SYNOPSIS
Objective
The purpose of this study was to provide a national estimate of the number of healthcare-associated infections (HAI) and deaths in United States hospitals.
Methods
No single source of nationally representative data on HAIs is currently available. The authors used a multi-step approach and three data sources. The main source of data was the National Nosocomial Infections Surveillance (NNIS) system, data from 1990–2002, conducted by the Centers for Disease Control and Prevention. Data from the National Hospital Discharge Survey (for 2002) and the American Hospital Association Survey (for 2000) were used to supplement NNIS data. The percentage of patients with an HAI whose death was determined to be caused or associated with the HAI from NNIS data was used to estimate the number of deaths.
Results
In 2002, the estimated number of HAIs in U.S. hospitals, adjusted to include federal facilities, was approximately 1.7 million: 33,269 HAIs among newborns in high-risk nurseries, 19,059 among newborns in well-baby nurseries, 417,946 among adults and children in ICUs, and 1,266,851 among adults and children outside of ICUs. The estimated deaths associated with HAIs in U.S. hospitals were 98,987: of these, 35,967 were for pneumonia, 30,665 for bloodstream infections, 13,088 for urinary tract infections, 8,205 for surgical site infections, and 11,062 for infections of other sites.
Conclusion
HAIs in hospitals are a significant cause of morbidity and mortality in the United States. The method described for estimating the number of HAIs makes the best use of existing data at the national level.
Healthcare-associated infections (HAIs) are a common cause of morbidity and mortality in the United States and are among the most common adverse events in healthcare.1 Recently, new emphasis on HAIs as a patient safety and public health problem has underscored the need for systematic HAI surveillance as part of a broad-based prevention and control strategy.2–4
As of March 2006, seven states have implemented mandatory reporting of HAIs by hospitals and other states are considering similar legislative mandates.5 At the national level, the Centers for Disease Control and Prevention's (CDC) National Nosocomial Infections Surveillance (NNIS) system, which was started in 1970 with 62 participating hospitals, expanded to over 300 acute care hospitals in 42 states by 2000. The NNIS system provided data on HAIs and was voluntary and confidential;6 benchmark rates were published for inter-hospital comparison.7 CDC's successor system to NNIS, the National Healthcare Safety Network (NHSN), is designed to facilitate participation by a larger number of hospitals and expand enrollment to other types of healthcare settings.8
Ideally, one source of HAI information would meet different needs for surveillance data. In practice, however, resource requirements and evolving needs and priorities have prompted more selective goal setting and surveillance efforts. The NNIS system reflects these changes. Comprehensive or “hospital-wide” surveillance was performed by at least half of NNIS hospitals through 1991. This enabled national estimates of all HAIs in hospitals to be made directly from NNIS data. However, interest in hospital-wide surveillance waned as more efficient, targeted surveillance emerged and new emphasis was placed on surveillance of high-risk, high-volume areas of hospital practice.9 NNIS added more specific components (e.g., intensive care units) and discontinued hospital-wide surveillance in 1998. While more targeted surveillance yielded many benefits for HAI prevention and control, the cessation of hospital-wide surveillance has complicated the task of estimating all HAIs in hospitals. Still, with the aid of historical NNIS data and data from other sources, we developed a multi-step approach to estimate the magnitude of HAIs and associated deaths in U.S. hospitals in 2002. The purposes of this article are to present national estimates of HAIs and to discuss the challenges and opportunities for improving national public health surveillance of HAIs.
METHODS
Definitions
An HAI was defined as a localized or systemic condition that (1) results from an adverse reaction to the presence of an infectious agent(s) or its toxin(s), (2) that occurs during a hospital admission, (3) for which there is no evidence the infection was present or incubating at admission, and (4) meets body site-specific criteria.6 Patient-days were defined as the total number of days that patients were in the hospital.
Data sources
The National Nosocomial Infections Surveillance (NNIS) system.
