL-6 mAb approach will determine the potential different biological effects of IL-6 ligand and receptor blockade.
NZB/W F1 mice spontaneously develop an autoantibody response against DNA and chromatin antigens as well as polyclonal hypergammaglobulinaemia and ultimately severe immune complex-mediated glomerulonephritis. These mice have been widely used as a model to study lupus nephritis.14 Our study is designed to investigate the effect of anti-IL-6 mAb treatment on disease development and the mechanisms by which anti-IL-6 mAb regulates autoimmune responses in NZB/W F1 mice.
Materials and methods
Antibodies and reagents
RPMI media, heat-inactivated fetal bovine serum, L glutamine, non-essential amino acids and sodium pyruvate were purchased from Invitrogen (Carlsbad, CA).
Phycoerythrin (PE)-conjugated anti-phosphorylated stat3 antibody, fluorescein isothiocyanate (FITC)-conjugated anti-CD4 antibody, cychrome-conjugated anti-B220 antibody were obtained from BD (BD Pharmingen, San Diego, CA). Neutralizing rat anti-IL-6 mAb (MAB406), and isotype control immunoglobulin G1 (IgG1) mAb (MAB005) were purchased from R & D Systems (Minneapolis, MN), and their endotoxin levels were 1·5 EU/mg and 0·97 EU/mg, respectively.
Animals and experimental protocol
NZB/W F1 mice aged 10–12 weeks were obtained from Jackson Laboratories (Bar Harbor, ME). On day 0, the study animals were randomly assigned to control or treatment groups (n = 15 per group). An intraperitoneal injection of saline, isotype control antibody (1 mg/mouse), or anti-mIL-6 mAb (1 mg/mouse) was administered weekly from 12 to 34 weeks of age. Animals were monitored weekly. Urine was collected via free catch (once every 3 weeks starting from 12 weeks of age) and stored at −80°. Blood was collected once every 3 weeks starting from 12 weeks of age and serum was stored at −80°. At final harvest, spleen, lymph nodes and kidneys were harvested into appropriate storage buffers before further analysis by in vitro functional assays. This study protocol was reviewed and approved by Centocor’s Institutional Animal Care and Use Committee.
Serum amyloid A (SAA) analysis by enzyme-linked immunosorbent assay (ELISA)
SAA levels were determined by ELISA (Biosource, Camarillo, CA) according to the manufacturer’s recommendations. Briefly, serum samples were diluted 1 : 200 in assay diluent and incubated with conjugated anti-mouse SAA antibody. Substrate tetramethylbenzidine was added, samples were read at OD450 nm and results were analysed using four-parameter fit to determine sample values.
Serum IL-6 analysis
Analysis of serum IL-6 levels was performed by incubation of 25 μl of serum with immobilized bead-bound antibodies in a 16-plex (Linco Research Inc., St Charles, MO) Luminex assay for quantitative determination of 16 cytokines according to the manufacturer’s recommendations. Briefly, samples were incubated with immobilized antibodies overnight at 4°, washed and detected by incubation with biotinylated detection antibody and streptavidin phycoerythrin. Samples were read on a Luminex 100 (Luminex Corporation, Austin, TX). Data were analysed using a non-linear fit equation.
Autoantibody analysis
Anti-dsDNA autoantibodies were determined by ELISA. The dsDNA-coated plates were purchased from DiaSorin (Stillwater, MN). Serum samples were diluted 1 : 100 in phosphate-buffered saline and incubated on plates at room temperature for 2 hr. Alkaline phosphatase-conjugated anti-mouse IgG (Southern Biotechnology Associates, Birmingham, AL) was added to the plate for 1 hr followed by incubation with p-nitrophenylphosphate substrate (Sigma, St Louis, MO) for 30 min and OD405 nm was measured. OD values from separate assays were normalized to serum sample from a 20-week-old MRL lpr/lpr mouse that was positive for anti-dsDNA autoantibody.
Histological analysis of kidney pathology
Kidneys were harvested when animals were 34 weeks old and immediately immersed in 0·7% periodate lysine paraformaldehyde (PLP) buffer composed of 0·1 m phosphate buffer, 0·7% paraformaldehyde, 75 mm l-lysine, and 10 mm NaIO4. The kidneys were embedded in paraffin blocks after overnight fixation with the PLP buffer. Preparation of 7-μm sections and staining with haematoxylin & eosin as well as periodic acid Schiff (PAS) were performed by routine methods. Samples were examined and scored for disease severity in a blinded fashion. Pathology was assessed using the World Health Organization (WHO) classifications.15
Flow cytometry analysis of T-cell and B-cell activation status
Mice were killed at 34 weeks of age and their spleens were removed and placed in cold RPMI-1640 medium supplemented with 10% fetal bovine serum (Invitrogen), 10 mg/ml gentamycin, 2 mm l-glutamine, and 0·1 mm 2-mercaptoethanol. Red blood cells were lysed in red blood cell lysing buffer on ice for 5 min. Splenocytes were stained with optimal concentrations of fluorochrome conjugated mAbs (5 × 105 cells in 200 μl of phosphate-buffered saline, 1% bovine serum albumin, and 0·1% sodium azide) in U-shaped microtitre plates at 4° for 30 min, and fixed with 1% paraformaldehyde. Samples were analysed on a FACSCalibur instrument (Becton Dickinson, Mountain View, CA). The following mAbs were used for analysis of T- and B-cell activation markers: anti-CD62L PE, anti-CD44 CyChrome, anti-CD4 FITC, anti-CD8 FITC, anti-CD25 PE, anti-CD21 FITC, anti-CD23 PE, anti-CD19 peridinin chlorophyll alpha protein (PerCP), anti-B220 FITC, anti-CD5 PE, anti-CD80 FITC, anti-CD86 PE, anti-CD4 Cy, and Fc blocker anti-CD16/32 (BD Pharmingen).
