Abstract
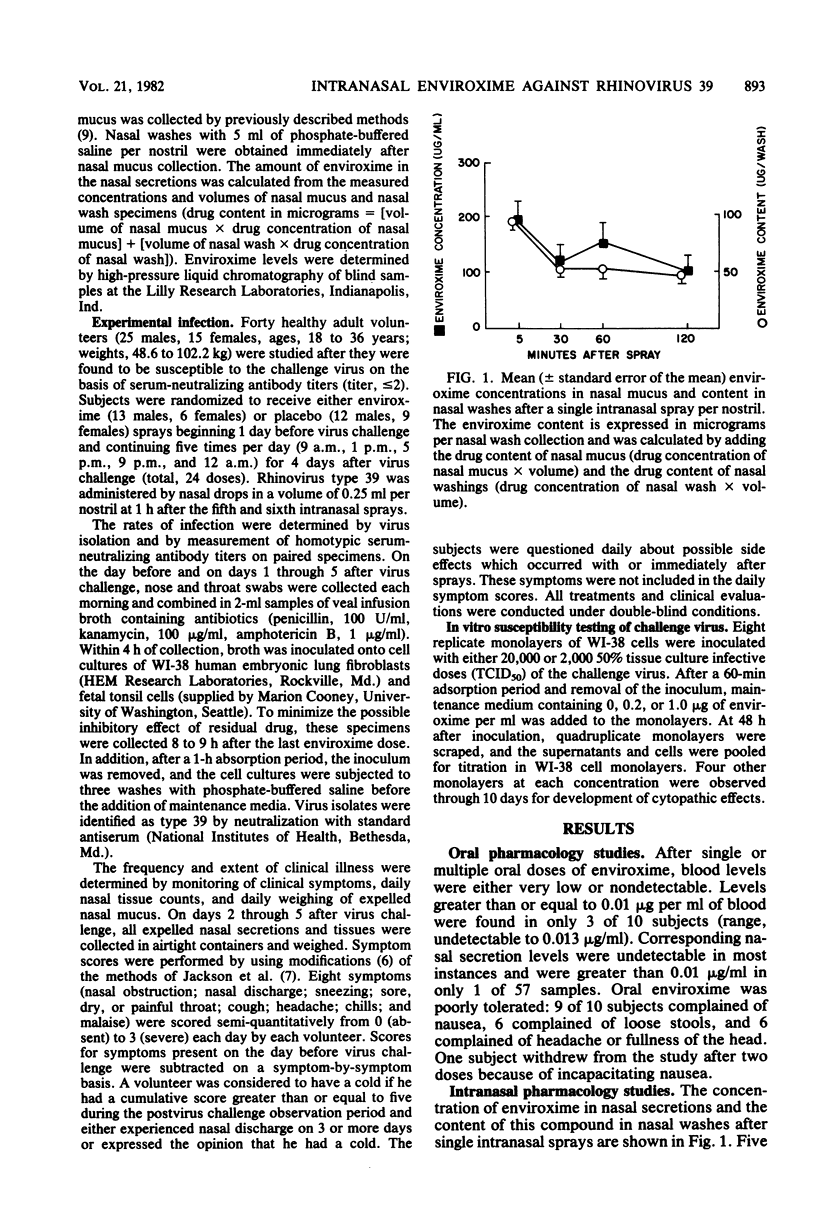
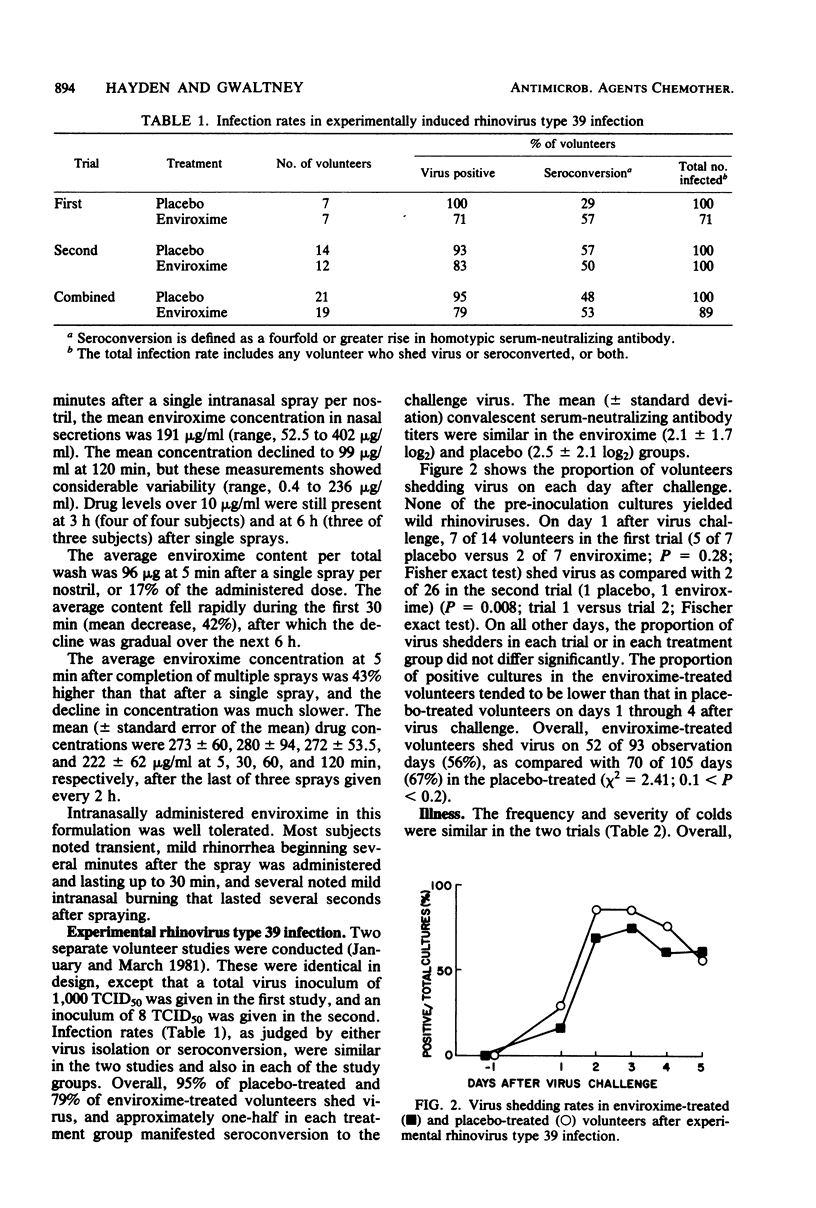
Intranasal administration of enviroxime by aerosol spray was associated with drug levels in nasal secretions that 1 h later averaged 750-fold higher than those inhibitory for rhinoviruses in vitro (0.2 microgram/ml). However, administration of intranasal enviroxime (one spray per nostril, five times per day) to susceptible volunteers, beginning 1 day before and continuing for 4 days after virus exposure, did not significantly reduce infection or illness due to experimentally induced rhinovirus type 39 infection. The combined results of two separate trials yielded an infection rate of 100% for 21 placebo-treated and 89% for 19 enviroxime-treated subjects. Approximately one-half of the volunteers in each group had seroconversion to the challenge virus. Overall, 52% of the placebo-treated and 53% of the enviroxime-treated subjects developed colds. No significant differences in symptom scores, nasal mucus weights, or numbers of nasal tissues used were observed between the two groups. Two-thirds of the enviroxime-treated volunteers noted intranasal irritation immediately after sprays, as compared with only one-third of the placebo-treated subjects.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen I., Camner P., Jensen P. L., Philipson K., Proctor D. F. A comparison of nasal and tracheobronchial clearance. Arch Environ Health. 1974 Nov;29(5):290–293. doi: 10.1080/00039896.1974.10666589. [DOI] [PubMed] [Google Scholar]
