Abstract
Class III multidrug resistance (MDR) P-glycoproteins (P-gp), mdr2 in mice and MDR3 in man, mediate the translocation of phosphatidylcholine across the canalicular membrane of the hepatocyte. Mice with a disrupted mdr2 gene completely lack biliary phospholipid excretion and develop progressive liver disease, characterized histologically by portal inflammation, proliferation of the bile duct epithelium, and fibrosis. This disease phenotype is very similar to a subtype of progressive familial intrahepatic cholestasis, hallmarked by a high serum γ-glutamyltransferase (γ-GT) activity. We report immunohistochemistry for MDR3 P-gp, reverse transcription-coupled PCR sequence analysis, and genomic DNA analysis of MDR3 from two progressive familial intrahepatic cholestasis patients with high serum γ-GT. Canalicular staining for MDR3 P-gp was negative in liver tissue of both patients. Reverse transcription-coupled PCR sequencing of the first patient’s sequence demonstrated a homozygous 7-bp deletion, starting at codon 132, which results in a frameshift and introduces a stop codon 29 codons downstream. The second patient is homozygous for a nonsense mutation in codon 957 (C → T) that introduces a stop codon (TGA). Our results demonstrate that mutations in the human MDR3 gene lead to progressive familial intrahepatic cholestasis with high serum γ-GT. The histopathological picture in these patients is very similar to that in the corresponding mdr2(−/−) mouse, in which mdr2 P-gp deficiency induces complete absence of phospholipid in bile.
Unidirectional transport of organic compounds across the hepatocyte canalicular membrane into bile is mediated by a set of primary active export pumps that belong to the superfamily of ATP-binding cassette (ABC) transporters (1). This set includes multidrug resistance genes and multidrug resistance-associated protein genes. In humans there are two MDR genes: MDR1 and MDR3 (2). MDR1 P-glycoprotein (P-gp) transports hydrophobic drugs and, when overexpressed, confers multidrug resistance to (tumor) cells (3). This protein is expressed in various tissues including the liver. In contrast, MDR3 P-gp is a phospholipid translocator (4–6) that is predominantly, if not exclusively, expressed in the canalicular membrane of the hepatocyte (7). The function of MDR3 has been elucidated by disruption of its homologue in the mouse, the mdr2 gene (8). Mdr2(−/−) knockout mice do not secrete phospholipids into bile and develop severe liver disease, characterized by inflammation of the portal tracts, proliferation of the bile ducts, and fibrosis (9). We have hypothesized that the disease is caused by bile in which the toxicity of detergent bile salts is not inactivated by phospholipids. This hypothesis was supported by the observation that the severity of the liver disease could be influenced by modulation of the hydrophobicity of the bile salt pool (10). Feeding of cholate, which is a relatively hydrophobic bile salt for mice, strongly aggravated the disease process, whereas ursodeoxycholate (UDCA), a hydrophilic noncytotoxic bile salt, halted it. Because the human bile salt pool is considerably more hydrophobic than that in the mouse, MDR3 deficiency could be expected to result in development of severe liver disease or even not be compatible with life (11).
Progressive familial intrahepatic cholestasis (PFIC) is a heterogeneous group of autosomal recessive liver disorders, characterized by early onset of cholestasis that progresses to cirrhosis and liver failure before adulthood (12, 13). PFIC can be divided in three subcategories: The first type is believed to be caused by defects in bile salt synthesis (14–16). The second type is thought to be caused by defective bile salt secretion and is also called Byler disease (17, 18). Two loci for Byler have been found and mapped to chromosome 18q21–q22 (19) and recently to chromosome 2 (20). Patients with bile salt synthesis defects and Byler patients have normal serum γ-glutamyltransferase (γ-GT) levels (21). A third type of PFIC patients can be distinguished from the other two types by a high serum γ-GT activity and liver histology that shows portal inflammation and ductular proliferation in an early stage (21, 22). These differences suggest that a distinct etiological mechanism underlies this third type of PFIC. In fact, the histological and biochemical characteristics of this subtype resemble the features of the mdr2(−/−) knockout mouse very closely. The recent finding that a patient with this PFIC subtype lacked MDR3 mRNA in the liver further substantiates that the third type may be caused by MDR3 deficiency (23). In this study we investigated whether a genetic defect in the MDR3 gene underlies the liver disorders in PFIC patients with high serum γ-GT activity.