Proliferation assays
Mixed lymphocyte reactions to assess T-cell proliferation were conducted by incubating 300 000 lymphocytes from NZB/W F1 mice with 500 000 irradiated splenocytes from C57BL/6 mice. B-cell stimulation was assessed using 300 000 splenocytes incubated with 1 μg/ml each of anti-IgM F(ab′)/anti-CD40 (BD Pharmingen) for 72 hr. Proliferation was assessed using ATPLite and results were collected as luminescence singles on a TopCount (PerkinElmer, Shelton, CT).
Ex vivo autoantibody production
Splenocytes were harvested from NZB/W F1 mice treated with saline, control antibody and anti-IL-6 mAb. Splenocytes (1 × 106/well) in triplicates were incubated in a 96-well flat bottom plate containing RPMI-1640 medium (Invitrogen) alone, 5 μg/ml anti-IgM F(ab′)/1 μg/ml anti-CD40 (Pierce/Pharmingen, San Diego, CA), anti-IgM F(ab′)/anti-CD40 and 6·25 ng/ml of mIL-6 (R & D Systems), or anti-IgM/anti-CD40 and 125 μg/ml anti-IL-6 mAb (R & D Systems) at 37° for 7 days. Cell culture supernatants were collected and analysed for anti-dsDNA and anti-Sm/RNP autoantibody levels by ELISA as described above.
Examination of stat3 phosphorylation by intracellular flow cytometry
Stat3 phosphorylation was determined by intracellular flow cytometry analysis following the manufacturer’s recommended protocol (Pharmingen). Splenocytes were harvested from C57BL/6 and NZB/W F1 mice; then 2 × 106 splenocytes per well were incubated in a 96-well round-bottom plate with or without murine IL-6 for 6 min. Briefly, the cells were then fixed in 2% paraformaldehyde at 37° for 10 min and washed twice before FITC-labelled anti-CD4 and cychrome-labelled anti-B220 or cychrome-labelled anti-CD19 mAbs were added for surface marker staining at room temperature for 30 min. The cells were then washed once and permeabilized in 90% methanol for 30 min on ice. After the cells were washed twice, PE-labeled anti-pstat3 (Pharmingen) was added to the cells for intracellular staining for 1 hr in the dark at room temperature. The cells were then washed twice again and resuspended for flow cytometric analysis.
Statistical analysis
Serum SAA, IL-6 and anti-dsDNA levels, ex vivo anti-dsDNA autoantibody production stimulated by anti-CD40 and anti-IgM, and B/T-cell proliferation were expressed as mean ± SEM. Statistical significance was determined by two-tailed analysis of variance by standard t-test. For statistical analysis of kidney pathologies, the incidence of severe disease was compared across groups by Fisher exact test with a Bonferroni adjustment of the nominal type I error to determine the variance among the treatment groups. Rank order histological data were analysed by anova with Dunn’s correction for multiple comparisons. p <0·05 were accepted as significant.
Results
Anti-IL-6 mAb reduced SAA levels in NZB/W F1 mice
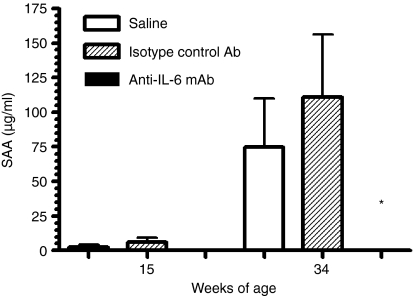
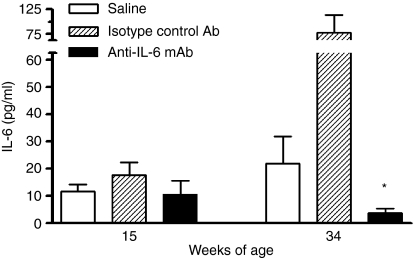
SAA is an acute-phase protein that can be induced by IL-6. To monitor whether anti-IL-6 treatment modifies the production of SAA as a marker of treatment effect, the level of SAA was examined throughout the study. SAA levels were significantly elevated as disease progressed in the control antibody-treated mice (Fig. 1), probably as a result of increased IL-6 production in these mice (Fig. 2). This elevated SAA was significantly reduced by anti-IL-6 mAb treatment throughout the study (Fig. 1). This result demonstrated that anti-IL-6 mAb treatment suppressed acute-phase reactant which had a role in anti-inflammation. The neutralizing effect of the anti-IL-6 mAb was maintained throughout the study. Anti-IL-6 mAb also suppressed the serum IL-6 level in this study (Fig. 2), which confirmed the efficacy of the antibody treatment.
Figure 1.
Anti-IL-6 mAb inhibited SAA production in NZB/W F1 mice. SAA levels measured by ELISA at 15 and 34 weeks of age are shown. *p < 0·05 versus saline and isotype control mAb-treated groups.
Figure 2.
Anti-IL-6 mAb suppressed serum IL6 level. Serum samples were analysed for IL-6 levels by Luminex at 15 and 34 weeks of age. *p < 0·05 versus saline and control mAb-treated groups.
