Abstract
The ability to measure human thymic output would be an invaluable tool for the study of the development of the naïve T cell repertoire, as well as naïve T cell regeneration after intensive cytotoxic chemotherapy or effective antiretroviral therapy of progressive HIV infection. We and others have demonstrated previously that quantification of T cell receptor rearrangement excision circles (TREC) within peripheral T cell populations provides insight into the frequency of recent thymic emigrants (RTE) and, therefore, into thymic function. However, measurement of RTE by this approach is complicated by the fact that TREC levels also are determined by turnover within the naïve T cell compartment. Here, we report a phenotypic approach to RTE measurement. We demonstrate that αE integrin (CD103) expression is up-regulated very late in thymic development on a subset of CD8+/CD4− thymocytes and also defines a distinct subset of naïve CD8+ T cells in the periphery. The latter subset is differentiated from circulating CD103+ mucosa-associated memory T cells by its naïve T cell phenotype (CD45RO−, CD62Lbright, CD27bright, CD11adim, CD95dim) and its high concentration of TREC. Indeed, sorted CD103+ naïve CD8+ cells display higher levels of TREC than their CD103− naïve counterparts, and these cells demonstrate an age-related decline in frequency that is enhanced significantly by thymectomy. The thymic dependence of this subset and the cells' relatively evanescent presence in the periphery suggest that these cells are a population of RTE and that quantification of their frequency in peripheral blood provides an estimate of the level of ongoing thymopoiesis.
Recent advances in cancer chemotherapy, stem cell transplantation, and antiretroviral therapy have highlighted the clinical importance of T lymphocyte regeneration. Studies in both humans and animal models indicate that T cell regeneration has two major components: (i) a relatively rapid memory T cell expansion that can reconstitute responses to previously encountered antigens and (ii) a slower redevelopment of the broad naïve T cell repertoire that is thought to be necessary for the reconstitution of immune responsiveness to the vast panoply of potential new antigens (1, 2). For conventional T cells, the latter component is thought to depend largely on the function of the thymus, an organ that progressively involutes with age and, therefore, has an uncertain capacity to fully reconstitute a damaged naïve T cell compartment in adulthood (1–10).
These considerations stress the potential clinical value of quantification of thymic function, an assessment that would allow evaluation of the kinetics and extent of the thymic contribution to immune reconstitution in individual subjects. In animal models, it has been possible to phenotypically identify recent thymic emigrants (RTE)—newly produced peripheral naïve T cells that retain some phenotypic signature of recent thymic maturation that distinguishes them from long-lived, remotely produced naïve T cells (11–13). These cells constitute the output of thymus, and, thus, their frequency in the periphery is a useful measure of thymic function (12). However, to date, this approach has not been applicable to the human system, because no such RTE phenotype has been reported for human T cells. Here, we demonstrate that αEβ7 integrin (CD103) expression is up-regulated on late CD8+/CD4−/CD3bright thymocytes in the human and appears to be retained on a distinct subset of naïve CD8+ T cells in the periphery that have the expected characteristics of RTE. Thus, it now is possible to use multiparameter flow cytometry to quantify a population of RTE as a measure of human thymic output, as well as to isolate this unique subset of newly produced CD8+ naïve T cells for further study.
Materials and Methods
Samples.
Adult and pediatric peripheral blood samples were obtained by venipuncture after informed consent following procedures approved by the Institutional Review Board of the University of Texas Southwestern Medical Center, Dallas. Some postthymectomy specimens were provided by B. Haynes at Duke University Medical Center, Durham, NC, following the guidelines of the Institutional Review Board of that institution. All postthymectomy subjects were thymectomized for treatment of myasthenia gravis, except for one patient whose thymus was removed as a result of multiple cardiac artery bypass grafting. Normal umbilical cord blood was provided by the obstetrics unit of Parkland Memorial Hospital, Dallas. Samples of normal pediatric thymus and hyperplastic tonsil (the former was removed during surgery for repair of cardiac malformations; the latter, for recurrent tonsillitis) were provided by the tissue acquisition service of the Department of Pathology at the University of Texas Southwestern Medical Center and Children's Medical Center of Dallas. Thymic and tonsillar tissue samples were stored in saline in the operating room and processed within 1 h of excision.
Cell Preparation and Staining.
Mononuclear cells were obtained from venous blood by density gradient sedimentation using Ficoll/Hypaque (Histopaque; Sigma). Mononuclear cells were obtained from tonsil and thymus by gentle teasing of tissue over wire mesh. Cells then were washed twice in PBS before either immunofluorescence staining or CD8 T cell purification and T cell receptor rearrangement excision circle (TREC) analysis. For staining, 1–2 × 106 cells were stained with combinations of four directly conjugated mAbs (see below) in ≈0.25 ml of PBS with 0.5% BSA (GIBCO) and 5 mM sodium azide for 20 min, followed by two washes in the same medium and resuspension in PBS with 1% paraformaldehyde. Stained cells were stored at 4°C in the dark until acquisition on the flow cytometer. CD8+ T cells were isolated from the mononuclear cell preparations by using the MACS magnetic bead separations system (Miltenyi Biotec, Auburn, CA) and then stored frozen at −80°C until TREC analysis was performed.
