SYNOPSIS
Objectives
Exposure to varicella zoster virus through close contact with people with chickenpox was suggested to boost specific immunity, reducing the risk of herpes zoster (HZ). Since the introduction of the varicella immunization program in the U.S. in 1995, varicella morbidity has decreased substantially. This article examines incidence and risk factors associated with self-reported HZ disease and whether exposure to chickenpox within the previous decade reduces the risk of shingles in this age group.
Methods
In 2004, a national random-digit dial telephone survey was used to obtain information on self-reported HZ disease, demographic characteristics, and exposure to children with chickenpox in the past decade. National estimates of the incidence of shingles disease were calculated.
Results
Incidence rate of self-reported HZ was 19 per 1,000 population per year. White individuals were 3.5 times more likely to report shingles than Hispanic individuals (p<0.01). Previous exposure to chickenpox did not protect against HZ disease in this population. Seven percent of adults ≥65 years of age reported exposure to children with chickenpox in the past decade.
Conclusions
Incidence of HZ among individuals ≥65 years of age in the U.S. may be higher than previously described in the literature, with whites being at higher risk for the disease. Currently, the potential contribution of exposure to chickenpox as a mechanism for maintaining cell-mediated immunity against HZ may be limited to a small percentage of the population. Vaccination against HZ may represent the best means of decreasing this disease burden.
Herpes zoster (HZ) disease, also known as shingles, presents as a painful cutaneous eruption affecting one or more dermatomes. The disease results from reactivation of varicella-zoster virus (VZV) dormant in dorsal-root ganglia since primary infection, usually in childhood.1 Virus reactivation may be associated with a decline in cell-mediated immunity, usually due to age or to immunosuppressive illness or treatment.2 The incidence of shingles increases with age, as do complications of the disease. The most debilitating is post-herpetic neuralgia, a persistent neuropathic pain syndrome. Currently in the United States, almost all adults ≥40 years of age show serological evidence of varicella infection and are, therefore, at risk of HZ disease.3
Re-exposure to VZV through close contact with people with chickenpox or shingles has been suggested to boost specific immunity, reducing the risk of VZV reactivation.2,4,5 Since the introduction of the varicella immunization program in 1995 in the U.S., varicella morbidity has been reduced by 70%–90%.6,7 The consequent reduced boosting of immunity may result in reactivation of VZV, leading to higher incidence of shingles among older adults. A vaccine to protect adults ≥60 years old from HZ disease and its complications has been licensed in the U.S. and is now recommended for use among adults ≥60 years old.8,9
National assessment of HZ disease incidence is essential to monitor the potential benefit of a HZ immunization program among older adults in the U.S. We looked at the U.S. population at greatest risk for zoster through a national survey to estimate HZ incidence in those ≥65 years of age. We examined risk factors associated with self-reported HZ disease and whether exposure to chickenpox within the previous decade reduces the risk of shingles in this age group.
METHODS
The National Adult Immunization Survey (NAIS) was designed to use the same sampling frame as the National Immunization Survey (NIS). The NIS uses list-assisted random-digit dialing and a large number of screening calls to identify households that contain an age-eligible child to provide estimates of immunization coverage for 19- to 35-month-olds.10 NAIS data collection from February 2, 2004, through May 27, 2004, included questions on influenza and pneumonia vaccinations, colorectal screening, and HZ disease. The following questions pertaining to HZ were asked during the telephone interview: (1) During the past ten years have you cared for or had other close contact with children while the children had chickenpox? (2) Have you had shingles in the past 12 months? Those responding affirmatively to the second question were asked, (3) Was this your first episode of shingles? Data were also collected on basic demographic characteristics and self-rated health status. Trained interviewers conducted the interviews in English or Spanish by using a computer-assisted telephone interview system. Proxy interviews were conducted for respondents unable to complete the interview due to illness, injury, or a language barrier.
The analysis included interviews from households with adults aged ≥65 years who were eligible for participation in the NAIS, regardless of whether an eligible child was identified for the NIS. All estimates were weighted to produce national estimates. Weighting adjustments included standard, large random-digit dialing survey adjustments to account for the exclusion of households without telephones, for households having multiple telephone lines, for nonresponse, and to adjust to known population control totals.