Anti-IL-6 mAb inhibited anti-dsDNA autoantibody production in serum
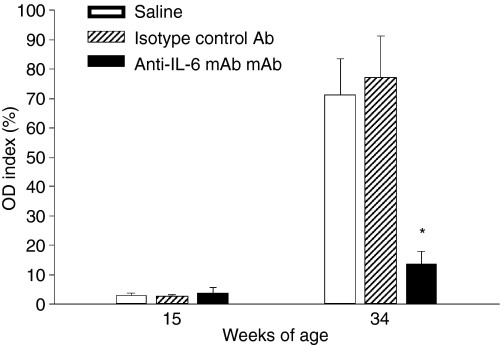
Next, we examined the effect of anti-IL-6 mAb treatment on anti-dsDNA autoantibody production because the presence of autoantibodies against dsDNA is a classic pathological marker of murine SLE. As serum levels of anti-dsDNA were monitored continuously over the course of the study, mice treated with saline and isotype control antibody exhibited increased levels of anti-dsDNA autoantibody over time (Fig. 3). In contrast, anti-IL-6 mAb treatment resulted in a significant inhibition of anti-dsDNA autoantibody production when mice were 34 weeks old at the termination of the study (Fig. 3).
Figure 3.
Anti-IL-6 mAb suppressed autoantibody production. Serum samples were analysed for anti-dsDNA autoantibody levels by ELISA at 15 and 34 weeks of age. OD index values represent individual data points normalized throughout the studies to a single positive control serum with anti-dsDNA. *p < 0·05 versus saline and control mAb-treated groups.
Anti-IL-6 mAb suppressed the progression of kidney pathology
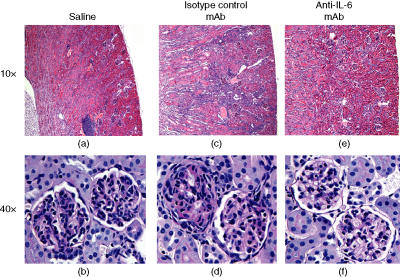
Glomerulonephritis is another pathological hallmark feature of murine SLE. To examine the effects of anti-IL-6 mAb treatment on kidney function and pathology, we examined kidney pathology histologically when mice were 34 weeks old. At 34 weeks of age, in all saline control mice, the periarterial lymphocytic infiltrate at the hilus and along the major branches of the renal artery, typical of NZB/W F1 mice, was observed (Fig. 4a). There was also evidence of glomerular disease characterized by an increase in mesangial cellularity, collapse of capillary lumina, thickened basement membranes and the presence of amorphous hyaline deposits (Fig. 4b). These characteristics were consistent with WHO Class III disease.15 Interstitial inflammation in the cortex was minimal and protein casts were seen only rarely. These histological changes were associated with an increase in urinary total protein/creatinine ratio (Table 1). Treatment with the isotype control mAb increased both the interstitial inflammation (Fig. 4c) and the extent and severity of glomerular disease consistent with WHO Class IV to V disease (Fig. 4d). In contrast, treatment with anti-IL-6 mAb had a beneficial effect on renal disease. Histopathological analysis showed that treatment with anti-IL-6 mAb significantly reduced the disease severity to that of WHO Class II to III disease (Fig. 4e,f). To allow statistical analysis of the histological data, the WHO Classifications were supplemented by rank order analysis. The mice were ranked from the least to the most severe and the rank data were analysed by anova. The worsening of the renal disease by treatment with isotype control mAb and the beneficial effect of the anti-IL-6 mAb were reflected in the percentage of animals exhibiting severe disease and the rank order of disease severity across the groups for glomerular disease (Table 1). There was also a trend for decreased severity of the periarterial inflammatory infiltrate.
Figure 4.
Histological analysis of kidney pathology. (a) Haematoxylin & esosin (H&E) section of a saline control mouse. Note prominent perivascular, predominantly lymphocytic infiltrates at the hilus (original magnification 10×). (b) PAS-stained section of a saline control mouse. Note thickened basement membranes and mesangial cell hyperplasia (original magnification 40×). (c) H&E section of an isotype control mouse. Note prominent perivascular, predominantly lymphocytic infiltrates at the hilus and extensive interstitial inflammatory infiltrates in the cortex (original magnification 10×). (d) PAS-stained section of an isotype control mouse. The extent of glomerular disease is greater than that seen in (b) (original magnification 40×). (e) H&E section of an anti-IL-6 mAb-treated mouse. Note lack of perivascular lymphocytic infiltrates at the hilus and interstitial inflammatory infiltrates in the cortex (original magnification 10×). (f) PAS-stained section of an anti-IL-6 mAb-treated mouse. The extent of glomerular disease is much less than that seen in (b) or (d) (original magnification 40×).
Table 1.
