Abstract
A necessary role for cytotoxic T lymphocytes in protection against Mycobacterium tuberculosis (MTB) has been suggested by studies of the β2-microglobulin-deficient mouse, which is unable to present antigens through MHC class I and class I-like molecules and invariably succumbs early after infection. To identify the relative contributions of distinct putative MHC class I-dependent cell populations in protection against tuberculosis, we compared a variety of gene-disrupted mouse strains for susceptibility to MTB infection. Among the strains tested, the most susceptible mice, as measured by survival time and bacterial loads, were the β2-microglobulin−/−, followed by transporter associated with antigen processing deficient (TAP1−/−), CD8α−/−, perforin−/−, and CD1d−/− mice. These findings indicated that (i) CD8+ T cells contribute to protection against MTB, and their protective activity is only partially dependent on perforin; (ii) β2-microglobulin-dependent T cell populations distinct from CD8+ T cells also contribute to anti-MTB immunity; and (iii) protective immune mechanisms are predominantly TAP-dependent, although TAP-independent mechanisms also contribute to protection. Because CD1d-deficient animals were fully resistant to MTB, other TAP-independent mechanisms must contribute to protection. We suggest here that both classical and nonclassical MHC class I-restricted T cells, distinct from CD1d-restricted cells, may be involved in protective immune responses against tuberculosis.
Keywords: CD8+ T cells, perforin, β2-microglobulin, transporter associated with antigen processing, CD1d
Tuberculosis and AIDS are the two leading causes of mortality from infectious diseases worldwide (1). Tuberculosis alone accounts for 3 million deaths per year (2). Because of the variability of protection imparted by the current Bacille Calmette–Guerin vaccine, ranging from none to 80% (3), there is an urgent need to develop better prophylactic strategies. To assess the efficacy of any new vaccine, it will be important to define those mechanisms necessary and sufficient for protective immunity to Mycobacterium tuberculosis (MTB). These immunological mechanisms may then be targeted specifically in new vaccines to induce host immune responses that more successfully protect individuals against this formidable pathogen.
MTB replicates primarily within host macrophages. Several studies have indicated an important role for macrophage activation induced by the cytokines IFN-γ (4) and tumor necrosis factor-α (5) in the control of MTB. It is well established that CD4+ T cells, activated through MHC class II molecules, are essential for protection against tuberculosis (6), in part by producing lymphokines such as IFN-γ (7). Control of this intracellular pathogen also seems to depend critically on the recognition and elimination of infected cells. Thus, it would be expected that MHC class I-restricted T cells are important for the generation of protective immune responses in MTB infection. MHC class I molecules are composed of a heavy α-chain and a light chain, β2-microglobulin (β2-m). MHC class I molecules are loaded with peptides actively transported from the cytosol into the endoplasmic reticulum by an ATP-dependent transporter associated with antigen processing (TAP) heterodimer. The class I MHC–peptide complex then travels through the Golgi apparatus onto the cell surface, where it presents the peptide to CD8+ T cells. This process is known as the classical MHC class I pathway. The nonclassical or MHC class Ib molecule, CD1, uses a TAP-independent pathway, whereby glycolipid antigens are loaded in CD1-containing MHC class II loading compartments. Other less well characterized MHC Ib molecules use TAP-dependent (8, 9) and/or TAP-independent (10–12) pathways for antigen processing. We have previously shown that β2-m-deficient (β2-m−/−) mice are highly susceptible to MTB infection (13). β2-m−/− mice have several important immune and nonimmune disorders, among them an almost total defect in the surface expression of MHC class I molecules and the lack of CD8+ cytotoxic T lymphocytes (14). Results from earlier adoptive transfer and in vivo T cell depletion studies have suggested that CD8+ T cells may be involved in the immune response to MTB (15–18). Recent in vitro experiments have identified CD8+ T cells that recognize MTB antigens in the context of both classical MHC class I (19) and nonclassical MHC class I molecules, such as human CD1b (20, 21) and other nonpolymorphic class Ib molecules (22, 23). Most recently, an increased susceptibility to MTB infection was reported in TAP1-deficient mice that was not apparent in CD1d-deficient animals (24). However, it remained unclear whether other TAP-independent mechanisms contribute to protection against tuberculosis.
