Abstract
Retroviruses have been widely used in gene transmission studies. In this paper, we show that nonviral apoptotic proteins can be displayed on viral membrane surfaces and that the displayed proteins can execute their normal effector functions. We introduced the genes encoding the apoptosis effector proteins, human CD95 ligand (hFasL) or human tumor necrosis factor-related apoptosis-inducing ligand (hTRAIL), into a cell line that packages Moloney murine leukemia virus vectors. Retrovirus preparations from these lines killed target cells efficiently, and target killing was prevented by Fas-Ig fusion protein or soluble TRAIL receptor (sDR5), respectively. We show that the virus preparation exhibiting Fas-specific cytotoxicity has the same density as a retrovirus, contains full-length FasL protein, and can be depleted of infectivity by immunoadsorption with anti-FasL antibody. This novel property of retroviruses—the display of functional effector proteins—may allow the custom design of reagents whose normal function requires their being embedded in a membrane.
C-type retroviruses and lentiviruses, such as Moloney murine leukemia virus and HIV, respectively, assemble their internal core components in the cytoplasm of an infected cell. The core migrates to the inner surface of the cytoplasmic membrane where it buds from the cell surrounded by viral-encoded envelope proteins embedded in a cell-derived lipid bilayer (for review see ref. 1). In most cases, membrane-embedded envelope protein is required for viral budding and likely forms a binding site for the core particle. Any requirement for other cellular proteins in the budding process has not been described.
Because the target specificity of retroviruses typically is determined by the viral-encoded envelope protein, several groups have attempted to alter specificity by altering the envelope protein, or creating a pseudotype. For example, one method of pseudotyping is to fuse single-chain antibodies to the amino terminus of the Env protein (2–4). Another method is to produced chimeric Env proteins containing a heterologous peptide such as that which binds integrin receptors, erythropoietin, or heregulin (5–7).
Although these studies have attempted to examine or alter viral host specificity by altering the envelope protein, few of them address whether foreign proteins can be directed to the viral membrane or whether once there, they would execute effector functions. The ability to display membrane effector proteins on viral particles may have many advantages over protein presentation on whole cells or simply on presentation of purified protein. Virus particles are less restricted than cells in accessing the target area, and virus particles diffuse more slowly than soluble proteins and could have a longer lifespan in the target area. In addition, many biological processes, including Fas/Fas ligand (FasL)-induced apoptosis, require multimerization of membrane proteins (8). Viruses carrying multiple copies of an effector protein could effectively activate such processes.
Materials and Methods
Production of Retroviral Packaging Cell Lines Carrying the Human FasL Gene.
The human FasL cDNA (hFasL) (9), kindly provided by S. Nagata (Department of Genetics, Osaka University Medical School, Osaka, Japan) and the human tumor necrosis factor-related apoptosis-inducing ligand (hTRAIL) cDNA (10), kindly provided by J. Tschopp (Institut De Biochimie, Université de Lausanne, Switzerland) were cloned into Moloney leukemia virus-derived pLXSN (GenBank accession no. M28248; ref. 11), which was kindly provided by A. Dusty Miller (Fred Hutchinson Cancer Research Center, Seattle, WA). This FasL construct, designated pMLV-hFasL, or the hTRAIL construct (pMLV-hTRAIL) was transfected with Lipofectamine (GIBCO) into the packaging cell line PE501 (a gift from A. Dusty Miller). Viral-laden supernatants from this cell line were recovered after 48 h and then used to infect the amphotrophic virus-producing PA317 packaging cell line (American Type Culture Collection). In this paper, we refer to the FasL-overexpressing packaging line as hFasL-PA317 and the hTRAIL-overexpressing packaging cell line as hTRAIL-PA317. A similarly prepared packaging cell line (krox-PA317) carrying the human krox gene (12) was used to prepare control virus throughout the study. Clones were selected and expanded with G418 (0.4 mg/ml; Geneticin, GIBCO).
Purification of Virus.
The hFasL-PA317 and krox-PA317 cells first were cultured in the presence of G418 to 80% confluence. The medium was replaced with culture medium without G418, and the cells were cultured for 2 days. The supernatant was collected for virus purification, and the cells were used separately as effector cells in cytotoxicity assays. The supernatant was first centrifuged at 12,000 rpm (Sorvall Superspeed) to remove cell debris [cell-free supernatant (CFS)] and then centrifuged at 25,000 rpm for 3 h with a Beckman ultracentrifuge with an SW30 rotor. The virus-containing pellet (VP) was resuspended in medium and sterilized by passing through a filter with a pore diameter of 0.45 μm . In some experiments, the resuspended virus preparation was further purified by centrifugation for 18 h at 20,000 rpm through a 20–65% sucrose gradient. Fractions collected from the gradient in 1-ml aliquots were analyzed for density, FasL cytotoxicity, and the ability to transfer G418 resistance by infection (11). The results were expressed as colony-forming units.