Kidney disease severity ranking at week 34
| Treatment | % Mice with moderate to severe renal disease (WHO Class III to IV) | Periarterial lymphocytic infiltrates median rank (interquartile range) | Glomerular disease median rank (interquartile range) |
|---|---|---|---|
| Saline | 60 | 11 (6·0–13·5) | 9·5 (7·5–11·5) |
| Isotype control mAb | 70 | 11 (6·3–13·3) | 13 (8·0–14·3) |
| Anti-IL-6 mAb | 101 | 5·5 (3·0–8·0) | 3·51 (2·0–6·0) |
Significantly different from Isotype Control mAb treated group
Anti-IL-6 mAb suppressed T-cell and B-cell activation
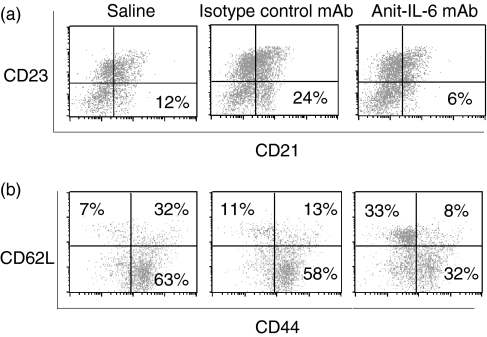
To investigate the mechanism by which anti-IL-6 mAb treatment suppressed murine SLE, we began to examine whether anti-IL-6 mAb was able to modulate the immune responses in autoimmune prone NZB/W F1 mice. Surface markers for T and B cells on splenocytes were examined to determine the activation status of these cells. Even though the activation markers of CD80 and CD86 levels on B cells were not significantly affected by any of the treatments, CD21+ CD23– marginal zone B cells were significantly reduced upon anti-IL-6 mAb treatment as compared to saline treatment when mice were 34 weeks old (Fig. 5a). Furthermore, CD44– CD62L+ naive T cells were significantly increased and conversely, CD44+ CD62L+ activated T cells were significantly reduced by anti-IL-6 mAb treatment at the end of the study (Fig. 5b). These observations suggested that anti-IL-6 mAb treatment suppresses B-cell differentiation and T-cell activation in NZB/W F1 lupus mice.
Figure 5.
Anti-IL-6 mAb reduced the number of activated B and T cells. (a) Total splenocytes were analysed with anti-CD19, anti-CD21 and anti-CD23 antibodies by flow cytometry to determine the population of CD21+ CD23– marginal zone B cells at 34 weeks of age. The cells were gated on CD19+ B cells. One representative out of five dot plots from each treatment group is shown. (b) Total splenocytes were analysed with anti-CD4, anti-CD62L and anti-CD44 antibodies by flow cytometry to determine the population of naive, activated and memory T cells at 34 weeks of age. The cells were gated on CD4+ T cells. One representative out of five dot plots from each treatment group was shown.
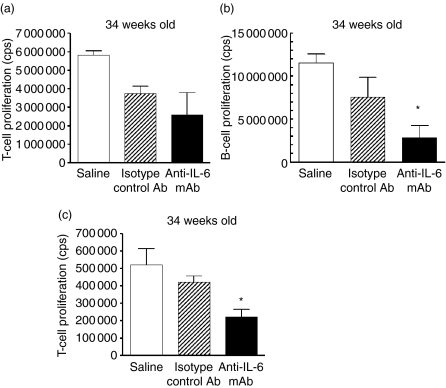
To further investigate whether anti-IL-6 mAb treatment affects the functions of T and B cells, antibody induced proliferation as well as mixed lymphocyte reaction (MLR) were performed to determine T-cell and B-cell responses under optimal and suboptimal stimulatory conditions using splenocytes isolated from various treatment groups. In vitro, T-cell proliferation stimulated with anti-CD3/anti-CD28 (data not shown) and anti-CD3 mAb alone were not significantly affected by in vivo anti-IL-6 mAb treatment as compared to the saline and isotype control mAb treatments when mice were 34 weeks old (Fig. 6a). In contrast, in vitro B-cell proliferation stimulated with anti-CD40/anti-IgM mAbs was significantly inhibited by in vivo anti-IL-6 mAb treatment as compared to the saline and isotype control antibody treatments when mice were 34 weeks old (Fig. 6b). These data indicated that T and B cells responded differently to anti-IL-6 mAb treatment, and T cells were capable of responding to optimal stimulation even after long-term in vivo anti-IL-6 mAb treatment. Moreover, anti-IL-6 mAb treatment significantly inhibited the MLR response as compared to saline and isotype control antibody treatments when mice were 34 weeks old (Fig. 6c), suggesting that anti-IL-6 mAb treatment reduced T-cell proliferation under physiological stimulations.
Figure 6.
Effect of anti-IL-6 mAb treatment on T-cell and B-cell responses at 34 weeks of age. (a) Anti-CD3-stimulated T-cell proliferation was examined in splenocytes harvested when mice were 34 weeks of age. ATPlite assay was performed to determine T-cell proliferation and the results are expressed as cells per count (cps). (b) B-cell proliferation induced by anti-CD40/anti-IgM antibodies was assessed in splenocytes harvested when mice were 34 weeks of age. ATPlite assay was performed and the results are expressed as cps. (c) T-cell proliferation in MLR was examined in splenocytes harvested when mice were 34 weeks of age. ATPlite assay was performed and the results are expressed as cps. *p < 0·05 versus saline and isotype control mAb-treated groups.