We postulate that the absence of CD8+ T cells and associated cytotoxic immune functions are responsible for the susceptibility of β2-m−/− mice to tuberculosis infection. We further ask whether the CD8+ T cell contribution is a cytotoxic role mediated by perforin and whether CD8+ T cells responded to antigens presented by the classical TAP-dependent pathway. To address these questions, we performed a direct comparison of susceptibility to virulent MTB by using multiple mouse strains defective in β2-m-dependent functions. We showed that, although CD8+ T cells are indeed involved in protection against MTB, they do not fully account for the increased susceptibility of β2-m−/− mice. Furthermore, by this approach, the relative importance of the various MHC I restricted immune mechanisms was revealed, based on the rank order of susceptibility of the different mutant strains.
Materials and Methods
Animals.
CD8α−/−, perforin−/−, β2-m−/−, and wild-type C57BL/6 control mice were purchased from The Jackson Laboratory. TAP1−/− mice and CD1d−/− mice have been described (25, 26). All gene-deficient mice used were either fully backcrossed or at least backcrossed six times to the C57BL/6 genetic background. Wild-type C57BL/6 mice were used as the control group. In one experiment, we used CD1d−/− mice backcrossed six times to the BALB/c background, and littermate CD1d+/+ BALB/c mice as controls. All mice were bred and maintained under specific pathogen-free conditions in our animal facilities. Mice were 3–4 months old at the onset of experimental infection.
Bacteria.
MTB strain Erdman (Trudeau Institute, Saranac Lake, NY) was harvested from the spleens of C57BL/6 mice and passaged twice in Middlebrook 7H9 broth (Difco) supplemented with 1% glycerol, 0.05% Tween-80 (Sigma), and 10% (vol/vol) oleic acid-albumin-dextrose-catalase (OADC). Aliquots from logarithmically growing cultures were frozen in 10% (vol/vol) glycerol in PBS. Representative vials were thawed and enumerated for viable colony forming units (CFU) on Middlebrook 7H10 agar plates (Difco) supplemented with 1% glycerol and 10% (vol/vol) OADC.
Animal Infections and Organ Harvesting.
Mice were injected i.v. into the lateral tail vein with either a low dose (2 × 105 or 1 × 105) or a high dose (1 × 106) of bacilli in 200 μl of PBS containing 0.05% Tween-80. Groups of four to five mice were killed 24 h after infection and at later time points. Bacterial burdens were determined from the lungs, liver, and spleen of each mouse. Organs were removed aseptically and homogenized in PBS-Tween by using a homogenizer (PRO 200, ProScientific, Monroe, CT). Four serial dilutions of organ homogenates were plated onto 7H10 agar (Difco) plates supplemented with 1% glycerol, and 10% (vol/vol) OADC. Plates were incubated at 37°C, and MTB colonies were counted 3 weeks later. In addition, groups of 12 to 30 mice from each strain were monitored for survival analysis.
Histologic Examination.
Lung, liver, and spleens were fixed in 10% (vol/vol) buffered formalin for 24 h, embedded in paraffin, and processed for histologic examination. Sections were stained with hematoxylin and eosin for histologic evaluation or with the Kinyoun method to visualize acid fast bacteria.
Statistical Analysis.
Survival intervals and bacterial burdens were summarized by median values and 25th–75th percentiles. Differences between mouse strains were analyzed by using the Kruskal–Wallis test for nonparametrical distributions.
