Abstract
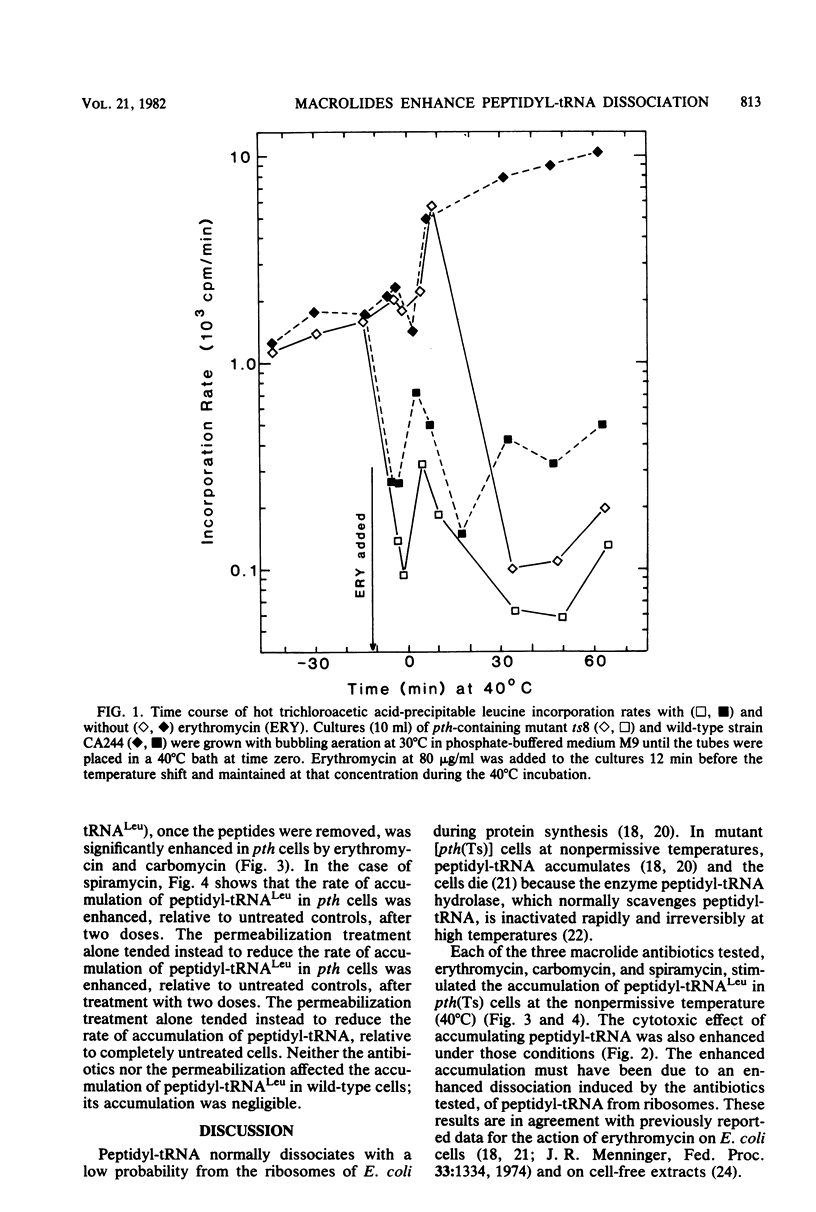
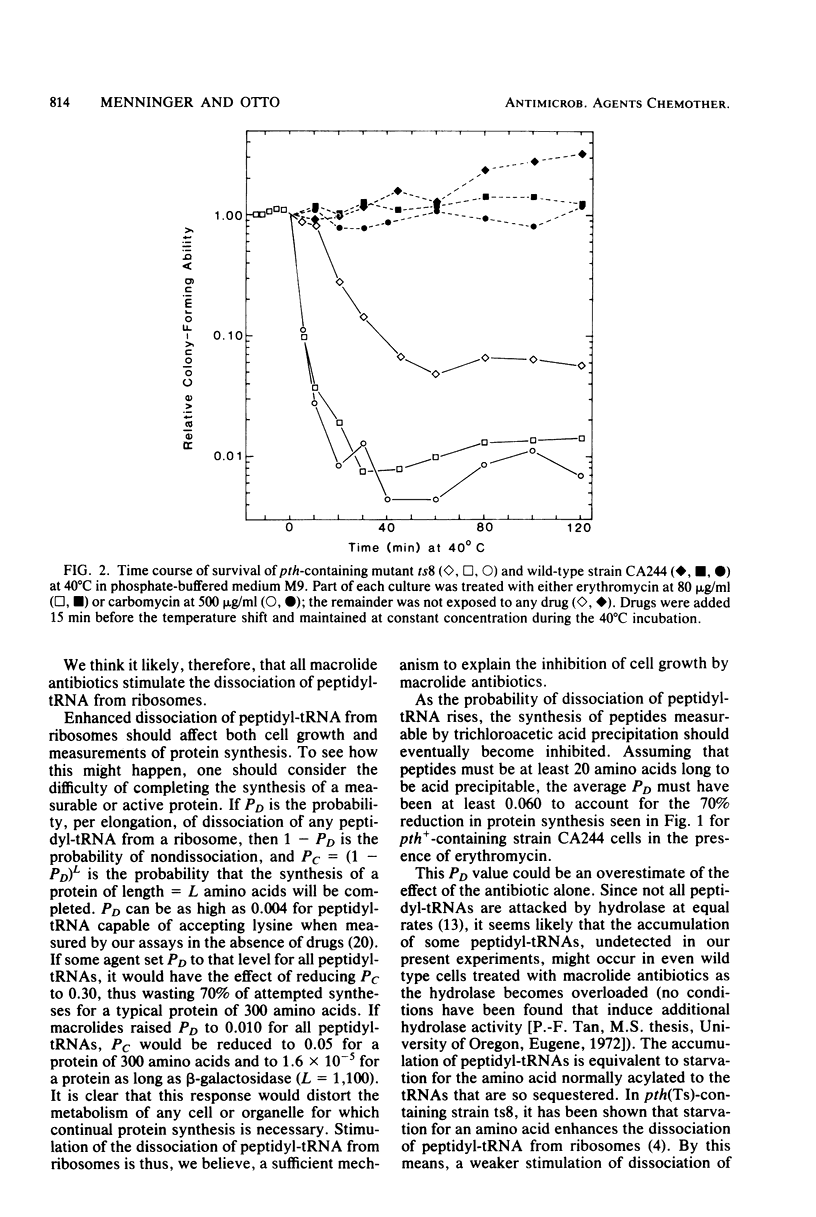
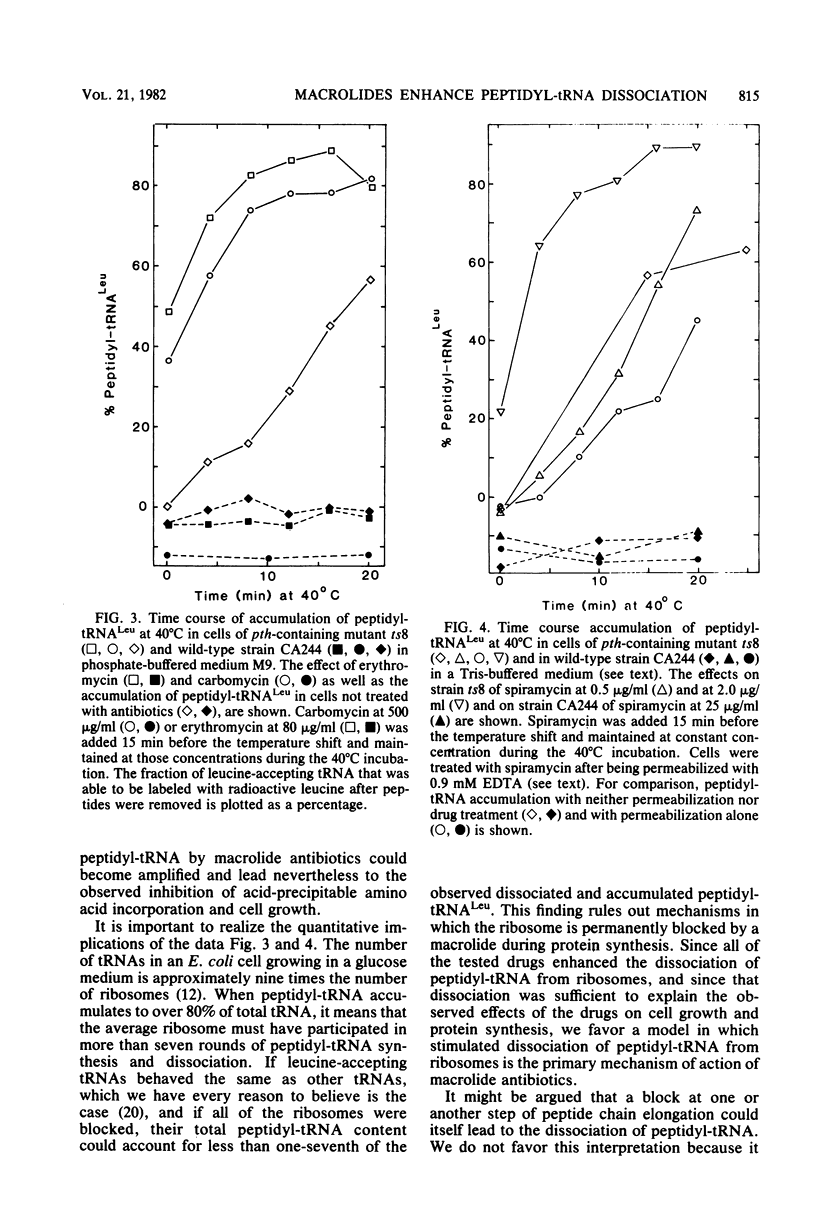
In mutant Escherichia coli with temperature-sensitive peptidyl-tRNA hydrolase (aminoacyl-tRNA hydrolase; EC 3.1.1.29), peptidyl-tRNA accumulates at the nonpermissive temperature (40 degrees C), and the cells die. These consequences of high temperature were enhanced if the cells were first treated with erythromycin, carbomycin, or spiramycin at doses sufficient to inhibit protein synthesis in wild-type cells but not sufficient to kill either mutant or wild-type cells at the permissive temperature (30 degrees C). Since peptidyl-tRNA hydrolase in he mutant cells is inactivated rapidly and irreversibly at 40 degrees C, the enhanced accumulation of peptidyl-tRNA and killing were the result of enhanced dissociation, stimulated by the antibiotics, of peptidyl-tRNA from ribosomes. The implications of these findings for inhibition of cell growth and protein synthesis are discussed. Certain alternative interpretations are shown to be inconsistent with the relevant data. Previous conflicting observations on the effects of macrolide antibiotics are explained in terms of our observations. We conclude that erythromycin, carbomycin, and spiramycin (and probably all macrolides) have as a primary mechanism of action the stimulation of dissociation of peptidyl-tRNA from ribosomes, probably during translocation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherly A. G., Menninger J. R. Mutant E. coli strain with temperature sensitive peptidyl-transfer RNA hydrolase. Nat New Biol. 1972 Dec 20;240(103):245–246. doi: 10.1038/newbio240245a0. [DOI] [PubMed] [Google Scholar]
- Cannon Michael, Burns Kay. Modes of action of erythromycin and thiostrepton as inhibitors of protein synthesis. FEBS Lett. 1971 Oct 15;18(1):1–5. doi: 10.1016/0014-5793(71)80392-7. [DOI] [PubMed] [Google Scholar]