The NNIS system was a voluntary network of U.S. hospitals collaborating with CDC to monitor HAIs from 1970–2005. Hospitals participating in NNIS provided acute care, had 100 or more beds, and a minimum of one full-time equivalent infection control practitioner for the first 100 occupied beds. Detailed methods of the NNIS system are described elsewhere.6 Hospitals participating in NNIS were not selected randomly and might not represent all acute care hospitals in the United States. We used data from 283 participating NNIS hospitals in 2002; these contributed 2.3 million patient-days of information from 678 intensive care units (ICUs).
National Hospital Discharge Survey (NHDS).
The NHDS is an annual CDC probability survey of characteristics of inpatients discharged from nonfederal short-stay hospitals in the United States. Methods for NHDS have been described extensively.10,11 Briefly, survey sampling is conducted in three stages. First, the geographic area (e.g., counties) is sampled; second, hospitals are selected within those geographic areas; and third, patient discharges are sampled within selected hospitals. Patient records are reviewed to collect information on characteristics of the patient (e.g., age, gender), procedures performed, diagnoses, and dates of admission and discharge. Patient-days are calculated by counting days from admission to discharge and summing the days for all patients during the year. In 2002, 445 hospitals participated in the survey and provided data on a weighted 37.5 million discharges. Of these discharges, 90% (33,726,611) were among adults and children and 10% (3,789,310) were among newborns.
American Hospital Association (AHA) Survey.
The AHA conducts an annual survey of hospitals and their characteristics (e.g., number of beds, discharges, services, occupancy).12 Participation in the AHA survey does not require membership in the AHA. Admissions and patient-day data are available for federal and nonfederal hospitals. In 2000, there were 5,800 hospitals in the AHA survey representing 34.9 million admissions and 236.4 million patient-days. Federal hospitals accounted for 13.2 million (5.6%) patient-days.
Estimate of HAIs in hospitals
To estimate infections, we created four subpopulations (newborns in high-risk nurseries, newborns in well-baby nurseries, adults and children in ICUs, and adults and children outside of ICUs) and grouped HAIs into five major sites (surgical site infections, bloodstream infections, pneumonia, urinary tract infections, and other sites combined).
Step 1. Estimate of HAI among newborns.
We stratified patient-days from NHDS among newborns into days spent in the high-risk nursery or the well-baby nursery according to the distribution of patient-days for these two subpopulations in NNIS hospital-wide surveillance. Then we calculated infection rates by major site of infection for the high-risk nursery using 2002 NNIS data from the high-risk nursery component, and in the well-baby nursery by major site of infection from 1990–1995 using NNIS hospital-wide surveillance data. Total infections were estimated by multiplying the number of NHDS patient-days by the corresponding NNIS infection rates for the high-risk nursery and well-baby nursery (number of patient-days × infection rate/patient-days = number of infections).
Step 2. Estimate of HAI among adults and children in ICUs.
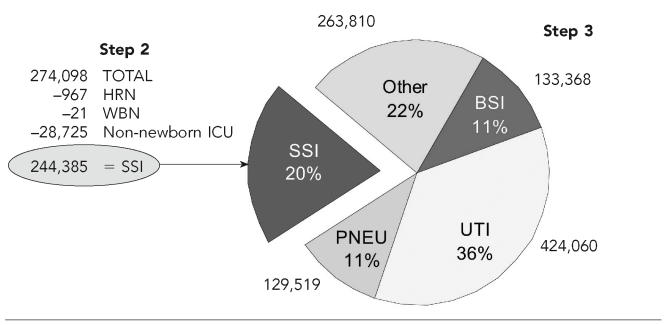
From NHDS, we obtained total patient-days nationally for adults and children and stratified these into ICU days and days outside of the ICU. For ICU patients, we calculated HAI rates by using NNIS ICU data for each major site of infection (number of patient-days × infection rate/patient-days = number of infections) (see Figure).
Figure.
Calculation of estimates of health care-associated infections in U.S. hospitals among adults and children outside of intensive care units, 2002
NOTES: From the total number of surgical site infections (SSI) obtained from the National Hospital Discharge Dataset and the National Nosocomial Infections Surveillance (NNIS) system, we subtracted the number of SSI among newborns and adults and children in intensive care units. The remaining SSI were among adults and children outside of intensive care units. From hospital-wide surveillance in NNIS, we had the distribution of infections by major site and calculated the corresponding number of infections for pneumonias (PNEU), urinary tract infections (UTI), bloodstream infections (BSI), and other sites.