Anti-IL-6 mAb inhibited autoantibody production from autoreactive B cells derived from NZB/W F1 mice
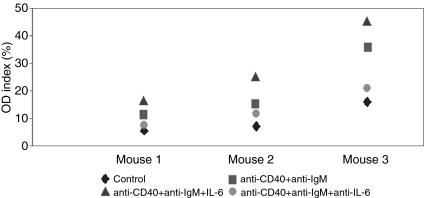
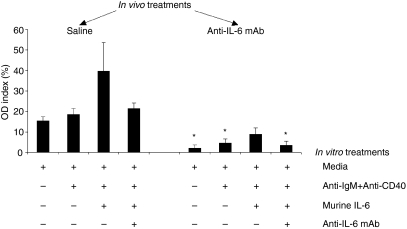
Anti-dsDNA autoantibody produced from autoreactive B cells is critical for SLE development. We therefore conducted ex vivo experiments to assess the ability of autoreactive B cells from the three in vivo treatment groups to generate anti-dsDNA autoantibody. First, we examined the anti-dsDNA autoantibody production by autoreactive B cells using splenocytes from three 16-week-old NZB/W F1 mice without any treatment. It was obvious that recombinant mouse IL-6 stimulated autoreactive B cells to produce anti-dsDNA autoantibody in culture, and the addition of anti-IL-6 mAb blocked the stimulatory effect of IL-6 (Fig. 7). The amount of anti-dsDNA autoantibody produced by autoreactive B cells varied among individual mice, this was consistent with the variability of disease development in this model. Next, we examined the effect of recombinant IL-6 and anti-IL-6 mAb treatments on the production of anti-dsDNA autoantibodies by autoreactive B cells from 34-week-old NZB/W F1 mice that were treated in vivo with saline, isotype control mAb, and anti-IL-6 mAb. Interestingly, autoreactive B cells from saline-treated NZB/W F1 mice produced anti-dsDNA autoantibodies in the presence or absence of recombinant murine IL-6, indicating the autoreactive nature of these cells in the NZB/W F1 mice (Fig. 8). Not surprisingly, in vitro anti-IL-6 mAb treatment seemed to inhibit recombinant IL-6 induced anti-dsDNA autoantibody production by these cells. More importantly, when recombinant mouse IL-6 was not added in the culture, anti-dsDNA autoantibody production by autoreactive B cells from anti-IL-6 mAb-treated NZB/W F1 mice was significantly inhibited as compared to that by autoreactive B cells from saline-treated mice (Fig. 8). Moreover, even the addition of strong B-cell stimulators such as anti-IgM and anti-CD40 with or without IL-6 did not induce these B cells to produce comparable levels of anti-dsDNA autoantibody as compared to the saline-treated mice (Fig. 8). This result was consistent with the serum anti-dsDNA autoantibody levels from the respective treatment groups. Anti-dsDNA autoantibody production by autoreactive B cells from the isotype control mAb-treated mice was between that of those cells from the saline-treated and anti-IL-6 mAb-treated mice (data not shown).
Figure 7.
Anti-dsDNA autoantibody produced by autoreactive B cells from 16-week-old NZB/W F1 mice. Splenocytes were cultured with media, anti-CD40/anti-IgM mAbs, anti-CD40/anti-IgM plus recombinant mouse IL-6, and anti-CD40/anti-IgM plus anti-IL-6 mAb in triplicates for 7 days before the supernatants were harvested and the anti-dsDNA autoantibody level was measured by ELISA. OD index values represent individual supernatants normalized to a single positive control serum with anti-dsDNA.
Figure 8.
Anti-IL-6 mAb inhibited autoantibody production from autoreactive B cells. Anti-dsDNA autoantibody produced by autoreactive B cells from 34-week-old NZB/W F1 mice treated with saline, isotype control mAb and anti-IL-6 mAb were examined in splenocytes cultured with media, anti-CD40/anti-IgM mAbs, anti-CD40/anti-IgM plus recombinant mouse IL-6, and anti-CD40/anti-IgM plus anti-IL-6 mAb and recombinant IL-6 in triplicates for 7 days before the supernatants were harvested and the anti-dsDNA autoantibody level was measured by ELISA. OD index values represent individual supernatants normalized to a single positive control serum with anti-dsDNA autoantibody. *p < 0·05 versus saline-treated group.
Anti-IL-6 mAb inhibited stat3 phosphorylation in T and B cells
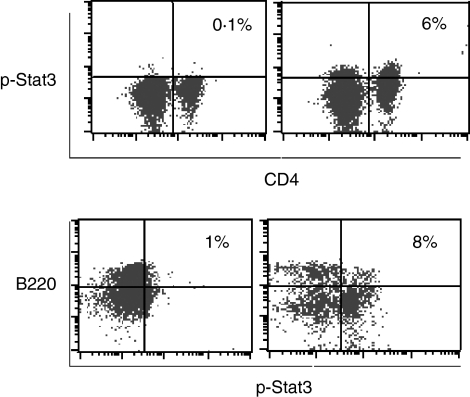
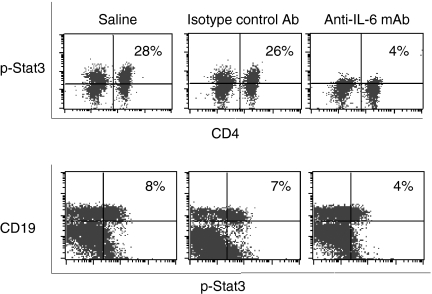
As demonstrated in previous studies,1 interaction between autoreactive T and B cells is critical for autoimmunity in SLE. Although autoreactive T and B cells are found in the general population, only certain individuals actually develop autoimmune diseases. This suggests that autoimmune-prone individuals have a lower threshold to self-antigen stimulation and that their T and B cells are more susceptible to activation. It is therefore possible that the altered internal signal transduction mechanisms for T-cell and B-cell activation in these autoimmune-prone individuals are a potential cause for autoimmunity. By treating the NZB/W F1 mice with anti-IL-6 mAb, we were able to inhibit T-cell and B-cell responses and the subsequent autoimmune disease. It was therefore a reasonable next step to investigate further the IL-6-dependent stat3 phosphorylation in T and B cells. We first compared the spontaneous stat3 phosphorylation in T and B cells of the NZB/W F1 mice and the naive C57BL/6 mice and noticed significant differences between the two strains of mice. NZB/W F1 mice had more active spontaneous stat3 phosphorylation in T and B cells at 16 (data not shown) and 22 weeks (Fig. 9) of age as compared to the age-matched normal C57BL/6 mice. This result confirmed that NZB/W F1 mice showed hyperactive signal transduction pathways as represented by the stat3 pathway. When T and B cells from the three treatment groups in our current study were examined, stat3 phosphorylation was decreased in T cells from anti-IL-6 mAb-treated mice at 34 weeks of age as compared to that from the saline and isotype control mAb treatment groups. Similarly, stat3 phosphorylation in B cells from the anti-IL-6 mAb-treated mice was decreased at 34 weeks of age as compared to that from the saline and isotype control mAb treatment groups (Fig. 10). These results demonstrate that anti-IL-6 mAb treatment counteracts the internal hyperactivation of T and B cells in lupus-prone NZB/W F1 mice, and subsequently controls the autoimmune responses in murine SLE.
