Abstract
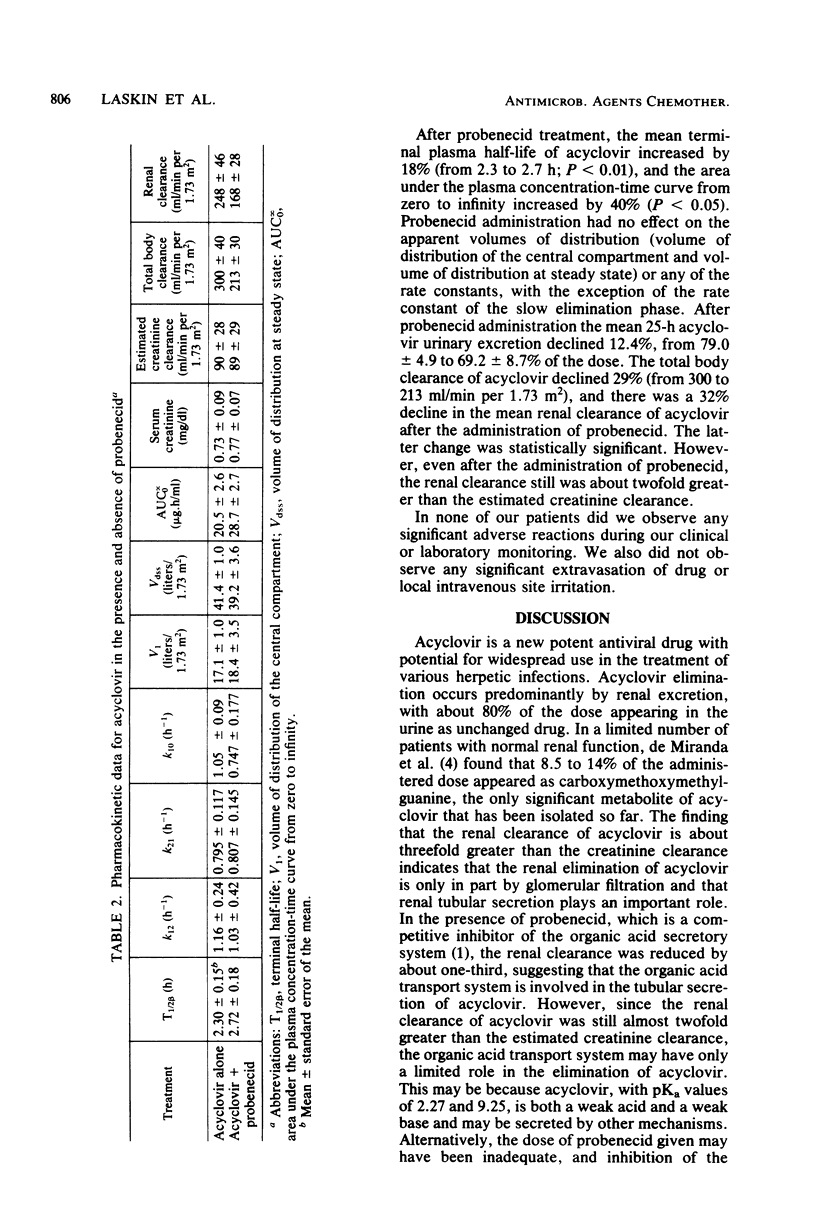
The effects of probenecid on the pharmacokinetics and renal clearance of acyclovir were studied in humans. Acyclovir (5 mg/kg) was given as a 1-h infusion to three volunteers with normal renal function both before and after oral administration of probenecid (1 g). The kinetics were well described by a two-compartment open model with zero-order infusion. The mean acyclovir concentrations at all time points after 1.0 h from the end of acyclovir infusion following probenecid administration were statistically higher than the corresponding mean acyclovir concentrations following the acyclovir infusion without probenecid administration. In the absence of probenecid, the renal clearance (248 +/- 80 ml/min per 1.73 m2) accounted for 83% of the total clearance (300 +/- 69 ml/min per 1.73 m2) and was almost threefold greater than the estimated creatinine clearance (90 +/- 48 ml/min per 1.73 m2). After probenecid administration, there was a 32% decline in renal clearance (248 to 168 ml/min per 1.73 m2; P less than or equal to 0.05), a 40% increase in the area under the curve (91.3 to 127.6 nmol.h/ml; P less than 0.05), and an 18% increase in the terminal plasma half-life (2.3 to 2.7 h; P less than 0.01). Although statistically significant, these effects due to the influence of probenecid probably have only limited clinical importance. In this study we confirmed that acyclovir is eliminated predominantly by renal clearance, both by glomerular filtration and tubular secretion; our results suggested that at least part of the tubular secretion is inhibited by probenecid.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEYER K. H., RUSSO H. F., TILLSON E. K., MILLER A. K., VERWEY W. F., GASS S. R. 'Benemid,' p-(di-n-propylsulfamyl)-benzoic acid; its renal affinity and its elimination. Am J Physiol. 1951 Sep;166(3):625–640. doi: 10.1152/ajplegacy.1951.166.3.625. [DOI] [PubMed] [Google Scholar]
- Brigden D., Bye A., Fowle A. S., Rogers H. Human pharmacokinetics of acyclovir (an antiviral agent) following rapid intravenous injection. J Antimicrob Chemother. 1981 Apr;7(4):399–404. doi: 10.1093/jac/7.4.399. [DOI] [PubMed] [Google Scholar]