HRN = high-risk newborns
WBN = well-baby nurseries
ICU = intensive care unit
SSI = surgical site infections
BSI = bloodstream infections
UTI = urinary tract infections
PNEU = pneumonia
Step 3. Estimate of HAIs among hospitalized adults and children outside of ICUs.
NNIS HAI rates outside of ICUs were likely underestimated from 1990 to 1995; therefore, we used a different method from those above to estimate HAIs among adults and children outside of ICUs. Specifically, we estimated the number of infections from a single major site and then used the distribution percentage for that site from NNIS hospital-wide surveillance to extrapolate to the total number of HAIs in adults and children outside of ICUs. We chose the surgical site for our calculations because the number of surgical procedures is available for the U.S. population in the NHDS. We multiplied the number of surgical procedures in the NHDS by the surgical site infection rate from NNIS 2002 surveillance. From this estimate, we subtracted surgical site infections among newborns and among adults and children in ICUs, which yielded the total number of such infections among hospitalized adults and children outside of ICUs, i.e., 244,385. Surgical site infections accounted for 20% of all HAIs in NNIS hospital-wide surveillance; thus, we used that percentage to estimate the number of infections for other body sites (see Figure).
Step 4. Adjustment to include federal hospitals.
Because NHDS does not include federal hospitals, we used AHA data to adjust the HAI estimate to reflect the burden of these infections in federal hospitals. To do this, we calculated an adjustment factor by which we multiplied the number of non-newborn patient-days and non-newborn infection estimates. The number of newborn patient-days in federal hospitals was minimal, so we did not adjust the newborn estimates. From the AHA survey of 2000, we took the number of patient-days in federal hospitals (11.6 million), added the number of nonfederal hospital patient-days among adults and children (192.4 million) and divided the sum by the nonfederal hospital patient-days among adults and children (192.4 million). The result was 1.06, which we used as a multiplier of the number of adult and children NHDS patient-days and infection estimates. The multiplier increased the number of HAI by 6%
Estimate of deaths
When patients with an HAI die during hospitalization at participating NNIS facilities, infection control professionals make an assessment of the relationship of the HAI to the death and classify the relationship as causal, contributory, not related, or unknown. If a patient has multiple HAIs and dies during hospitalization, the infection control professional makes an assessment of each infection separately. For this estimate, we included deaths in which the HAI caused or contributed to the death, and refer to these as deaths associated with HAIs. Using NNIS infection data from 1999 to 2003, we calculated the percentage of patients with an HAI who died and the percentage of those whose death was associated with their HAI. We then multiplied the percentages within each of the four subpopulations by the estimate of patients with an HAI derived through steps 1–4.
RESULTS
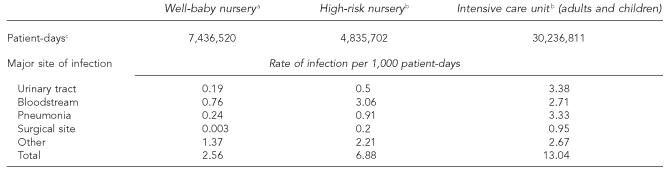
The total number of patient-days from NHDS in 2002 was 176.4 million. Adults and children accounted for 93.1% of patient-days (30.2 million in ICUs and 133.9 million outside ICUs) and newborns for 6.9% (7.4 million in well-baby nurseries, and 4.8 million in high-risk nurseries). The infection rate per 1,000 patient-days was highest in ICUs (13.0), followed by high-risk nurseries (6.9), and well-baby nurseries (2.6) (Table 1).
Table 1.
