Abstract
Bloom's syndrome is a genetic disorder characterized by increased incidence of cancer and an immunodeficiency of unknown origin. The BLM gene mutated in Bloom's syndrome encodes a DNA helicase involved in the maintenance of genomic integrity. To explore the role of BLM in the immune system, we ablated murine Blm in the T-cell lineage. In the absence of Blm, thymocytes were severely reduced in numbers and displayed a developmental block at the β-selection checkpoint that was partially p53 dependent. Blm-deficient thymocytes rearranged their T-cell receptor (TCR) β genes normally yet failed to survive and proliferate in response to pre-TCR signaling. Furthermore, peripheral T cells were reduced in numbers, manifested defective homeostatic and TCR-induced proliferation, and produced extensive chromosomal damage. Finally, CD4+ and CD8+ T-cell responses were impaired upon antigen challenge. Thus, by ensuring genomic stability, Blm serves a vital role for development, maintenance, and function of T lymphocytes, suggesting a basis for the immune deficiency in Bloom's syndrome.
Genomic integrity is essential for the survival of all organisms as its impairment results in dysregulated growth, cell death, or tumor development. Indeed, multiple molecular mechanisms are used to prevent the accumulation of mutations and to maintain the structural integrity of chromosomes during genome replication (49). Mutations in the components of genome maintenance pathways such as TP53, BRCA1/2, or ATM are frequently associated with tumor development; in addition, they often affect normal development, proliferation, and/or survival of many cell types (49), including cells of the immune system (12).
One of the striking examples of failed genomic maintenance is provided by Bloom's syndrome (BS), a rare human autosomal recessive disorder (14). The gene mutated in BS, BLM (7), encodes an ATP-dependent DNA helicase that belongs to an evolutionarily conserved family of RecQ helicases (1). The BLM protein is preferentially expressed in actively dividing cells (48), and in the mouse, high Blm mRNA levels are found in the thymus, spleen, ovary and testis (4). BLM is believed to suppress illegitimate homologous recombination (HR) and to remove roadblocks that cause replication forks to stall (1). Not surprisingly therefore, BS is characterized by marked genomic instability and is associated with growth retardation, sun-induced erythema, impaired fertility, and greatly increased predisposition to all types of cancer (14).
Notably, BS patients also manifest an immune deficiency that often leads to life-threatening infections (15). Patients usually present with low concentrations of one or more plasma immunoglobulin isotypes (26, 50) and fail to develop delayed-type hypersensitivity (14). Some patients exhibit decreased numbers of CD4+ T lymphocytes (50) and impaired T-cell proliferation in vitro (26, 50). Together with an impaired T helper cell function in BLM patients (47), these data suggest a defect in the T-cell lineage. However, the cellular basis of the immune deficiency in BS is poorly understood, and the function of BLM in the immune system has not been defined (12).
During their development in the thymus, T cells undergo successive stages of commitment, proliferation, and selection to ultimately generate a functional but (largely) self-tolerant T-cell receptor (TCR) repertoire. Several critical developmental events take place at the CD4− CD8− double-negative (DN) stage of thymocyte development. First, the earliest progenitors (DN1; CD25− CD44+) commit to the T-cell lineage and differentiate into DN2 (CD25+ CD44+) cells. At the subsequent transition to the DN3 (CD25+ CD44−) stage, VDJ rearrangement of the T-cell receptor β (Tcrb) locus is initiated. Surface expression by DN3 thymocytes of the pre-TCR complex, consisting of a functional TCRβ chain together with the invariant pre-Tα protein, marks a major checkpoint in early T-cell development termed β-selection. It is necessary and sufficient to induce the transition to the subsequent DN4 (CD25− CD44−) stage, when cells undergo a major proliferative expansion and eventually differentiate into CD4+ CD8+ double-positive (DP) thymocytes (51). The failure of β-selection due to an inability to assemble or express a functional TCRβ chain, impaired pre-TCR expression or signaling, or defective response to pre-TCR signals results in a developmental block and eventual apoptosis of thymocytes at the DN3 stage (9, 20, 31, 35, 45). DP thymocytes rearrange the T-cell receptor α (Tcra) locus and undergo positive and negative selection events to generate mature CD4+ CD8− or CD4− CD8+ single-positive (SP) thymocytes, which migrate into the periphery (13). In the context of exerting their effector functions, mature peripheral T cells are capable of antigen-induced proliferation but may also undergo homeostatic proliferation in case of lymphopenia, e.g., as a consequence of reduced thymic output (16).
Several tumor suppressor genes regulate T-cell development and function. For instance, the loss of p53 allows T cells to bypass the β-selection checkpoint (2, 19, 27, 38) and predisposes to the development of thymic lymphomas (6). Also, the loss of Atm leads to thymus hypoplasia with smaller numbers of mature thymocytes and a reduced peripheral T-cell compartment (8). Finally, a T-cell-specific deletion of Brca1 results in a severe block of early thymocyte development at the β-selection checkpoint (31). Interestingly, V(D)J recombination appears to be intact in all cases, suggesting other important roles for these genes in lymphocyte development.
To begin defining the role of BLM in the immune system, we set out to study the development and function of Blm-deficient T lymphocytes. A germ line-constitutive deletion of Blm previously generated in our lab results in embryonic lethality at embryonic day 13.5 (E13.5) due to severe anemia (4). Therefore, we used a conditional Blm allele (34) to delete Blm specifically in the T-cell lineage. We now report that thymocyte development is severely blocked at the β-selection checkpoint, accounting for severely reduced thymocyte numbers in conditional knockout mice. Although Blm is dispensable for VDJ recombination of the Tcrb genes, we found it to be essential for maintenance, for genome integrity, and ultimately for the function of T lymphocytes. Our data highlight the importance of genomic maintenance mechanisms in the adaptive immune system and provide a likely explanation for the immune deficiency in BS patients.
MATERIALS AND METHODS
Mice.
The Blmnull allele (Blmtm1Ches) with a disrupted Blm exon 8 has been described previously (4). The Blmflox allele (Blmtm4Ches) used in this study contains a loxP-flanked exon 8 (3) and is similar to the previously described allele Blmtm2Ches (34), except that the Neo resistance cassette had been deleted. cis-acting replication element (Cre)-mediated deletion of exon 8 leads to a frameshift and a premature termination of Blm translation (Blmtm3Ches; see reference 34). pTaCre mice were generated by introducing Cre recombinase into the first coding exon of pTa within a bacterial artificial chromosome (BAC) (42). The resulting pTaCre clone was microinjected to generate transgenic animals, and a line harboring two transgene copies was derived and used for further experiments. Other lines included lckCre and CD4Cre mice (29) (kind gifts from C. B. Wilson), the αβTCR AND transgenic line (28), the lckBcl-2 transgenic line (44) (both from Jackson Laboratories), Rag2−/− mice (45) (Taconic), and Trp53−/− mice (6), which have been maintained in this lab for several years. All mice were kept in specific pathogen-free facilities, and all experiments were approved by the institutional review board at the animal facility of the Harvard Medical School.
Blm deletion PCR.
