Abstract
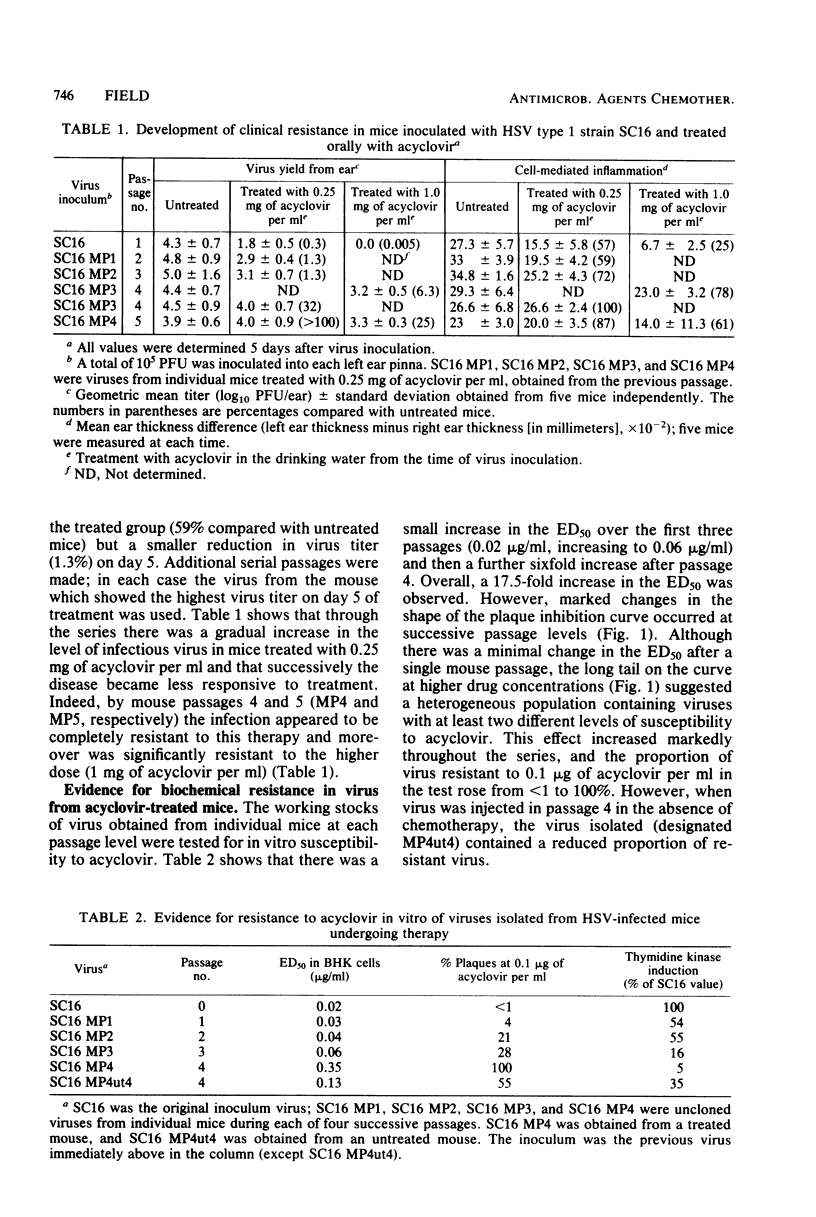
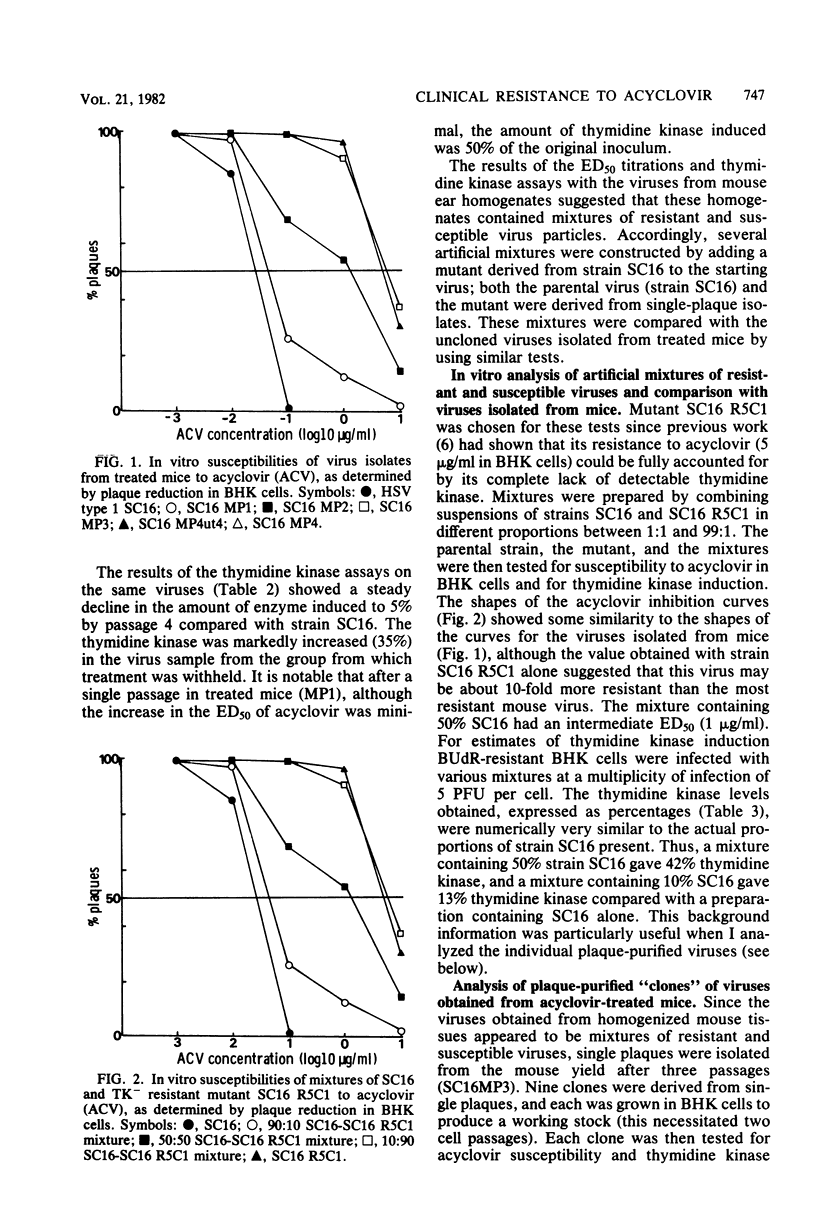
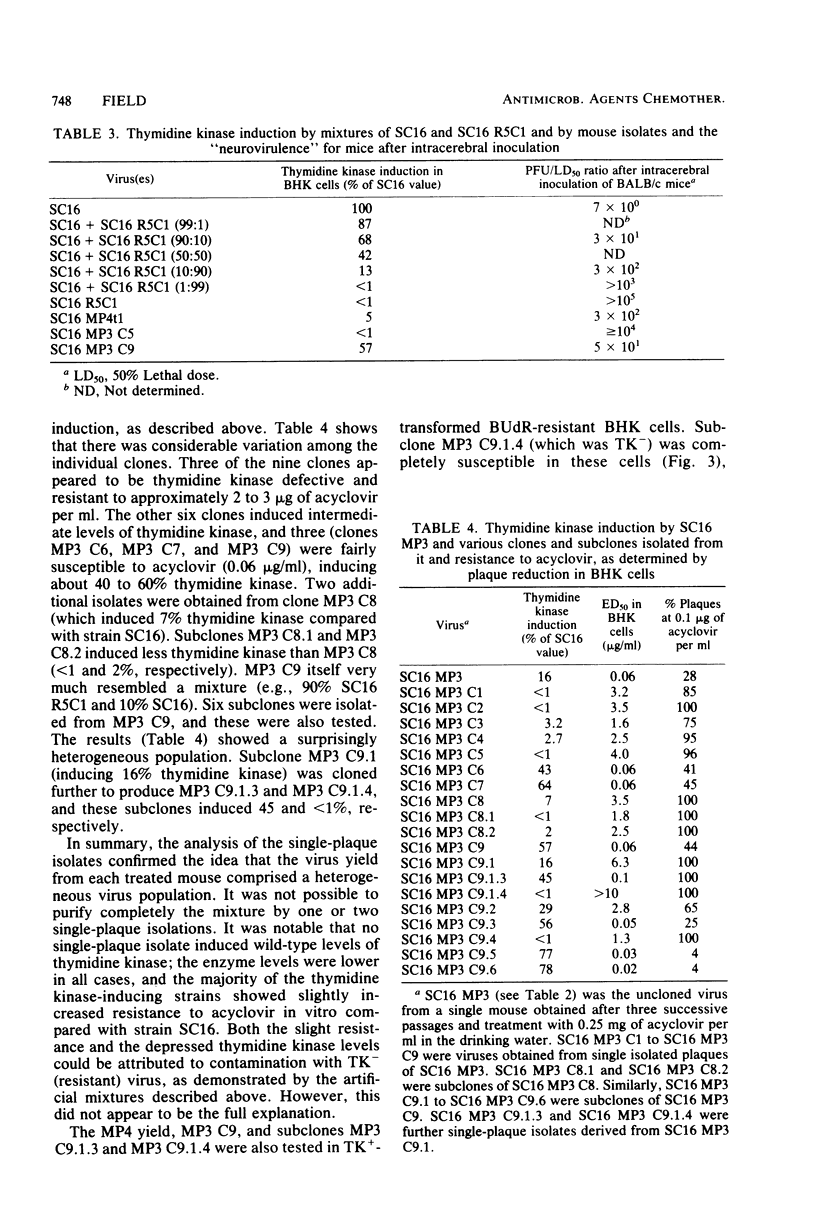
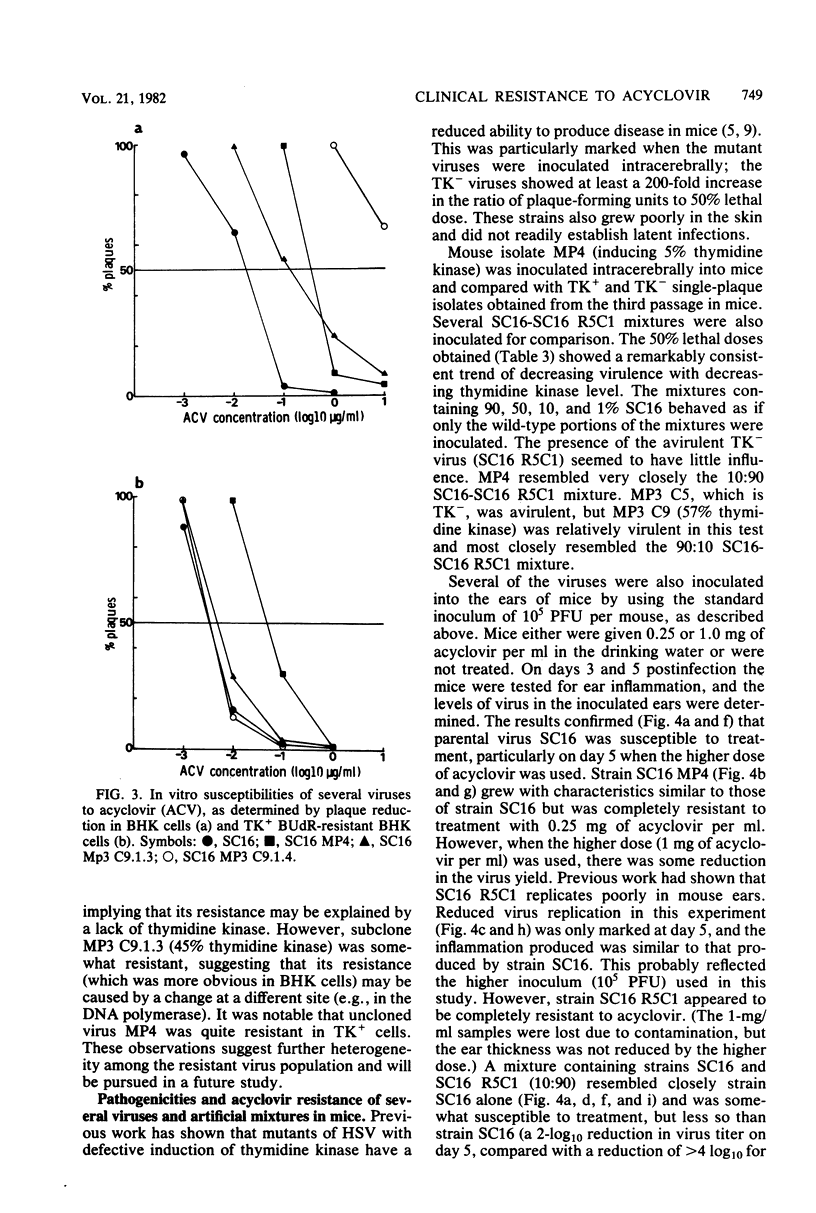
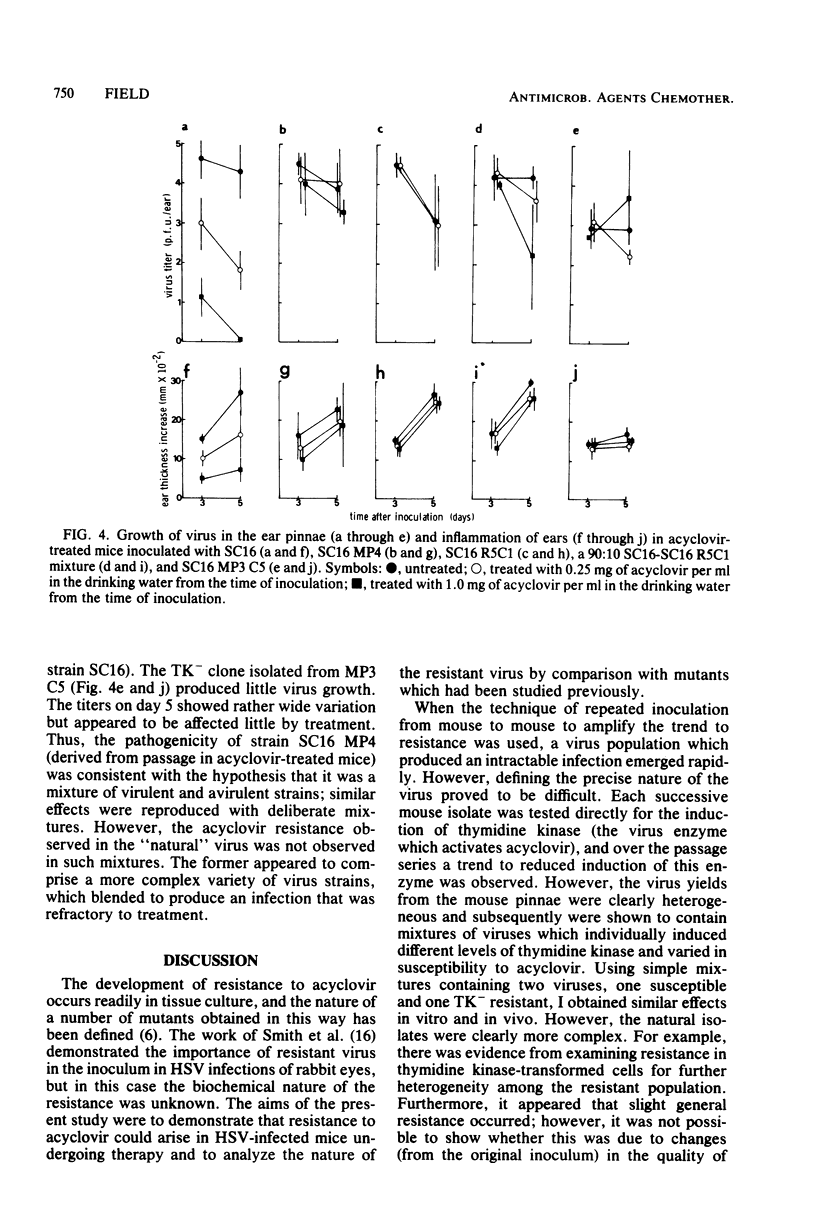
Mice inoculated in the ear pinna with herpes simplex virus were treated effectively by including 1 mg of acyclovir per ml in the drinking water. During a 5-day course of treatment the development of resistance was not readily apparent. However, when a suboptimal therapeutic dose was used and virus was repeatedly inoculated into further mice undergoing therapy, the infection became completely refractory to treatment by passage 4. Some of the viruses isolated exhibited reduced ability to induce thymidine kinase, and this appeared to account at least in part for the development of resistance. However, the viruses isolated from the tissues of such mice comprised complex mixtures of strains with widely differing in vitro susceptibilities to acyclovir. The properties of these virus yields gave an indication of the likely nature of resistance to nucleoside analogs