FasL Cytotoxicity.
The human T lymphoma cell line Jurkat expresses both human Fas and TRAIL receptors and is sensitive to FasL and hTRAIL. We enhanced the TRAIL-mediated cytotoxicity by the addition of 6 μg/ml Polybrene (our unpublished results). LB27.4 is a mouse B lymphoma hybridoma that expresses Fas and is sensitive to FasL. Target cells (2 × 104), labeled with Na251CrO4 as previously described (13), were mixed with various sample preparations. In some experiments, either Fas-Ig fusion protein (14) or soluble DR5 (sDR5; provided by Y. Chan, University of Pennsylvania, Philadelphia) was added (10). After culture for 5 h, culture supernatants were collected, and the radioactivity released to the supernatant was counted with a γ counter. Cells cultured in the absence of cytotoxic reagent were used as background controls, where background is always <20% of the total cpm added. The radioactivity released by cells cultured in the presence of 0.5% Nonidet P-40 was used as a reference for total cell death. The cytotoxic activity was determined by the formula: (cpm of sample − cpm of background)/(cpm of total release − cpm of background). All experiments were carried out in duplicate and repeated two to six times. The quantitative expression of the cytotoxic activity of the samples was presented as lytic units/ml. A lytic unit was defined as the amount of cytotoxic material required to cause 20% specific lysis in the 5-h 51Cr-release assay. The fluorescence in situ assay of cytotoxicity was carried out by using the Live/Dead Assay Kit from Molecular Probes, following the protocol suggested by the manufacturer, except that we used a concentration of ethidium bromide 3-fold higher than suggested.
Western Blot Analysis for FasL.
The association of FasL protein with virus preparations and packaging cell lines was determined by Western blot analysis. Virus particles in culture supernatant were pelleted by ultracentrifugation, resuspended in PBS (pH 7.3), pelleted, and resuspended again in PBS. Control samples from the krox-PA317 and PA317 packaging lines were prepared similarly. All three cell lines were harvested by washing with PBS, scraping cells into PBS on ice, pelleting in a Microfuge (Beckman), and washing again twice with PBS before pelleting and resuspending in 150 μl of TKM buffer (10 mM Tris⋅HCl, pH 7.6/10 mM KCl/5 mM MgCl2). Fresh phenylmethylsulfonyl fluoride (PMSF) dissolved in 95% ethanol was added to a final concentration of 100 μg/ml. Nonidet P-40 and SDS were added to final concentrations of 0.5% and 0.1%, respectively. The resuspension was passed 10 times through a 25-gauge needle, and 2 μl of Triton X-100 was added. Protein concentrations of all samples were determined by using the bicinchoninic acid method (Pierce), and Western blotting was performed as described (15). Detection of FasL protein used monoclonal anti-human FasL antibody G247.4 (PharMingen) and the enhanced chemi-luminescence kit (Amersham), following the manufacturer's protocol.
Immunoadsorption.
Biotinylated NOK-2 anti-FasL mAb (2 μg), kindly provided by S. Kobayashi (Hokkaido, Japan) or isotype control mAb (biotinylated anti-2,4,6,-trinitrophenyl mouse IgG2a) were mixed with 200 μl of VP for 2 h at 4°C. The mixture was then incubated for 2 h with 30 μl of agarose beads conjugated with avidin (Sigma). Supernatants were collected after centrifugation and were analyzed for FasL cytotoxicity and infectability. Infectability was assayed with NIH 3T3 cells as described (11). After infection, G418-resistant colonies were fixed for 5 min with methanol, stained for 5 min with Giemsa, destained briefly with deionized water, and counted. Results are expressed as colony-forming units of conditioned supernatant.
Results and Discussion
FasL-Expressing Packaging Lines and Their Conditioned Supernatant Are Cytotoxic.