Results and Discussion
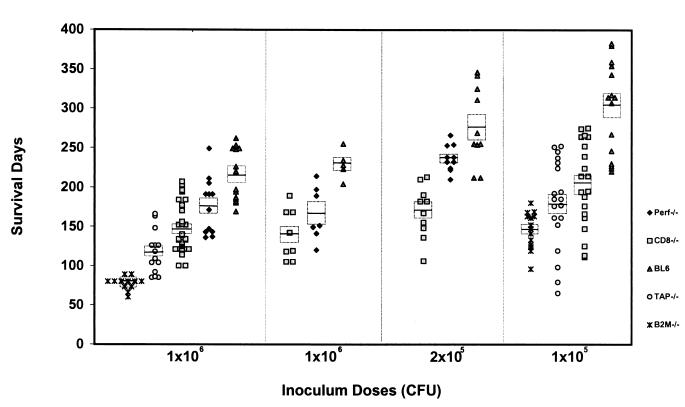
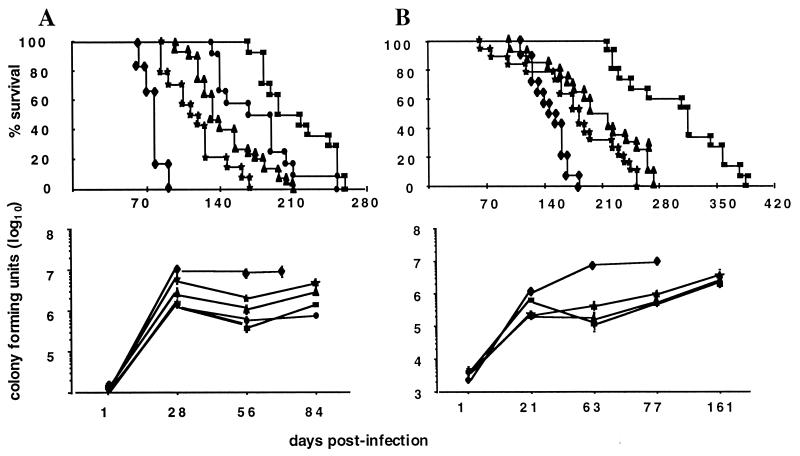
We sought to address the question of which T cell functions and specificities associated with cytotoxic T cell activity are involved in protection against experimental tuberculosis. β2-m−/− mice are highly susceptible to MTB infection (13). To define the specific immune defect(s) of β2-m−/− mice responsible for their vulnerability to tuberculosis, selected gene knockout mouse strains were infected with MTB. An accurate assessment of the relative importance of the different immune mechanisms necessitated direct comparison within the same experiment. Groups of mice deficient in the β2-m, TAP1, CD8α, or perforin molecules and wild-type controls were concurrently infected with 106 bacilli and followed for disease progression. The mean survival times for all mice with targeted gene-disruptions were significantly shorter than those of the wild-type animals (P < 0.005 for all comparisons; Fig. 1). β2-m−/− mice were the most susceptible group, followed by TAP1−/− mice, CD8α−/− mice, perforin−/− mice, and wild-type animals (Fig. 2A). Paired analysis indicated that the differences between all mutant strains were statistically significant (P < 0.005). Of particular interest was the striking difference in survival found between β2-m−/− and CD8α−/− mice (P < 0.0001). Indeed, CD8α−/− mice survived 2 months longer than β2-m−/− mice. Differences between β2-m−/− and TAP1−/− mice were also highly significant (P < 0.0001). This trend was confirmed in a subsequent experiment in which the mice were challenged with a 10-fold lower inoculum of MTB (Fig. 2B). An alternative measurement of susceptibility to MTB is bacterial burdens in the vital organs. The numbers of CFU in all organs harvested were consistent with the survival pattern. The highest bacterial burden was present in the β2-m−/− mice, followed by TAP1−/−, CD8α−/−, perforin−/−, and wild-type mice. These differences were most obvious in the lung, where they became apparent as early as 21 days after infection and continued to increase until the death of the animal (Fig. 2B).
Figure 1.
Survival of gene-deficient mice after receiving different doses of MTB. Results of four independent experiments with high (1 × 106) and low infectious doses (2 × 105 and 1 × 105) are displayed. Each marker represents survival days after infection of an individual mouse. The horizontal lines indicate the mean survival times, and the rectangles represent SEM. The number of mice per strain varies between 7 and 30. The mean survival times for all mouse strains are significantly different from each other in all experiments (P < 0.05).
Figure 2.
Simultaneous MTB infection of mouse strains deficient in selective β2-m-dependent functions. Survival and lung burden of β2-m−/− (⧫), TAP1−/− (★), CD8α−/− (▴), perforin−/− (●), and wild-type C57BL/6 (■) mice infected i.v. with (A) a high inoculum (1 × 106 CFU) or (B) a low inoculum (1 × 105 CFU) of MTB. (Upper) The percentage of survival of groups of 14 to 30 mice per strain. The differences in survival between all strains are statistically significant. (Lower) The lung burden of MTB at different time points after infection. Results are expressed as the means CFUs ± SEM of five mice per time point and per strain.