Flow Cytometric Analysis and Sorting.
Six-parameter flow cytometric analysis was performed on a two-laser FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems) by using FITC, phycoerythrin (PE), peridinin chlorophyll protein (PerCP), and allophycocyanin (APC) as the four fluorescent parameters. Direct fluorochrome conjugates of the following mAbs were used: CD1a, CD2, CD3, CD4, CD5, CD8, CD10, CD11a, CD25, CD27, CD38, HLA-DR, CD45RO, CD62L, CD69, CD71, and CD95 (Becton Dickinson Immunocytometry Systems); CD103 and CD11a (Coulter); CD45RO (Dako); anti-T cell receptor (TCR) -αβ and -γδ (Caltag, South San Francisco, CA). For routine analysis of putative RTE, mononuclear cell preparations were stained for CD103 (FITC), CD62L (PE), CD8 (PerCP), and CD45RO (APC), and 150,000 events were acquired gated on CD8 expression and a light scatter gate designed to include only viable small lymphocytes. List mode multiparameter data files (each file with forward scatter, orthogonal scatter, and four fluorescent parameters) were analyzed by using the paint-a-gateplus software program (Becton Dickinson Immunocytometry Systems). The criteria for delineating memory and naive T cells have been described previously (10, 14). Briefly, the naïve subset comprises a distinct, homogeneous cluster of CD27bright, CD45RO−, CD62L+, CD11adim, CD95dim cells, whereas diverse memory subsets comprise the remainder.
Five- or six-parameter fluorescence-activated cell sorting was performed on (i) peripheral blood mononuclear cells from four euthymic adult subjects stained with CD103(FITC), CD62L(PE), CD8(biotin/streptavidin-PE/Cy5 tandem), and CD45RO(APC) and (ii) thymocytes stained with CD103(FITC), CD8(PE), and CD4 (APC) by using a FACSVantage SE (Becton Dickinson Immunocytometry Systems). Sorted populations were analyzed for sort purity and stored at −80°C until TREC analysis.
Quantification of TREC.
Signal-joint TREC were quantified in purified CD8+ T cells or sorted CD8+ T cell subsets by quantitative competitive–PCR (QC-PCR), as described previously (15), or real-time PCR. Purified or sorted cells were lysed in 100 μg/ml proteinase K (Boehringer Mannheim) for 1 h at 56°C and then 10 min at 95°C at 107 cells/ml. Real-time quantitative PCR was performed by using the 5′ nuclease (TaqMan) assay on 5 μl of cell lysate with an ABI7700 system (Perkin–Elmer) and the primers cacatccctttcaaccatgct and gccagctgcagggtttagg and probe FAM′acacctctggtttttgtaaaggtgcccact′TAMRA (MegaBases, Chicago). PCR reactions contained 0.5 units of Platinum Taq polymerase (GIBCO), 3.5 mM MgCl2, 0.2 mM dNTPs, 500 nM of each primer, 150 nM probe, and Blue-636 reference (MegaBases). Conditions were 95° for 5 min and then 95° for 30 s and 60° for 1 min for 40 cycles. A standard curve was plotted and TREC values for samples were calculated by the ABI7700 software. Samples were analyzed in duplicates or triplicates, which never varied by more than 10% from each other, and the result was averaged. This assay was validated by analyzing known standards and unknown samples in parallel with the QC-PCR assay. Cell lysates had been checked for consistency of DNA content by using a β-actin control PCR.
Statistical Analysis.
The Mann–Whitney U Test was used for unpaired comparison of putative RTE and TREC levels between populations of remote athymic subjects and euthymic subjects (age range, 19–73 years). The paired Student's t test was used for paired comparisons of TREC levels in CD103+ and CD103−, CD8 single positive thymocytes and peripheral naïve CD8+ T cells. Statistical analyses were performed by using statview 5.0 software (SAS Institute, Cary, NC).
Results and Discussion
CD103 Is Highly Expressed on Mature CD8+/CD4− Thymocytes and Defines a Subset of Naïve Phenotype CD8+ T Cells in the Peripheral Immune System.