PATIENTS AND METHODS
Patient 1.
The first patient (B.K.) is a Turkish boy of healthy consanguinous parents and has one nonaffected sister. There are no other family members reported with liver disease. He has suffered from recurrent bouts of jaundice since the age of 3 months, when he presented with severe icterus, diarrhea, fever, and pruritus. At the age of 3 years, he was first presented at the university children’s hospital in Hamburg with hepatosplenomegaly, elevated serum liver enzymes, increased γ-GT activity (6 times normal), and a high serum bile acid concentration (50 times normal). Prothrombin time was 50% (normal is >70%). Examination of a liver biopsy of this patient revealed nonspecific portal inflammation, extensive portal fibrosis, and cirrhosis. There was no response to UDCA treatment. Orthotopic liver transplantation was performed at the age of 3.5 years because of complications of liver cirrhosis. Extrahepatic bile ducts were patent at time of liver transplantation. Presently at the age of 6 years he is in a good clinical condition.
Patient 2.
The second patient (A.B.) is a North-African boy of first-cousin parents. The four older siblings are unaffected. No other patients with liver disease have been reported in this family, except for the mother who has experienced episodes of cholestasis during pregnancy. Since the age of 8 months, he has suffered from recurrent episodes of severe pruritus. He presented at the Bicêtre Hospital in Paris at the age of 3 years with hepatosplenomegaly, strongly increased serum γ-GT activity (38 times normal), and increased serum bile acids (16 times normal), whereas his serum aspartate and alanine aminotransferase activities were only mildly elevated. Prothrombin time was <10% but normalized after vitamin K1 injection. Liver histology revealed ductular proliferation and extensive portal fibrosis. Normal intra- and extrahepatic bile ducts were visualized by cholangiography. Despite the start of UDCA treatment at 4 years of age, liver enzymes and liver histology further deteriorated toward end-stage liver disease with ascites and severe jaundice. He underwent a liver transplantation at the age of 9 years and is now in good clinical condition.
Liver Material.
The livers of the two PFIC patients with high serum γ-GT activity were studied after transplantation. Livers of two PFIC patients with normal serum γ-GT levels served as controls. Normal human control liver was obtained from surgical pathology specimens. All liver material, used in this study, was snap-frozen in liquid nitrogen immediately after resection/transplantation and stored at −70°C.
Immunohistochemistry.
Cytokeratin 7 was stained as a marker for bile duct epithelium by standard immunohistochemical procedures (24). For detection of canalicular proteins the following antibodies were used: monoclonal antibody C219 (Dako) that binds all P-gps (MDR1, MDR3, and “sister of P-gp”) and monoclonal antibody M2III-6 that is directed against canalicular multispecific organic anion transporter (cMOAT) (25). MDR3 P-gp was detected with the specific rabbit polyclonal antibody α-REG1, raised against the N-terminal part of the MDR3 P-gp (amino acids 2–58) (7). Cryostat sections (3-μm-thick) were air-dried and fixed in acetone for 10 min at room temperature. After a preincubation with 10% normal goat serum, sections were incubated with the primary antibody (1:10 diluted) overnight at 4°C. Endogenous peroxidase activity was blocked with 0.1% NaN3/0.03% H2O2. Second antibodies were horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG2a to detect C219 and cMOAT and HRP-conjugated goat anti-rabbit for α-REG1 (both at a 1:100 dilution; Dako), to which 10% normal human AB0 serum was added. HRP-based signal amplification was applied by using the FITC-tyramide procedure (NEN Life Science Products, Boston, USA), followed by incubation with HRP-conjugated rabbit anti-fluorescein isothiocyanate (1:100 dilution; Dako). Finally, slides were developed for HRP activity by using 5 mg/ml 3-amino-9-ethyl carbazole and 0.03% H2O2. To control for background, incubations without primary antibody were performed.
RNA Extraction, Reverse Transcription-Coupled PCR (RT–PCR), and Sequence Analysis.