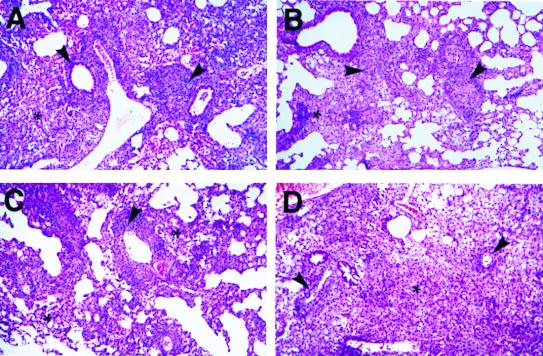
Consistent with the bacteriological burden, lung histopathology showed a progressive granulomatous pneumonia with multifocal necrosis that positively correlated with the duration of infection and the importance of the mouse genetic immunodeficiency as defined by survival and bacterial burdens described above. Thus, pathology was most extensive in the β2-m−/− mice and the least pronounced in the perforin−/− mice (Fig. 3). Conversely, the perivascular, peribronchial, and interstitial lymphocytic infiltrations, typically observed in MTB infected-lungs, decreased as the immunodeficiency became more severe. Thus, wild-type and perforin−/− mice showed a pronounced lymphocytic infiltration (Fig. 3A) compared with moderate levels observed in CD8α−/− mice (Fig. 3B) and TAP1−/− mice (Fig. 3C). The lymphocytic infiltration in β2-m−/− mice was markedly attenuated (Fig. 3D).
Figure 3.
Lung histopathology of wild-type, CD8α−/−, TAP1−/−, and β2-m−/− mice at 4 weeks after challenge with 106 MTB. (A) Wild-type mice have an interstitial pneumonitis composed of pronounced perivascular lymphocytic infiltration (arrowheads) accompanied by a diffuse histiocytic macrophage infiltrate (asterisk). (B) CD8−/− mice present moderate perivascular and interstitial lymphocytic infiltration (arrowheads) and a prominent histiocytic cell response (asterisk). (C) TAP−/− mice show modest numbers of perivascular lymphocytes (arrowhead) and manifest histiocytic cell infiltration (asterisk). (D) β2-m−/− mice have an extensive pneumonitis, which is predominantly composed of diffused histiocytic infiltration (asterisk), and markedly lower numbers of perivascular lymphocytes (arrowheads).
Our findings establish that both CD8+ T cells and the perforin molecule contribute to protection against MTB. It has been reported previously that the perforin molecule does not play a role in the early protective response to MTB (27, 28). Our results were consistent with this finding but suggest that perforin contributes to protection in the late phases of infection. Yet, the increased susceptibility of CD8α−/− mice compared with that of perforin−/− mice indicates that the role or roles of MTB-specific CD8+ T cells are not limited to perforin-dependent cytotoxicity. These results were confirmed in additional experiments, in which CD8α−/− mice, perforin−/− mice, and wild-type C57BL/6 mice were inoculated with different challenge doses of MTB. Again, CD8α−/− mice showed significantly reduced mean survival times in comparison with perforin−/− mice (Fig. 1). Concordantly, the number of bacilli retrieved from the lung, spleen, and liver 56 days after infection with 106 MTB was highest in CD8α−/− mice (8.8 × 105 CFUs), followed by perforin−/− mice (3.3 × 105 CFUs; P = 0.009) and wild-type controls (2 × 105 CFUs; P = 0.028). Histological examination indicated that the perivascular lymphoid aggregates were reduced and more diffuse in CD8α−/− mice compared with those of perforin−/− or wild-type animals (data not shown). Based on these findings, we conclude that perforin-independent lytic mechanisms (29) or production of lymphokines, such as IFN-γ (30), are likely to be involved in CD8+ T cell-associated protective immune responses to MTB infection.
The greater resistance to infection observed in CD8α−/− mice compared with β2-m−/− mice clearly indicates that additional protective mechanisms are lacking in the latter strain. This intriguing finding may have several possible explanations. Classical MHC class I molecules depend on TAP to transport peptides from the cytosol into the endoplasmic reticulum where the assembly with the MHC molecule occurs, whereas several nonclassical MHC class I molecules are capable of loading their antigens in a TAP-independent fashion (10–12, 31). The critical involvement of classical TAP-dependent MHC class I presentation in protection in vivo was shown by the short survival time of TAP1−/− mice (Fig. 2) and confirmed in an independent experiment in which survival of TAP1−/− mice was again significantly shorter than that of wild-type controls (data not shown). Because most CD8+ T cells are thought to be restricted by classical MHC class I molecules and therefore require TAP for presentation of protein antigens, we predicted that TAP1−/− mice would be as susceptible as the CD8α−/− mice to MTB infection. Unexpectedly, a significant difference in susceptibility was detected between TAP1−/− and CD8α−/− mice (P < 0.005 ). These results may reflect the involvement of a yet to be defined TAP-dependent CD8− cell population. Alternatively, the absence of TAP molecules might have led to a more complete depletion of MHC I-dependent cells because of the lack of antigen presentation for positive selection, whereas disruption of the CD8α gene, resulting in the absence of the CD8 receptor, might still allow generation of some functional MHC class I-restricted CD4− CD8− (double-negative) T cells. Analogous differences have been observed previously for mice with disrupted MHC class II and CD4 genes (7). Indeed, mice deficient for the CD4 receptor have significant numbers of double-negative T cells producing IFN-γ in a MHC class II-restricted manner in response to specific antigen (32).