- Caplan A. B., Menninger J. R. Tests of the ribosomal editing hypothesis: amino acid starvation differentially enhances the dissociation of peptidyl-tRNA from the ribosome. J Mol Biol. 1979 Nov 5;134(3):621–637. doi: 10.1016/0022-2836(79)90370-x. [DOI] [PubMed] [Google Scholar]
- Cundliffe E. Antibiotics and polyribosomes. II. Some effects of lincomycin, spiramycin, and streptogramin A in vivo. Biochemistry. 1969 May;8(5):2063–2066. doi: 10.1021/bi00833a042. [DOI] [PubMed] [Google Scholar]
- Cundliffe E., McQuillen K. Bacterial protein synthesis: the effects of antibiotics. J Mol Biol. 1967 Nov 28;30(1):137–146. doi: 10.1016/0022-2836(67)90249-5. [DOI] [PubMed] [Google Scholar]
- Ennis H. L. Polysome metabolism in Escherichia coli: effect of antibiotics on polysome stability. Antimicrob Agents Chemother. 1972 Mar;1(3):197–203. doi: 10.1128/aac.1.3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairclough R. H., Cantor C. R. The distance between the anticodon loops of two tRNAs bound to the 70 S Escherichia coli ribosome. J Mol Biol. 1979 Aug 25;132(4):575–586. doi: 10.1016/0022-2836(79)90375-9. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Ishitsuka H., Kaji A. Comparative studies on the mechanism of action of lincomycin, streptomycin, and erythromycin. Biochem Biophys Res Commun. 1969 Oct 22;37(3):499–504. doi: 10.1016/0006-291x(69)90943-7. [DOI] [PubMed] [Google Scholar]
- Kössel H. Purification and properties of peptidyl-tRNA hydrolase from Escherichia coli. Biochim Biophys Acta. 1970 Mar 19;204(1):191–202. doi: 10.1016/0005-2787(70)90502-2. [DOI] [PubMed] [Google Scholar]
- Mao J. C., Robishaw E. E. Effects of macrolides on peptide-bond formation and translocation. Biochemistry. 1971 May 25;10(11):2054–2061. doi: 10.1021/bi00787a014. [DOI] [PubMed] [Google Scholar]