Rates of healthcare-associated infections in newborns and adults and children by site of infection, National Nosocomial Infections Surveillance (NNIS) system
From NNIS hospital-wide surveillance, 1990–1995
From NNIS surveillance 2002, high-risk nursery and ICU component
From the National Hospital Discharge Survey (NHDS) for the U.S. population in non-federal hospitals
We estimated 274,098 surgical site infections in the U.S. population for procedures monitored in the NNIS system, or about two surgical site infections per 100 procedures (Figure). Of these, 244,385 surgical site infections were among adults and children outside of ICUs. Knowing that the estimate of surgical site infections was 244,385 and that these were approximately 20% of all infections, we estimated that for adults and children outside of ICUs, there were 424,060 urinary tract infections, 129,519 pneumonias, 133,368 bloodstream infections, and 263,810 other infections. The estimated total HAIs among adults and children in hospitals but outside of ICUs was 1,195,142.
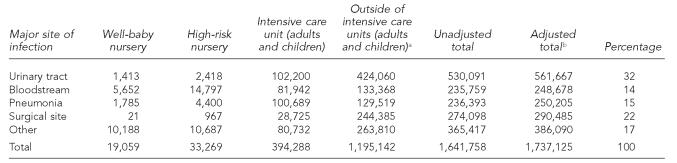
We estimated 33,269 HAIs among newborns in high-risk nurseries, 19,059 among newborns in well-baby nurseries, 394,288 among adults and children in ICUs, and 1,195,142 among adults and children outside of ICUs (Table 2). The total number of HAIs among these subpopulations (1,641,758), adjusted to include federal facilities, was 1,737,125 HAI in the United States for 2002. This number represents a rate of 9.3 infections per 1,000 patient-days or 4.5 per 100 admissions in 2002.
Table 2.
Estimated number of healthcare-associated infections in U.S. hospitals by subpopulation and major site of infection, United States, 2002
See proportions applied from description in Figure.
Adjusted for inclusion of federal hospitals by multiplying non-newborn values by 1.06
Among the 1.7 million patients with an HAI in 2002, there were 155,668 deaths, of which 98,987 were caused by or associated with the HAI. The percentage of patients whose deaths were associated with an HAI varied by major site and subpopulation. The lowest percentage was 0% of infants in well-baby nurseries with urinary tract infections, bloodstream infections, and surgical site infections. The highest percentage of patients with an HAI whose death was associated with the infection was among adults and children in ICUs, where the percentage varied from 11% for surgical site infections to 25% for bloodstream infections. The number of deaths associated with HAIs by major site combining the subpopulations was greatest for pneumonia (35,967) and bloodstream infections (30,665). An estimated 13,088 deaths were associated with urinary tract infections, 8,205 with surgical site infections, and 11,062 with infections of other sites.
DISCUSSION
We estimate that 1.7 million HAIs occurred in U.S. hospitals in 2002 and were associated with approximately 99,000 deaths. The number of HAIs exceeded the number of cases of any currently notifiable disease,13 and deaths associated with HAIs in hospitals exceeded the number attributable to several of the top ten leading causes of death reported in U.S. vital statistics.14 These estimates are sobering and reinforce the need for improved prevention and surveillance efforts.
These estimates have several limitations. We used 1990s data from hospital-wide surveillance for estimates in 2002 in two areas: infection rates in well-baby nurseries and the distribution of infections by major site. Similar data are not available for a more recent time period. The impact of using old infection rates in well-baby nurseries is minimal because the rate was the lowest among the subpopulations and the total number of infections (19,059) was only 1.1% of the total number of infections. The distribution by major site of infection has a large impact on our estimates because adults and children outside of ICUs accounted for 68.8% of all HAIs. However, there is a lack of data in the United States to suggest that the distribution has changed since the 1990s. In other countries, recent studies provide support for using at least 20% for surgical site infections as a percentage of infections by site.15–17
We may have underestimated the total number of HAIs because surgical site infections are likely underreported in the NNIS system. Most surgical site infections become evident after discharge,18,19 and the completeness and accuracy of post-discharge surveillance is variable in NNIS hospitals. Since we extrapolated from the number of surgical site infections among adults and children outside of ICUs to other infection sites in the same population using NNIS infection rates, the total number might be underestimated. In addition, the decrease in the average hospital length of stay over time might have increased the chance of missing post-discharge surgical site infections.20 There may be other factors, however, such as a higher rate of infections that might result in an overestimate of infections. Therefore, we acknowledge a lack of precision in our estimates.