All analyses were conducted using SAS, release 9.111 and SUDAAN, release 9.0.0.12 Wald chi-square tests were used to test for associations between having a first episode of shingles in the previous 12 months and demographic characteristics, as well as visit to a doctor, health status, and exposure to children with chickenpox; post-hoc tests were also performed. We compared the responses of those interviewed in Spanish with those interviewed in English as well as those of proxy with non-proxy respondents. Percentages are reported with 95% confidence intervals. A two-sided significance level of 0.05 was adopted for all statistical tests.
RESULTS
The response rate for the 2004 NAIS was 51.4%, with a total of 3,675 interviews completed among adults aged ≥65 years. The 240 proxy interview respondents were older than non-proxy respondents (68.3% ≥75 years of age vs. 44.0%; p< 0.001), were more likely to report fair or poor health status (51.4% vs. 21.8%; p< 0.001), and were less likely to report the first episode of HZ in the past 12 months (0.6% vs. 1.9%; p=0.02). Thus, to avoid potential (recall) bias we excluded proxy interviews from the analysis.
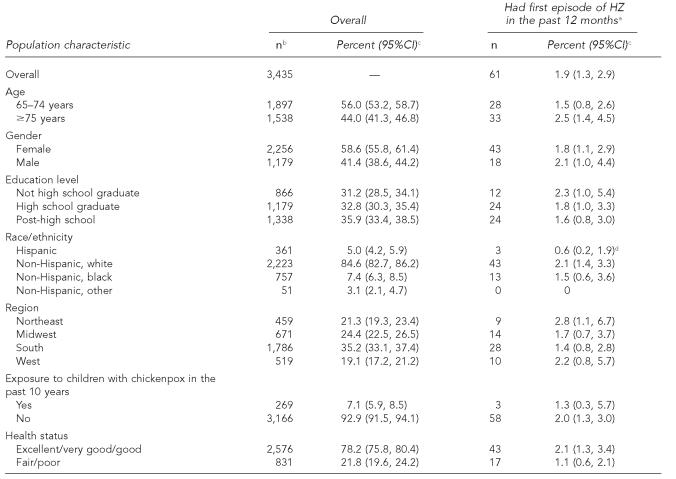
Overall, 61 out of 3,435 respondents reported having a first episode of HZ in the past 12 months (weighted percent of 1.9%), translating to an incidence of 19 episodes/1,000 individuals/year. Population characteristics are summarized in the Table.
No statistically significant differences in reported first episode of HZ by age, gender, education level, region of residency, exposure to children with chickenpox in the last decade, or health status were found. Reported first episode of HZ disease did vary by race/ethnicity (p=0.04). The incidence of HZ among whites was 3.5 times that of the disease incidence among Hispanic respondents (2.1% vs. 0.6% respectively; p=0.009) while no statistically significant difference was found between Hispanic and black respondents or black and white respondents. Seventy-eight percent of people interviewed (2,576) self-rated their health status as being good to excellent. There was no statistically significant difference in incidence of HZ among those reporting they were in excellent/very good/good health compared with those in fair/poor health (Table). Of 361 Hispanic respondents, 49.1% were interviewed in Spanish. There were no statistically significant differences between Hispanics interviewed in Spanish and those interviewed in English in reported incidence of HZ, exposure to a child with chickenpox in the past 10 years, gender, region, or health status.
Table.
Herpes zoster incidence among adults ≥65 years of age by population characteristics, National Adult Immunization Survey, United States, 2004
Respondents reporting having HZ in the past 12 months but it was not their first episode are included in the denominator along with those not having HZ in the past 12 months.
Unweighted sample size; characteristic-specific total may be lower due to missing values. Proxy respondents are excluded.
Weighted percent (95% confidence interval)
p< 0.05 by Wald chi-square test of association; non-Hispanic other excluded from this analysis.
CI = confidence interval
DISCUSSION
In a national survey of the U.S. population aged ≥65 years, we found that the annual incidence of self-reported HZ was 19/1,000 population. The incidence of HZ disease among Hispanics was statistically significantly lower than the rate estimated among non-Hispanic-whites. We did not find that previous exposure to children with chickenpox in the past decade was protective against HZ in this age group.
