Abstract
Productive engagement of T cell receptors (TCRs) by cognate ligand (major histocompatibility complex plus peptide) leads to proliferation, differentiation, and the elaboration of effector functions. Altered peptides generated by single amino acid substitutions in the antigenic peptide have diverse effects on the outcome of the T cell response. We have generated an altered peptide (Q144) from an autoantigenic peptide of myelin proteolipid protein 139–151 by a single amino acid substitution (from tryptophan to glutamine) in the primary TCR contact at position 144 that is capable of inducing CD4+ T cell responses in H-2s mice. By using a Q144-specific T cell clone (Q1.1B6), we see a hierarchy in T cell proliferation and cytokine production with various position 144 substituted peptides and have identified a peptide (L144) that hyperstimulates this T cell clone. In contrast to Q144, L144 induces maximal proliferation at 7 logs lower antigen concentration, induces greater cell death at higher antigen dose, and induces the secretion of cytokines not detected following stimulation with the cognate ligand. This heteroclitic T cell response associated with changes in cytokine profile was observed with several other T cell clones of different specificities. The L144 peptide also induces costimulation independent proliferation and cytokine production from the Q1.1B6 T cell clone. We describe this as a superagonist response. Such responses may have a role in the initiation of autoimmunity by promoting a proinflammatory environment following ligation of a cross-reactive TCR on autoreactive T cells.
Keywords: costimulation, superagonists, T cell differentiation
The effector functions of T cells are dictated, to a large extent, by the cytokines produced by the cell following activation and differentiation (1). Cytokines can initiate, propagate, or regulate tissue-specific autoimmune injury. In a number of autoimmune disease models, including experimental autoimmune encephalomyelitis (EAE), T helper type 1 (Th1) cells that secrete pro-inflammatory cytokines [interleukin 2 (IL-2), interferon γ (IFN-γ), and tumor necrosis factor β (TNF-β)] induce autoimmunity (2), whereas Th2 cells (secreting IL-4 and IL-10) can protect from EAE, although they do not always do so (3–6). The phenotype of the CD4+ T cell response in human disease has also been correlated with the outcome of infection and the autoimmune process (7). Much of the experimental work in models of autoimmunity has focused on the immune response to specific peptide ligands (cognate ligands), but it is also known that subtle modification of these antigens [to produce altered peptide ligands (APLs)] can have profound effects on the outcome of disease (8–11).
How APLs alter the course of autoimmune disease has been an area of intense interest in recent years, and the in vitro effects of some altered peptides may begin to explain their in vivo functions. APLs have been shown to mediate T cell receptor (TCR) antagonism (12), induce T cell anergy (13), and partially activate T cell clones (14, 15). Some of our recent work (16, 17) and that of others (18) has suggested that APLs can affect T cell differentiation and therefore the Th1/Th2 balance may determine disease outcome. In an EAE model induced with proteolipid protein (PLP) peptide 139–151 (W144), we have identified peptide analogs that protect animals from disease (11, 16). For at least one analog in which the tryptophan at position 144 has been replaced with glutamine (Q144), the ability to transfer protection appears to be a function of a subset of T cells that are cross-reactive and respond to both Q144 and the native PLP peptide W144 (16). Therefore the cross-reactive nature of these responses seems to be critical to their effects in vivo.
Because of the biological significance of cross-reactive T cells in our experimental system, we characterized the response of cross-reactive T cell clones derived by immunization with Q144. We found a hierarchy in the response of clones specific for various altered ligands. Heteroclitic proliferative responses were associated with changes in the cytokine profile of the responding clones, and at equivalent peptide concentrations induced a more pro-inflammatory environment than the cognate ligand. We have characterized the response of one clone, Q1.1B6, in detail and have shown that the heteroclitic ligand demonstrates a hierarchy in the induction of secretion of different cytokines. Furthermore, activation with the “superagonist” ligand was less costimulation dependent than activation with the cognate ligand. The existence of similar superagonist ligands in nature may be important in the induction and/or regulation of autoimmune disease.
MATERIALS AND METHODS
Generation of T Cell Clones and Peptide Antigens.
T cell clones were derived from female SJL mice (H-2s) immunized with 100 μg of peptide antigen Q144 in complete Freund’s adjuvant as described (16). Q1.1B6 was cloned twice and maintained in culture by stimulation with irradiated syngeneic spleen cells and Q144 (15 μM) every 1–2 months. Other clones were derived as described (17, 19). Peptide antigens with C terminal amides were synthesized by Richard Laursen (Boston University) on a MilliGen model 9050 synthesizer by using fluorenylmethoxycarbonyl chemistry. The peptides described have the sequences HSLGKWLGHPDKF (proteolipid protein 139–151/W144), HSLGKLLGHPDKF (L144), HSLGKRLGHPDKF (R144), HSLGKQLGHPDKF (Q144), HSLGKALGHPDKF (A144), and HSLGKLLGRPDKF (L144/R147). Substitutions are shown in bold.