For the semiquantitative analysis of Blm deletion, a three-primer PCR assay using primers A (5′-GCTGCTATAGCACTGATCGGTACC-3′), B (5′-TGGTGGGTAAACATTCCTCAGTGG-3′), and C (5′-ATAGGCGTATCACGAGGCCCTTTCG-3′) was used. Primers A and B amplify a 330-bp band from the floxed allele, while primers A and C amplify a 120-bp band from the null allele. To assess the sensitivity, spiking experiments were performed in which genomic DNA from two mammary myoepithelioma cell lines bearing exclusively either the floxed or the deleted allele (3) was quantitated by UV spectroscopy and mixed in ratios ranging from 1,000:1 to 1:1,000. Fifty nanograms of the blended DNA was used as a template and amplified using a Taq DNA polymerase in storage buffer A system (Promega). An initial denaturation step at 94°C for 4 min was followed by 35 cycles of 94°C for 1 min, 58°C for 1 min, and 72°C for 1.5 min, and a final 10-min incubation at 72°C. The assay was shown to detect ≥10% of each allele and to correlate closely with the Southern analysis (see Fig. S2 in the supplemental material; also data not shown).
Flow cytometry and magnetic separation.
Single-cell suspensions prepared from thymus, spleen, and lymph nodes were surface labeled with the following fluorescently conjugated monoclonal antibodies (MAbs): anti-CD3ɛ, anti-CD4, anti-CD8α, anti-CD11b, anti-CD19, anti-CD25, anti-CD27, anti-CD44, anti-CD45, anti-CD62L, anti-TCRγ/δ, anti-Vα 2, anti-Vα 3.2, anti-Vα 8.3, anti-Vα 11.1/11.2, anti-major histocompatibility complex class II (I-A/I-E), and anti-B220 (eBioscience or BD Pharmingen). Analyses were done on a FACScalibur cytometer using CellQuest software, and cell sorting was performed on a FACSVantage or a FACSAria cell sorter (BD Immunocytometry Systems). DN1-4 thymocytes were defined as lineage (CD3, CD4, CD8, CD11b, B220)-negative unless otherwise noted. Intracellular TCRβ staining was essentially done as described previously (54), using phycoerythrin-coupled anti-TCRβ MAb. Intracellular stains with a hamster immunoglobulin G isotype control antibody (BD Pharmingen) were consistently negative. For sorting experiments, lymphoid cells from two mice per genotype were pooled. Magnetic separation was done by labeling with the respective biotin-conjugated antibodies, followed by streptavidin-coupled microbeads using a Mini-MACS system (Miltenyi Biotec).
PCR, Southern, and sequence analysis of Tcrb rearrangements.
DN3 (lineage− CD44− CD25+) thymocytes were sorted from lineage-depleted thymocyte fractions, and 10 ng of genomic DNA was used for the amplification of Dβ2→ Jβ2 and Vβ→ Dβ2Jβ2 rearrangements. The oligonucleotides specific for Dβ2, Vβ4, Vβ10, and Vβ17 (forward) and Jβ2 (reverse) were as described previously (10), whereas the forward primer for Vβ8.2 and the oligonucleotides for the Thy-1 loading control were from reference 54. The PCR parameters were calibrated to remain in the linear amplification range and were as follows: 94°C for 2 min and 80°C for 5 min (during which Taq was added), followed by 20 (Thy1) or 28 (TCRβ) cycles of 94°C for 1 min, 61°C for 1.5 min, and 72°C for 2.5 min, and a final 5-min incubation at 72°C. The products were separated, blotted, and hybridized with the Jβ2 internal probe as described previously (54). To analyze the fidelity of TCRβ VDJ joints, the PCR products corresponding to the Vβ10Dβ2Jβ2.1 and Vβ17Dβ2Jβ2.7 rearrangements were cloned using a TOPO TA cloning kit (Invitrogen) and randomly sequenced.
Cell cycle analysis and apoptosis assays.
Cell cycle analyses were carried out using a fluorescein isothiocyanate (FITC) bromodeoxyuridine (BrdU) flow kit (BD Pharmingen) according to the manufacturer's instructions. Mice were injected intraperitoneally with 1 mg BrdU in phosphate-buffered saline (PBS), their thymi were harvested after 45 min, and thymocytes were stained for cell surface markers, BrdU and DNA content using 7-amino-actinomycin (7-AAD). Annexin V and terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining were performed employing a TACS annexin V-FITC kit (Trevigen) and an APO-DIRECT kit (BD Pharmingen), respectively, using 7-AAD instead of propidium iodide for DNA content analysis.
Proliferation assays.
Splenocytes were depleted of CD8+, CD19+, and CD11b+ cells by magnetic separation, yielding ∼80% CD4+ T lymphocytes. The purified cells were labeled with 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes) in serum-free Dulbecco's minimal essential medium for 15 min at 37°C and cultured either alone or on plate-bound anti-CD3 and anti-CD28 antibodies (FG purified; eBioscience) at 5 × 104 or 1 × 105 cells/well in 96-well dishes. Cells were harvested after 4 or 6 days, and CD4+ cells were analyzed for CFSE fluorescence or annexin V binding by flow cytometry.
Immunizations and in vitro restimulation cultures.
Hind footpads of 8-, 11-, 37-, 44-, and 50-week-old Cre+Blmnull/flox conditional knockout (CKO) and control mice were injected with 100 μg ovalbumin (OVA; grade VI; Sigma), emulsified in complete Freund's adjuvant. After 7 days, popliteal and inguinal lymph nodes (LN) were harvested. CFSE-labeled LN cells were restimulated with 450 μg OVA at 3 × 106 cells/well in 24-well dishes. After 6 days, cells were stained with anti-CD4 and anti-CD8 antibodies and analyzed for CFSE fluorescence by flow cytometry.
Cytogenetic analyses and sister chromatid exchange assay.
Cytogenetic and sister chromatid exchange (SCE) analyses were performed on splenic CD4+ T cells that had been purified by magnetic separation and cultured on anti-CD3/anti-CD28-coated plates for 3 to 5 days (see above). Giemsa-stained metaphase spreads were prepared by standard procedures, and SCE assays were done as described previously (4), with 10 μM BrdU added for the last 42 h of culture.
Statistics.
Average values ± standard deviations are given, and P values were calculated by employing the two-sample Student's t test (unpaired, two-tailed, assuming unequal variances) using Microsoft Excel software.
RESULTS
Blm is required for early T-cell development.
For the tissue-specific targeting of Blm, we used a loxP-flanked (floxed) Blm allele (3) that is rendered nonfunctional (Δ) by Cre-mediated recombination (see Fig. S1 in the supplemental material). Indeed, mice homozygous for the Δ-allele recapitulate the embryonic lethality (34) observed in the constitutive knockout mice (4). CKO animals were generated by mating Blmflox/flox mice to mice harboring the constitutive null (4) and wild-type alleles of Blm in addition to a Cre transgene (Cre+Blmnull/wt). This strategy facilitated a direct comparison of the Cre+ Blmnull/flox CKO mice to their Cre+Blmwt/flox (Cre+ control), Blmnull/flox, or Blmwt/flox littermate controls. Because the latter two groups were essentially identical (data not shown), they were regarded as the same group (Cre− control) in all experiments.
To delete Blm in T lymphocytes, we utilized two strains that mediate Cre recombination early in the T-cell lineage. In addition to the well-characterized lckCre strain (29, 54), we utilized a novel strain in which Cre recombinase is expressed from the pre-T-cell receptor alpha (pTa) gene within a BAC transgene. We found that the pTaCre strain mediates a more consistent recombination early in thymocyte development, particularly when the recombined cells are strongly counterselected (B. Reizis and P. Leder, unpublished data). Indeed, Southern hybridization revealed a nearly complete Blm deletion in the thymus, whereas it was low or absent in most other organs of CKO animals (see Fig. S1 in the supplemental material). Furthermore, PCR-based analysis of Blm deletion confirmed a robust recombination in all thymocyte subsets, including the earliest committed progenitors at the DN2 stage; in contrast, the great majority of thymic epithelial cells retained the unrecombined allele (see Fig. S2 in the supplemental material). Finally, Western blotting revealed the almost complete absence of Blm protein in purified αβTCR thymocytes from pTαCre+ CKO mice (see Fig. S2 in the supplemental material).