Age is a major risk factor for HZ and almost half of all cases of HZ disease occur in people >60 years of age.2,8,12–18 The incidence of HZ shown in this study is higher than estimates previously reported among individuals ≥65 years old in the U.S. (range: 4–13 per 1,000 population).8,13–18 Although true differences between populations may influence rates of HZ disease, differences in rates observed across studies are more likely to be dependent on study methods, including definition of the study population, case ascertainment, and selection of medical specialties or health care systems capturing cases. Most published data have relied on automated administrative databases that record medical information, which are prone to coding errors and may lead to misclassification of HZ cases.16–18 Moreover, studies derived from retrospective medical records abstraction could underestimate HZ incidence by not including individuals who do not seek medical attention for a mild episode of shingles. The higher HZ incidence estimated in our study, however, is unlikely to result from the substantial reduction in varicella disease burden after introduction of the vaccination program. The literature has documented an increase in age-specific HZ disease incidence even before the introduction of the varicella vaccine.14,16–18
A United Kingdom study has also described a protective effect associated with ethnicity among people born in countries with evidence of late-onset of varicella (e.g., countries in Central America).19 The same study showed black adults to have lower risk for HZ than white adults. In North Carolina, a study among older adults estimated black adults to be four times less likely than white adults to experience HZ (p< 0.0001).13 In our study, although black adults had lower HZ incidence than white adults, this association was not statistically significant. Hypothesized reasons for the lower risk of zoster among different racial/ethnic groups may include true differences in the immune response to VZV, or in lifetime occurrence of varicella.13,19,20 Fifty percent of Hispanics were interviewed in Spanish, which could indicate that they immigrated to the U.S. later in life, and therefore may have acquired infection at adolescence or adulthood in their country of origin. There were no differences in HZ incidence between Hispanic individuals interviewed in Spanish and those interviewed in English, suggesting that language was not the reason for differences between groups.
A number of studies have either directly or indirectly examined the role of contacts with individuals infected with VZV in both immunocompromised and healthy populations, suggesting that multiple contacts with chickenpox cases appear to lower the risk of zoster during adulthood.2,4,5,19 We did not find such an association, either because the protective effect of exposures occurred >10 years previously or because there may not be a true association between exposure to chickenpox and occurrence of HZ in this age group. Interestingly, only 7% of interviewees reported exposure to children with chickenpox in the past decade, which is likely to reflect the substantial reduction in varicella disease morbidity in the U.S. since introduction of the vaccine in 1995. Rates of exposure to children with chickenpox may have been higher during the pre-vaccine era.
Our study has some limitations. First, NAIS is a telephone survey; although statistical adjustments compensate for nonresponse, some bias may remain.21,22 Second, a larger sample would have yielded narrower confidence intervals, which could allow for detection of statistical significance on the effect of exposure to chickenpox and various demographic characteristics on zoster incidence. Third, we did not collect information on the severity of disease presentation, which could have facilitated estimates of the contribution of mild HZ cases to disease incidence rates. Finally, we could not validate the accuracy of self-reporting in our study. One study found self-report of shingles among older adults to be accurate, with a positive predictive value of 96.7% and no false negative reports.23 Misclassification of HZ disease due to other rashes could have played a role in disease incidence. In the zoster vaccine trial, 24% of suspected HZ cases were not confirmed as such by laboratory testing.8 A similar degree of misclassification would reduce our estimate to an annual incidence of 15/1,000 population..
In conclusion, HZ causes considerable disease among adults ≥65 years of age in the U.S., and the disease incidence may be higher than previously described in the literature, with white adults being at higher risk for the disease. It is likely that, due to the successful implementation of the varicella vaccination program in the U.S., only a small percentage of the elderly population are currently exposed to chickenpox, which would limit any potential contribution of exposure to chickenpox as a mechanism for maintaining cell-mediated immunity against HZ. Vaccination against HZ represents the best means of decreasing disease burden from HZ in this population. National surveys like the NAIS may be a valuable tool to monitor national HZ disease incidence and the uptake of new vaccines designed for older individuals in the U.S.
Acknowledgments
The authors are grateful to Dr. Marc-Alain Widdowson and Dr. Jane Seward for their useful comments on earlier versions of this manuscript.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention, U.S. Department of Health and Human Services.