CD103 (αE integrin) originally was designated as the mucosal lymphocyte antigen because of its highly selective expression on memory T cells within the lamina propria and epithelium of the mucosa of the alimentary tract and within the lung (16, 17). The purpose of this expression pattern became apparent when it was determined that a major function of this integrin was to serve as the T cell counterreceptor for E-cadherin on mucosal epithelial cells (18). We and others defined a small subset of peripheral blood CD103+ (predominantly CD8+) memory T cells (1–3%) with a homing receptor phenotype [e.g., l-selectin (CD62L) negative] consistent with this subset being a recirculating component of mucosa-associated T cells (16, 19). CD103 expression also was noted on a small subset of thymocytes in this early work (19), but naïve T cell expression of CD103 was not recognized and the significance of CD103 expression in the thymus was not resolved.
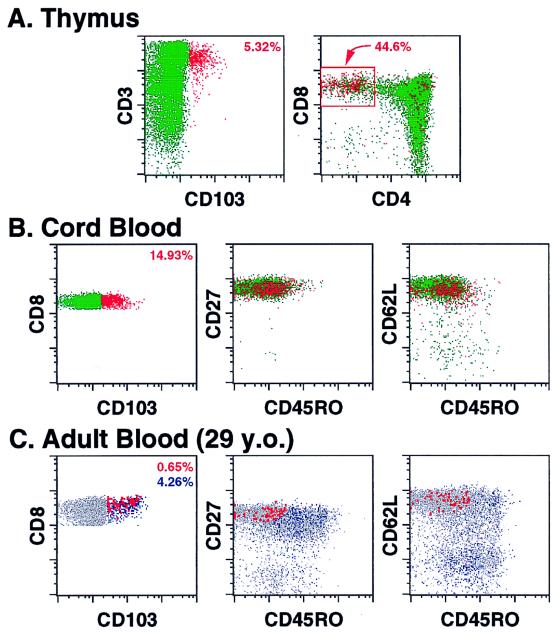
In our efforts to define a phenotypic signature of RTE, we began by looking for unique phenotypic characteristics of the most mature thymocytes (20). As shown in Fig. 1A, we noted that CD103 defines a small subset (median = 4.4%, range = 2.1–5.4%, n = 8) of thymocytes that were almost entirely CD3bright, CD4−, CD8+. This CD103+ subset has other phenotypic features of mature thymocytes—CD1−, CD10−, CD45RAbright, CD45ROdim, CD27bright—and was predominantly TCR-αβ+/γδ− (data not shown). When quantified within the CD3bright, CD8+, CD4− subset, CD103 was expressed by a median of 26% of these cells (Fig. 1A), suggesting that induction of this antigen might be a very late event for a large fraction of CD8 single positive thymocytes about to be exported to the periphery.
Figure 1.
Multiparameter flow cytometric analysis of CD103 expression among thymocytes and peripheral blood CD8+ T cells. (A) Thymocytes were examined for their correlated expression of CD3, CD4, CD8, and CD103. Five thousand events, gated on viable cells, are shown in each plot. CD103+ events are colored red (with the percentage of these cells given in the upper right corner of the left plot), whereas CD103− events are colored green. The boxed area in the right plot delineates mature CD3bright, CD8 single positive thymocytes with the percentage of CD103+ within this population indicated. (B and C) Mononuclear cells from umbilical cord blood (B) and adult blood (C) were examined for their correlated expression of (i) CD8, CD103, CD45RO, and CD27 and (ii) CD8, CD103, CD45RO, and CD62L. Five thousand (B) or 10,000 (C) events, gated on viable CD8+ small lymphocytes, are shown in each plot. In these analyses, the CD8 vs. CD103 profiles and the percent positive for the delineated subsets essentially were identical for the two staining combinations. In B, CD103+ events are colored red (with the percentage of these cells given in the upper right corner of the left plot), whereas CD103− events are colored green. Note that cord blood CD8+ T cells are almost entirely naïve in phenotype (CD27bright, CD62Lbright, CD45RO−). In C, CD103+ events falling within the naïve cell cluster (putative RTE) are enlarged and colored red, whereas all other CD103+ events (memory) are colored blue (with the respective percentage of these two populations given in the upper right corner of the left plot in the same color).
To assess the relative proliferative history of the CD103-defined CD8 single positive thymocyte subsets, we performed TREC analysis on sorted cell populations. The TREC measured in this study are stable DNA episomes generated during the process of TCR gene rearrangement in ≈70% of TCR-αβ+ T cells (15). After formation, TREC are not replicated and, therefore, are progressively diluted by cell expansion. Thus, differences in TREC/unit cells between two T cell populations reflect differences in the proliferative history of each population subsequent to TCR rearrangement. As shown in Table 1, the TREC content of both CD103+ and CD103−, CD8 single positive thymocyte populations was considerably higher than that of peripheral blood T cells in the same subjects (which, at the age of these individuals, would be predominantly naïve in phenotype). This finding indicates that the CD103+ subset in thymus indeed does represent a stage of thymopoiesis and not the subset of mature, peripheral memory T cells that have been reported to recirculate to the thymic medulla in animal models (21, 22). Interestingly, in three of the four samples tested, the CD103− thymocyte subset had TREC levels three to four times higher than that of the CD103+ subset, which is consistent with the latter subset having undergone one to two additional divisions since TCR rearrangement. These data are consistent with the CD103+ subset representing a more mature thymocyte subset, although they do not prove a direct linear relationship (see below).