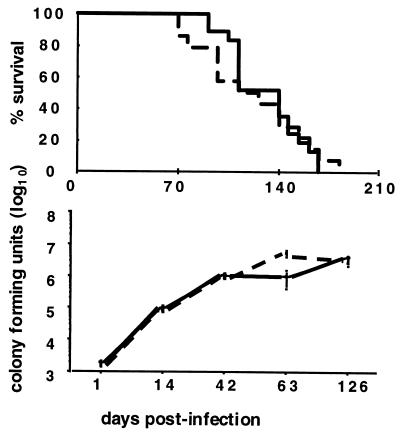
Another striking finding was the substantially higher resistance of TAP1−/− mice compared with that of β2-m−/− mice. These data indicate the involvement of TAP-independent mechanisms in the protective response to tuberculosis. T cells may recognize MTB antigens presented in a TAP-independent fashion by CD1 and other nonpolymorphic MHC class Ib molecules, as previously suggested by in vitro studies with human (22, 23, 33, 34) and murine cells (35, 36). To evaluate the contribution of the only functional CD1 gene in the mouse, CD1d−/− and CD1d+/+ littermate controls were challenged with MTB. This experiment showed no differences in survival or in lung burden at 126 days of the onset of infection, although a higher number of CFUs were detected in the lungs of CD1d−/− animals 63 days after infection (Fig. 4). Finally, a high-dose challenge with MTB of C57BL/6 mice with gene disruptions in the CD1d, CD8α, TAP1, or β2-m molecules confirmed the lack of increased susceptibility to tuberculosis of CD1d−/− mice compared with wild-type controls (P = 0.455) and confirmed the same rank order of susceptibility of the remaining mutants (data not shown). These results indicated that the CD1d molecule in mice is not essential for protection against MTB infection. While this manuscript was in preparation, Behar et al. (24) reported increased susceptibility to MTB infection in the TAP-deficient mice but not in the CD1d-deficient mice. These results are consistent with ours but failed to indicate whether specificities other than TAP-dependent classical class I MHC-restricted T cells contribute to protection to tuberculosis.
Figure 4.
Survival and lung burden of CD1d−/− and CD1d+/+ mice with MTB infection. CD1d−/− (dashed line) mice and CD1d+/+ (solid line) littermates, both from the sixth backcross to BALB/c, were infected i.v. with 105 CFU of MTB. (Upper) The percentages of survival time of groups of 14 and 18 mice per strain do not show any difference. (Lower) The lung burden from 1 to 125 days after infection. Five mice per strain were analyzed at each time point. Results are expressed as the mean CFUs ± SEM.
Because the CD1d-restricted pathway cannot explain the difference in susceptibility found between β2-m−/− and TAP1−/− mice, alternative mechanisms must be considered. Potential candidates are T cells restricted by other nonpolymorphic MHC class Ib proteins. Non-T cell factors are also deficient in β2-m−/− mice. Because of the absence of neonatal Fc receptor (37), another MHC-like molecule, β2-m−/− mice have an increased Ig catabolism (38) and reduced circulating Ig levels. β2-m−/− mice also have alterations in natural killer cell development and function (39); however, beige mice defective for natural killer cells showed no increased susceptibility to MTB infection (40). Finally, β2-m−/− mice develop nonimmune disorders, such as an iron overload (41), and the requirement for iron in mycobacterial metabolism (42) could potentially add to their greater susceptibility to infection.