- Matzke A. J., Barta A., Kuechler E. Mechanism of translocation: relative arrangement of tRNA and mRNA on the ribosome. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5110–5114. doi: 10.1073/pnas.77.9.5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke A. J., Barta A., Kuechler E. Photo-induced crosslinking between phenylalanine transfer RNA and messenger RNA on the Escherichia coli ribosome. Eur J Biochem. 1980 Nov;112(1):169–178. doi: 10.1111/j.1432-1033.1980.tb04998.x. [DOI] [PubMed] [Google Scholar]
- Menninger J. R. Accumulation of peptidyl tRNA is lethal to Escherichia coli. J Bacteriol. 1979 Jan;137(1):694–696. doi: 10.1128/jb.137.1.694-696.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menninger J. R. Peptidyl transfer RNA dissociates during protein synthesis from ribosomes of Escherichia coli. J Biol Chem. 1976 Jun 10;251(11):3392–3398. [PubMed] [Google Scholar]
- Menninger J. R. Ribosome editing and the error catastrophe hypothesis of cellular aging. Mech Ageing Dev. 1977 Mar-Apr;6(2):131–142. doi: 10.1016/0047-6374(77)90014-8. [DOI] [PubMed] [Google Scholar]
- Menninger J. R. The accumulation as peptidyl-transfer RNA of isoaccepting transfer RNA families in Escherichia coli with temperature-sensitive peptidyl-transfer RNA hydrolase. J Biol Chem. 1978 Oct 10;253(19):6808–6813. [PubMed] [Google Scholar]
- Menninger J. R., Walker C., Tan P. F. Studies on the metabolic role of peptidyl-tRNA hydrolase. I. Properties of a mutant E. coli with temperature-sensitive peptidyl-tRNA hydrolase. Mol Gen Genet. 1973 Mar 19;121(4):307–324. doi: 10.1007/BF00433230. [DOI] [PubMed] [Google Scholar]
- Müller K., Lüdemann H. D., Jaenicke R. Reconstitution of lactic dehydrogenase from pig heart after reversible high-pressure dissociation. Biochemistry. 1981 Sep 15;20(19):5411–5416. doi: 10.1021/bi00522a009. [DOI] [PubMed] [Google Scholar]