Total RNA was extracted from liver by the acid-phenol single step method (26). cDNA synthesis was carried out with 5 μg of total RNA and random hexamer primers with SuperScript reverse transcriptase (Life Technologies), at 45°C for 1 h, followed by 1 min 100°C.
The human MDR3 cDNA was amplified from both patients and control cDNA by using two sets of MDR3-specific primers: nucleotides −32 to −9, 5′-CCTGCCAGACACGCGCGAGGTTC-3′ (MDR3–1 sense), and nucleotides 2044–2069, 5′-CTTCAAGTCCATCGGTTTCCACATC-3′ (MDR3–1 antisense); nucleotides 1912–1936, 5′-CAGTCAGAAGAATTTGAACTAAATG-3′ (MDR3–2 sense), and nucleotides 3829–3853, 5′-CTGTAGCAAAAGTTCATAAGTTCTG-3′ (MDR3–2 antisense). PCRs were carried out in a Perkin–Elmer GeneAmp PCR system 2400, in 1× Taq polymerase buffer (Life Technologies)/1.5 mM MgCl2/all four dNTPs (each at 0.5 mM)/each primer at 400 nM/0.5 unit of Taq polymerase. All reactions were denaturated at 94°C for 5 min and subjected to 35 cycles with an annealing temperature of 56°C. Each cycle started with 30 s at 94°C, 30 s at the indicated annealing temperature, and 150 s at 72°C. The PCR was terminated after an extension step at 72°C for 7 min. These PCR fragments were excised from agarose gel, purified, ligated into the TA-cloning plasmid pCRII (Invitrogen), and transformed into INVαF′ competent cells (Invitrogen). White colonies were picked and grown overnight, and plasmid DNA was isolated by using the alkaline lysis method. Of each patient, at least 10 clones were pooled and both strands were sequenced with the dideoxynucleotide chain-termination method (27), by using 15-nucleotide-long internal primers. Analysis for both patients has been performed in Amsterdam and Bicêtre.
Genomic DNA Analysis.
Genomic DNA was isolated from leukocytes and the region containing the mutation was amplified, by using primers that encompass the mutation. For patient one (B.K.), both parents, and his sister, we used as a sense primer, 5′-CTCAGGATTGGTGCTGGAGTTC-3′ (residues 360–382), and as antisense primer, 5′-CGTGGCTACTGCTTGAAAG-3′ (residues 585–603). The resulting 1.3-kb PCR fragment, containing intron 6 (1.15 kb), was cleaved with SalI. Single-strand conformation polymorphism (SSCP) analysis was performed on the 69-bp restriction fragment encompassing the 7-bp deletion (28). After denaturation, DNA was directly cooled on ice and electrophoresed at 4°C. DNA was stained with a standard silver staining protocol (Bio-Rad). For patient two (A.B.) and parents, we used as the sense primer 5′-GCAGAAGGCACACATCTATG-3′ (residues 2793–2812) and as the antisense primer 5′-AGAATAACATCTCTGAAGCG-3′ (residues 2906–2934), after which restriction analysis with TaqI was performed.