Figure 9.
Spontaneous stat3 phosphorylation in T and B cells from 22-week-old C57BL/6 and NZB/W F1 mice. Splenocytes were cultured with complete culture media for 6 min at 37°. Surface markers CD4, CD19 and intracellular phosphorylated stat3 were determined by flow cytometry. Representatives of two experiments were shown.
Figure 10.
Anti-IL-6 mAb inhibited stat3 phosphorylation in T and B cells. Stat3 phosphorylation in T and B cells from saline, isotype control antibody, and anti-IL-6 mAb-treated NZB/W F1 mice was examined in splenocytes cultured with 40 ng/ml of recombinant mouse IL-6 for 6 min at 37°. Surface markers, CD4, CD19 and intracellular phosphorylated stat3 were determined by flow cytometry. Representatives of CD4+ T cells from 24-week-old mice and CD19+ B cells from 34-week-old mice are shown.
Discussion
Over the last three decades, there has been a lack of major breakthrough in SLE treatment despite intensive investigation of the disease and its mechanisms. Genetic predisposition, environmental factors and complicated interactions of the immune system all contribute to the complexity of this autoimmune disease. With the characterization of several animal models, however, investigators were able to focus on the mechanisms of action underlying the interactions of the immune system. Combined efforts from multiple investigators have proposed a model of autoreactive T-cell and B-cell interactions in the initiation and progression of SLE.1 Based on the model, a treatment that simultaneously targets both T-cell and B-cell compartments would be greatly beneficial for SLE patients. Our studies were designed to investigate the effects and mechanisms of anti-IL-6 mAb treatment in inhibiting autoreactive T-cell and B-cell interactions.
IL-6 is believed to be a critical modulator in various inflammatory diseases with a broad range of bioactivities on diverse target cells.3 Elevated levels of IL-6 have been found in patients with various inflammatory and autoimmune diseases.16 Anti-IL-6R mAb treatment has been shown to be effective in clinical trials of RA, Castleman’s disease, juvenile idiopathic arthritis and Crohn’s disease while anti-IL-6R mAb treatment in lupus and multiple myeloma are currently ongoing.17 In addition, elevated levels of IL-6 have also been found in various animal models of autoimmunity.16 These data suggest that IL-6 and IL-6-dependent signalling pathways are potential targets for autoimmune therapy.
In both lupus patients and animal lupus models, elevated IL-6 has been associated with B-cell hyperactivity, autoantibody production and immunopathology.18 In vitro neutralization of IL-6 or IL-6R resulted in significantly diminished IL-6 and anti-DNA autoantibody by B cells from SLE patients.18 Anti-IL6 therapy has been suggested to be beneficial in SLE patients in a recent comprehensive review.19 In our studies, anti-IL-6 mAb treatment not only significantly inhibited in vivo anti-dsDNA autoantibody production in NZB/W F1 mice, but also significantly inhibited ex vivo anti-dsDNA autoantibody production by anti-IgM/anti-CD40-stimulated B cells as compared to that from saline-treated animals. Although the inhibitory effect could be the result of a reduction of hypergammaglobulinaemia, it would still suggest that anti-IL-6 mAb treatment is beneficial for the disease. Furthermore, anti-IL-6 mAb treatment significantly inhibited T-cell proliferation in MLR as well as B-cell proliferation induced by anti-IgM/anti-CD40 antibodies in cultures.
Increased urinary excretion of IL-6 in patients with active lupus nephritis and higher in situ expression of IL-6 in lupus nephritis along the glomeruli and the tubules indicates that IL-6 may play an important role in the pathogenesis of mesangial proliferative glomerulonephritis.18 Both the residential mesangial cells and the infiltrated lymphocytes (macrophages and T cells) in the kidney contribute to the excessive IL-6 level in SLE autoimmune responses and the subsequent kidney damage. Upon administration of anti-IL-6 mAb in our studies, the disease severity of glomerulonephritis was significantly reduced in NZB/W F1 mice.
Based on the previously proposed model of autoreactive T-cell and B-cell interaction for SLE autoimmunity by multiple investigators1 small amounts of autoreactive B cells that have a low threshold for self antigens may initiate the autoimmune responses in lupus-prone humans or animals, which results in the clonal expansion of autoreactive B cells as well as autoreactive T cells, and subsequently the amplification of specific autoimmune responses that lead to the loss of tolerance. Therefore, it would be beneficial for a therapy to block the initial autoreactive responses as well as the activation of the downstream cascades. We believe that anti-IL-6 mAb treatment may be a therapeutic approach that accomplishes this goal because IL-6 has such profound and diverse effects on both T and B cells as well as other cell types critical for SLE autoimmune responses, and our data in these studies support that view.