- Cunningham R. F., Israili Z. H., Dayton P. G. Clinical pharmacokinetics of probenecid. Clin Pharmacokinet. 1981 Mar-Apr;6(2):135–151. doi: 10.2165/00003088-198106020-00004. [DOI] [PubMed] [Google Scholar]
- Gehan E. A., George S. L. Estimation of human body surface area from height and weight. Cancer Chemother Rep. 1970 Aug;54(4):225–235. [PubMed] [Google Scholar]
- Laskin O. L., Longstreth J. A., Saral R., de Miranda P., Keeney R., Lietman P. S. Pharmacokinetics and tolerance of acyclovir, a new anti-herpesvirus agent, in humans. Antimicrob Agents Chemother. 1982 Mar;21(3):393–398. doi: 10.1128/aac.21.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawer G. E., Lucas S. B., Knowles B. R., Stirland R. M. Computer-assisted prescribing of kanamycin for patients with renal insufficiency. Lancet. 1972 Jan 1;1(7740):12–15. doi: 10.1016/s0140-6736(72)90005-0. [DOI] [PubMed] [Google Scholar]
- Quinn R. P., de Miranda P., Gerald L., Good S. S. A sensitive radioimmunoassay for the antiviral agent BW248U [9-(2-hydroxyethoxymethyl)guanine]. Anal Biochem. 1979 Oct 1;98(2):319–328. doi: 10.1016/0003-2697(79)90148-9. [DOI] [PubMed] [Google Scholar]
- Schaeffer H. J., Beauchamp L., de Miranda P., Elion G. B., Bauer D. J., Collins P. 9-(2-hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature. 1978 Apr 13;272(5654):583–585. doi: 10.1038/272583a0. [DOI] [PubMed] [Google Scholar]
- Sedman A. J., Wagner J. G. CSTRIP, a fortran IV computer program for obtaining initial polyexponential parameter estimates. J Pharm Sci. 1976 Jul;65(7):1006–1010. doi: 10.1002/jps.2600650713. [DOI] [PubMed] [Google Scholar]
- Spector S. A., Connor J. D., Hintz M., Quinn R. P., Blum M. R., Keeney R. E. Single-dose pharmacokinetics of acyclovir. Antimicrob Agents Chemother. 1981 Apr;19(4):608–612. doi: 10.1128/aac.19.4.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Miranda P., Good S. S., Laskin O. L., Krasny H. C., Connor J. D., Lietman P. S. Disposition of intravenous radioactive acyclovir. Clin Pharmacol Ther. 1981 Nov;30(5):662–672. doi: 10.1038/clpt.1981.218. [DOI] [PubMed] [Google Scholar]
- de Miranda P., Whitley R. J., Blum M. R., Keeney R. E., Barton N., Cocchetto D. M., Good S., Hemstreet G. P., 3rd, Kirk L. E., Page D. A. Acyclovir kinetics after intravenous infusion. Clin Pharmacol Ther. 1979 Dec;26(6):718–728. doi: 10.1002/cpt1979266718. [DOI] [PubMed] [Google Scholar]