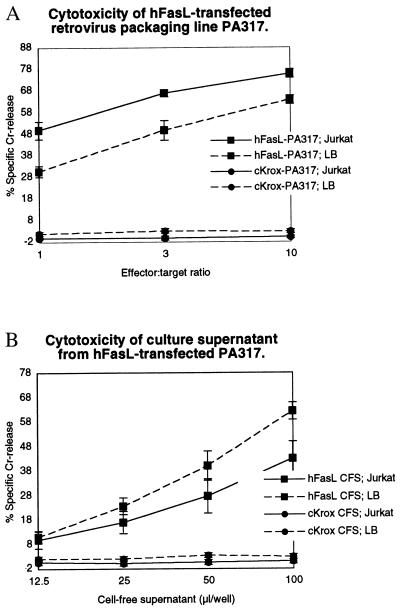
To test the hypothesis that cytotoxic proteins could be displayed on retroviruses and could execute their functions, we introduced a hFasL retroviral vector into the PA317 Moloney murine leukemia virus-packaging cell line to generate six G418-resistant, retrovirus-producing clonal cell lines. Each of these lines rapidly killed Fas receptor-bearing Jurkat target cells in a 5-h cytotoxicity assay (data not shown). The cell line that showed the strongest cytotoxicity (hereafter referred to as hFasL-PA317) was used for all subsequent studies. Fig. 1 shows that preparations of both cells or CFS from hFasL-PA317 express strong and acute cytotoxicity against two Fas-expressing targets: Jurkat, a human T lymphoma line, and LB27.4, a mouse B cell hybridoma. In contrast, the same preparations from a control line, krox-PA317, were not cytotoxic.
Figure 1.
Cytotoxicity expressed by hFasL-PA317 packaging cells and culture supernatant. (A) The percentage of 51Cr released after cell death was measured in an acute assay after incubation of the FasL packaging cell line or the control cKrox packaging cell line with the indicated target cell at various effector:target cell ratios. (B) As in A, except the cell-free culture supernatant was incubated with the target cells instead of the packaging cell line.
CFS Contains Two Distinct Cytotoxic Fractions.
Previous studies have shown that cells that express the FasL protein exhibit two forms of cytotoxicity. The first form is mediated by the full-length transmembrane FasL (16–18). The second is mediated by a soluble FasL (sFasL) proteolytically cleaved by metalloproteinase digestion from the transmembrane form and released into the culture medium. Because the culture supernatant of hFasL-PA317 may contain both sFasL and virus particles, either might be responsible for the strong expression of cytotoxicity by the CFS of the hFasL-PA317 cell line. To attempt to distinguish these two possibilities, we used ultracentrifugation to fractionate the CFS into virus-free supernatant (VFS) and a VP. Both VFS and a resuspended VP were examined for FasL cytotoxicity and the ability to transfer G418 resistance (Table 1). Whereas the VP fraction was highly efficient at transferring the neo gene and rendering cells G418-resistant, the VFS fraction contained no such activity. Both the VP fraction and the VFS fraction expressed cytotoxicity against the LB27.4 target, suggesting that the VFS does indeed contain sFasL. Because the resuspended VP was washed twice, its cytotoxicity was not likely caused by contamination of free sFasL. Furthermore, the pelleted virus preparation contained 43–49% of the total cytotoxic activity in the original CFS. The association of such a high cytotoxic activity with the VP cannot be accounted for by the simple contamination with sFasL.
Table 1.
Expression of FasL cytotoxicity and transfer of G418 resistance by fractions of hFasL-PA317 supernatant
| Exp. | Total units in fraction (% of CFS activity)
|
|||
|---|---|---|---|---|
| CFS | VFS | VP | ||
| FasL cytotoxicity, units* | 1 | 1,225 (100%) | 525 (43%) | 525 (43%) |
| 2 | 1,330 (100%) | 490 (37%) | 600 (45%) | |
| 3 | 1,440 (100%) | 560 (39%) | 700 (49%) | |
| Transfection activity, cfu† | 1 | 3.9 × 106 (100%) | 0 (0%) | 1.0 × 106 (26%) |
| 2 | 9.5 × 106 (100%) | 0 (0%) | 3.5 × 106 (37%) | |
Culture supernatant (35 ml) from the hFasL-PA317 virus-packaging cell line was used to prepare CFS, VP, and VFS as described in Materials and Methods.
One unit of FasL cytotoxicity is defined as the amount required to cause 20% specific 51Cr release in a 5-h 51Cr-release assay against LB27.4 targets.
† cfu, colony-forming unit; defined as the unit required to transfer G418 resistance in NIH 3T3 cells and to produce one viable colony.
The acute nature of the cytotoxicity suggests that it was unlikely that FasL cytotoxicity was caused by expression of viral-encoded FasL in the target cell. To formally exclude this possibility, LB27.4 targets were treated with actinomycin D (2 μg/ml) for 30 min. This treatment blocked 99% of de novo RNA synthesis but did not inhibit target death induced by the resuspended VP (data not shown).