Table 1.
Quantification of TREC within thymocyte subpopulations (CD103+ and CD103− CD8 single positive thymocytes) and paired peripheral blood samples
| Subject | Age | CD103−
|
CD103+
|
Blood
|
|---|---|---|---|---|
| TREC/1 × 105 CD8+ thymocytes | TREC/1 × 105 CD8+ thymocytes | TREC/1 × 105 T cells | ||
| 1 | 5 years | 103,642 | 37,000 | 4,674 |
| 2 | 4 months | 29,435 | 10,352 | 7,774 |
| 3 | 6 years | 62,009 | 15,527 | NA |
| 4 | 3 months | 41,397 | 40,463 | 3,866 |
NA, not available.
We next analyzed whether CD103 was expressed by naïve CD8+ T cells in the periphery. We initially focused on cord blood T cells, which are composed almost exclusively of naïve T cells and, because of active T lymphopoiesis in the second and third trimester of gestation (23), would be expected to contain a high frequency of RTE. Indeed, as shown in Fig. 1B, CD103 expression was found on a significant fraction of naïve CD8+ T cells in cord blood (median = 12.2%; range = 6.4–20%, n = 8). After birth, antigen exposure leads to accumulation of memory T cells, including the mucosa-associated, CD103+ memory subset. Examination of pediatric and adult blood (n = 62) by four-color flow cytometry indeed did confirm the appearance of this CD103+ memory subset, but often also demonstrated a discrete subset of CD103+, CD8+ T cells with a naïve—CD45RO−, CD45RA+, CD27bright, CD95dim, CD11adim, CD62L+—phenotype (Fig. 1C and data not shown). Although various combinations of these markers could define a similar CD8+, CD103+ naïve subset, the combination of CD45RO and CD62L (along with CD103 and CD8) proved the most efficient at separating the CD103+ naïve subset from the predominantly CD62L−, CD103+ memory subset and was used for the quantitative analyses presented below. In five adult subjects analyzed for CD103+ naïve CD8+ T cells in two to four separate blood draws, the coefficient of variations for these determinations was less than 7% (data not shown). The CD103+ naïve cells displayed light-scatter properties consistent with small lymphocytes and lacked expression of activation markers such as CD25, CD71, CD69, and HLA-DR. Moreover, consistent with the thymocyte data, these cells were predominantly TCR-αβ+/γδ−. CD103 was not expressed significantly by naïve CD4+ T cells in cord, pediatric, or adult blood (data not shown).
The CD103+, activation marker-negative, CD8+ T cell subset was not restricted to peripheral blood. As shown in Table 2, paired samples of peripheral blood and tonsil specimens from pediatric subjects revealed similar to up to 5-fold-higher frequencies of these cells in tonsil. Thus, like the overall population of naïve T cells, CD103-bearing naïve CD8+ cells appear to recirculate through, and localize within, secondary lymphoid tissues.
Table 2.
Frequency of CD103+ naïve and total naïve CD8+ T cells in matched peripheral blood and tonsil
| Subject | Age (years) | Blood
|
Tonsil
|
||
|---|---|---|---|---|---|
| % total naïve | % CD103+ naive | % total naïve | % CD103+ naive | ||
| 1 | 7 | 79 | 0.12 | 61 | 0.54 |
| 2 | 3 | 59 | 0.67 | 86 | 0.76 |
| 3 | 6 | 57 | 2.98 | 58 | 3.24 |
| 4 | 9 | 76 | 0.27 | 63 | 1.36 |
CD103+ Memory T Cells Contain Highly Reduced Numbers of TREC, Whereas CD103+ Naïve Cells Contain Higher Levels of TREC than the CD103− Naïve Subset.