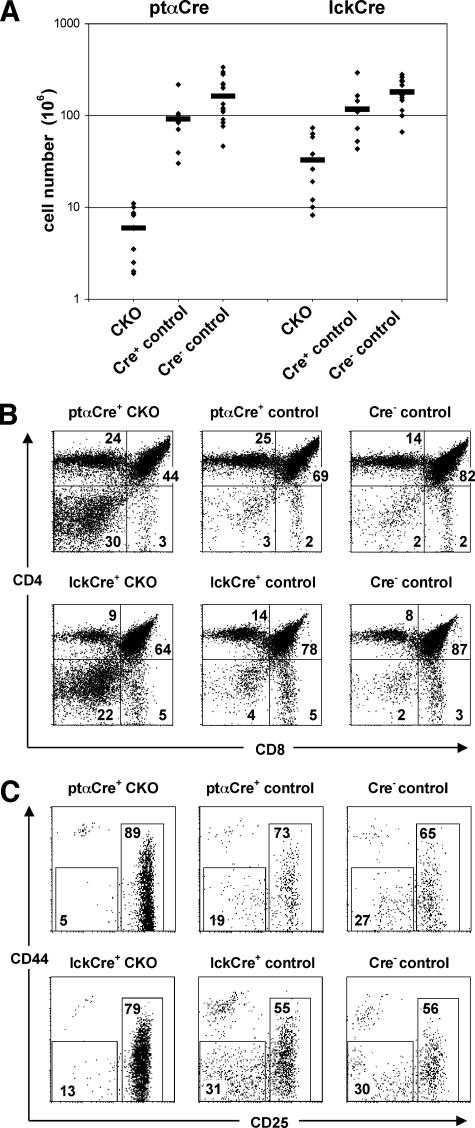
The thymi of young adult pTαCre+ CKO mice were very small and contained less than 1/15 as many cells ([6 ± 3.4] × 106 cells; n = 10) as their Cre+ control ([91 ± 53] × 106 cells; n = 9) or their Cre− control ([162 ± 89] × 106cells; n = 15) littermates (Fig. 1A). Consistent with the T-cell-specific deletion, the development of B lymphocytes and other lineages in the CKO bone marrow was normal (data not shown). A similar albeit less pronounced hypocellularity was also observed in lckCre+ CKO thymi ([33 ± 24] × 106 cells; n = 10) compared to the Cre+ control ([116 ± 72] × 106 cells; n = 10) or Cre− control ([179 ± 70] × 106 cells; n = 11) littermates. Analysis of major thymocyte subsets in both strains revealed an increase up to 10-fold in the proportion of DN thymocytes, suggesting a major block at the DN-to-DP transition (Fig. 1B). Within the DN cell population, we observed an accumulation of cells at the DN3 stage and a profound decrease in the subsequent DN4 subset (Fig. 1C). Accordingly, the absolute numbers of DN2 + 3 cells were only modestly reduced in the pTαCre+ CKO thymi, whereas all later stages (DN4, DP, CD4SP, and CD8SP) were decreased by up to 95% (Table 1). Because of the robust deletion and strong phenotype produced by the pTaCre strain, we focused on the pTαCre+ CKO animals for most subsequent experiments. Altogether, the data from both the lckCre and pTaCre strains indicate that the development of Blm-deficient thymocytes is blocked at the β-selection checkpoint.
FIG. 1.
Defective T-cell development in the absence of Blm. (A) Numbers of total thymocytes from 5- to 6-week-old ptαCre+ CKO, ptαCre+ control, Cre− control (from pTaCre cross), lckCre+ CKO, lckCre+ control, and Cre− control (from lckCre cross) mice. Each diamond represents an individual mouse. Average values are indicated by horizontal bars. The differences between the ptαCre+ CKO or the lckCre+ CKO mice and their respective controls are statistically significant (P, ≤0.01). (B and C) Flow cytometric analysis of total thymocytes of ptαCre+ CKO (top panels) or lckCre+ CKO (bottom panels) and their respective littermate control mice. (B) CD8 versus CD4 expression of total thymocytes; (C) CD25 versus CD44 expression of thymocytes gated on lineage (CD3, CD4, CD8, CD11b, B220)-negative cells.
TABLE 1.
Absolute numbers of lymphocytes in thymus, spleen, and lymph nodesa
| Organ | ptαCre+ CKO (n = 10) | ptαCre+ control (n = 9) | Cre− control (n = 15) |
|---|---|---|---|
| Thymus | |||
| DN2 + 3 | 0.71 ± 0.41 | 1.4 ± 1.6 | 1.6 ± 1.5 |
| DN4 | 0.07 ± 0.05 | 0.68 ± 1.1 | 0.95 ± 0.78*** |
| DP | 3.3 ± 2.7 | 69.0 ± 47.1** | 109 ± 52.6*** |
| CD4SP | 0.86 ± 0.59 | 13.4 ± 4.9*** | 38.3 ± 39.3** |
| CD8SP | 0.30 ± 0.17 | 2.9 ± 1.4*** | 2.8 ± 1.2*** |
| TCRγδ | 0.11 ± 0.08 | 0.44 ± 0.31* | 0.61 ± 0.50** |
| Spleen | |||
| CD4+ | 4.7 ± 2.9 | 22.8 ± 9.3*** | 23.7 ± 9.0*** |
| CD8+ | 2.0 ± 1.3 | 10.2 ± 4.2*** | 8.7 ± 5.5*** |
| CD19+ | 26.6 ± 8.5 | 40.1 ± 19.9 | 29.9 ± 11.4 |
| TCRγδ | 0.47 ± 0.32 | 0.66 ± 0.37 | 0.73 ± 0.25 |
| Lymph nodes | |||
| CD4+ | 4.8 ± 3.0 | 21.8 ± 11.4** | 27.7 ± 9.2*** |
| CD8+ | 2.1 ± 1.1 | 8.8 ± 4.3** | 8.2 ± 5.6*** |
| CD19+ | 10.1 ± 4.3 | 7.7 ± 5.2 | 8.3 ± 4.0 |
| TCRγδ | 0.33 ± 0.13 | 0.41 ± 0.40 | 0.37 ± 0.20 |
Absolute numbers (106 ± SD) of the indicated cell populations are shown. DN cells were CD4− CD8−, DP were CD4+ CD8+, CD4SP were CD4+ CD8−, and CD8SP were CD4− CD8+. Asterisks indicate the P values (CKO versus Cre+ control and Cre− control mice, respectively). *, P = 0.05 to 0.01; **, P = 0.01 to 0.001; ***, P < 0.001. n, number of mice.
Blm-deficient thymocytes rearrange their Tcrb genes normally and receive a β-selection signal.