- Andersen I., Lundqvist G. R., Proctor D. F. Human nasal mucosal function in a controlled climate. Arch Environ Health. 1971 Dec;23(6):408–420. doi: 10.1080/00039896.1971.10666029. [DOI] [PubMed] [Google Scholar]
- Aoki F. Y., Crowley J. C. Distribution and removal of human serum albumin-technetium 99m instilled intranasally. Br J Clin Pharmacol. 1976 Oct;3(5):869–878. doi: 10.1111/j.1365-2125.1976.tb00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLong D. C., Reed S. E. Inhibition of rhinovirus replication in in organ culture by a potential antiviral drug. J Infect Dis. 1980 Jan;141(1):87–91. doi: 10.1093/infdis/141.1.87. [DOI] [PubMed] [Google Scholar]
- Gwaltney J. M., Jr, Moskalski P. B., Hendley J. O. Interruption of experimental rhinovirus transmission. J Infect Dis. 1980 Dec;142(6):811–815. doi: 10.1093/infdis/142.6.811. [DOI] [PubMed] [Google Scholar]
- JACKSON G. G., DOWLING H. F., SPIESMAN I. G., BOAND A. V. Transmission of the common cold to volunteers under controlled conditions. I. The common cold as a clinical entity. AMA Arch Intern Med. 1958 Feb;101(2):267–278. doi: 10.1001/archinte.1958.00260140099015. [DOI] [PubMed] [Google Scholar]
- Phillpotts R. J., Jones R. W., Delong D. C., Reed S. E., Wallace J., Tyrrell D. A. The activity of enviroxime against rhinovirus infection in man. Lancet. 1981 Jun 20;1(8234):1342–1344. doi: 10.1016/s0140-6736(81)92520-4. [DOI] [PubMed] [Google Scholar]
- Powell K. R., Shorr R., Cherry J. D., Hendley J. O. Improved method for collection of nasal mucus. J Infect Dis. 1977 Jul;136(1):109–111. doi: 10.1093/infdis/136.1.109. [DOI] [PubMed] [Google Scholar]