Our data clearly show that CD8+ T cells contribute to the in vivo protective immune response to MTB but that their functional activity does not depend entirely on perforin-mediated cytotoxicity. Further, our results indicate that the absence of CD8+ T cells does not fully account for the increased susceptibility of β2-m−/− mice to infection, pointing to contributions of additional protective components. The β2-m-dependent populations involved in MTB immune protection include both TAP-dependent and TAP-independent mechanisms. Finally, we determined that the immune contributions made by mouse CD1d-restricted cells do not explain the difference between β2-m−/− and TAP1−/− mice in susceptibility to MTB infection, suggesting a role for other previously unrecognized TAP-independent MHC pathways.
Acknowledgments
We are grateful to Alvin Watford, Bing Chen, XiaoJuan Wang, and Ilona Breiterene for their contribution to the breeding of gene-deficient mice and Biosafety Level 3 technical assistance and to Andrea Cooper and Ian Orme for generously sharing their unpublished data. This work was supported by National Institutes of Health Grants AI 07118 and 23545 and by the Howard Hughes Medical Institute.
Abbreviations
- MTB
Mycobacterium tuberculosis
- β2-m
β2-microglobulin
- TAP
transporter associated with antigen processing
- OADC
oleic acid-albumin-dextrose-catalase
- CFU
colony forming unit
References
- 1.World Health Organization. The World Health Report 1999. Geneva: WHO/OMS; 1999. [Google Scholar]
- 2.Bloom B R. Nature (London) 1992;358:538–539. doi: 10.1038/358538b0. [DOI] [PubMed] [Google Scholar]
- 3.Bloom B R, Fine P E M. In: The BCG Experience: Implications for Iuture Vaccines Against Tuberculosis. Bloom B R, editor. Washington DC: ASM; 1994. pp. 531–557. [Google Scholar]
- 4.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flynn J, Goldstein M, Chan J, Triebold K, Pfeffer K, Lowenstein C, Schreiber R, Mak T, Bloom B. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 6.Flory C M, Hubbard R D, Collins F M. J Leukocyte Biol. 1992;51:225–229. doi: 10.1002/jlb.51.3.225. [DOI] [PubMed] [Google Scholar]
- 7.Caruso A M, Serbina N, Klein E, Triebold K, Bloom B R, Flynn J L. J Immunol. 1999;162:5407–5416. [PubMed] [Google Scholar]
- 8.Chiu N M, Chun T, Fay M, Mandal M, Wang C R. J Exp Med. 1999;190:423–434. doi: 10.1084/jem.190.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aldrich C J, DeCloux A, Woods A S, Cotter R J, Soloski M J, Forman J. Cell. 1994;79:649–658. doi: 10.1016/0092-8674(94)90550-9. [DOI] [PubMed] [Google Scholar]
- 10.Lenz L L, Dere B, Bevan M J. Immunity. 1996;5:63–72. doi: 10.1016/s1074-7613(00)80310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tompkins S M, Kraft J R, Dao C T, Soloski M J, Jensen P E. J Exp Med. 1998;188:961–971. doi: 10.1084/jem.188.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tilloy F, Treiner E, Park S, Garcia C, Lemonnier F, de la Salle H, Bendelac A, Bonneville M, Lantz O. J Exp Med. 1999;189:1907–1921. doi: 10.1084/jem.189.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flynn J L, Goldstein M M, Triebold K J, Koller B, Bloom B R. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koller B, Marrack P, Kappler J W, Smithies O. Science. 1990;248:1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 15.Orme I M, Collins F M. Cell Immunol. 1984;84:113–120. doi: 10.1016/0008-8749(84)90082-0. [DOI] [PubMed] [Google Scholar]
- 16.Muller I, Cobbold S, Waldmann H, Kaufmann S H E. Infect Immun. 1987;55:2037–2041. doi: 10.1128/iai.55.9.2037-2041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orme I M. J Immunol. 1987;138:293–298. [PubMed] [Google Scholar]
- 18.Hubbard R, Flory C, Collins F. Infect Immun. 1991;59:2012–2016. doi: 10.1128/iai.59.6.2012-2016.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufmann S H E. Rev Infect Dis. 1989;11:S448–S454. doi: 10.1093/clinids/11.supplement_2.s448. [DOI] [PubMed] [Google Scholar]