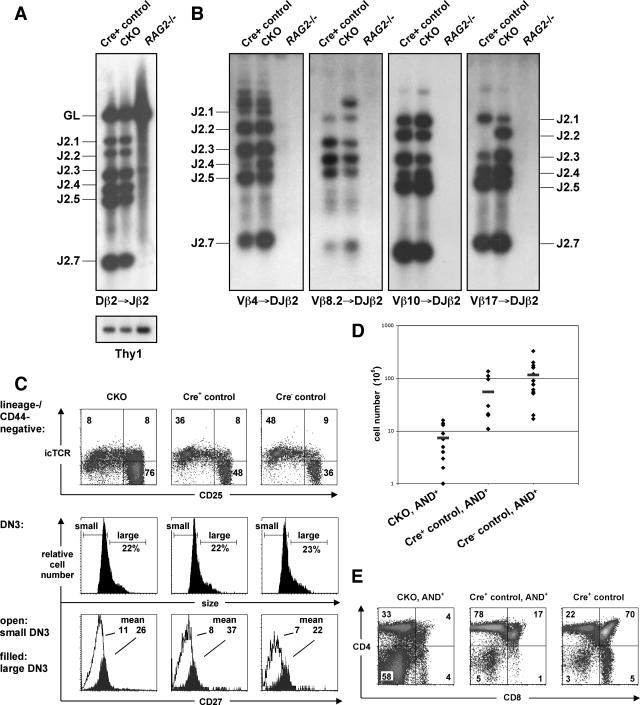
The observed developmental block at the DN3-to-DN4 transition could arise from a defect in Tcrb rearrangement, from pre-TCR signaling, or from proliferation and survival of β-selected thymocytes. Because BLM is involved in the maintenance of genome integrity, we first tested the extent and outcome of Tcrb gene rearrangement in the absence of Blm. To this end, DN3 thymocytes from pTαCre+ CKO and Cre+ control mice were examined for DJβ or VDJβ rearrangements by genomic PCR. As shown in Fig. 2A and B, sorted CKO DN3 thymocytes manifested normal levels of all rearrangement products. Furthermore, sequencing of the amplified VDJ products revealed similar frequencies of productive in-frame rearrangements as well as comparable complementarity-determining region 3 lengths and N nucleotide additions (see Table S1 in the supplemental material). Although no other developmental blocks were apparent in CKO mice, we looked for evidence of potentially abnormal TCRα locus rearrangement. Staining of DP thymocytes with all commercially available anti-TCRVα antibodies revealed similar frequencies of TCRVα usage by CKO and Cre+ control thymocytes (see Fig. S3 in the supplemental material).
FIG. 2.
Intact Tcrb rearrangements and β-selection in CKO mice. (A and B) VDJ rearrangements were PCR amplified from DN3 thymocytes sorted from CKO, Cre+ control, and Rag2−/− mice. PCRs detected Dβ2→ Jβ2 rearrangements, the Thy1 gene as a loading control (A), or Vβ→ DJβ2 rearrangements (B). Products were hybridized with a probe corresponding to the Jβ2.7 segment. The “GL” band represents the Dβ2-Jβ2 locus in germ line configuration, while the other bands represent the indicated rearrangements. (C) DN3/DN4 thymocytes (lineage− CD44−) from CKO and control mice were analyzed for their intracellular expression of TCRβ protein (upper row), and lineage− CD44− CD25+ DN3 cells of the same mice for their forward scatter (i.e., size; middle row). The percentages of large DN3 cells (L cells) are given. Histograms in the lower row depict surface CD27 levels (stained before permeabilization) of the same cells, and the mean fluorescence intensities are given (open histograms, gated on small DN3 cells; filled histograms, gated on L cells). (D) Numbers of total thymocytes from CKO (n = 10), Cre+ control (n = 8), and Cre− control (n = 13) mice carrying the AND TCR transgene. The differences between mutant mice and their respective controls are statistically significant (P ≤ 0.05). (E) Flow cytometric analysis of CD8 versus CD4 expression of total thymocytes from AND+ CKO and control mice.
Upon receiving a signal from the pre-TCR, DN3 thymocytes increase in size and commence DNA synthesis (23) as well as up-regulate the expression of CD27 (46). As illustrated in Fig. 2C, the fraction of large DN3 cells (L cells) in CKO mice (21% ± 4%; n = 12) was similar to that of Cre+ control (23% ± 9%; n = 11) and Cre− control (17% ± 6%; n = 18) littermates. Furthermore, L cells upregulated CD27 expression to an extent similar to that of CKO and control mice. Also, on a per cell basis, the levels of intracellular TCRβ protein in CKO and control DN3 cells were comparable (Fig. 2C). Thus, Blm-deficient thymocytes appear to rearrange their TCRβ chain genes and pass the β-selection checkpoint yet fail in their immediate response to the latter.
If the pre-TCR assembly or signaling were intact in Blm-deficient thymocytes, the resulting developmental block would not be rescued by a rearranged TCR transgene. To test this possibility, we introduced the rearranged AND αβTCR transgene (28) into Blm-deficient mice. Despite the presence of the TCR transgene, Blm-deficient thymocytes were still reduced in total numbers (Fig. 2D) and exhibited a severe block at the DN stage (Fig. 2E). Thus, a TCR transgene failed to rescue the development of Blm-deficient T cells, confirming an impairment in their developmental progression following β-selection.
Impaired proliferation and survival of Blm-deficient thymocytes.
Because Blm appeared dispensable for pre-TCR assembly and signaling, we tested whether it might play a role in the proliferation and/or survival of β-selected thymocytes. To this end, cell cycle profiles of thymocyte populations were analyzed after in vivo short-term labeling with BrdU. In CKO mice, we observed a major reduction of DNA synthesis in DN3 L cells and particularly in DN4 cells, the two populations undergoing a proliferative burst following pre-TCR signaling (Fig. 3A). Instead, elevated proportions of cells was found in the G0/G1 phase of the cell cycle. In contrast, the proportion of cycling DP cells was slightly elevated in the mutant thymi, whereas the proliferation of SP thymocytes was comparable between mutants and controls (Fig. 3A and Table 2). Next, we assessed the extent of cell death in major thymocyte populations. Notably, annexin V labeling revealed increased apoptosis in all subpopulations of Blm-deficient thymocytes (Fig. 3B and Table 2), including DN3 L cells and DN4, DP, and particularly CD4SP cells. The same trend of increased apoptosis was observed using the TUNEL assay, which detects later stages of apoptosis (Fig. 3C and Table 2). These results indicate that Blm-deficient thymocytes exhibit an impaired proliferative capacity as well as excessive cell death, particularly during the proliferative expansion following β-selection.
FIG. 3.
Impaired proliferation and increased apoptosis in thymocytes of CKO mice. (A) Cell cycle profiles of thymocyte subpopulations from CKO and Cre− control mice after short-term in vivo labeling with BrdU. The percentages of cells in S phase (BrdU+) and G0/G1 phase (BrdU− 7-AADlo) are shown. (B) Detection of early apoptotic cells of the indicated 7-AAD− thymocyte subsets from CKO and Cre− control mice as revealed by annexin V staining. (C) Detection of late apoptotic cells of the indicated thymocyte subsets from CKO and Cre+ control mice using TUNEL. The data displayed are representative of four independent experiments.
TABLE 2.