Infection rates from NNIS hospitals might be different than those in other hospitals. NNIS hospitals are frequently larger, more likely to be affiliated with academic institutions, and located in the mid- and south-Atlantic regions of the United States.21 Rates of HAIs from NNIS hospitals cannot be applied to other healthcare settings.
Finally, our death estimate is limited in that attributable mortality is often difficult to determine from a patient's records. Even for experts, it can be problematic to determine whether patients die from their infection or from their co-morbidities.22
Other methods might be useful to estimate national burden including prevalence surveys and use of surrogate data. Annual prevalence surveys are used to measure the burden of HAIs in many countries. For example, prevalence per 100 admissions was 9.1 in Greece in 1999,15 8.0 in Denmark in 1999,16 7.0 in Spain in 1997,17 5.1 in Norway in 2002,23 and 4.6 in Slovenia in 2001.24 A disadvantage to annual prevalence surveys is that trends might reflect changes in case ascertainment over time rather than true changes in prevalence.25 In addition, data from annual prevalence surveys is less useful for prevention at the facility level. In the United States, prevalence surveys could be used periodically to supplement surveillance data to estimate HAIs in hospitals.
There are several examples of using surrogate data from administrative records for surveillance purposes (e.g., the International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes).26 An evaluation of administrative data to identify injuries in children demonstrated high correlation, sensitivity, and specificity.27 Previously unrecognized differences in sepsis by race were described at the national level using ICD-9-CM codes;28 however, the frequency of HAIs has been more difficult to capture using administrative data. Some HAIs are captured in estimates using the Agency for Healthcare Research and Quality patient safety indicators to estimate patient safety events during Medicare hospitalizations29 and by the mandatory reporting system in Pennsylvania.30 Evaluations of these surrogate systems have so far indicated low sensitivity31,32 and low predictive value.31 An evaluation of the performance of five different measures of bloodstream infections demonstrated improved performance of clinical indicators over administrative indicators.33 In general, the difficulty may be related to the need to determine if the infection is associated with the delivery of healthcare services. Standardized definitions and methods are features associated with the success of HAI surveillance.8
In 1995, CDC estimated that 1.9 million HAIs occurred in U.S. hospitals.34 In 2002, we estimated 1.7 million HAIs. Direct comparison of these estimates should be avoided because both are based on the same hospital-wide surveillance data. However, our estimates of surgical site infections do not depend on hospital-wide data and might be useful to compare. In 1995, we estimated that there were 269,268 surgical site infections, or 2.21% of surgical procedures monitored in NNIS. In 2002, we estimated there were 274,268 surgical site infections, or 1.96% of procedures monitored.
New attention to HAIs and advances in information technology could lead to greater participation of hospitals in organized surveillance efforts.35 At CDC, the evolution of the NNIS system into the NHSN has provided a web-based platform that could help address the need for HAI data at the local, state, and national levels.
Acknowledgments
The authors are indebted to the NNIS/NHSN system participants for their ongoing efforts to monitor infections and improve patient safety.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Leape LL, Brennan TA, Laird N, Lawthers AG, Localio AR, Barnes BA, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324:377–84. doi: 10.1056/NEJM199102073240605. [DOI] [PubMed] [Google Scholar]
- 2.Leape LL, Berwick DM. Five years after To Err is Human: what have we learned? JAMA. 2005;293:2384–90. doi: 10.1001/jama.293.19.2384. [DOI] [PubMed] [Google Scholar]
- 3.Burke JP. Infection control–a problem for patient safety. N Engl J Med. 2003;348:651–6. doi: 10.1056/NEJMhpr020557. [DOI] [PubMed] [Google Scholar]
- 4.Leape LL. Reporting of adverse events. N Engl J Med. 2002;347:1633–8. doi: 10.1056/NEJMNEJMhpr011493. [DOI] [PubMed] [Google Scholar]
- 5.Association for Professionals in Infection Control and Epidemiology (APIC) Government advocacy. [cited 2005 Sep 22]. Available from: URL: http://www.apic.org/Content/NavigationMenu/GovernmentAdvocacy/MandatoryReporting/Abouttheissue/about_the_issue.htm.