in humans and suggested some difficulties which may be encountered when clinical specimens are analyzed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burns W. H., Saral R., Santos G. W., Laskin O. L., Lietman P. S., McLaren C., Barry D. W. Isolation and characterisation of resistant Herpes simplex virus after acyclovir therapy. Lancet. 1982 Feb 20;1(8269):421–423. doi: 10.1016/s0140-6736(82)91620-8. [DOI] [PubMed] [Google Scholar]
- Crumpacker C. S., Schnipper L. E., Marlowe S. I., Kowalsky P. N., Hershey B. J., Levin M. J. Resistance to antiviral drugs of herpes simplex virus isolated from a patient treated with acyclovir. N Engl J Med. 1982 Feb 11;306(6):343–346. doi: 10.1056/NEJM198202113060606. [DOI] [PubMed] [Google Scholar]
- Darby G., Field H. J., Salisbury S. A. Altered substrate specificity of herpes simplex virus thymidine kinase confers acyclovir-resistance. Nature. 1981 Jan 1;289(5793):81–83. doi: 10.1038/289081a0. [DOI] [PubMed] [Google Scholar]
- Darby G., Larder B. A., Bastow K. F., Field H. J. Sensitivity of viruses to phosphorylated 9-(2-hydroxyethoxymethyl)guanine revealed in TK-transformed cells. J Gen Virol. 1980 Jun;48(Pt 2):451–454. doi: 10.1099/0022-1317-48-2-451. [DOI] [PubMed] [Google Scholar]
- Field H. J., Bell S. E., Elion G. B., Nash A. A., Wildy P. Effect of acycloguanosine treatment of acute and latent herpes simplex infections in mice. Antimicrob Agents Chemother. 1979 Apr;15(4):554–561. doi: 10.1128/aac.15.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Darby G. Pathogenicity in mice of strains of herpes simplex virus which are resistant to acyclovir in vitro and in vivo. Antimicrob Agents Chemother. 1980 Feb;17(2):209–216. doi: 10.1128/aac.17.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Darby G., Wildy P. Isolation and characterization of acyclovir-resistant mutants of herpes simplex virus. J Gen Virol. 1980 Jul;49(1):115–124. doi: 10.1099/0022-1317-49-1-115. [DOI] [PubMed] [Google Scholar]
- Field H. J., De Clercq E. Effects of oral treatment with acyclovir and bromovinyldeoxyuridine on the establishment of maintenance of latent herpes simplex virus infection in mice. J Gen Virol. 1981 Oct;56(Pt 2):259–265. doi: 10.1099/0022-1317-56-2-259. [DOI] [PubMed] [Google Scholar]