REFERENCES
- 1.Weller TH. Varicella and herpes zoster. Changing concepts of the natural history, control, and importance of a not-so-benign virus. N Engl J Med. 1983;309:1362–8. doi: 10.1056/NEJM198312083092306. [DOI] [PubMed] [Google Scholar]
- 2.Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58:9–20. doi: 10.1177/003591576505800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kilgore PE, Kruszon-Moran D, Seward JF, Jumaan A, Van Loon FP, Forghani B, et al. Varicella in Americans from NHANES III: implications for control through routine immunization. J Med Virol. 2003;70(Suppl 1):S111–8. doi: 10.1002/jmv.10364. [DOI] [PubMed] [Google Scholar]
- 4.Gershon AA, LaRussa P, Steinberg S, Mervish N, Lo SH, Meier P. The protective effect of immunologic boosting against zoster: an analysis in leukemic children who were vaccinated against chickenpox. J Infect Dis. 1996;173:450–3. doi: 10.1093/infdis/173.2.450. [DOI] [PubMed] [Google Scholar]
- 5.Thomas SL, Wheeler JG, Hall AJ. Contacts with varicella or with children and protection against herpes zoster in adults: a case-control study. Lancet. 2002;360:678–82. doi: 10.1016/S0140-6736(02)09837-9. [DOI] [PubMed] [Google Scholar]
- 6.Decline in annual incidence of varicella—selected states, 1990–2001. MMWR Morb Mortal Wkly Rep. 2003;52(37):884–5. [PubMed] [Google Scholar]
- 7.Seward JF, Watson BM, Peterson CL, Mascola L, Pelosi JW, Zhang JX, et al. Varicella disease after introduction of varicella vaccine in the United States, 1995–2000. JAMA. 2002;287:606–11. doi: 10.1001/jama.287.5.606. [DOI] [PubMed] [Google Scholar]
- 8.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–84. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (US) National Immunization Program. Shingles (herpes zoster) vaccine. [cited 2006 Dec 21]. Available from: URL: http://cdc.gov/nip/vaccine/zoster/default.htm.
- 10.Zell ER, Ezzati-Rice T, Battaglia MP. National Immunization Survey: the methodology of a vaccination surveillance system. Public Health Rep. 2000;115:65–77. doi: 10.1093/phr/115.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.SAS Institute. SAS: release 9.1. Cary (NC): SAS Institute; 2002. [Google Scholar]
- 12.Research Triangle Institute. SUDAAN: release 9.0.0. Research Triangle Park (NC): 2004. [Google Scholar]
- 13.Schmader K, George LK, Burchett BM, Pieper CF, Hamilton JD. Racial differences in the occurrence of herpes zoster. J Infect Dis. 1995;171:701–4. doi: 10.1093/infdis/171.3.701. [DOI] [PubMed] [Google Scholar]
- 14.Ragozzino MW, Melton LJ, 3rd, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore) 1982;61:310–6. doi: 10.1097/00005792-198209000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Richards P. Shingles in one family practice. Arch Fam Med. 1996;5:42–6. doi: 10.1001/archfami.5.1.42. [DOI] [PubMed] [Google Scholar]
- 16.Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med. 1995;155:1605–9. [PubMed] [Google Scholar]
- 17.Mullooly JP, Riedlinger K, Chun C, Weinmann S, Houston H. Incidence of herpes zoster, 1997–2002. Epidemiol Infect. 2005;133:245–53. doi: 10.1017/s095026880400281x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jumaan AO, Yu O, Jackson LA, Bohlke K, Galil K, Seward JF. Incidence of herpes zoster, before and after varicella-vaccination-associated decreases in the incidence of varicella, 1992–2002. J Infect Dis. 2005;191:2002–7. doi: 10.1086/430325. [DOI] [PubMed] [Google Scholar]
- 19.Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis. 2004;4:26–33. doi: 10.1016/s1473-3099(03)00857-0. [DOI] [PubMed] [Google Scholar]
- 20.Dworkin RH. Racial differences in herpes zoster and age at onset of varicella. J Infect Dis. 1996;174:239–41. doi: 10.1093/infdis/174.1.239. [DOI] [PubMed] [Google Scholar]
- 21.Frankel MR, Srinath KP, Hoaglin DC, Battaglia MP, Smith PJ, Wright RA, Khare M. Adjustments for non-telephone bias in random-digit-dialing surveys. Stat Med. 2003;22:1611–26. doi: 10.1002/sim.1515. [DOI] [PubMed] [Google Scholar]
- 22.Smith PJ, Battaglia MP, Huggins VJ, Hoaglin DC, Roden A, Khare M, et al. Overview of the sampling design and statistical methods used in the National Immunization Survey. Am J Prev Med. 2001;20(4 Suppl):17–24. doi: 10.1016/s0749-3797(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 23.Schmader K, George LK, Newton R, Hamilton JD. The accuracy of self-report of herpes zoster. J Clin Epidemiol. 1994;47:1271–6. doi: 10.1016/0895-4356(94)90132-5. [DOI] [PubMed] [Google Scholar]