Memory T cells have significantly lower TREC than naïve T cells in keeping with antigen-driven clonal expansion at the time of and after the naïve-to-memory cell transition (15, 24, 25). Within long-lived naïve T cell populations (26), normal homeostatic mechanisms appear to decrease TREC concentrations over time such that high and low TREC concentrations imply a naive cell population containing more recently produced T cells (e.g., RTE) and remotely produced T cells, respectively (15). To assess the relative proliferative history of the various naïve and memory CD8+ populations defined by CD103, we sorted young adult CD8+ T cells on the basis of CD103, CD62L, and CD45RO into four subsets, CD103+ naïve, CD103− naïve, CD103+ memory, and CD103− memory, using CD62L and CD45RO to define the naïve and memory subsets (see Fig. 1C). In all four subjects examined (Table 3), TREC levels were higher in the CD103+ naïve subset as compared with the naïve cells lacking CD103 (significant at P = 0.026), suggesting that this naïve subpopulation had undergone fewer cell divisions since TCR rearrangement than the other defined subpopulations. As predicted, both memory populations demonstrated lower levels of TREC than either naïve population, although the CD103+ memory cells consistently contained the lowest numbers of TREC, suggesting that these mucosa-associated memory cells have undergone, on average, a higher turnover than the other circulating memory cells. Taken together, these data have two important implications. First, they unequivocally demonstrate that the CD103+ T cells with a naïve phenotype are, in fact, naïve and are not memory cells that have reacquired a naïve phenotype or otherwise contaminate the naïve cell cluster. Second, they suggest that the CD103+ naïve subset, on average, has undergone less turnover than their CD103− counterparts, a finding suggestive of more recent production and, therefore, consistent with this population representing RTE. Although these data, along with the TREC analysis of the CD103+ and CD103−, CD8 single positive thymocytes described above, are consistent with a maturation sequence going from CD103− thymocytes → CD103+ thymocytes → CD103+ naïve cells → CD103− naïve cells, they do not prove a linear relationship because we cannot rule out the possibility that these two subsets exist in parallel.
Table 3.
Quantification of TREC within CD103-defined CD8+ T cell subsets
| Subject | % CD8+ peripheral blood T cells
|
TREC/1 × 105 CD8+ peripheral blood T cells
|
||||||
|---|---|---|---|---|---|---|---|---|
| Age | Total naïve | CD103+ naive | Total CD8 cells | CD103+ naïve | CD103− naïve | CD103− memory | CD103+ memory | |
| 1 | 23 | 52 | 0.71 | 7,860 | 14,878 | 10,948 | 2,880 | <10 |
| 2 | 25 | 70 | 0.95 | 22,611 | 38,766 | 28,466 | 14,074 | 10,126 |
| 3 | 30 | 59 | 0.78 | 8,415 | 14,305 | 8,658 | 5,237 | 2,440 |
| 4 | 31 | 72 | 0.38 | 12,715 | 22,056 | 9,822 | 188 | NA |
NA, not available.
Frequencies of CD103+ Naïve T Cells Decline in an Age-Dependent Fashion in Euthymic Subjects and Are Significantly Diminished After Thymectomy.
In mice, thymic output is highest during fetal and early postnatal life and declines with age—almost 20-fold from 3–5 weeks of age to 26 weeks (27). In humans, the lymphoepithelial cellularity of thymus is maximal in early childhood and, thereafter, is gradually, but progressively, replaced by adipose tissue (≈85% decreased by age 40; >95% by age 80) (5, 9). In keeping with this physical involution, regeneration of naïve T cells after chemotherapy has been demonstrated to be age-dependent, with children much more efficient at this regeneration than even young adults (7).
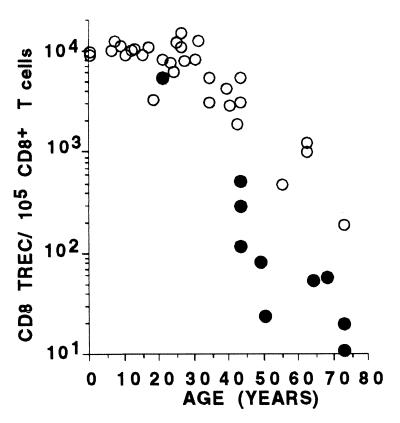
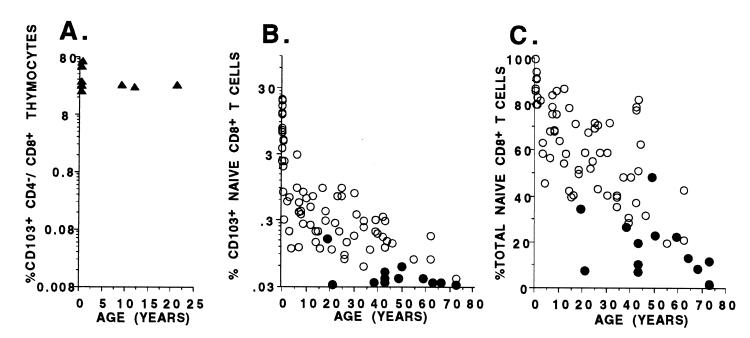
These data strongly support a decline in thymic function with age that should be reflected in the frequency of circulating RTE. Indeed, we have reported previously that the number of TREC within both the CD4+ and CD8+ T cell subsets shows an exponential decline of ≈1.5 logs from birth to 73 years of age (15). TREC analysis on the larger euthymic cohort studied here (Fig. 2) confirms this decline and, with additional samples from the pediatric age group, demonstrates a lag in the first two decades of life in which TREC decline little, if at all. As shown in Fig. 3B, analysis of the CD103+, CD8+ naïve subset in peripheral blood samples from these same individuals demonstrates that this subset also shows an age-dependent, ≈2-log decline in frequency but, importantly, with a different pattern than that observed for TREC. In contrast to the initial lag observed with TREC decline, the decline in the frequency of CD103+ naïve CD8+ T cells demonstrates an approximately 1-log decay in the first year of life followed by an additional 1-log decay from year 2 to the eighth decade. This kinetic difference in the decline of TREC and CD103+, CD8+ naïve T cell frequencies with age might be explained by the fact that TREC decline after a decrease in thymic output requires proliferative dilution of the TREC and, therefore, turnover within the naïve population. Although T cell turnover studies have not been performed in human infants, naïve cells in general are thought to turnover relatively slowly (26), such that TREC decline might lag months, perhaps years, behind the actual decline in thymic output. It should also be emphasized that the decline in both CD8+ T cell TREC and CD103+, CD8+ naïve T cells is much more dramatic than for the overall CD8+ naïve subset (shown by using a linear scale in Fig. 3C), indicating that changes in the former precede and likely predict changes in the latter, and not the reverse.