Proliferation and Cytokine Assays.
Rested T cell clones (1–2 × 104 cells per well) were activated with irradiated (3,000 rads) syngeneic splenic antigen-presenting cells (APCs; 5 × 105 per well) and peptide antigens or anti-TCR or anti-CD3 (PharMingen) antibody. Proliferation was assessed by pulsing the cells with [3H-methyl]thymidine 1 μC/well (1 Ci = 37 GBq) 48 h after activation. The cells were harvested 18 h later, and the incorporated radioactivity was measured in triplicate wells. Supernatants were collected 40 h after activation and diluted 1:2; then cytokine concentrations were measured by specific capture ELISA according to the manufacturers instructions (PharMingen) as described (16). To assess the response to fixed APCs and different peptides, syngeneic splenic APCs were irradiated and an aliquot of cells was fixed with 75 mM ECDI (CalBiochem-Novabiochem) for 1 h as described (20). The fixed and unfixed APCs were washed extensively and used with Q144 or L144 at 60 μM to activate Q1.1B6. Blockade of the CD28/CTLA4 pathway was performed with human CTLA4-Ig (Genetics Institute, Cambridge, MA) compared with activation in the presence of a control fusion protein.
TCR cDNA Cloning.
cDNAs encoding TCR-α and -β chains were isolated by inverse PCR essentially as described (21). In brief, poly(A)+ mRNA was isolated from 106 cells, primed with oligo(dT) 12–18 and transcribed to first-strand cDNA with SuperScript II RT (GIBCO/BRL). Double-stranded cDNA was synthesized by using a cDNA synthesis system (GIBCO/BRL) and subsequently blunt ended with T4 DNA polymerase (New England Biolabs). The cDNA was circularized by T4 DNA ligase (New England Biolabs) and subjected to PCR (94°C for 1 min, 55–57°C for 1 min, and 72°C for 2 min for 30 cycles) by using two Cα- (AAGAGACCAACGCCACCTAC, GCTGTCCTGAGACCGAGGAT) or Cβ-(GCACAATCCTCGAAACCACT, GATGGCTCAAACAAGGAGAC) specific primers. PCR products were ligated into plasmid pCR2.1 Vector (Invitrogen) and the recombinant DNA was electroporated into competent Escherichia coli XL1-Blue MRF′ (Stratagene). Positive transformants were identified by colony hybridization by using 32P-labeled Cα- or Cβ-specific internal oligonucleotides, respectively, as probes. Their DNA was subsequently isolated and sequenced.
Measurement of Peptide Binding to I-As.
I-As molecules were prepared by affinity chromatography from cell lysates derived from the B cell lymphoma LS102.9 (H-2dxs), and the binding of various peptides was measured in a competition assay with a radiolabeled peptide as described (22, 23). The concentration of peptide needed to inhibit binding by 50% was calculated from this assay.
RESULTS
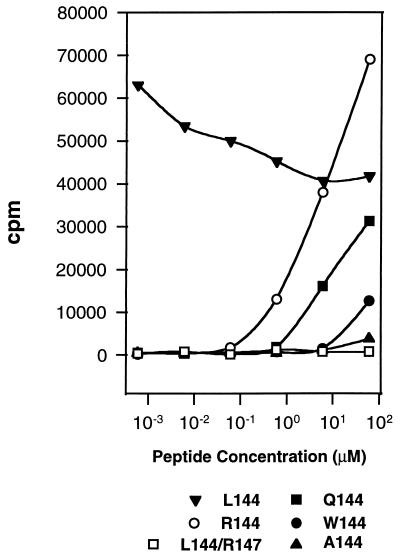
The Q1.1B6 clone was generated from SJL mice immunized with Q144 and responds to this peptide in the context of I-As. To probe the fine specificity of the response of this clone we activated it with a number of different position 144 substituted peptides, all of which have a similar affinity for I-As, and measured the proliferative response (Fig. 1). With these peptides we could define a hierarchy of responses. Two analogs (L144, R144) elicited proliferation at lower concentrations than the cognate ligand (Q144), whereas higher concentrations of two others (W144, A144) were needed to induce a response. No proliferation was detected with the double substituted analog L144/R147. These APLs could be ranked relative to each other in terms of potency in the proliferation assay and the complete hierarchy of response was found to be L144 > R144 > Q144 > A144 ≥ W144. The response to L144 was particularly striking, because even at 6 × 10−4 μM the proliferation induced by the peptide had not reached a maximum and at higher peptide concentrations the peptide appeared to inhibit T cell growth. This heteroclitic behavior was noted with L144 synthesized at two different facilities and was T cell specific because the same L144 was nonantigenic with other independently derived Q144 specific T cell clones (data not shown).