SAA is known to be up-regulated by IL-6 in various inflammatory and autoimmune diseases and the increased production of SAA can be inhibited by anti-IL-6 treatment. Since stat3 signalling is involved in SAA induction20 and IL-6 can activate the stat3 signalling pathway, the IL-6-dependent signalling pathway naturally becomes the focus of our investigation. It has been well established that stat3 signalling is associated with cell growth, survival, differentiation and migration. 21 Stat3 signalling appears to regulate the survival and activation of T cells and B-cell differentiation into antibody-forming plasma cells.22 Stat3 signalling also plays a critical role for antigen-presenting cell-mediated T-cell activation and tolerance.23 Although IL-6 is not the only cytokine that activates stat3, it is a major contributor for stat3 phosphorylation in various autoimmune diseases because of its wide range of target cell types in the inflammatory responses. Elevated stat3 phosphorylation has not only been found in the synovial tissue of RA patients, but also in ankle joints of mice with antigen-induced and collagen-induced arthritis16 and in the lungs during acute lung injury.24 We demonstrated that spontaneous stat3 phosphorylation occurred more frequently in T and B cells from NZB/W F1 mice, and mIL-6 induced significantly higher stat3 phosphorylation in T and B cells. In vivo anti-IL-6 mAb treatment significantly blocked the IL-6-induced stat3 phosphorylation in both T and B cells. However, we should point out that anti-IL-6 mAb treatment did not completely eliminate the hyper stat3 phosphorylation in autoimmune-prone mice, probably because stat3 signalling is regulated by other cytokines such as IL-10. Furthermore stat3 phosphorylation is unlikely to be the only signalling event that is associated with lupus autoimmunity development.
There were two concerns related to the chronic treatment of anti-IL-6 mAb before we conducted the studies. One was whether the injection of anti-IL-6 mAb would suppress the normal immune function of the animals, and the other was the possible development of immune response against the rat anti-murine IL-6 mAb, which might abrogate the effectiveness of the treatment over time. Results from our in vitro T-cell and B-cell proliferation assays indicated that the T cells were fully capable of activation under optimal stimulatory conditions while B cells may be inhibited after long-term treatment of anti-IL-6 mAb. The inhibitory effect of anti-IL-6 mAb treatment on T cells was only seen in suboptimal stimulatory conditions without costimulatory molecules. To address the second concern, we measured the mouse anti-rat antibody production in the serum, and the result showed only minimal production of the mouse anti-rat antibody in the anti-IL-6 mAb-treated animals as compared to that in the saline-treated animals (data not shown). Taken together, long-term treatment with anti-IL-6 mAb generated limited suppression of the normal immune function and minimal immune response against the rat mAb used, which could be a result of the repetitive application of relatively high doses of the mAb. This is consistent with the recent finding of IL-6-inhibited Treg cell generation and anti-IL-6 mAb treatment could help the Treg cells to regulate autoimmune responses.25
In summary, our results suggest that anti-IL-6 mAb treatment could inhibit autoreactive T-cell and B-cell responses against self-antigens while maintaining the normal immune responses to foreign antigens. Our study demonstrates the benefits of anti-IL-6 mAb treatment for murine SLE with minimal risk of immune suppression. Our results agree with the previously proposed model of autoreactive T-cell and B-cell interaction in the regulation of SLE autoimmunity. Strategies to manipulate IL-6 and IL-6-dependent stat3 signalling pathways are novel therapeutic approaches for SLE and other autoimmune diseases.
World Health Organisation Morphologic Classification of Lupus Nephritis (1995 Revised Version)
| Class | |
|---|---|
| I. | Normal glomeruli |
| a. Nil (by all techniques) | |
| b. Normal on light microscopy but deposits see on electron or immunofluorescence microscopy | |
| II. | Pure mesangial alterations (mesangiopathy) |
| a. Mesangial widening, mild hypercellularity, or both | |
| b. Moderate hypercellularity | |
| III. | Focal segmental glomerulonephritis (associated with mild or moderate mesangial alterations) |
| a. Active necrotizing lesions | |
| b. Active and sclerosing lesions | |
| c. Sclerosing lesions | |
| IV. | Diffuse glomerulonephritis (severe mesangial, endocapillary, or mesangiocapillary proliferation, and/or extensive subendothelial deposits. Mesangial deposits are present invariably and subepithelial deposits often, and may be numerous) |
| a. Without segmental lesions | |
| b. With active necrotizing lesions | |
| c. With active and sclerosing lesions | |
| d. With sclerosing lesions | |
| V. | Diffuse membranous glomerulonephritis |
| a. Pure membranous glomerulonephritis | |
| b. Associated with lesions of category II (a or b) | |
| VI. | Advanced sclerosing glomerulonephritis |
Acknowledgments
The authors are grateful to Patricia Rafferty, Dorie Makropoulos, Kim Shamberger and Laura Mcalonan for their excellent technical assistance. The authors thank Xiaosha Zhang for his statistical analysis of the kidney pathological findings.