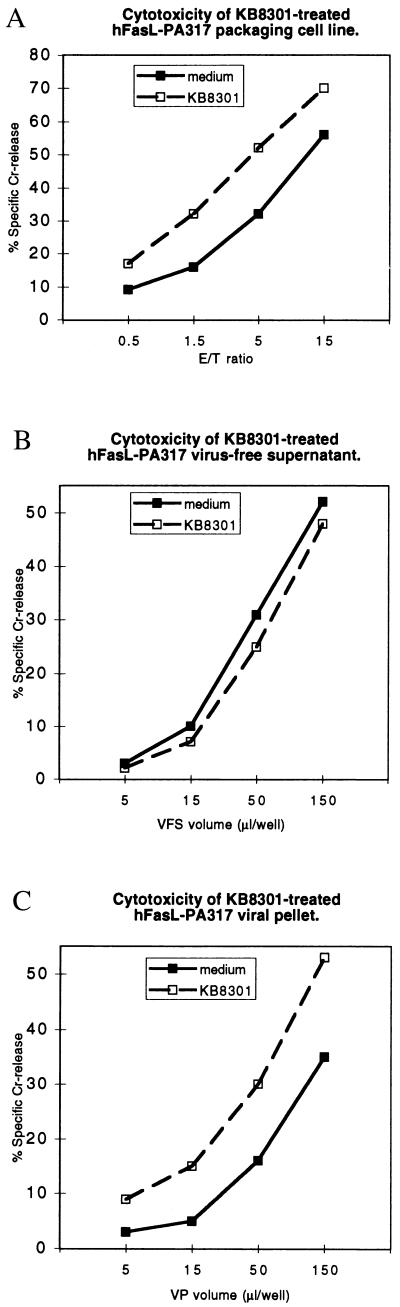
Metalloproteinase Inhibitor KB8301 Does Not Inhibit Cytotoxicity of FasL VP.
The metalloproteinase responsible for the generation of soluble FasL from the transmembrane form can be inhibited by KB8301 ([4-(N-hydroxyamino)-2R-isobutyl-3S-methylsuccinyl]-l-3-(5,6,7,8,-tetrahydro-1-naphthyl)alanine N-methylamide) (16). KB8301 at 10 μM can also significantly enhance the expression of membrane FasL (16). We prepared culture supernatant from hFasL-PA317 in the presence of 10 μM KB8301. Fig. 2 shows that treatment with inhibitor significantly increased the cytotoxic activity of hFasL-PA317 cells. Interestingly, the cytotoxicity of the resuspended VP also was increased significantly. In contrast, the cytotoxicity present in the VFS was reduced modestly. This finding suggested that cytotoxicity arising from the packaging cell line or from the virus preparation could be increased by increasing the amount of membrane-bound FasL. In addition, the result suggests that cytotoxicity from the VFS preparation is, at least in part, caused by sFasL and that blocking the proteolytic release of membrane-bound FasL to sFasL can reduce this cytotoxicity.
Figure 2.
Culture supernatant from KB8301-treated hFasL-PA317 cells expresses FasL cytotoxicity. The hFasL-PA317 cells were cultured in the presence or absence of 10 μM KB8301 for 2 days. The FasL-PA317 packaging cells (A), the VFS (B), and the resuspended VP (C) in its original sample volume were used in 5-h cytotoxicity assays against LB27.4 targets. E/T ratio, effector-to-target ratio.
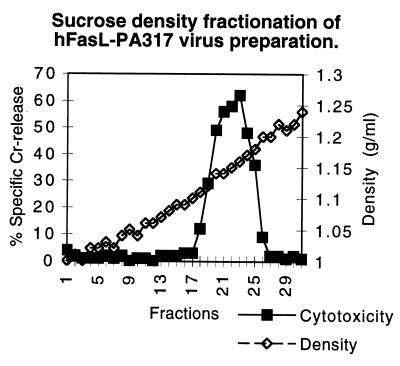
Cytotoxicity Is Associated with Particles Having the Same Density as Retrovirus.
To correlate the cytotoxicity in the virus preparation with virus particles per se, we measured the density of the cytotoxic particles. Culture supernatant from the hFasL-PA317 retrovirus packaging line was pelleted by ultracentrifugation to sediment virus. Approximately 50% of the original cytotoxic activity was recovered in the pelleted fraction. We then resuspended the pellet in a small volume and subjected it to ultracentrifugation through a 20–65% sucrose gradient. Each fraction was measured for density and FasL cytotoxic activity against the LB27.4 target (Fig. 3). A single peak was observed for the expression of FasL cytotoxicity. The fractions expressing maximal cytotoxic activity were located at a density between 1.14 g/ml and 1.16 g/ml, which is consistent with the retrovirus density reported in the literature (19, 20).