- 20.Stenger S, Mazzaccaro R J, Uyemura K, Cho S, Barnes P F, Rosat J P, Sette A, Brenner M B, Porcelli S A, Bloom B R, et al. Science. 1997;276:1684–1687. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- 21.Stenger S, Hanson D A, Teitelbaum R, Dewan P, Niazi K R, Froelich C J, Ganz T, Thoma-Uszynski S, Melian A, Bogdan C, et al. Science. 1998;282:121–125. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 22.Lewinsohn D M, Alderson M R, Briden A L, Riddell S R, Reed S G, Grabstein K H. J Exp Med. 1998;187:1633–1640. doi: 10.1084/jem.187.10.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Canaday D H, Ziebold C, Noss E H, Chervenak K A, Harding C V, Boom W H. J Immunol. 1999;162:372–379. [PubMed] [Google Scholar]
- 24.Behar S M, Dascher C C, Grusby M J, Wang C-R, Brenner M B. J Exp Med. 1999;189:1973–1980. doi: 10.1084/jem.189.12.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Kaer L, Ashton-Rickardt P G, Ploegh H L, Tonegawa S. Cell. 1992;71:1205–1214. doi: 10.1016/s0092-8674(05)80068-6. [DOI] [PubMed] [Google Scholar]
- 26.Mendiratta S K, Martin W D, Hong S, Boesteanu A, Joyce S, Van Kaer L. Immunity. 1997;6:469–477. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- 27.Laochumroonvorapong P, Wang J, Liu C, Ye W, Moreira A L, Elkon K B, Freedman V H, Kaplan G. Infect Immun. 1997;65:127–132. doi: 10.1128/iai.65.1.127-132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cooper A M, D'Souza C, Frank A A, Orme I M. Infect Immun. 1997;65:1317–1320. doi: 10.1128/iai.65.4.1317-1320.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lammas D A, Stober C, Harvey C J, Kendrick N, Panchalingam S, Kumararatne D S. Immunity. 1997;7:433–444. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- 30.Tascon R E, Stavropoulos E, Lukacs K V, Colston M J. Infect Immun. 1998;66:830–834. doi: 10.1128/iai.66.2.830-834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brutkiewicz R R, Bennink J R, Yewdell J W, Bendelac A. J Exp Med. 1995;182:1913–1919. doi: 10.1084/jem.182.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Locksley R M, Reiner S L, Hatam F, Littman D R, Killeen N. Science. 1993;261:1448–1451. doi: 10.1126/science.8367726. [DOI] [PubMed] [Google Scholar]
- 33.Chan J, Fujiwara T, Brennan P, McNeil M, Turco S J, Sibille J-C, Snapper M, Aisen P, Bloom B R. Proc Natl Acad Sci USA. 1989;86:2453–2457. doi: 10.1073/pnas.86.7.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan J, Fan X, Hunter S W, Brennan P J, Bloom B R. Infect Immun. 1991;59:1755–1761. doi: 10.1128/iai.59.5.1755-1761.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delibero G, Flesch I, Kaufmann S H E. Eur J Immunol. 1988;18:59–66. doi: 10.1002/eji.1830180110. [DOI] [PubMed] [Google Scholar]
- 36.Bouwer H G, Bai A, Forman J, Gregory S H, Wing E J, Barry R A, Hinrichs D J. Infect Immun. 1998;66:2814–2817. doi: 10.1128/iai.66.6.2814-2817.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abou-Zeid C, Smith I, Grange J M, Ratliff T L, Steele J, Rook G A W. J Gen Microbiol. 1988;134:531–538. doi: 10.1099/00221287-134-2-531. [DOI] [PubMed] [Google Scholar]
- 38.Israel E J, Wilsker D F, Hayes K C, Schoenfeld D, Simister N E. Immunology. 1996;89:573–578. doi: 10.1046/j.1365-2567.1996.d01-775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao N, Bix M, Zijlstra M, Jaenisch R, Raulet D. Science. 1991;253:199–202. doi: 10.1126/science.1853205. [DOI] [PubMed] [Google Scholar]
- 40.Cooper A, Roberts A, Rhoades E, Callahan J, Getzy D, Orme I M. Immunology. 1995;84:423–432. [PMC free article] [PubMed] [Google Scholar]
- 41.Santos M, Clevers H, de Sousa M, Marx J J. Blood. 1998;91:3059–3065. [PubMed] [Google Scholar]
- 42.De Voss J J, Rutter K, Schroeder B G, Barry C E., III J Bacteriol. 1999;181:4443–4451. doi: 10.1128/jb.181.15.4443-4451.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]