Figure 2.
CD8 TCR excision circles per 105 CD8+ T cells in peripheral blood (CD8 TREC) as a function of age in euthymic (○) and remotely (>3-year) athymic (●) subjects. Age 0 samples refer to umbilical cord blood.
Figure 3.
Quantification of CD103-defined subsets among mature CD8+ thymocytes and peripheral blood CD8+ naïve T cells. (A) Percentage (log scale) of CD8+/CD4−/CD3+ thymocytes expressing CD103 in thymi removed from subjects ranging in age from 6 weeks to 20 years. (B) Percentage (log scale) of CD8 T cells expressing a naïve phenotype (CD62L+, CD45RO−) and CD103 from the peripheral blood of euthymic from birth to 73 years of age (○) and from remotely (>3-year) athymic individuals from ages 20 to 73 years (●). (C) Percentage (linear scale) of total naïve T cells (CD27+, CD62L+, CD45RO−) with the CD8+ subset from peripheral blood from the same individuals as shown in B.
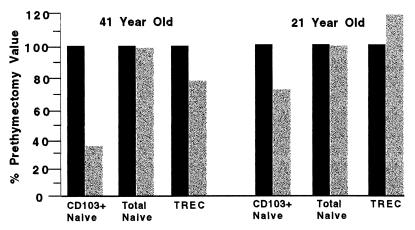
Consistent with our previous report (15), TREC levels within CD8+ T cells of an expanded cohort of remotely thymectomized subjects (>3 years, n = 13; age range, 19–73 years) were reduced significantly in comparison with a cohort of euthymic subjects covering the same age range (Fig. 2; P = 0.0004). Concurrently measured frequencies of CD103+ naïve CD8+ T cells were also diminished significantly in these remote athymics (Fig. 3B; P < 0.0001), supporting the dependence of this subset on continued thymic function. We also had the opportunity to study two subjects (ages 21 and 41 years) at the time of and 6 months after thymectomy for frequency of total naïve CD8+ T cells, CD103+, CD8+ naïve cells, and CD8+ TREC (Fig. 4). As shown in the figure, CD103+ naïve CD8+ T cells decreased 30% and 71%, respectively, in these two patients over the follow-up period, whereas TREC levels increased 21% and decreased less than 22%, respectively, and the total fraction of naïve CD8+ T cells declined <6%. Three additional subjects examined before and up to 7 months after thymectomy for TREC levels confirm lag times of at least this time period for TREC decline (B. Haynes, Duke University, personal communication). Taken together, these data, along with the data indicating a rapid decline in CD103+ naïve CD8+ cells in the first year of life, strongly suggest that CD103 expression on peripheral naïve cells is (or the peripheral CD103+ naïve cells themselves are) transient relative to the overall population of naïve cells and that the maintenance of this population in the periphery is dependent on, and likely proportional to, ongoing thymic output.
Figure 4.
Relative percentage of CD103+ naïve and total naïve T cells within the CD8+ subset and the relative number of CD8 TREC per 105 CD8+ T cells in two subjects at the time of (solid bars) and 6 months after (shaded bars) thymectomy.
Although the kinetics of decline in CD103 expression by naive T cells (or CD103+ naïve cells) in the peripheral immune system is not defined precisely, the apparent lag of TREC decline in early life and after thymectomy relative to quantification of the CD103+ naive CD8+ subset also suggests that the TREC assay may be relatively insensitive to declining thymic function, perhaps, as mentioned above, because of the requirement for cell turnover for TREC decline to become manifest. Measurement of phenotypically defined “RTE” therefore may provide more accurate data than TREC content in following declines in thymic output, particularly early in life when thymic output is high. On the other hand, late in life, normal frequencies of CD103+ cells within the CD8+ naïve subset are low (0.1–0.2%) relative to detection limits (≈0.03%), and thus measurement of TREC may be more informative in such subjects. Indeed, the observation that the magnitude of the differences between euthymic and remote athymic subjects is larger with TREC analysis than with phenotypic analysis ( compare Figs. 2 and 3b) is likely due to the small analytical range of the latter assay in older subjects.