Figure 1.
Hierarchy of the T cell proliferative response of the T cell clone Q1.1B6 to different altered peptide ligands: L144 > R144 > Q144 >W144 ≥ A144 ≫ L144/R147. T cell clones were activated with peptide antigens at the concentrations shown. Proliferation was assessed 48 h after activation.
To characterize the functional response further we measured cytokines in the supernatants of cells activated with various ligands (Table 1). The phenotype of the Q1.1B6 clone was Th0, because activation with Q144 stimulated the production of both IFN-γ and IL-4. Ligands lower in the hierarchy (A144, W144) induced the same cytokines as Q144 but in lower amounts, and W144 induced relatively more IFN-γ than IL-4. Surprisingly, the analogs that hyperstimulated the clones also induced the secretion of detectable levels of IL-2. L144 consistently stimulated IL-2 production, and activation with R144 also elicited lower levels of this cytokine. To confirm that the differences in detectable IL-2 reflected differences in its induction we assessed the amount of IL-2 mRNA following stimulation by 50 μM Q144 or L144 with quantitative reverse transcription–PCR by using a cytokine mimic (24). This demonstrated a 100-fold increase in IL-2 mRNA levels after activation with L144 compared with Q144 (data not shown). These results were confirmed by intracytoplasmic staining of IL-2 (data not shown). IL-10 was detected in some experiments following activation with L144 and R144 but was never detected following activation by the cognate ligand or the other analogs (data not shown). This hyperstimulation is characterized by a heteroclitic proliferative response and the secretion of cytokines not detected following activation by the cognate ligand. We describe such hyperstimulatory ligands as superagonists.
Table 1.
The pattern and amounts of cytokines produced by the Q1.1B6 clone changes following stimulation with different peptide analogs despite similar binding affinities for MHC class II
| APL | Sequence | Relative MHC binding | Proliferation, Δcpm | Δ cytokine, pg/ml
|
||
|---|---|---|---|---|---|---|
| IFN-γ | IL-2 | IL-4 | ||||
| Q144 | HSLGKQLGHPDKF | 0.6 | 84,250 | 2,650 | <50 | 1,090 |
| A144 | HSLGKALGHPDKF | 0.6 | 22,200 | 730 | <50 | 370 |
| L144 | HSLGKLLGHPDKF | 0.8 | 29,420 | 13,770 | 1,490 | 9,420 |
| R144 | HSLGKRLGHPDKF | 0.8 | 48,650 | 8,890 | 490 | 2,550 |
| W144 | HSLGKWLGHPDKF | 1.0 | 16,103 | 2,160 | <50 | 100 |
| L144/R147 | HSLGKLLGRPDKF | 1.3 | 37 | <100 | <50 | <25 |
The data are the mean values from four or five independent experiments at antigen concentrations tested between 6 and 60 μM. Values >3× background are shown in bold.
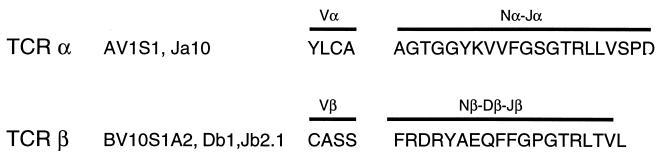
To exclude the possibility that the response we observed was caused by the expression of multiple TCRs, we identified the TCR genes expressed by Q1.1B6 by inverse PCR. In-frame rearrangements corresponding to AV1S1Ja10 and BV10S1A2-Db1, Jb2.1 were identified for TCR-α and -β, respectively (Fig. 2). An additional out-of-frame rearrangement was identified for TCR-α. No other TCR-α rearrangements were detected among 32 PCR clones examined. Expression of TCR-β chains in Q1.1B6 was examined further by PCR by using primers specific for all reported Vβ sequences (25). This confirmed the presence of Vβ10, and no additional rearrangements were detected (data not shown). This shows that the clone expressed only one functional TCR-α and -β chain gene.
Figure 2.
TCR utilization by the Q1.1B6 clone. TCR V, D, and J segments and the predicted amino acid sequence of V(D)J regions of TCR-α and -β chains from T cell clone Q1.1B6 are shown. Assignments to V, D, and J segments are based on Arden et al. (40).