RESULTS
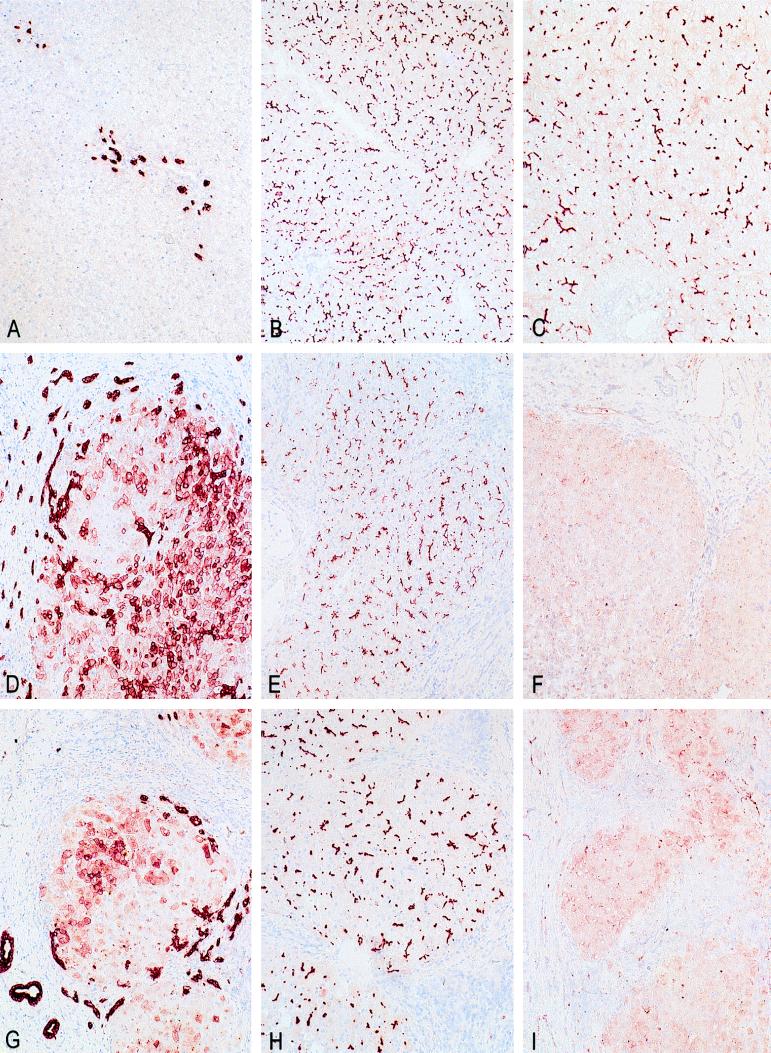
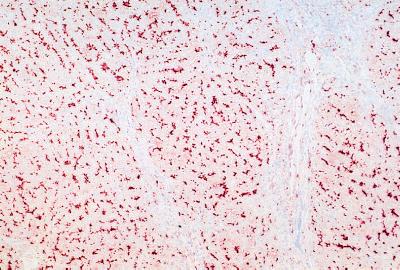
The biochemical and histological features of the two patients under study fall in the third group of PFIC patients with high serum γ-GT activity. Histological examination and immunohistochemical staining with cytokeratin 7 of the resected livers of both patients revealed extensive bile duct proliferation and ductular metaplasia of hepatocytes near the portal tract (Fig. 1 D and G) (24). Both patients had developed complete cirrhosis with portal bridging fibrosis. In both patients a defect in bile salt synthesis was excluded. Serum bile salt analysis by HPLC showed normal concentrations of cholate (41%) and chenodeoxycholate (59%) species. No abnormal bile salt species could be detected in urine of patient 2 via fast atom bombardment MS (16). Immunohistochemistry was performed on frozen liver sections with a polyclonal antibody against MDR3 P-gp, α-REG1 (7). The epitope for α-REG1 resides in the N-terminal part of the MDR3 P-gp (codon 2–58). In normal human control liver (Fig. 1C) and in PFIC patients with normal serum γ-GT activity (Fig. 2), the antibody stained the canalicular membrane of the hepatocyte throughout the entire liver lobule. In contrast, liver sections of both patients showed complete absence of canalicular staining (Fig. 1 F and I). As control, other canalicular transporters were stained. The monoclonal antibody C219, which is directed to all P-gps including MDR1, stained the canalicular membrane in livers of both patients (Fig. 1 E and H), in human control liver (Fig. 1B), and in livers of PFIC patients with normal γ-GT levels (data not shown). A monoclonal antibody against the canalicular multispecific organic anion transporter cMOAT (M2III-6) (25) also stained the canalicular membrane in all liver specimens (data not shown). This strongly suggests that both patients specifically lack MDR3 P-gp.
Figure 1.
Immunohistochemical staining of control and patient livers. (A–C) Normal human control liver. (D–F) Patient 1. (G–I) Patient 2. (A, D, and G) Immunohistochemical detection of cytokeratin 7 in bile duct epithelium cells. Both patient livers have extensive ductular proliferation (dark staining) and metaplasia of hepatocytes to a ductular phenotype near the portal tract (light staining). (B, E, and H) Immunostaining with C219, a monoclonal antibody against all mammalian P-gps. All livers demonstrate canalicular staining. (C, F, and I) Immunostaining with α-REG1 (a polyclonal antibody against MDR3 P-gp). In contrast to normal human liver (C), both patient livers (F and I) reveal absence of canalicular staining for MDR3 P-gp.