Abbreviations
- ELISA
enzyme-linked immunosorbent assay
- ERK
extracellular signal related kinase
- FITC
fluorescein isothiocyanate
- IL-6
interleukin-6
- IL-6Rα
IL-6 receptor α
- JAK1
janus kinase 1
- mAb
monoclonal antibody
- MAPK
mitogen-activated protein kinase
- MLR
mixed lymphocyte reaction
- NZB/W
New Zealand Black/White
- OA
osteoarthritis
- PAS
periodic acid Schiff
- PE
phycoerythrin
- PerCP
peridinin chlorophyll alpha protein
- PLP
periodate lysine paraformaldehyde
- RA
rheumatoid arthritis
- SAA
serum amyloid A
- SLE
systemic lupus erythematosus
- stat3
signal transducers and activators of transcription 3
References
- 1.Shlomchik MJ, Craft JE, Mamula MJ. From T to B and back again: positive feedback in systemic autoimmune disease. Nat Rev. 2001;1:147–53. doi: 10.1038/35100573. [DOI] [PubMed] [Google Scholar]
- 2.Kishimoto T. Interleukin-6: from basic science to medicine – 40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 3.Hirano T, Nakajima K, Hibi M. Signaling mechanisms through gp130: a model of the cytokine system. Cytokine Growth Factor Rev. 1997;8:241–52. doi: 10.1016/s1359-6101(98)80005-1. [DOI] [PubMed] [Google Scholar]
- 4.Hirano T, Ishihara K, Hibi M. Roles of Stat3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–56. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 5.Shouda T, Yoshida T, Hanada T, et al. Induction of the cytokine signal regulator SOCS3/CIS3 as a therapeutic strategy for treating inflammatory arthritis. J Clin Invest. 2001;108:1781–8. doi: 10.1172/JCI13568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mihara M, Nishimoto N, Ohsugi Y. The therapy of autoimmune diseases by anti-interleukin-6 receptor antibody. Expert Opin Biol Ther. 2005;5:683–90. doi: 10.1517/14712598.5.5.683. [DOI] [PubMed] [Google Scholar]
- 7.Sasai M, Saeki Y, Ohshima S, et al. Delayed onset and reduced severity of collagen-induced arthritis in interleukin-6-deficient mice. Arthritis Rheum. 1999;42:1635–43. doi: 10.1002/1529-0131(199908)42:8<1635::AID-ANR11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 8.Ohshima S, Saeki Y, Mima T, et al. Interleukin 6 plays a key role in the development of antigen-induced arthritis. Proc Natl Acad Sci USA. 1998;95:8222–6. doi: 10.1073/pnas.95.14.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samoilova EB, Horton JL, Hilliard B, Liu TS, Chen Y. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis. Roles of IL-6 in the activation and differentiation of autoreactive T cells. J Immunol. 1998;161:6480–6. [PubMed] [Google Scholar]
- 10.Richards HB, Satoh M, Jennette JC, Okano T, Kanwar YS, Reeves WH. Disparate T cell requirements of two subsets of lupus-specific autoantibodies in pristane-treated mice. Clin Exp Immunol. 1999;115:547–53. doi: 10.1046/j.1365-2249.1999.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linker-Israeli M, Deans RJ, Wallace DJ, Prehn J, Ozeri-Chen T, Kinenberg JR. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J Immunol. 1991;147:117–23. [PubMed] [Google Scholar]
- 12.Mihara M, Takagi N, Takeda Y, Ohsugi Y. IL-6 receptor blockage inhibits the onset of autoimmune kidney disease in NZB/W F1 mice. Clin Exp Immunol. 1998;112:397–402. doi: 10.1046/j.1365-2249.1998.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finck BK, Chan B, Wofsy D. Interleukin 6 promotes murine lupus in NZB/NZW F1 mice. J Clin Invest. 1994;94:585–91. doi: 10.1172/JCI117373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morel L, Tian XH, Croker BP, Wakeland EK. Epistatic modifiers of autoimmunity in a murine model of lupus nephritis. Immunity. 1999;11:131–9. doi: 10.1016/s1074-7613(00)80088-6. [DOI] [PubMed] [Google Scholar]
- 15.Weening JJ, D’Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65:521–30. doi: 10.1111/j.1523-1755.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 16.Ishihara K, Hirano T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002;13:357–68. doi: 10.1016/s1359-6101(02)00027-8. [DOI] [PubMed] [Google Scholar]
- 17.Nishimoto N, Kishimoto T. Inhibition of IL-6 for the treatment of inflammatory diseases. Current Opin Pharmacol. 2004;4:386–91. doi: 10.1016/j.coph.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Tackey E, Lipsky PE, Illei GG. Rationale for interleukin-6 blockade in systemic lupus erythematosus. Lupus. 2004;13:339–43. doi: 10.1191/0961203304lu1023oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anolik JH, Aringer M. New treatments for SLE. Cell-depleting and anti-cytokine therapies. Best Prac Res Clin Rheumatol. 2005;19:859–78. doi: 10.1016/j.berh.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Hagihara K, Nishikawa T, Isobe T, Song J, Sugamata Y, Yoshizaki K. IL-6 plays a critical role in the synergistic induction of human serum amyloid A (SAA) gene when stimulated with proinflammatory cytokines as analyzed with an SAA isoform real-time quantitative RT-PCR assay system. Biochem Biophys Res Comms. 2003;314:363–9. doi: 10.1016/j.bbrc.2003.12.096. [DOI] [PubMed] [Google Scholar]
- 21.Hirano T, Ishihara K, Hibi M. Roles of stat3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–56. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 22.Ivashkiv LB, Hu X. Signaling by stats. Arthritis Res Ther. 2004;6:159–68. doi: 10.1186/ar1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng F, Wang H, Cuenca A, et al. A critical role for stat3 signaling in immune tolerance. Immunity. 2003;19:425–36. doi: 10.1016/s1074-7613(03)00232-2. [DOI] [PubMed] [Google Scholar]
- 24.Gao H, Guo R, Speyer CL, et al. Stat3 activation in acute lung injury. J Immunol. 2004;172:7703–12. doi: 10.4049/jimmunol.172.12.7703. [DOI] [PubMed] [Google Scholar]
- 25.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]