- Otaka T., Kaji A. Mode of action of bottromycin A2. Release of aminoacyl- or peptidyl-tRNA from ribosomes. J Biol Chem. 1976 Apr 25;251(8):2299–2306. [PubMed] [Google Scholar]
- Otaka T., Kaji A. Release of (oligo) peptidyl-tRNA from ribosomes by erythromycin A. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2649–2652. doi: 10.1073/pnas.72.7.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S. Antibiotics as probes of ribosome structure: binding of chloramphenicol and erythromycin to polyribosomes; effect of other antibiotics. Antimicrob Agents Chemother. 1974 Mar;5(3):255–267. doi: 10.1128/aac.5.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S., Nakagawa A., Omura S. Effect of leucomycins and analogues on binding [14C ]erythromycin to Escherichia coli ribosomes. Antimicrob Agents Chemother. 1974 Nov;6(5):606–612. doi: 10.1128/aac.6.5.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters M., Yarus M. Transfer RNA selection at the ribosomal A and P sites. J Mol Biol. 1979 Nov 5;134(3):471–491. doi: 10.1016/0022-2836(79)90364-4. [DOI] [PubMed] [Google Scholar]
- Tai P. C., Wallace B. J., Davis B. D. Selective action of erythromycin on initiating ribosomes. Biochemistry. 1974 Oct 22;13(22):4653–4659. doi: 10.1021/bi00719a029. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Teraoka H. Binding of erythromycin to Escherichia coli ribosomes. Biochim Biophys Acta. 1966 Jan 18;114(1):204–206. doi: 10.1016/0005-2787(66)90272-3. [DOI] [PubMed] [Google Scholar]
- Wurmbach P., Nierhaus K. H. Codon-anticodon interaction at the ribosomal P (peptidyl-tRNA)site. Proc Natl Acad Sci U S A. 1979 May;76(5):2143–2147. doi: 10.1073/pnas.76.5.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]