Figure 2.
Immunohistochemical detection of MDR3 P-gp in a PFIC patient with normal serum γ-GT activity. Canalicular staining of MDR3 P-gp with α-REG1 demonstrates a normal expression level of this protein. Note the portal fibrosis without ductular proliferation as revealed by hematoxylin staining.
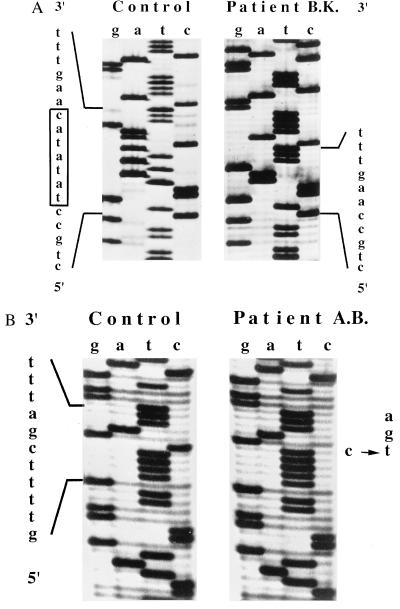
RT–PCR was performed on liver RNA of both patients. Sequence analysis of the RT–PCR products covering the complete coding sequence of patient one (B.K.) revealed a homozygous 7-nucleotide deletion starting at codon 132 (TATATAC). This deletion results in a frameshift (Fig. 3A) and subsequent introduction of a stop codon 29 codons downstream. Sequence analysis of RT–PCR products from the second patient (A.B.) revealed a nonsense mutation in codon 957 (C → T), which introduces a stop codon (TGA) (Fig. 3B).
Figure 3.
Parts of the MDR3 cDNA sequences of patient 1 (B.K.) (A) and patient 2 (A.B.) (B) encompassing the mutations. Normal sequence is depicted left of the sequence of each patient. The box in A indicates the 7-bp deletion (TATATAC) of patient B.K. starting at codon 132, which results in a frameshift and subsequent stop codon 29 codons downstream. The arrow in B indicates the single base-pair substitution in codon 957 (C → T) in patient A.B. that results in a stop codon (CGA → TGA).
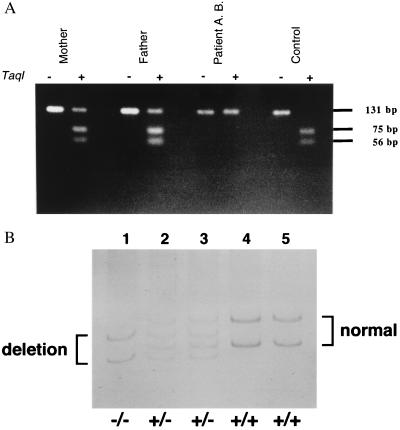
Although sequence analysis of RT–PCR products suggests that both patients are homozygous for their mutations, it cannot be excluded that these patients are genetic compounds having the combination of a spontaneously mutated allele with a null allele. Therefore, genomic DNA analysis from leukocytes of both patients and their first-degree relatives was performed to clarify the inheritance pattern. The mutation in patient two deletes a TaqI restriction site (5′-TCGA-3′, where the boldface type is the mutated nucleotide C → T). This could be used to confirm the homozygous state of the patient and the heterozygous state of both parents with amplified genomic DNA (Fig. 4A). In patient one, the deletion did not lead to gain or loss of a restriction site. Therefore, homozygosity of the patient (−/−), heterozygosity of the parents (+/−), and homozygosity of the sister (+/+) was confirmed by performing PCR–SSCP analysis on genomic DNA (Fig. 4B). These data confirm the homozygosity of both patients for the detected mutation, and in addition, clearly demonstrate that PFIC has a recessive inheritance pattern.
Figure 4.