Cell subpopulations assayed for actively dividing and apoptotic cellsa
| Strain | % of cells in S phase ± SD
|
% of annexin V+ cells ± SD
|
% TUNEL+ cells ± SD
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of mice | L cells | DN4 | DP | CD4SP | CD8SP | No. of mice | L cells | DN4 | DP | CD4SP | CD8SP | No. of mice | DN | DP | CD4SP | |
| PtαCre+ CKO | 8-12 | 30.8 ± 9.7 | 22.4 ± 7.7 | 6.3 ± 1.7 | 1.8 ± 1.3 | 4.6 ± 4.7 | 9 | 5.6 ± 3.1 | 55.9 ± 6.4 | 6.7 ± 4.9 | 18.6 ± 9.2 | 5.4 ± 2.7 | 8 | 3.3 ± 1.1 | 0.83 ± 0.21 | 0.18 ± 0.08 |
| ptαCre+ control | 9-13 | 42.0 ± 8.7* | 41.0 ± 10.5*** | 4.5 ± 1.4** | 1.2 ± 0.9 | 4.5 ± 4.5 | 10 | 2.3 ± 1.3* | 54.7 ± 8.0 | 2.1 ± 0.8* | 4.3 ± 2.4** | 3.6 ± 1.6 | 9 | 3.0 ± 1.2 | 0.42 ± 0.12*** | 0.10 ± 0.03* |
| Cre− control | 7 | 50.1 ± 9.2** | 48.6 ± 10.9*** | 4.8 ± 0.8* | 1.0 ± 0.3 | 3.1 ± 1.8 | 16 | 1.5 ± 0.8** | 43.3 ± 10.6** | 1.6 ± 0.3* | 3.2 ± 1.1*** | 2.8 ± 1.7* | 9 | 3.0 ± 1.3 | 0.30 ± 0.09*** | 0.11 ± 0.04* |
Percentages of cells in the S phase and cells positive for annexin V and TUNEL. For details see the legend for Fig. 3. Asterisks indicate the P values (CKO versus Cre+ control and Cre− control mice, respectively). *, P = 0.05 to 0.01; **, P = 0.01 to 0.001; ***, P < 0.001.
The tumor suppressor protein p53 was shown to mediate thymocyte apoptosis in the absence of a pre-TCR signal, particularly in situations of defective DNA repair or DNA damage accumulation (2, 19, 20, 27, 31, 38). We therefore tested whether the defective development of Blm-deficient thymocytes might be rescued by a p53 deficiency using Trp53-mutant lckCre+ CKO mice. As illustrated in Fig. 4A, the thymic cellularities of p53-deficient lckCre+ CKO mice were increased almost threefold compared to those of Trp53-heterozygous CKO littermates, corresponding to a 55% rescue. Importantly, the observed rescue correlated with a relief of the developmental block at the DN3 stage (Fig. 4B). These results indicate that p53-mediated apoptosis or cell cycle arrest following β-selection contributes to the developmental block of Blm-deficient thymocytes.
FIG. 4.
Partial rescue of the Blm-dependent thymic developmental block by inactivation of the Trp53 or a Bcl-2 transgene. (A) Numbers of total thymocytes from 5- to 6-week-old Trp53−/− and Bcl2 transgenic mice as well as their respective Trp53+/− and Bcl2− controls. Average thymic sizes (± standard deviations) and numbers of analyzed animals are given above the plots. (B) Flow cytometric analysis of total thymocytes of lckCre+ p53+/− CKO and lckCre+ p53−/− CKO mice and their respective littermate controls. The experiments were done as in Fig. 1B and C. The absolute thymocyte numbers of individual mice are indicated.
Enforced expression of the prosurvival protein Bcl-2 can rescue certain genetic defects associated with impaired T-cell development (33). We therefore crossed the lck promoter-driven Bcl-2 transgene (44) onto the pTαCre+ CKO mice. Although the overall thymocyte numbers remained relatively low (Fig. 4A), they were increased threefold in the lck-Bcl-2+ CKO mice compared to those in the transgene-negative CKO mice. In contrast to the p53 deficiency, however, the Bcl-2 transgene did not rescue the DN3 block but instead increased the absolute numbers of all thymocyte subsets (data not shown). Thus, increased resistance to apoptosis appears beneficial to thymocytes that lack Blm.
Decreased T-cell numbers and counterselection of Blm-deficient T cells in the periphery of CKO mice.
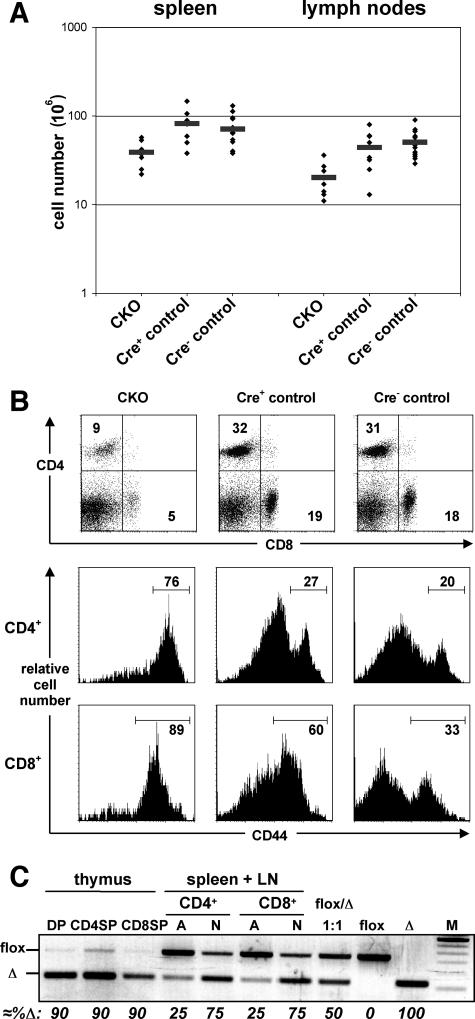
Consistent with the defective thymic development, the peripheral T-cell compartment was severely reduced in the pTαCre+ CKO mice. The spleens and LN contained 45 to 60% fewer cells (Fig. 5A), with decreased fractions of both CD4+ and CD8+ T cells (Fig. 5B). In both organs, the absolute numbers of CD4+ and CD8+ T cells were four to five times lower than in control littermates, whereas B-cell as well as TCRγδ-cell numbers were comparable (Table 1). Interestingly, peripheral T lymphocytes were enriched in cells bearing high levels of the activation marker CD44 (Fig. 5B), suggesting that these cells were undergoing homeostasis-driven proliferation (16). Indeed, in vivo labeling with BrdU revealed a sevenfold increase in proliferating (BrdU+) T cells in the CKO mice, whereas <1% of B lymphocytes incorporated BrdU in both groups of mice (not shown).
FIG. 5.
T-cell hypocellularity and counterselection of Blm-deficient T lymphocytes in the periphery of Blm mutants. (A) Numbers of total splenocytes and lymph node cells from CKO (n = 9), Cre+ control (n = 8), and Cre− control (n = 15) 5- to 6-week-old mice are displayed. The differences between the Blm mutant mice and their respective controls are statistically significant (P, <0.05). (B) Total splenocytes of 5- to 6-week-old CKO, Cre+ control, and Cre− control mice were analyzed for CD4 and CD8 expression by flow cytometry. The histograms in rows 2 and 3 show CD44 expression of splenocytes gated on CD4+ and CD8+ cells, respectively. (C) The deletion of Blm exon 8 in sorted thymocyte and peripheral T-cell populations from CKO mice were determined by semiquantitative PCR. Peripheral CD4+ and CD8+ T cells of pooled splenocytes and lymph node cells were separated into either activated (A, CD44+ CD62L−) or naive (N, CD44− CD62L+) cells. M, 100-bp ladder (highlighted band, 600 bp).