Summary.
Unlike animal models in which thymocytes can be labeled directly and then followed as they emigrate to the periphery (13, 26), confirmation of RTE status among human T cells is difficult, and necessarily indirect. However, we describe here a novel naïve CD8+ T cell subset, defined by CD103 expression, that displays key predicted characteristics of RTE: (i) phenotypic linkage to the most mature thymic subset, (ii) relatively high TREC expression, and (iii) an age- and thymectomy-associated decline in frequency, suggesting that this subset is short-lived in the periphery without continued thymic output. This subset may not comprise all CD8+ RTE—we cannot rule out the possibility that the thymus exports both CD103+ and CD103−, CD8+ cells—but these characteristics suggest that the CD103+ naive subset is at least one major population of RTE.
The CD103+ naïve phenotype is particular to CD8+ T cells only, perhaps reflecting a late thymic-differentiation step unique to MHC class I-restricted cells. It should be emphasized that this limitation to CD8+ T cells does not compromise the usefulness of this phenotype in assessing thymic function. An operational thymus cannot selectively bias production of T cells toward the CD4+ or CD8+ subsets—the relative production of these two subsets is controlled by endogenous factors (genetic and/or related to selection mechanisms) and is not thought to be subject to extrinsic regulation (28, 29). Thus, the CD103+, CD8+ RTE production likely would be a consistent fraction of total RTE production and therefore would reflect overall thymic output. In keeping with this, the frequency of TREC within CD4+ and CD8+ T cell populations precisely correlates and provides almost identical information (ref. 15 and data not shown).
Although it will be necessary to accumulate more data in informative clinical situations to more precisely define the kinetic parameters of the CD103-defined RTE population (e.g., prospectively studied cohorts of thymectomized subjects or subjects receiving thymic transplants), the available data strongly suggest that quantification of CD103-defined RTE (as compared with TREC assessment) provides unique information on thymic function, particularly situations in which thymic output is declining more rapidly than TREC levels can be diluted by peripheral naïve cell turnover. Indeed, the data presented here suggest the possibility of a more precipitous decline in thymic function in the first year of life than suggested by either TREC or thymic morphometric analysis (9). Finally, it should be noted that the development of phenotypic criteria for RTE identification will allow further phenotypic characterization of RTE as well as the flow cytometric isolation of these cells for specific analysis of their functional capabilities and TCR repertoire.
Acknowledgments
We acknowledge the midwifery service of Parkland Memorial Hospital, the cardiothoracic surgery service and histotechnologists at Children's Medical Center of Dallas for assistance with pediatric sample acquisition, B. Ferguson-Darnell and J. Beckham for assistance with cell sorting, and B. J. Hill for assistance with TREC assays. We acknowledge Dr. B. Haynes and J. Thomasch at Duke University Medical Center, Durham, NC, and Dr. R. Barohn and L. Camperlingo, R.N., at the University of Texas Southwestern Medical Center, Dallas, for acquisition of samples from adult athymic individuals. This work was supported by National Institutes of Health Grant R21 AI44758. R.A.K. is an Elizabeth Glaser Scientist of the Pediatric AIDS Foundation.