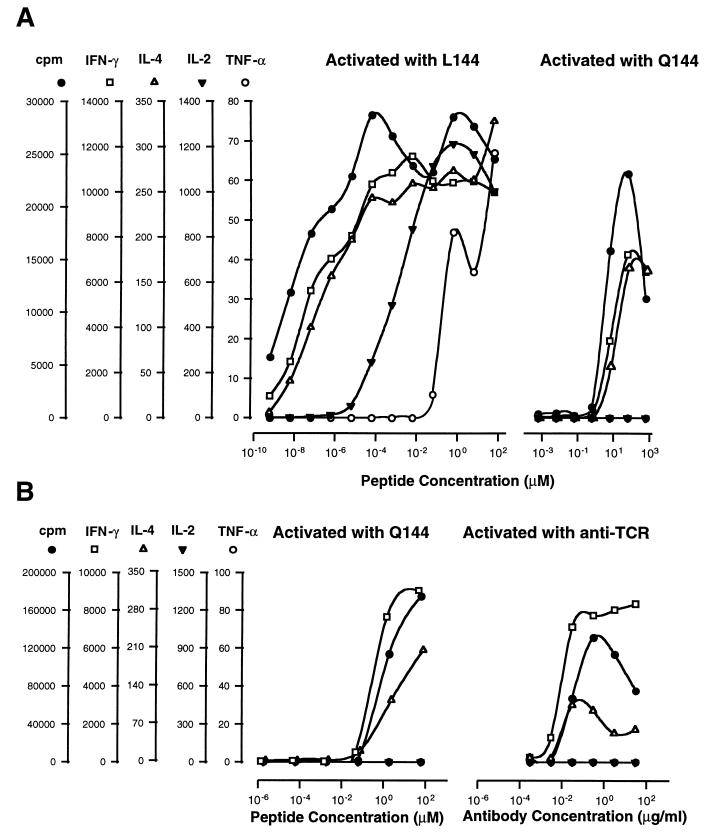
To test whether the effects of the superagonist peptide could be mimicked by increasing concentrations of the cognate ligand, we compared the response of Q1.1B6 to L144 over a broad dose range from 6 × 10−10 to 6 × 101 μM peptide, with the response to Q144, in terms of proliferation and cytokine production. These experiments revealed a hierarchy in the induction of different cytokines with their half-maximal production occurring at very different concentrations of L144 (Fig. 3A). The most sensitive measure of T cell response was proliferation that was detectable a 6 × 10−10 μM L144 compared with 6 × 10−1 μM Q144. IFN-γ and IL-4 were the first cytokines detected. Their production reached a maximum within 1–2 logs of the concentration which elicited maximum proliferation. In the case of L144, further increases in peptide to 6 × 10−5 μM led to detectable IL-2 secretion and at the highest doses of L144 TNF-α could also be detected. The T cells were confirmed to be the source of TNF-α by demonstrating its detection from T cells stimulated by fixed APCs (see below). In contrast, in the same experiment the response to Q144 fell within a narrow concentration range (6 × 10−1 to 6 × 102 μM) and only IFN-γ and IL-4 were detected. Neither at maximal proliferation nor at the highest antigen concentration tested (6 × 102 μM of Q144) were IL-2 or TNF-α detected. Although the difference in reactivity to Q144 and L144 may be quantitative and not qualitative, to achieve with Q144 the stimulation we see with L144, we estimate that theoretically it would be necessary to dissolve 1 g of Q144 in 1 ml of medium to elicit IL-2 and 1 kg of peptide in 1 ml of medium to elicit TNF-α.
Figure 3.
Dose response of Q1.1B6 with agonist peptide (Q144), superagonist peptide (L144), and anti-TCR antibodies (H57.597). (A) L144 and Q144 differ in proliferation and pattern of cytokines produced from the Q1.1B6 clone over a wide dose range, following activation by antigen and APCs. (B) The proliferation and cytokine production to anti-TCR antibody most closely resembles the response to the cognate Q144 ligand. All samples in each set were tested on the same plate. Background proliferation was <400 cpm. Background cytokine production was below the limit of detection of the assays. One representative experiment of at least four is shown.
We then determined whether activation of Q1.1B6 with anti-TCR or anti-CD3 antibody plus APCs, with the potential to cross-link all the available TCRs, resembled more closely the response to Q144 or L144. With either antibody the proliferation reached a maximum comparable to that induced by Q144 and we detected IFN-γ and IL-4 but not TNF-α or IL-2. The data for anti-TCR-antibody is shown in comparison with Q144 peptide (Fig. 3B). We conclude that over the concentration range used, anti-TCR or anti-CD3 antibody was unable to elicit superagonist responses.