Since Blm deletion in the thymus is highly efficient but not absolute (see Fig. S2 in the supplemental material), we followed the fate of Blm-positive T cells in the periphery, where pTαCre expression is turned off, using our Blm deletion PCR assay. Although the great majority of thymocytes had deleted Blm (Fig. 5C), a prominent band corresponding to the nonrecombined floxed allele was apparent in peripheral naïve CD4+ and CD8+ T cells. Strikingly, however, these Blm-proficient cells were dominant among peripheral T cells with an activated phenotype (Fig. 5C). These data show that the residual T cells with a functional Blm allele undergo homeostatic proliferation, whereas Blm-deficient T cells are counterselected during this process.
Reduced proliferation of Blm-deficient peripheral T cells and impaired T-cell immunity in CKO mice.
To examine Blm-deficient peripheral T cells, it was imperative to circumvent the early block of thymocyte development and enforce Cre recombination in the periphery. To this end, we employed the CD4Cre strain that mediates a late-onset deletion in thymocytes and a constitutive recombination in peripheral CD4+ T cells (29, 54). Indeed, thymic cellularity and subset distribution were normal in the CD4Cre+ CKO mice (data not shown). However, the absolute numbers of both CD4+ and CD8+ peripheral T cells were reduced approximately twofold in these mice compared to that in controls ([15 ± 4.0] × 106, n = 12 versus [27 ± 8.5] × 106, n = 17; P = 0.00003), while B-cell numbers did not differ significantly (data not shown). These data suggest that the overall survival of Blm-deficient peripheral T cells is impaired even in a situation where the thymic output is normal.
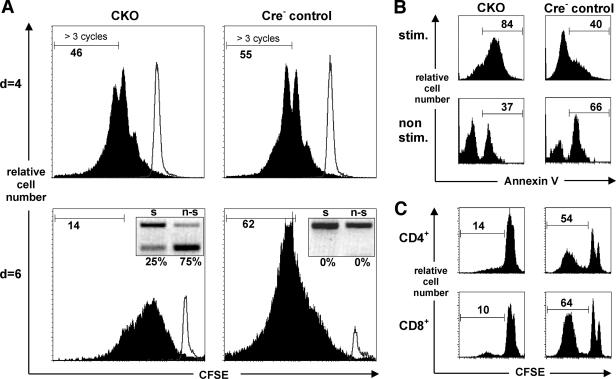
To directly analyze T-cell proliferation in the absence of Blm, we examined TCR-induced proliferation of peripheral T cells from CD4Cre+ CKO mice. To this end, CD4+ splenic T cells were purified, labeled with the vital dye CFSE, and stimulated with anti-CD3/anti-CD28 MAb in vitro. During the initial days of culture, the proliferation of Blm-deficient T cells was only marginally different from that of controls (Fig. 6A). However, after 6 days in culture, the proliferation of CKO T cells was significantly delayed, while the frequency of annexin V-positive apoptotic cells was increased (Fig. 6A and B). Furthermore, a pronounced selection against Blm-mutant T lymphocytes was observed in these cultures (Fig. 6A, 6-day insets), mirroring the counterselection observed in vivo (Fig. 5C). Therefore, our data indicate that mature Blm-deficient T cells initially respond to the proliferative signal yet fail to sustain prolonged proliferation.
FIG. 6.
Blm mutant mice show impaired T-cell responses in vivo and a reduced proliferative capacity of peripheral T cells. (A and B) Purified splenic CD4+ T cells from young CD4Cre+ CKO and Cre− control mice were labeled with CFSE and cultured alone or on plate-bound anti-CD3 plus anti-CD28 antibodies. Cells were harvested at day 4 or 6, as indicated, and analyzed for CFSE stain gating on CD4+ cells (A). Two independent experiments are shown. Inserts show genomic PCR analysis of Blm exon 8 deletion (s, stimulated; n-s, nonstimulated). (B) Annexin V staining of the cells (CD4+, 7-AAD−) harvested at day 6, displayed in panel A. The results shown are representative of five independent experiments (filled histograms or “stim,” stimulated; open histograms or “non stim,” nonstimulated). Note that CKO as well as control cells underwent considerable apoptosis in the nonstimulated cultures. (C) Nine-month-old CKO and Cre+ control mice were immunized with OVA emulsified in complete Freund's adjuvant. After 1 week, draining LN were harvested, and LN cell suspensions were labeled with CFSE and restimulated in vitro with OVA for six days, after which CD4+ and CD8+ T cells were analyzed for CFSE stain by flow cytometry. The histograms show the fraction of cells that had divided more than twice and are representative of eight and nine CKO and control mice, respectively.
To measure the antigen-specific T-cell response in vivo, we immunized CD4Cre+ CKO and control mice with OVA and measured OVA-induced T-cell proliferation in the regional LN using CFSE dilution. As illustrated in Fig. 6C, the fraction of responding T cells (i.e., those that divided more than twice) was significantly low in CKO compared to that in control cultures (CD4+, 27% ± 15% versus 53% ± 15%, P = 0.003; CD8+, 25% ± 17% versus 59% ± 15%, P = 0.0005; with n = 8 and n = 9 for CKO and control mice, respectively). Collectively, these data indicate that both developing and mature T cells require the Bloom's helicase for normal proliferation and survival, translating into a defective T-cell immunity in CKO mice.
Blm-deficient T lymphocytes accumulate chromosomal damage.
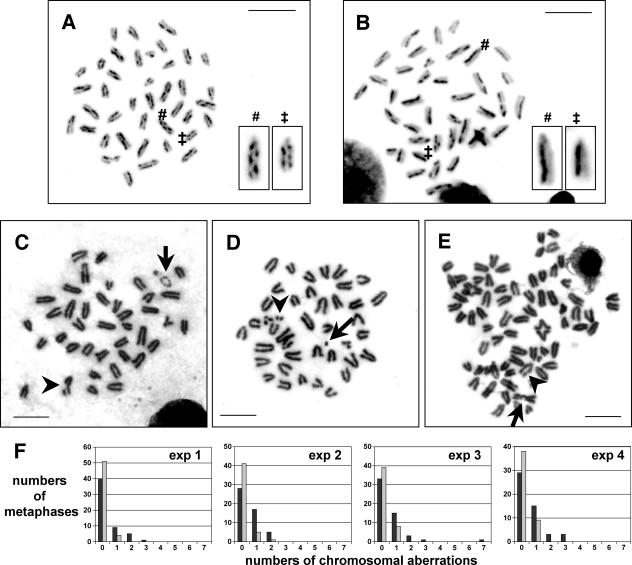
Blm-mutant cells show elevated levels of homologous recombination events (4, 14) and increased genomic instability (14, 34). We therefore tested the integrity of the T-cell genome in the absence of Blm. First, we analyzed T cells for a hallmark feature of Blm deficiency, elevated levels of SCE. Indeed, proliferating peripheral CD4+ T lymphocytes from CD4Cre+ CKO mice accumulated four times as many SCE events on average (2.4 ± 0.4 SCEs per chromosome; n = 636 chromosomes; 57 to 110 SCEs per metaphase) (Fig. 7A) than cells from Cre+ control mice (0.58 ± 0.2 SCEs per chromosome; n = 642 chromosomes; 11 to 40 SCEs per metaphase; P = 10−13) (Fig. 7B). To investigate chromosome integrity, metaphase spreads of proliferating CD4+ T cells were examined for chromosomal structural abnormalities. In four independent experiments, the fraction of aneuploid cells was consistently higher in the absence of Blm. Furthermore, Blm-deficient T cells harbored three to six times as many aberrant chromosome structures (e.g., small marker chromosomes, chromosome breaks, or translocations) as did control cells (Fig. 7C through F; also see Table S2 in the supplemental material). These findings reveal a prominent genome instability in Blm-deficient T cells, which likely provides the basis for their impaired proliferation and survival by inducing cell cycle arrest and apoptosis in a largely p53-dependent manner.