- 6.Horan TC, Gaynes RP. Surveillance of nosocomial infections. In: Mayhall CG, editor. Hospital epidemiology and infection control. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 1659–702. [Google Scholar]
- 7.National Nosocomial Infections Surveillance (NNIS) Am J Infect Control. 2004;32:470–85. doi: 10.1016/S0196655304005425. Report, data summary from January 1992 through June 2004 issued October 2004. [DOI] [PubMed] [Google Scholar]
- 8.Tokars JI, Richards C, Andrus M, Klevens M, Curtis A, Horan T, et al. The changing face of surveillance for health care-associated infections. Clin Infect Dis. 2004;39:1347–52. doi: 10.1086/425000. [DOI] [PubMed] [Google Scholar]
- 9.Sartor C, Edwards JR, Gaynes RP, Culver DH. Evolution of hospital participation in the National Nosocomial Infections Surveillance System, 1986 to 1993. Am J Infect Control. 1995;23:364–8. doi: 10.1016/0196-6553(95)90267-8. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (US) National Center for Health Statistics 2002 NHDS data file documentation. [cited 2005 Mar 11]. Available from: URL: http://www.cdc.gov/nchs/about/major/hdasd/nhdssam.htm.
- 11.Dennison C, Pokras R. Design and operation of the National Hospital Discharge Survey: 1988 redesign. Vital Health Stat. 2000;1(39):1–42. [PubMed] [Google Scholar]
- 12.American Hospital Association. Chicago: Health Forum LLC; 2005. Hospital statistics 2004. [Google Scholar]
- 13.Summary of notifiable diseases—United States, 2002. MMWR Morb Mortal Wkly Rep. 2004;51(53):1–84. [PubMed] [Google Scholar]
- 14.Deaths: leading causes for 2001. Natl Vital Stat Rep. 2003;52(9) Table E. [PubMed] [Google Scholar]
- 15.Starakis I, Marangos M, Gikas A, Pediaditis I, Bassaris H. Repeated point prevalence survey of nosocomial infections in a Greek university hospital. J Chemother. 2002;14:272–8. doi: 10.1179/joc.2002.14.3.272. [DOI] [PubMed] [Google Scholar]
- 16.Christensen M, Jepsen OB. Reduced rates of hospital-acquired UTI in medical patients Prevalence surveys indicated effect of active infection control programmes. J Hosp Infect. 2001;47:36–40. doi: 10.1053/jhin.2000.0847. [DOI] [PubMed] [Google Scholar]
- 17.Vaqué J, Rosselló J, Arribas L. Prevalence of nosocomial infections in Spain: EPINE study 1990–1997EPINE Working Group. J Hosp Infect. 1999;43(Suppl):S105–11. doi: 10.1016/s0195-6701(99)90073-7. [DOI] [PubMed] [Google Scholar]
- 18.Manian F, Meyer L. Adjunctive use of monthly physician questionnaires for surveillance of surgical site infections after hospital discharge and in ambulatory surgical patients: report of a seven-year experience. Am J Infect Control. 1997;25:390–4. doi: 10.1016/s0196-6553(97)90084-5. [DOI] [PubMed] [Google Scholar]
- 19.Sands K, Vineyard G, Platt R. Surgical site infections occurring after hospital discharge. J Infect Dis. 1996;173:963–70. doi: 10.1093/infdis/173.4.963. [DOI] [PubMed] [Google Scholar]
- 20.National Center for Health Statistics. Hyattsville (MD): 2005. [cited 2005 Sep 22]. Health, United States, 2004 with chartbook on trends in the health of Americans with special feature on drugs. Available from: URL: http://www.cdc.gov/nchs/hus.htm. [PubMed] [Google Scholar]
- 21.Richards C, Emori TG, Edwards J, Fridkin S, Tolson J, Gaynes R. Characteristics of hospitals and infection control professionals participating in the National Nosocomial Infections Surveillance system 1999. Am J Infect Control. 2001;29:400–3. doi: 10.1067/mic.2001.118408. [DOI] [PubMed] [Google Scholar]