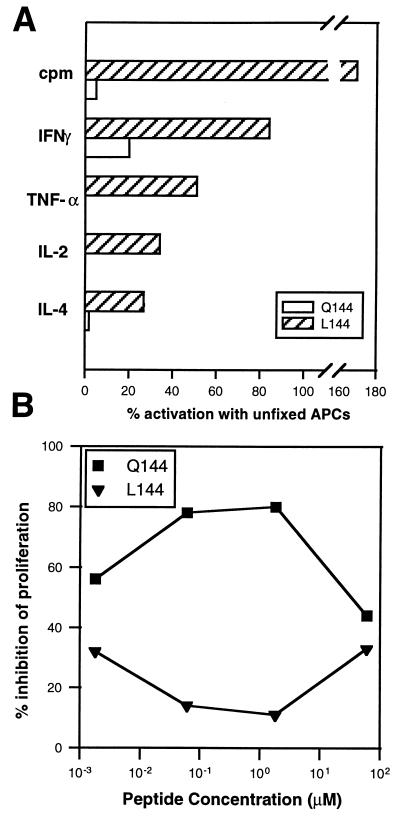
Because the normal activation of T cell clones requires cognate ligand (signal 1) and costimulation in the form of B7 (CD80/CD86)-CD28 mediated signaling (signal 2) (26, 27), we wished to determine whether superagonists had the same requirement. Activation with cognate ligand in the absence of signal 2 leads to T cell anergy (28), a process thought to limit activation of T cells by self antigens (29). If superagonists have different requirements for costimulation, they may have the potential to overcome this checkpoint in self-tolerance. To assess this we activated rested T cell clones with ECDI-fixed and non-fixed APCs. We found that on activation with fixed APCs, Q144 was only able to elicit low levels of IFN-γ secretion and did not induce significant proliferation or IL-4 production. Under the same fixation conditions L144 induced proliferation and production of all the cytokines detectable on stimulation with unfixed APCs (Fig. 4A). The average levels of proliferation were actually higher following activation by L144 on fixed APCs because of a reduction in the high-dose inhibition of growth seen with this peptide (Fig. 4A). To determine the specific role of the costimulation via CD28/CTLA4 we blocked activation with human CTLA4-Ig. At high concentrations of antigen this blockade had little effect following activation with either Q144 or L144. At lower antigen concentrations activation by Q144, but not by L144 was significantly reduced by the CTLA4-Ig but not control fusion protein (Fig. 4B). These experiments have been confirmed by using CHO cells transfected with I-As alone, or I-As with B7 costimulatory molecules. Activation with Q144 requires costimulation whereas activation with L144 does not (A. Murtaza and V.K.K., unpublished data). We conclude that stimulation with the superagonist ligand L144 has less stringent costimulatory requirements than activation with the cognate ligand Q144. In fact, in some cases the presentation of L144 by costimulation-deficient APCs may actually induce greater expansion of Q1.1B6 compared with presentation on costimulation competent APCs.
Figure 4.
Q1.1B6 is less costimulation dependent when activated with L144 compared with Q144. (A) Syngeneic splenic APCs were irradiated and an aliquot of cells was fixed with ECDI. Washed ECDI-fixed and unfixed-APCs were used to activate the Q1.1B6 T cell clone in a proliferation assay with 100 μg/ml of the L144 or Q144 peptides. The data are the mean of four experiments. (B) The Q1.1B6 was activated by syngeneic splenic APCs in the presence of human CTLA4-Ig or a control fusion protein at a concentration of 10–25 μg/ml, to determine percent inhibition. The data are the mean of two experiments.
To address the issue of whether heteroclitic T cell responses with changes in cytokine profile were only seen in one clone, Q1.1B6, or whether other clones would show similar hierarchies and changes in cytokine patterns, we analyzed T cell clones generated independently from mice immunized with altered peptide ligands (L144/R147 and Q144). We identified four clones from two additional clonings that produced significant amounts of additional cytokines (IL-2 and IFN-γ) on activation with the hyperstimulatory altered ligand, but not the immunizing ligand (Table 2). This demonstrates that several different clones from different clonings and of different specificities, shifted their cytokine profile toward the production of Th1 cytokines on activation with heteroclitic ligands. It also shows that this behavior is not restricted to Q1.1B6 but can also be observed with other T cell clones.
Table 2.