Figure 3.
Sucrose density gradient analysis of resuspended VP. VP prepared by ultracentrifugation was resuspended and applied to a sucrose gradient (20–65%). After centrifugation, 1-ml fractions were collected. Density was determined by weight and volume measurements. Cytotoxicity was determined by using LB27.4 targets.
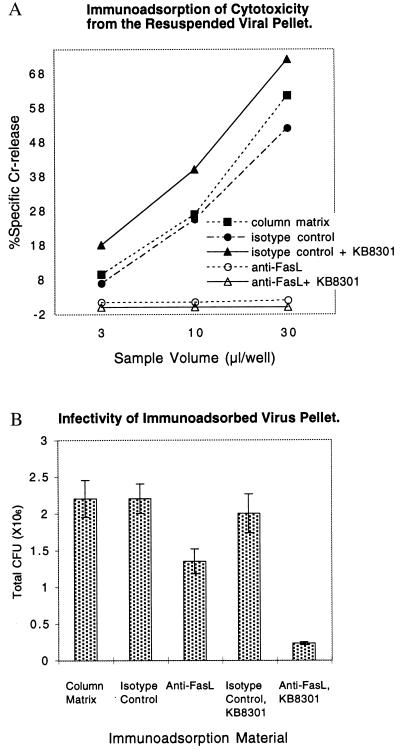
Immunoadsorption of Virus with Immobilized Anti-FasL mAb.
As a further demonstration that FasL is associated with the virus, we tested the ability of immobilized anti-FasL mAb (NOK2; IgG2a subclass) to bind infectious virus from a VP preparation of the hFasL-PA317 packaging line. Immobilized mouse IgG2a anti-2,4,6,-trinitrophenyl mAb and column matrix with no immobilized antibody were used as controls. Fig. 4A shows the cytotoxicity of VP material after immunoadsorption. NOK2 anti-FasL mAb completely removed the FasL cytotoxicity from a preparation of VP. When the resuspended VP was adsorbed with either immobilized IgG2a anti-2,4,6,-trinitrophenyl mAb or the column matrix itself, there was no retention of cytotoxicity of the virus preparation. The NOK2 anti-FasL mAb also completely removed the cytotoxicity from a VP prepared from hFasL-PA317 packaging cells treated with protease inhibitor KB8301.
Figure 4.
Immunoadsorption with anti-FasL depletes the cytotoxicity and infectivity of the resuspended VP. Both VP from untreated hFasL-PA317 cells and VP from cells treated with KB8301 were resuspended in culture medium. They were mixed with biotinylated anti-FasL mAb (NOK-2), biotinylated anti-2,4,6,-trinitrophenyl mAb, or culture medium for 2 h at 4°C. The mixtures were then incubated with avidin-conjugated beads for 2 h. The recovered supernatants were examined for cytotoxicity against LB27.4 target cells (A) and for infectivity (B). Infectivity was determined by counting NIH 3T3 surviving colonies exposed to G418 as described in Table 1.
The infectivity of the VP preparations after adsorption was determined. Fig. 4B shows that ≈40% of the viral infectivity was depleted with the anti-FasL antibody (column 3), and no depletion was observed in the VP subjected to depletion by column matrix (column 1) or matrix bound to an isotype control antibody (column 2). Furthermore, 90% of the viral infectivity was depleted with the anti-FasL antibody when the virus was prepared from hFasL-PA317 cells cultured in the presence of KB8301 (Fig. 4B, column 5 vs. column 4). In addition, infectivity could be demonstrated when FasL VP was adsorbed with wells coated with anti-FasL mAb. As controls, infectivity was not observed with krox VP or wells coated with isotype control (data not shown). It should be noted that the FasL VP contains not only active vector but also inactive vector and vesicles. The precise proportion of the active vector amongst these particles is not determined.
Western Analysis of the FasL Packaging Line and Virus Preparation.