- Field H. J., Wildy P. The pathogenicity of thymidine kinase-deficient mutants of herpes simplex virus in mice. J Hyg (Lond) 1978 Oct;81(2):267–277. doi: 10.1017/s0022172400025109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H., McMillan A., Darby G. The sensitivity of acyclovir-resistant mutants of herpes simplex virus to other antiviral drugs. J Infect Dis. 1981 Feb;143(2):281–285. doi: 10.1093/infdis/143.2.281. [DOI] [PubMed] [Google Scholar]
- Hill T. J., Field H. J., Blyth W. A. Acute and recurrent infection with herpes simplex virus in the mouse: a model for studying latency and recurrent disease. J Gen Virol. 1975 Sep;28(3):341–353. doi: 10.1099/0022-1317-28-3-341. [DOI] [PubMed] [Google Scholar]
- Klemperer H. G., Haynes G. R., Shedden W. I., Watson D. H. A virus-specific thymidine kinase in BHK-21 cells infected with herpes simplex virus. Virology. 1967 Jan;31(1):120–128. doi: 10.1016/0042-6822(67)90015-3. [DOI] [PubMed] [Google Scholar]
- Nash A. A., Field H. J., Quartey-Papafio R. Cell-mediated immunity in herpes simplex virus-infected mice: induction, characterization and antiviral effects of delayed type hypersensitivity. J Gen Virol. 1980 Jun;48(Pt 2):351–357. doi: 10.1099/0022-1317-48-2-351. [DOI] [PubMed] [Google Scholar]
- Schaeffer H. J., Beauchamp L., de Miranda P., Elion G. B., Bauer D. J., Collins P. 9-(2-hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature. 1978 Apr 13;272(5654):583–585. doi: 10.1038/272583a0. [DOI] [PubMed] [Google Scholar]
- Schnipper L. E., Crumpacker C. S. Resistance of herpes simplex virus to acycloguanosine: role of viral thymidine kinase and DNA polymerase loci. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2270–2273. doi: 10.1073/pnas.77.4.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. O., Kennell W. L., Poirier R. H., Lynd F. T. In vitro and in vivo resistance of herpes simplex virus to 9-(2-hydroxyethoxymethyl)guanine (acycloguanosine). Antimicrob Agents Chemother. 1980 Feb;17(2):144–150. doi: 10.1128/aac.17.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]