T cell clones from several clonings show heteroclitic responses associated with changes in their cytokine profile
| Clone | Immunizing antigen | Activating antigen | Cytokine concentration (pg/ml)
|
||
|---|---|---|---|---|---|
| IFN-γ | IL-2 | IL-4 | |||
| LR.1B2 | L144/R147 | L144/R147 | <100 | 370 | >3,200 |
| L144 | 2,500 | 4,250 | >3,200 | ||
| LR.1C1 | L144/R147 | L144/R147 | <100 | <50 | 2,710 |
| L144 | <100 | 370 | >3,200 | ||
| QW.3F4 | Q144 | Q144 | <100 | 70 | 1,560 |
| L144 | 780 | 325 | 1,910 | ||
| QW.9F8 | Q144 | Q144 | <100 | <50 | 1,850 |
| A144 | 200 | 1,550 | 2,030 | ||
Superagonist ligands induce both IL-2 and IFN-γ in cells compared with activation with the immunizing peptide. Values > background are shown in bold.
DISCUSSION
APLs have been shown to mediate a number of different functional outcomes. These outcomes include the dominant-negative effects of T cell antagonism (12), peptide induced T cell anergy (13), and the partial activation (partial agonist) states demonstrated by the initiation of cytokine secretion in the absence of proliferation (14, 15). All these outcomes are likely to be caused by a suboptimal interaction between TCR and peptide major histocompatibility complex (MHC). By using ligands with different MHC binding affinities, it has been possible to demonstrate that increased peptide/MHC density on APCs can lead to signals which induce Th1 cytokines (30, 31), and such ligands can induce the Th1 cytokine IFN-γ from a Th0 T cell clone. By using human T cell clones, superagonist ligands that induce heteroclitic proliferative responses from autoreactive T cells with changes in the patterns of signaling have recently been described (ref. 32; B. Hemmer and R. Martin, personal communication). We have now described a ligand with nearly identical affinity for MHC that hyperstimulates T cell activation, induces the production of cytokines not detected following activation with the cognate ligand, and has an enhanced capacity to induce high dose inhibition of proliferation. This behavior is not unique to this clone because similar responses have been seen in other clones generated by us (Table 2) and others, in independently derived murine (33) and human (B. Hemmer and R. Martin, personal communication) T cells. This leads us to propose a model in which the TCR/MHC/peptide avidity necessary to elicit a T cell response that is optimal for growth has both a lower and an upper threshold. Ligands with avidities on either side of this window are unable to initiate efficient T cell expansion either because they deliver a stimulus that is too weak or because they rapidly cause activation-induced cell death. This may serve to improve the fidelity of T cell recognition based on low affinity interactions between TCR and MHC/peptide, by providing a mechanism to neutralize the effects of high-avidity cross-reactive interactions in the periphery.
Can all TCRs respond to a range of different peptides and are T cells generally cross-reactive? The evidence that this is a common phenomenon is compelling (34). Furthermore, a recent study (35) of human T cell clones by using a random peptide library approach suggests that ligands which are heteroclitic can be generated fairly readily. Therefore, in considering whether the responses of Q1.1B6 are unusual, the important question is whether TCR interaction with cognate ligand usually induces a medium strength signal into the T cell or whether other ligands more commonly induce a maximal signal, therefore greatly reducing the likelihood of the existence of superagonist ligands for the majority of T cells. Although selection for maximal signal into the T cell cannot occur by affinity maturation of the TCR, it is possible that “affinity selection” of the T cell response can occur at the population level. The profoundly restricted T cell response to pigeon cytochrome c in B10.BR mice (36) may be an example of such a process. If foreign antigens do preferentially select high avidity/high signal strength T cells, then the existence of T cells with receptors that have potential superagonist ligands in the environment may be restricted to clones that have lower avidity interactions with their cognate ligands. This type of low avidity interaction is believed to characterize autoreactive TCRs, because high avidity autoreactive TCRs are deleted from the repertoire by negative thymic selection (37, 38). Therefore, low avidity autoreactive T cells may be the cells most likely to encounter a superagonist ligand in the form of a peptide generated from a foreign organism.
These experiments also show that the induction of cytokines from Q1.1B6 is a hierarchical process, in that the concentrations of L144 necessary to elicit half-maximal production of IL-2 and TNF-α are much higher than the concentration necessary to elicit half-maximal production of IFN-γ or IL-4. However, the enormous amounts of Q144 theoretically necessary to induce the same responses as L144, and the likely upper limit on the number of class II molecules that can be loaded (39), lead us to conclude that in functional terms the two ligands produce phenotypically different responses over a wide range of antigen concentrations. Thus at a particular antigen concentration the same T cell, stimulated by different ligands, can produce a dramatically different cytokine milieu. This may effect the differentiation of naive T cells, leading to Th2 or Th1 responses depending on the activating ligand. It might also alter the responses of memory T cells and therefore may be important for initiating autoimmune reactions. For example, a self-reactive Th2 cell, activated by a cross-reactive viral superagonist peptide, might undergo a change in phenotype and become an autoaggressive Th1 cell that could traffic to a target organ and trigger an inflammatory reaction.