FIG. 7.
Elevated numbers of SCEs and high loads of chromosomal damage in Blm-deficient T cells. (A and B) Representative metaphase spreads from purified splenic CD4+ T cells treated with BrdU after 2 days of anti-CD3/anti-CD28-induced proliferation. SCE assays were performed 42 h later. The metaphase shown in panel A, derived from a CD4Cre+ CKO mouse, shows 89 SCEs in 37 analyzed chromosomes (2.4 SCEs/chromosome), while 26 SCEs were found in 38 analyzed chromosomes in the metaphase from a Cre+ control mouse (0.7 SCEs/chromosome) displayed in panel B. (C through E) Metaphase spreads of purified splenic CD4+ T cells from CD4Cre+ CKO mice after 5 days of anti-CD3/anti-CD28-induced proliferation with examples of chromosome breaks (panel C and E, arrowheads), a prematurely condensed chromosome (panel C, arrow), a small marker chromosome (panel D, arrow), double minutes (panel D, arrowhead), and a translocation (panel E, arrow). The metaphase shown in panel E had undergone endoreduplication. (F) Chromosomal aberrations were scored on metaphase spreads of cells from CKO (dark bars) and control cultures (light bars) in four separate experiments as described in panels C through E. See Table S2 in the supplemental material for summary and statistics.
A hallmark feature of BS is a greatly elevated risk for developing the range of malignancies seen in the general population, including lymphomas (14). We therefore observed a cohort of 130 mice for the possible development of lymphomas or enhanced mortality (see Fig. S4 in the supplemental material). After a period of 20 months, 17/26 (65%) pTαCre+ CKO mice were still alive and only 3 of the 7 autopsied animals harbored a lymphoma (with 2/9 having died of an unknown cause). Of the two lymphomas for which genotyping results could be obtained, only one had the deleted Blm allele. Seven of the 17 CKO mice that were sacrificed at the age of 20 months had developed small tumors, none of them thymic lymphomas. On the other hand, 88/104 (85%) of the Cre+ control and Cre− control mice survived to this age, none of them harboring a thymic lymphoma. Thus, despite their genomic instability, Blm-deficient T cells do not manifest a pronounced capacity for malignant transformation.
DISCUSSION
In this paper, we report severely disturbed development and maintenance of αβ T-lineage cells in the absence of the Bloom's syndrome helicase. While mice with a hypomorphic mutation of Blm are viable (30, 34), a complete germ line deletion of Blm is embryonic lethal (4). Thus, to study the role of Blm in the T-cell lineage, we induced deletion of a floxed Blm allele specifically in T lymphocytes by employing three T-cell-specific Cre-expressing mouse lines (29, 54). In particular, we used a newly generated Cre strain in which Cre recombinase is expressed from the pTa locus in a BAC transgene. The pTa gene is strongly upregulated in the newly committed DN2 thymocytes (43), and this expression pattern is fully recapitulated by BAC reporter transgenes (17, 42). Indeed, the pTaCre transgene mediated an efficient early deletion of the floxed Blm allele already at the DN2 stage (see Fig. S2 in the supplemental material), which in turn produced a more severe phenotype than the lckCre transgene (Fig. 1). Of note, we found that the pTaCre transgene in neutral reporter crosses, e.g., in Cre+ controls, often produces widespread Cre recombination in multiple tissues (see Fig. S1 in the supplemental material), suggesting its transient activation early in embryonic development. However, in CKO mice, these early recombinant cells appear to be counterselected, yielding a largely T-cell-specific deletion of Blm; notably, thymic epithelial cells were nondeleted, corroborating the cell-autonomous nature of the thymic phenotype observed (see Fig. S1 and S2 in the supplemental material). Therefore, the pTaCre strain may be useful in situations where a robust early recombination in the T-cell lineage is desirable. Since Cre expression itself may sometimes cause phenotypic effects due to its nuclease activity (5), we included Blm-proficient Cre+ controls in all experiments. In addition, the phenotype in the periphery of pTαCre+ CKO mice (Fig. 5C), where Cre expression is turned down in T cells, argues against a major Cre effect.
In both lckCre+ and pTαCre+ CKO mice, we found dramatically reduced thymic cellularities and a profound block at the β-selection checkpoint. Nevertheless, the recombination of Tcrb genes appeared normal, and indeed, the developmental block could not be rescued by a rearranged TCR transgene. Thus, our results suggest that Blm is dispensable for V(D)J recombination. Indeed, two previous studies reported a normal frequency and fidelity of VDJ coding junction formation in BS cells (24, 41). The joining of RAG-dependent double strand breaks is mediated by the nonhomologous end joining (NHEJ) pathway of DNA repair (49). Although increased levels of aberrant NHEJ-dependent double strand break repair were found in BS cells, they appear to have resulted from a compensatory shift away from the default homologous recombination pathway toward the intrinsically error-prone NHEJ pathway in the absence of BLM (11). Thus, our data support the notion that BLM is not involved in the NHEJ pathway and that the immune deficiency in BS is not secondary to the defective rearrangement of antigen receptor genes.
While Tcrb recombination and pre-TCR signaling appeared normal in Blm-deficient thymocytes, the proliferative response to the latter was strongly impaired. The proliferation of β-selected thymocytes between the DN3 and DP stages involves six to eight cell divisions and is primarily responsible for the generation of the T-cell compartment (40). Therefore, defective proliferation of Blm-deficient thymocytes at this stage is consistent with the observed severe reduction of thymocyte numbers. In addition, we documented increased cell death of Blm-deficient thymocytes, both following β-selection and later in development such as at the CD4SP stage. Similarly, mature Blm-deficient T cells were indeed generated and persisted in the quiescent state (Fig. 5C and as observed in the CD4Cre+ CKO mice) yet were counterselected during homeostatic proliferation. Furthermore, Blm-deficient mature T cells were impaired in their proliferation and survival following TCR signaling in vitro (Fig. 6A and B), resulting in impaired T-cell responses after antigen challenge (Fig. 6C). Altogether, our data reveal an essential role for Blm in the proliferation and survival of both developing and mature T lymphocytes, with its deletion leading to defective T-cell immunity.
The p53 protein is a key player in the response to stress impinging on DNA replication and cell division, and its activation may lead to cell cycle arrest and/or apoptosis (49). In the thymus, p53 is thought to mediate the apoptosis of DN3 thymocytes that failed to receive the pre-TCR signal (19, 20). Indeed, we found that the Trp53 deletion substantially rescued both the reduced cellularity and the developmental block caused by the absence of Blm. However, a contribution of p53-independent cell death to the observed phenotype is expected as physical and functional cooperation of the p53 and BLM proteins was shown to be necessary for normal induction of p53-dependent apoptosis (52). The incomplete rescue is likely due to the involvement of additional mechanisms such as FADD-dependent death receptor signaling in controlling thymocyte survival at this stage of development (39). On the other hand, Bcl-2 overexpression failed to rescue the block at the DN3 stage, consistent with its inability to prevent thymocyte death caused by defective pre-TCR signaling (20, 32). Collectively, these results provide genetic evidence for a prominent yet not exclusive role of apoptosis in the impeded development of Blm-deficient thymocytes.