Genomic DNA analysis that confirms the homozygosity of both patients and heterozygosity of their parents. (A) TaqI restriction analysis of amplified genomic DNA of patient 2 (A.B.) and his parents. In normal human genomic DNA the TaqI digestion of the 131-bp PCR fragment results in two fragments with a length of 75 and 56 bp. In patient 2, the homozygous mutation destroys this restriction site and no fragments are observed. In his heterozygous parents, a combination of the two cleaved fragments and the intact PCR product is observed. (B) PCR–SSCP analysis of genomic DNA, containing the 7-bp mutation, of patient 1 (B.K.) and his family. Denaturated DNA was loaded and electrophoresed in a 15% acrylamide/N, N′-methylenebisacrylamide (50:1, wt/wt) gel at 4°C. Patient 1 (lane 1), father (lane 2), mother (lane 3), sister (lane 4), and control (lane 5). In patient 1, only the deleted forms (sense and antisense) are observed. Both parents show the wild-type and the deleted forms, indicating that they are heterozygotes. The patient’s sister is homozygous for the wild-type form.
DISCUSSION
This report describes the defects in a human MDR gene and emphasizes the important physiological role of MDR3 P-gp. MDR3 deficiency, as identified in these two children, induces severe progressive intrahepatic cholestasis that becomes manifest within 1 year after birth and progresses toward hepatic failure. In both patients described herein, the mutation gives rise to a truncated protein. No MDR3 P-gp could be detected in both patients (Fig. 1 F and I) by using the polyclonal antibody α-REG1, which binds to the N terminus of the protein. The (near) absence of any protein can be explained in two ways. The truncated protein may be broken down very rapidly after synthesis giving rise to extremely low steady-state levels of the protein. Alternatively, the premature stop codon may lead to instability of MDR3 mRNA. The latter explanation is supported by the finding of a nearly absent signal for MDR3 mRNA upon Northern blotting of liver of patient 2 (data not shown).
It has been demonstrated unambiguously that deficiency of the mdr2 P-gp in the mouse completely abrogates biliary phospholipid secretion. The human MDR3 gene has a very high degree of homology with the murine mdr2 gene, suggesting that it serves the same function. This hypothesis was recently confirmed by the transgenic expression of the MDR3 gene in mdr2 knockout mice, which restores phospholipid secretion completely (A.J. Smith, personal communication). Thus, it can be concluded that absence of MDR3 P-gp in man also leads to a defect in phospholipid secretion. Indeed, bile of another patient with the same disease phenotype (PFIC with high γ-GT) was reported to lack biliary phospholipid (less than 1 mM) but to have a normal bile salt concentration (103 mM; J.M.L.d.V., unpublished observations).
Mdr2 (+/−) mice, with a maximal phospholipid secretion of 60% of controls, do not develop liver disorders (8, 9). Hence, relatives of these patients, which are heterozygous for MDR3, are not likely to develop any liver disorders, although mild alterations in liver histology cannot be exluded. For example, the mother of patient 2 experienced recurrent episodes of cholestasis during pregnancy. The detergent action of simple bile salt micelles, which is normally inactivated by phospholipids, solubilizes the membranes of bile duct epithelial cells lining the biliary tree (11). This explains the histological features, characteristic for high serum γ-GT PFIC patients: inflammatory reactive changes, proliferation of the bile duct epithelium, and ductular metaplasia (24). It could well be that the high serum activity of the apical ectoenzyme γ-GT results from the continuous exposure of the biliary epithelium and the bile canaliculi to the hydrophobic bile salts, the detergent action of which is not longer inactivated by phospholipids. This could lead to increased solubilization of γ-GT from the membrane that could be regurgitated through the paracellular pathway (16, 17). Other feasible explanations are increased expression and/or mistargeting of the enzyme to the basolateral membrane that could lead to higher γ-GT activity in serum.