The less stringent requirements for costimulation that Q1.1B6 activated by L144 demonstrates may be particularly important for the initiation of autoreactivity. In organ specific autoimmunity, self-antigens may commonly be presented on nonprofessional APCs expressing MHC/peptide but no costimulatory molecules (29). If during an acute infection nonprofessional APCs within a target organ present peptides that act as superagonists for autoreactive T cells, cells that normally would be rendered anergic may be activated. The release of pro-inflammatory cytokines would subsequently up-regulate costimulation and lead to the recruitment of autoantigen specific T cells from the pool of circulating precursor T lymphocytes.
In summary, self-peptides may not act as superagonists because the self-reactive population bearing high-affinity TCR is deleted during thymic ontogeny. Superagonist ligands are more likely to be generated during infection at which time they could alter T cell differentiation, affecting the regulatory immune mechanisms that maintain peripheral tolerance, and induce autoimmunity.
Acknowledgments
We thank Heather Finnerty for DNA sequencing, Jim Lederer for reagents and help with reverse transcription–PCR, and Vadim Turchin for technical assistance. This work was supported by the National Institutes of Health (NS30843, R01NS35685, P01AI39671–01A1), the National Multiple Sclerosis Society (RG2571, RG2320), and the Howard Hughes Medical Institute (A.M.C.). L.B.N. is a Post Doctoral Fellow of the National Multiple Sclerosis Society.
ABBREVIATIONS
- APC
antigen presenting cell
- APL
altered peptide ligand
- EAE
experimental autoimmune encephalomyelitis
- IFN
interferon
- MHC
major histocompatibility complex
- TCR
T cell receptor
- Th
T helper
- IL
interleukin
- TNF
tumor necrosis factor
- ECDI
[1-ethyl-3-(3′-dimethylaminopropyl) carbodiimide]
Footnotes
References
- 1.Paul W E, Seder R A. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 2.Kuchroo V K, Martin C A, Greer J M, Ju S T, Sobel R A, Dorf M E. J Immunol. 1993;151:4371–4382. [PubMed] [Google Scholar]
- 3.Chen Y, Kuchroo V K, Inobe J, Hafler D A, Weiner H L. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 4.Khoruts A, Miller S D, Jenkins M K. J Immunol. 1995;155:5011–5017. [PubMed] [Google Scholar]
- 5.Kuchroo V K, Prabhu Das M, Brown J A, Ranger A M, Zamvil S S, Sobel R A, Weiner H L, Nabavi N, Glimcher L H. Cell. 1995;80:707–718. doi: 10.1016/0092-8674(95)90349-6. [DOI] [PubMed] [Google Scholar]
- 6.Nicholson L B, Kuchroo V K. Curr Opin Immunol. 1996;8:837–842. doi: 10.1016/s0952-7915(96)80013-6. [DOI] [PubMed] [Google Scholar]
- 7.Romagnani S. Annu Rev Immunol. 1994;12:227–257. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- 8.Wraith D C, McDevitt H O, Steinman L, Acha-Orbea H. Cell. 1989;57:709–715. doi: 10.1016/0092-8674(89)90786-1. [DOI] [PubMed] [Google Scholar]
- 9.Wauben M H M, Boog C J P, Van der Zee R, Joosten I, Schlief A, Van Eden W. J Exp Med. 1992;176:667–677. doi: 10.1084/jem.176.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brocke S, Gijbels K, Allegretta M, Ferber I, Piercy C, Blankenstein T, Martin R, Utz U, Karin N, Mitchell D J, Veromaa T, Waisman A, Gaur A, Conlon P, Ling N, Fairchild P J, Wraith D C, O’Garra A, Fathman C G, Steinman L. Nature (London) 1996;379:343–346. doi: 10.1038/379343a0. [DOI] [PubMed] [Google Scholar]
- 11.Kuchroo V K, Greer J M, Kaul D, Ishioka G, Franco A, Sette A, Sobel R A, Lees M B. J Immunol. 1994;153:3326–3336. [PubMed] [Google Scholar]
- 12.De Magistris M T, Alexander J, Coggeshall M, Altman A, Gaeta F C A, Grey H M, Sette A. Cell. 1992;68:625–634. doi: 10.1016/0092-8674(92)90139-4. [DOI] [PubMed] [Google Scholar]
- 13.Sloan-Lancaster J, Evavold B D, Allen P M. Nature (London) 1993;363:156–159. doi: 10.1038/363156a0. [DOI] [PubMed] [Google Scholar]