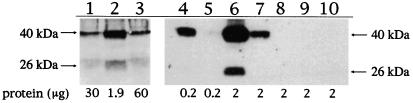
To provide further evidence that the retrovirus contains FasL protein, we performed Western analysis using an anti-FasL antibody (G247.4; IgG1 subclass). We first examined hFasL-PA317 total cell lysate (Fig. 5, lanes 1 and 3) and a VP lysate (lane 2). Two anti-FasL-reactive bands at 40 kDa and 26 kDa were detected for total cell lysates as previously described in other hFasL-transfected cell lines (21). Both bands were enriched significantly in the virus preparation. The virus preparation and the hFasL-PA317 cell preparation were then extensively washed with PBS before the preparation of new protein extracts to ensure the removal of any protein nonspecifically adsorbed to the cells or virus particles. The results (lanes 4–10) showed that the purified VP (lanes 4 and 6) still expressed both bands when 2 μg of protein was loaded (lane 6). When 0.2 μg of VP protein was loaded, only the 40-kDa FasL was detected (lane 4), indicating that it is the major form. The results also show that the purified virus contained highly enriched FasL protein as compared with total cell lysate (compare VP and cells, respectively, in lanes 1 and 2, lanes 4 and 5, and lanes 6 and 7). The specificity of the assay was shown by the absence of anti-FasL reactive bands when using parallel preparations of krox-PA317 cells (lane 8), VP of krox-PA317 (lane 9), or PA317 cells (lane 10). The data demonstrate that both full-length FasL and sFasL are associated with the virus preparation and suggest that some of the cleaved FasL could remain associated with the cells or the virus despite extensive washing.
Figure 5.
Both VP and hFasL-PA317 cells express full-length and short forms of hFasL. Western analyses were carried out by using anti-FasL antibody against the following preparations: FasL VP (lanes 2, 4, and 6); FasL-PA317 packaging cell line (lanes 1, 3, 5, and 7); the cKrox control VP (lane 9); the cKrox-PA317 packaging cell line (lane 8); and the starting PA317 packaging cell line (lane 10). Lanes 1–3 and 4–10 were independent experiments. Protein amounts loaded are indicated. The size of anti-FasL-reactive bands was estimated based on molecular mass standard. The 40-kDa band corresponded to the full-length FasL, and the 26-kDa band corresponded to sFasL (21).
Virus Packaged in Cell Lines That Overexpress hTRAIL Also Exhibit Cytotoxicity.
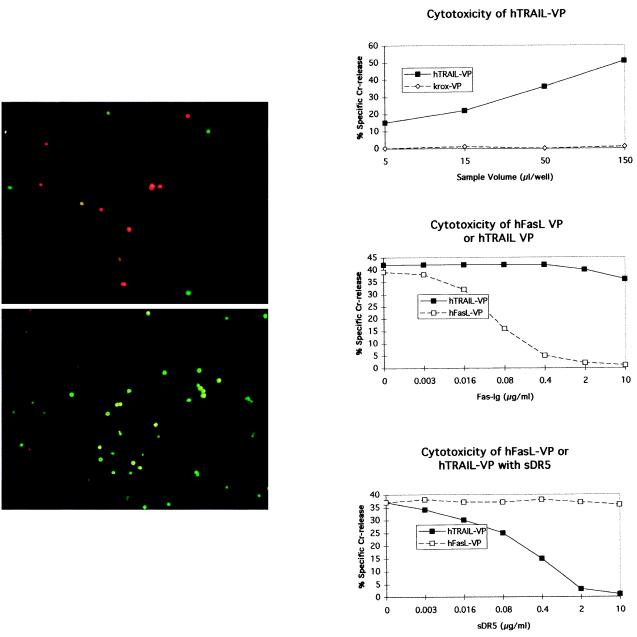
To examine whether the viral display of a type II cytotoxic membrane protein was limited to FasL or might be a more general phenomenon, we produced a packaging cell line that overexpressed the hTRAIL (22). Viral preparations of conditioned supernatant from the hTRAIL-PA317 packaging cell line were made. Fig. 6 A and B show that Jurkat target cells are sensitive to hTRAIL VP. This sensitivity is shown by the uptake of the red fluorescent ethidium dye by apoptotic cells (Fig. 6A). By contrast, targets treated with control krox VP were viable and took up the green fluorescent vital dye (Fig. 6B). Moreover, hTRAIL VP killed 51Cr-labeled Jurkat cells in a dose-dependent manner (Fig. 6C). The killing by hTRAIL VP was specifically and completely inhibited by sDR5. sDR5 did not inhibit the killing mediated by FasL VP (Fig. 6E). Conversely, Fas-Ig fusion protein inhibited the cytotoxicity of FasL VP but did not effect the cytotoxicity of hTRAIL VP (Fig. 6D).
Figure 6.