In the mdr2(−/−) mouse model, it was observed that feeding the noncytotoxic bile salt UDCA led to a complete replacement of the endogenous bile salt pool with UDCA and that this halted the progression of liver disease (10). In addition, UDCA has been shown to be effective in a variety of pediatric hepatobiliary diseases (29). In PFIC patients with high serum γ-GT, only 46% responded to treatment with UDCA (30). One explanation for nonresponsiveness could be that, in contrast to the mouse (10), UDCA treatment of humans only leads to a partial replacement of the bile salt pool. In addition, the UDCA treatment has been applied in a relatively late stage of the disease, when cholestasis had been manifest for a longer period. We hypothesize, however, that nonresponders have a complete defect in phospholipid secretion and that the partial UDCA replacement is insufficient to reduce the increased bile salt toxicity in phospholipid-free bile of these patients. Patients who do respond to UDCA treatment (29, 30) may have a partial defect and the residual phospholipid concentration in bile, combined with a partial UDCA replacement, may be sufficient to reduce the bile salt toxicity below a critical threshold. The fact that our two patients have a complete defect and did not respond to UDCA treatment is in line with this hypothesis. Early detection of a (partial) MDR3 defect in PFIC patients with high serum γ-GT might increase the chance of response toward UDCA treatment. Nowadays, this can be achieved by means of bile sampling, which is possible within 2 months after birth. If bile analysis reveals a diminished or absent phospholipid concentration, further analysis for MDR3 deficiency should be performed at a molecular level.
Acknowledgments
We thank Piet Borst and Sander Smith (Netherlands Cancer Instititute) for kindly supplying the α-REG1 antibody. We thank Nike Claessen for the help with the immunohistochemistry, Conny Bakker for performing the SSCP analysis, and Kenneth Setchell for the fast atom bombardment MS. We thank Piet Borst, Bert Groen, and Johan Offerhaus for critically reading the manuscript. This work was supported by the Dutch Organization for Scientific Research, Project 902-23-097, and by grants from Mutuelle Générale de l’ Education Nationale, Paris, France, and Association Française contres le Myopathies, Paris, France.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: P-gp, P-glycoprotein; PFIC, progressive familial intrahepatic cholestasis; γ-GT, γ-glutamyltransferase; UDCA, ursodeoxycholate; RT–PCR, reverse transcription-coupled PCR; HRP, horseradish peroxidase; SSCP, single-strand conformation polymorphism.
References
- 1.Keppler D, Arias I M. FASEB J. 1997;11:15–18. doi: 10.1096/fasebj.11.1.9034161. [DOI] [PubMed] [Google Scholar]
- 2.Borst P, Schinkel A H, Smit J J M, Wagenaar E, van Deemter L, Smith A J, Eijdems E W, Baas F, Zaman G J. Pharmacol Ther. 1993;60:289–299. doi: 10.1016/0163-7258(93)90011-2. [DOI] [PubMed] [Google Scholar]
- 3.Gottesman M M, Pastan I. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 4.Smith A J, Timmermans-Hereijgers J L P M, Roelofsen B, Wirtz K W A, Blitterswijk W J, Smit J J M, Schinkel A H, Borst P. FEBS Lett. 1994;354:263–266. doi: 10.1016/0014-5793(94)01135-4. [DOI] [PubMed] [Google Scholar]
- 5.Reutz S, Gros P. Cell. 1994;77:1071–1081. doi: 10.1016/0092-8674(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 6.Van Helvoort A, Smith A J, Sprong H, Fritzsche I, Schinkel A H, Borst P, Van Meer G. Cell. 1996;87:507–517. doi: 10.1016/s0092-8674(00)81370-7. [DOI] [PubMed] [Google Scholar]
- 7.Smit J J M, Schinkel A H, Mol C A A M, Majoor D, Mooi W J, Jongsma A P M, Lincke C R, Borst P. Lab Invest. 1994;71:638–649. [PubMed] [Google Scholar]