- 14.Evavold B D, Allen P M. Science. 1991;252:1308–1310. [PubMed] [Google Scholar]
- 15.Racioppi L, Ronchese F, Matis L A, Germain R N. J Exp Med. 1993;177:1047–1060. doi: 10.1084/jem.177.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicholson L B, Greer J M, Sobel R A, Lees M A, Kuchroo V K. Immunity. 1995;3:397–405. doi: 10.1016/1074-7613(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 17.Nicholson L B, Murtaza A, Hafler B P, Sette A, Kuchroo V K. Proc Natl Acad Sci USA. 1997;94:9279–9284. doi: 10.1073/pnas.94.17.9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfeiffer C, Stein J, Southwood S, Ketelaar H, Sette A, Bottomly K. J Exp Med. 1995;181:1569–1574. doi: 10.1084/jem.181.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prabhu Das M, Nicholson L B, Greer J M, Kuchroo V K. J Exp Med. 1997;186:867–876. doi: 10.1084/jem.186.6.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenkins M K, Schwartz R H. J Exp Med. 1987;165:302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uematsu Y, Wege H, Straus A, Ott M, Bannwarth W, Lanchbury J, Panayi G, Steinmetz M. Proc Natl Acad Sci USA. 1991;88:8534–8538. doi: 10.1073/pnas.88.19.8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Sullivan D, Sidney J, Appella E, Walker L, Phillips L, Colon S M, Miles C, Chesnut R W, Sette A. J Immunol. 1990;145:1799–1808. [PubMed] [Google Scholar]
- 23.Franco A, Southwood S, Arrhenius T, Kuchroo V K, Grey H M, Sette A, Ishioka G Y. Eur J Immunol. 1994;24:940–946. doi: 10.1002/eji.1830240424. [DOI] [PubMed] [Google Scholar]
- 24.Lederer J A, Perez V L, DesRoches L, Kim S M, Abbas A K, Lichtman A H. J Exp Med. 1996;184:397–406. doi: 10.1084/jem.184.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Hara R M, Byrne M C, Kuchroo V K, Nagelin A, Whitters M J, Jayaraman S, Henderson S L, Dorf M E, Collins M. J Immunol. 1995;154:2075–2081. [PubMed] [Google Scholar]
- 26.Linsley P S, Ledbetter J A. Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- 27.Viola A, Lanzavecchia A. Science. 1996;273:104–106. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz R H. Cell. 1992;71:1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 29.Matzinger P. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 30.Kumar V, Bhardwaj V, Soares L, Alexander J, Sette A, Sercarz E. Proc Natl Acad Sci USA. 1995;92:9510–9514. doi: 10.1073/pnas.92.21.9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaturvedi P, Yu Q, Southwood S, Sette A, Singh B. Int Immunol. 1996;8:745–755. doi: 10.1093/intimm/8.5.745. [DOI] [PubMed] [Google Scholar]
- 32.Vergelli M, Hemmer B, Kalbus M, Vogt A B, Ling N, Conlon P, Coligan J E, McFarland H F, Martin R. J Immunol. 1997;158:3746–3752. [PubMed] [Google Scholar]
- 33.Itoh Y, Germain R N. J Exp Med. 1997;186:757–766. doi: 10.1084/jem.186.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kersh G J, Allen P M. Nature (London) 1996;380:495–498. doi: 10.1038/380495a0. [DOI] [PubMed] [Google Scholar]
- 35.Hemmer B, Fleckenstein B T, Vergelli M, Jung G, McFarland H F, Martin R, Wiesmuller K H. J Exp Med. 1997;185:1651–1659. doi: 10.1084/jem.185.9.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McHeyzer-Williams M G, Altman J D, Davis M M. Curr Opin Immunol. 1996;8:278–284. doi: 10.1016/s0952-7915(96)80068-9. [DOI] [PubMed] [Google Scholar]
- 37.Nossal G J V. Cell. 1994;76:229–239. doi: 10.1016/0092-8674(94)90331-x. [DOI] [PubMed] [Google Scholar]
- 38.von Boehmer H. Cell. 1994;76:219–228. doi: 10.1016/0092-8674(94)90330-1. [DOI] [PubMed] [Google Scholar]
- 39.Dadaglio G, Nelson C A, Deck M B, Petzold S J, Unanue E R. Immunity. 1997;6:727–738. doi: 10.1016/s1074-7613(00)80448-3. [DOI] [PubMed] [Google Scholar]
- 40.Arden B, Clark S P, Kabelitz D, Mak T W. Immunogenetics. 1995;42:501–530. doi: 10.1007/BF00172177. [DOI] [PubMed] [Google Scholar]