Abbreviations
- TREC
T cell receptor rearrangement excision circle(s)
- RTE
recent thymic emigrants
- PE
phycoerythrin
- TCR
T cell receptor
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.070061597.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.070061597
References
- 1.Mackall C L, Gress R E. Immunol Rev. 1997;157:61–72. doi: 10.1111/j.1600-065x.1997.tb00974.x. [DOI] [PubMed] [Google Scholar]
- 2.Mackall C L, Hakim F T, Gress R E. Immunol Today. 1997;18:245–251. doi: 10.1016/s0167-5699(97)81664-7. [DOI] [PubMed] [Google Scholar]
- 3.Davis C M, McLaughlin T M, Watson T J, Buckley R H, Schiff S E, Hale L P, Haynes B F, Markert M L. J Clin Immunol. 1997;17:167–175. doi: 10.1023/a:1027382600143. [DOI] [PubMed] [Google Scholar]
- 4.George A J, Ritter M A. Immunol Today. 1996;17:267–272. doi: 10.1016/0167-5699(96)80543-3. [DOI] [PubMed] [Google Scholar]
- 5.Marusic M, Turkalj-Kljajic M, Petrovecki M, Uzarevic B, Rudolf M, Batinic D, Ugljen R, Anic D, Cavar Z, Jelic I, et al. Clin Exp Immunol. 1998;111:450–456. doi: 10.1046/j.1365-2249.1998.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCune J M, Loftus R, Schmidt D K, Carroll P, Webster D, Swor-Yim L B, Francis I R, Gross B H, Grant R M. J Clin Invest. 1998;101:2301–2308. doi: 10.1172/JCI2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mackall C L, Fleisher T A, Brown M R, Andrich M P, Chen C C, Feuerstein I M, Horowitz M E, Magrath I T, Shad A T, Steinberg S M, et al. N Engl J Med. 1995;332:143–149. doi: 10.1056/NEJM199501193320303. [DOI] [PubMed] [Google Scholar]
- 8.Berzins S P, Boyd R L, Miller J F. J Exp Med. 1998;187:1839–1848. doi: 10.1084/jem.187.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinmann G G, Klaus B, Muller-Hermelink H K. Scand J Immunol. 1985;22:563–575. doi: 10.1111/j.1365-3083.1985.tb01916.x. [DOI] [PubMed] [Google Scholar]
- 10.Collins R H, Jr, Sackler M, Pitcher C J, Waldrop S L, Klintmalm G B, Jenkins R, Picker L J. Exp Hematol. 1997;25:147–159. [PubMed] [Google Scholar]
- 11.Berzins S P, Davey G M, Randle-Barrett E S, Malin M A, Classon B J, Fraser S, Boyd R L. J Immunol. 1999;162:5119–5126. [PubMed] [Google Scholar]
- 12.Kong F, Chen C H, Cooper M D. Immunity. 1998;8:97–104. doi: 10.1016/s1074-7613(00)80462-8. [DOI] [PubMed] [Google Scholar]
- 13.Hosseinzadeh H, Goldschneider I. J Immunol. 1993;150:1670–1679. [PubMed] [Google Scholar]
- 14.Kern F, Khatamzas E, Surel I, Frommel C, Reinke P, Waldrop S L, Picker L J, Volk H. Eur J Immunol. 1999;29:2908–2915. doi: 10.1002/(SICI)1521-4141(199909)29:09<2908::AID-IMMU2908>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Douek D C, McFarland R D, Keiser P H, Gage E A, Massey J M, Haynes B F, Polis M A, Haase A T, Feinberg M B, Sullivan J L, et al. Nature (London) 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 16.Cerf-Bensussan N, Jarry A, Brousse N, Lisowska-Grospierre B, Guy-Grand D, Griscelli C. Eur J Immunol. 1987;17:1279–1285. doi: 10.1002/eji.1830170910. [DOI] [PubMed] [Google Scholar]
- 17.Picker L J, Martin R J, Trumble A, Newman L S, Collins P A, Bergstresser P R, Leung D Y. Eur J Immunol. 1994;24:1269–1277. doi: 10.1002/eji.1830240605. [DOI] [PubMed] [Google Scholar]
- 18.Cepek K L, Parker C M, Madara J L, Brenner M B. J Immunol. 1993;150:3459–3470. [PubMed] [Google Scholar]
- 19.Picker L J, Terstappen L W, Rott L S, Streeter P R, Stein H, Butcher E C. J Immunol. 1990;145:3247–3255. [PubMed] [Google Scholar]
- 20.Terstappen L W, Huang S, Picker L J. Blood. 1992;79:666–677. [PubMed] [Google Scholar]
- 21.Agus D B, Surh C D, Sprent J. J Exp Med. 1991;173:1039–1046. doi: 10.1084/jem.173.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michie S A, Kirkpatrick E A, Rouse R V. J Exp Med. 1988;168:1929–1934. doi: 10.1084/jem.168.5.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haynes B F, Heinly C S. J Exp Med. 1995;181:1445–1458. doi: 10.1084/jem.181.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Picker L J, Treer J R, Ferguson-Darnell B, Collins P A, Buck D, Terstappen L W. J Immunol. 1993;150:1105–1121. [PubMed] [Google Scholar]
- 25.Poulin J-F, Viswanathan M N, Harris J M, Komanduri K V, Weider E, Ringuette N, Jenkins M, McCune J M, Sekaly R-P. J Exp Med. 1999;190:479–486. doi: 10.1084/jem.190.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLean A R, Michie C A. Proc Natl Acad Sci USA. 1995;92:3707–3711. doi: 10.1073/pnas.92.9.3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scollay R G, Butcher E C, Weissman I L. Eur J Immunol. 1980;10:210–218. doi: 10.1002/eji.1830100310. [DOI] [PubMed] [Google Scholar]
- 28.van Meerwijk J P, Bianchi T, Marguerat S, MacDonald H R. J Immunol. 1998;160:3649–3654. [PubMed] [Google Scholar]
- 29.Gabor M J, Scollay R, Godfrey D I. Eur J Immunol. 1997;27:2986–2993. doi: 10.1002/eji.1830271135. [DOI] [PubMed] [Google Scholar]