Resuspended VP from hTRAIL-PA317 expresses specific cytotoxicity. The hTRAIL-PA317 cell line was generated as described in Materials and Methods. Both hTRAIL VP (A) and cKrox control VP (B) were cultured with Jurkat target cells for 5 h, followed by the addition of dyes. Dye uptake was evaluated 1 h later. Red fluorescent ethidium homodimer dye is taken up by apoptotic cells, and green calcein acetoxymethyl ester is taken up by living cells. (C) A dose–response curve of cytotoxicity of the hTRAIL VP against 51Cr-labeled Jurkat targets. (D) Cytotoxicity of hFasL VP but not hTRAIL VP is inhibited by Fas-Ig. (E) The cytotoxicity of hTRAIL VP but not hFasL VP is sensitive to a soluble form of the hTRAIL DR5 receptor (sDR5).
We have also overexpressed FasL in cell lines that do not express packaging proteins (data not shown). These cell lines secreted low levels of FasL-carrying vesicles that could be pelleted in sufficient quantity to be cytotoxic. However, the overexpression of FasL in packaging cell lines allowed us to purify significantly higher levels of cytotoxic particles. Furthermore, when producing cytotoxic particles, the ability to manipulate production of viruses and assay their concentrations is a significant advantage over relying on the spontaneous release of membranous vesicles.
Taken together, these data demonstrate that the retroviral preparation can be used to directly mediate an effector function. Fig. 4 in particular shows that not only can a large fraction of viral infectivity be removed with an anti-FasL antibody, but nearly all of the infectious particles can be removed if prepared in the presence of an inhibitor of FasL proteolysis, KB8301. The fact that this approach is successful with both FasL and hTRAIL suggests that other membrane-bound effector molecules, not limited to cytotoxic function and especially members of the nerve growth factor receptor/tumor necrosis factor superfamily, may also work. Future work will need to be done to produce packaging cell lines carrying the membrane forms of tumor necrosis factor and CD40 ligand to determine whether these viruses express cell-activating activity. Furthermore, although others have fused type I membrane proteins to the viral envelope protein, we show in this paper a means by which to incorporate type II membrane proteins into the viral membrane. This technology offers expanded potential as a powerful tool to study many membrane proteins and their functions. Conversely, we could use anti-FasL mAb to manipulate the infectivity of the virus preparation. The infectivity of FasL VP could be increased by NOK1 mAb in a dose-dependent manner by up to 500% (our unpublished results). In addition to providing evidence for FasL expression on virus particles, the results further suggest that vector activity could be manipulated through antibodies against the nonviral protein.
Finally, a number of reports have indicated that cells chronically infected with HIV-1 cause or accelerate Fas/FasL-mediated cytotoxicity of CD4+ or CD8+ T lymphocytes (23–25). Depletion of these cells is a contributing factor in the etiology of AIDS. Although the mechanism by which this occurs is still under extensive investigation, some reports indicate that it is the envelope protein of HIV-1, gp120, perhaps in conjunction with the viral-encoded protein Tat, that facilitates the activation of the Fas death pathway by inducing the expression of FasL (18, 23, 26). Our report opens another possibility, namely that the virus might mediate the cell-death signal by inheriting FasL protein after budding from a highly activated cell. Our study suggests that such a cytotoxic retrovirus may exist during infection under physiological conditions, and further study will need to be done to investigate this possibility.
Acknowledgments
We thank Gloria Higgins and A. Dusty Miller for their generosity in providing advice, retrovirus protocols, vectors, and packaging lines; Dr. S. Kobayashi for biotinylated NOK2; Dr. Y. Chan for purified sDR5; Dr. S. Nagata for the hFasL cDNA; Dr. J. Tschopp for the hTRAIL cDNA; and Michael Nicholas for expert technical assistance. This work was supported by National Institutes of Health Grant AI-36938 (to S.-T.J. and S.J.) and a grant from the Scleroderma Foundation (to D.S.). S.J. also is supported by the Ministry of Education, Science, and Culture of Japan.