- 8.Smit J J M, Schinkel A H, Oude Elferink R P J, Groen A K, Wagenaar E, van Deemter L, Mol C A A M, Ottenhoff R, van der Lugt N M, van Roon M A, van der Valk M A, Offerhaus G J A, Berns A J M, Borst P. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- 9.Mauad T H, van Nieuwkerk C M J, Dingemans K P, Smit J J M, Schinkel A H, Notenboom G, van den Bergh Weerman M A, Verkruisen R P, Groen A K, Oude Elferink R P J, van der Valk M A, Borst P, Offerhaus G J A. Am J Pathol. 1994;145:1237–1245. [PMC free article] [PubMed] [Google Scholar]
- 10.van Nieuwkerk C M J, Oude Elferink R P J, Groen A K, Ottenhoff R, Tytgat G N J, Dingemans K P, van den Bergh Weerman M A, Offerhaus G J A. Gastroenterology. 1996;111:165–171. doi: 10.1053/gast.1996.v111.pm8698195. [DOI] [PubMed] [Google Scholar]
- 11.Oude Elferink R P J, Tytgat G N J, Groen A K. FASEB J. 1997;11:19–28. doi: 10.1096/fasebj.11.1.9034162. [DOI] [PubMed] [Google Scholar]
- 12.Alonso E M, Snover D C, Montag A D, Freese K, Whitington P F. J Pediatr Gastroenterol Nutr. 1994;18:128–133. doi: 10.1097/00005176-199402000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Whitington P F, Freese D K, Alonso E M, Schwarzenberg S J, Sharp H L. J Pediatr Gastroenterol Nutr. 1994;18:134–141. doi: 10.1097/00005176-199402000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Clayton P T, Leonard J V, Lawson A M, Setchell K D R, Andersson S, Egestad B, Sjövall J. J Clin Invest. 1987;79:1031–1038. doi: 10.1172/JCI112915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Setchell K D R, Suchy F J, Welsh M B, Zimmer-Nechemias L, Heubi J, Balistreri W F. J Clin Invest. 1988;82:2148–2157. doi: 10.1172/JCI113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacquemin E, Setchell K D R, O’Connor N C, Estrada A, Maggiore G, Schmitz J, Hadchouel M, Bernard O. J Pediatr. 1994;125:379–384. doi: 10.1016/s0022-3476(05)83280-9. [DOI] [PubMed] [Google Scholar]
- 17.Jacquemin E, Dumont M, Bernard O, Erlinger S, Hadchouel M. Eur J Pediatr. 1994;153:424–428. doi: 10.1007/BF01983406. [DOI] [PubMed] [Google Scholar]
- 18.Clayton R J, Washington D C, Iber F L, Ruebner M D, McKusick V A. Am J Dis Child. 1969;117:112–124. [PubMed] [Google Scholar]
- 19.Carlton V E H, Knisely A S, Freimer N B. Hum Mol Genet. 1995;4:1049–1053. doi: 10.1093/hmg/4.6.1049. [DOI] [PubMed] [Google Scholar]
- 20.Strautnieks S S, Kagalwalla A F, Tanner M S, Knisely A S, Bull L, Freimer N, Kocoshis S A, Gardiner R M, Thompson R J. Am J Hum Genet. 1997;61:630–633. doi: 10.1086/515501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maggiore G, Bernard O, Riely C A, Hadchouel M, Lemonnier A, Alagille D. J Pediatr. 1991;111:251–252. doi: 10.1016/s0022-3476(87)80079-3. [DOI] [PubMed] [Google Scholar]
- 22.Maggiore G, Bernard O, Hadchouel M, Lemonnier A, Alagille D. J Pediatr Gastroenterol Nutr. 1991;12:21–26. doi: 10.1097/00005176-199101000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Deleuze J, Jacquemin E, Dubuisson C, Cresteil D, Dumont M, Erlinger S, Bernard O, Hadchouel M. Hepatology. 1996;23:904–908. doi: 10.1002/hep.510230435. [DOI] [PubMed] [Google Scholar]
- 24.Van Eyken P, Sciot R, Desmet V J. Histopathology. 1989;15:125–135. doi: 10.1111/j.1365-2559.1989.tb03060.x. [DOI] [PubMed] [Google Scholar]
- 25.Paulusma C C, Bosma P J, Zaman G J R, Bakker C T M, Otter M, Scheffer G L, Scheper R J, Borst P, Oude Elferink R P J. Science. 1996;271:1126–1128. doi: 10.1126/science.271.5252.1126. [DOI] [PubMed] [Google Scholar]
- 26.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 27.Sanger F, Nicklen S, Coulson A R. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orita M, Suzuki Y, Sekiya T, Hayashi K. Genomics. 1989;5:874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- 29.Balistreri W F. J Pediatr Gastroenterol Nutr. 1997;24:573–589. doi: 10.1097/00005176-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 30.Jacquemin E, Hermans D, Myara A, Habes D, Debray D, Hadchouel M, Sokal E M, Bernard O. Hepatology. 1997;25:519–523. doi: 10.1002/hep.510250303. [DOI] [PubMed] [Google Scholar]