- 22.Hiatt HH, Barnes BA, Brennan TA, Laird NM, Lawthers AG, Leape LL, et al. A study of medical injury and medical malpractice. N Engl J Med. 1989;321:480–4. doi: 10.1056/NEJM198908173210725. [DOI] [PubMed] [Google Scholar]
- 23.Eriksen HM, Iversen BG, Aavitsland P. Prevalence of nosocomial infections in hospitals in Norway, 2002 and 2003. J Hosp Infect. 2005;60:40–5. doi: 10.1016/j.jhin.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 24.Klavs I, Bufon Luznik T, Skerl M, Grqic-Vitek M, Lejko Zwpanc T, Dolinsek M, et al. Prevalence of and risk factors for hospital-acquired infections in Slovenia—results of the first national survey, 2001. J Hosp Infect. 2003;54:149–57. doi: 10.1016/s0195-6701(03)00112-9. [DOI] [PubMed] [Google Scholar]
- 25.Sartor C, Delchambre A, Pascal L, Drancourt M, De Micco P, Sambuc R. Assessment of the value of repeated point-prevalence surveys for analyzing the trend in nosocomial infections. Infect Control Hosp Epidemiol. 2005;26:369–73. doi: 10.1086/502554. [DOI] [PubMed] [Google Scholar]
- 26.Kostylova A, Swaine B, Feldman D. Concordance between childhood injury diagnoses from two sources: an injury surveillance system and a physician billing claims database. Inj Prev. 2005;11:186–90. doi: 10.1136/ip.2004.006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Public Health Service and Health Care Financing Administration. 4th ed. Washington: Public Health Service; 1991. International Classification of Diseases, 9th Revision, Clinical Modification. [Google Scholar]
- 28.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 29.Healthgrades Inc. Second annual patient safety in Ameri can hospitals report. [Accessed on September 22, 2005]. http://www.healthgrades.com/media/dms/pdf/HealthGradesNinthAnnualHospitalQualityinAmericaStudy.pdf.
- 30.Pennsylvania Health Care Cost Containment Council (PHC4) Hospital-acquired infections in Pennsylvania. [cited 2006 Dec 14];PHC4 Research Briefs. 2005 5 Available from: URL: http://www.phc4.org. [Google Scholar]
- 31.Sherman E, Heydon K, Teszner E, Alexander S, Rettig S, St. John KH, et al. Alexandria, VA: Final Program of the Society for Healthcare Epidemiology of America; 2005. Apr 9-12, Mandatory reporting of healthcare-associated infections: a long way to go; p. 136. Abstract #304. [Google Scholar]
- 32.Julian KG, Brumback A, Chicora M, et al. Alexandria, VA: Final Program of the Society for Healthcare Epidemiology of America; 2005. Apr 9-12, Collaborative approaches to mandatory nosocomial infection reporting in Pennsylvania; p. 136. Abstract #303. [Google Scholar]
- 33.Braun BI, Kritchevsky SB, Kusek L, Wong ES, Solomon SL, Steele L, et al. Comparing bloodstream infection rates: the effect of indicator specifications in the evaluation of processes and indicators in infection control (EPIC) study. Infect Control Hosp Epidemiol. 2006;27:14–22. doi: 10.1086/498966. Epub 2006 Jan 6. [DOI] [PubMed] [Google Scholar]
- 34.Weinstein RA. Nosocomial infection update. Emerg Infect Dis. 1998;4:416–20. doi: 10.3201/eid0403.980320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aspden P, Corrigan JM, Wolcott J, Erickson SM, editors. Committee on Data Standards for Patient Safety. Patient safety: achieving a new standard for care. [cited 2005 Oct 3]. Available from: URL: http://www.iom.edu/report.asp?id=16663. [PubMed]