Abbreviations
- FasL
Fas ligand
- hFasL
human FasL
- sFasL
soluble FasL
- CFS
cell-free supernatant
- hTRAIL
human tumor necrosis factor-related apoptosis-inducing ligand
- VP
viral pellet
- VFS
virus-free supernatant
- sDR5
soluble hTRAIL receptor DR5
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.070049197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.070049197
References
- 1.Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Plainview, NY: Cold Spring Harbor Lab. Press; 1997. [PubMed] [Google Scholar]
- 2.Chu T H, Dornburg R. J Virol. 1995;69:2659–2663. doi: 10.1128/jvi.69.4.2659-2663.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Somia N V, Zoppe M, Verma I M. Proc Natl Acad Sci USA. 1995;92:7570–7574. doi: 10.1073/pnas.92.16.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marin M, Noel D, Valsesia-Wittman S, Brockly F, Etienne-Julan M, Russell S, Cosset F L, Piechaczyk M. J Virol. 1996;70:2957–2962. doi: 10.1128/jvi.70.5.2957-2962.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valsesia-Wittmann S, Drynda A, Deleage G, Aumailley M, Heard J M, Danos O, Verdier G, Cosset F L. J Virol. 1994;68:4609–4619. doi: 10.1128/jvi.68.7.4609-4619.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasahara N, Dozy A M, Kan Y W. Science. 1994;266:1373–1376. doi: 10.1126/science.7973726. [DOI] [PubMed] [Google Scholar]
- 7.Han X, Kasahara N, Kan Y W. Proc Natl Acad Sci USA. 1995;92:9747–9751. doi: 10.1073/pnas.92.21.9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suda T, Hashimoto H, Tanaka M, Ochi T, Nagata S. J Exp Med. 1997;186:2045–2050. doi: 10.1084/jem.186.12.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takahashi T, Tanaka M, Inazawa J, Abe T, Suda T, Nagata S. Int Immunol. 1994;6:1567–1574. doi: 10.1093/intimm/6.10.1567. [DOI] [PubMed] [Google Scholar]
- 10.Schneider P, Thome M, Burns K, Bodmer J L, Hofmann K, Kataoka T, Holler N, Tschopp J. Immunity. 1997;7:831–836. doi: 10.1016/s1074-7613(00)80401-x. [DOI] [PubMed] [Google Scholar]
- 11.Miller A D, Miller D G, Garcia J V, Lynch C M. Methods Enzymol. 1993;217:581–599. doi: 10.1016/0076-6879(93)17090-r. [DOI] [PubMed] [Google Scholar]
- 12.Widom R L, Culic I, Lee J, Korn J H. Gene. 1997;198:407–420. doi: 10.1016/s0378-1119(97)00360-0. [DOI] [PubMed] [Google Scholar]
- 13.Matsui K, Omura S, Cui H, Schauer S L, Sonenshein G E, Ju S T. Eur J Immunol. 1997;27:2269–2278. doi: 10.1002/eji.1830270922. [DOI] [PubMed] [Google Scholar]
- 14.Ju S T, Panka D J, Cui H, Ettinger R, el-Khatib M, Sherr D H, Stanger B Z, Marshak-Rothstein A. Nature (London) 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 15.Davis L G, Dibner M D, Battey J F, editors. Basic Methods in Molecular Biology. New York: Elsevier; 1986. [Google Scholar]
- 16.Kayagaki N, Kawasaki A, Ebata T, Ohmoto H, Ikeda S, Inoue S, Yoshino K, Okumura K, Yagita H. J Exp Med. 1995;182:1777–1783. doi: 10.1084/jem.182.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oyaizu N, Adachi Y, Hashimoto F, McCloskey T W, Hosaka N, Kayagaki N, Yagita H, Pahwa S. J Immunol. 1997;158:2456–2463. [PubMed] [Google Scholar]
- 18.Uchiyama J, Kishi S, Yagita H, Matsuzaki S, Koga Y. Arch Virol. 1997;142:1771–1785. doi: 10.1007/s007050050196. [DOI] [PubMed] [Google Scholar]
- 19.Dinowitz M, Lie Y S, Low M A, Lazar R, Fautz C, Potts B, Sernatinger J, Anderson K. Dev Biol Stand. 1992;76:201–207. [PubMed] [Google Scholar]
- 20.Yaniv A, Gotlieb-Stematsky T, Vonsover A, Perk K. Int J Cancer. 1980;25:205–211. doi: 10.1002/ijc.2910250207. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka M, Suda T, Takahashi T, Nagata S. EMBO J. 1995;14:1129–1135. doi: 10.1002/j.1460-2075.1995.tb07096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pitti R M, Marsters S A, Ruppert S, Donahue C J, Moore A, Ashkenazi A. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 23.Westendorp M O, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin K M, Krammer P H. Nature (London) 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 24.Chen H, Yip Y K, George I, Tyorkin M, Salik E, Sperber K. J Immunol. 1998;161:4257–4267. [PubMed] [Google Scholar]
- 25.Boirivant M, Viora M, Giordani L, Luzzati A L, Pronio A M, Montesani C, Pugliese O. J Clin Immunol. 1998;18:39–47. doi: 10.1023/a:1023235803948. [DOI] [PubMed] [Google Scholar]
- 26.Accornero P, Radrizzani M, Care A, Mattia G, Chiodoni C, Kurrle R, Colombo M P. FEBS Lett. 1998;436:461–465. doi: 10.1016/s0014-5793(98)01127-2. [DOI] [PubMed] [Google Scholar]