The defective proliferation and survival of Blm-deficient thymocytes and peripheral T cells is consistent with the known cellular functions of BLM. Indeed, BLM is thought to facilitate DNA synthesis by removing “roadblocks” for replication forks (1). Therefore, the loss of BLM appears to hamper DNA replication as observed in BS cells (21) and consequently slows down cell division. More importantly, we demonstrate a high level of genome instability in Blm-deficient T cells, which likely contributes to their defective growth. In fact, one of the central functions of BLM is the suppression of excessive and/or illegitimate homologous recombination (25, 37). This function of BLM appears to be facilitated by its interactions with multiple proteins involved in genome maintenance, like topoisomerase IIIα or BRCA1 (1, 25, 53), and accounts for the hyperrecombination phenotype of BS cells. The latter is evident in the greatly increased number of sister chromatid exchanges, the hallmark of BLM deficiency (14), as observed here in cultured Blm-deficient T cells (Fig. 7A and B). Furthermore, BS cells are known to accumulate high loads of chromosomal aberrations (14), a feature mirrored in proliferating Blm-deficient T cells in our mouse model (Fig. 7C through F). Thus, our results indicate that Blm is a critical modulator of DNA replication and chromosome integrity in both developing and mature T lymphocytes.
The phenotype of the Blm CKO mice described here resembles that of a T-cell-specific deletion of Brca1 (31). Indeed, the latter resulted in the impaired proliferation and survival of thymocytes and mature T cells accompanied by chromosomal instability, whereas V(D)J recombination was normal. Moreover, the defective development of Brca1-deficient thymocytes was rescued on a Trp53-null background, albeit to a larger extent than observed here (31). It was shown that BLM interacts with BRCA1 within the BRCA1-associated genome surveillance complex, a multienzyme complex containing tumor suppressors and DNA damage repair proteins (53). Notably, neither the loss of Blm nor that of Brca1 increases the incidence of T-cell malignancies (as seen in reference 31 and this report), despite the well-established tumor suppressor function of both genes (22). This is likely due to the severely reduced thymic cellularity and the proliferative capacity of Blm-deficient or Brca1-deficient DN thymocytes, which substantially limit the pool of potentially transformed cells. Indeed, an increased incidence of lymphoma development was observed in mice with a hypomorphic allele of Blm which is more permissive for T-cell development, albeit rarely at a young age (30, 34). Furthermore, highly penetrant T-cell lymphomas arise in the absence of other tumor suppressor proteins such as p53 and Atm, neither of which cause a defect at the DN-to-DP transition (6, 8). Altogether, our data are consistent with the function of Blm and Brca1 in a common genome surveillance pathway and underscore the role of tumor suppressor proteins in the regulation of normal lymphocyte development and function.
Humans possess five RecQ helicases, three of which (BLM, WRN, and RECQ4) are associated with genomic instability disorders (Bloom's, Werner's, and Rothmund-Thomson syndromes, respectively [1]). However, a prominent immune deficiency is observed only in BS (1), suggesting a unique function of the BLM helicase in the immune system. The results of early work in BS patients indicated a functional impairment in both B- and T-cell lineages (14, 26, 47, 50). In view of our data, it appears likely that the immune deficiency in BS is based to a large extent on a T-cell defect. Indeed, decreased thymic output and reduced antigen-driven proliferation of Blm-deficient T cells undermine their ability to mount effective immune responses, as shown here for a model antigen-specific immune response. The possible functions of BLM helicase in other immune cell lineages and their contribution to the immune deficiency in BS patients remain to be elucidated in further studies.
Supplementary Material
Acknowledgments
We thank Jennifer McMenamin and Montserrat Michelman for excellent technical help, Chris Wilson for lckCre and CD4Cre mice, and members of the Leder and Rajewsky laboratories for helpful support and discussions.
This work was partially supported by the HHMI.
Footnotes
Published ahead of print on 8 January 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Bachrati, C. Z., and I. D. Hickson. 2003. RecQ helicases: suppressors of tumorigenesis and premature aging. Biochem. J. 374:577-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogue, M. A., C. Zhu, E. Aguilar-Cordova, L. A. Donehower, and D. B. Roth. 1996. p53 is required for both radiation-induced differentiation and rescue of V(D)J rearrangement in scid mouse thymocytes. Genes Dev. 10:553-565. [DOI] [PubMed] [Google Scholar]
- 3.Chester, N., H. Babbe, J. Pinkas, C. Manning, and P. Leder. 2006. Mutation of the murine Bloom's syndrome gene produces global genome destabilization. Mol. Cell. Biol. 26:6713-6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chester, N., F. Kuo, C. Kozak, C. D. O'Hara, and P. Leder. 1998. Stage-specific apoptosis, developmental delay, and embryonic lethality in mice homozygous for a targeted disruption in the murine Bloom's syndrome gene. Genes Dev. 12:3382-3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Alboran, I. M., R. C. O'Hagan, F. Gartner, B. Malynn, L. Davidson, R. Rickert, K. Rajewsky, R. A. DePinho, and F. W. Alt. 2001. Analysis of C-MYC function in normal cells via conditional gene-targeted mutation. Immunity 14:45-55. [DOI] [PubMed] [Google Scholar]
- 6.Donehower, L. A., M. Harvey, B. L. Slagle, M. J. McArthur, C. A. Montgomery, Jr., J. S. Butel, and A. Bradley. 1992. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356:215-221. [DOI] [PubMed] [Google Scholar]
- 7.Ellis, N. A., J. Groden, T. Z. Ye, J. Straughen, D. J. Lennon, S. Ciocci, M. Proytcheva, and J. German. 1995. The Bloom's syndrome gene product is homologous to RecQ helicases. Cell 83:655-666. [DOI] [PubMed] [Google Scholar]
- 8.Elson, A., Y. Wang, C. J. Daugherty, C. C. Morton, F. Zhou, J. Campos-Torres, and P. Leder. 1996. Pleiotropic defects in ataxia-telangiectasia protein-deficient mice. Proc. Natl. Acad. Sci. USA 93:13084-13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fehling, H. J., A. Krotkova, C. Saint-Ruf, and H. von Boehmer. 1995. Crucial role of the pre-T-cell receptor alpha gene in development of alpha beta but not gamma delta T cells. Nature 375:795-798. [DOI] [PubMed] [Google Scholar]
- 10.Gartner, F., F. W. Alt, R. Monroe, M. Chu, B. P. Sleckman, L. Davidson, and W. Swat. 1999. Immature thymocytes employ distinct signaling pathways for allelic exclusion versus differentiation and expansion. Immunity 10:537-546. [DOI] [PubMed] [Google Scholar]
- 11.Gaymes, T. J., P. S. North, N. Brady, I. D. Hickson, G. J. Mufti, and F. V. Rassool. 2002. Increased error-prone nonhomologous DNA end-joining—a proposed mechanism of chromosomal instability in Bloom's syndrome. Oncogene 21:2525-2533. [DOI] [PubMed] [Google Scholar]
- 12.Gennery, A. R., A. J. Cant, and P. A. Jeggo. 2000. Immunodeficiency associated with DNA repair defects. Clin. Exp. Immunol. 121:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Germain, R. N. 2002. T-cell development and the CD4-CD8 lineage decision. Nat. Rev. Immunol. 2:309-322. [DOI] [PubMed] [Google Scholar]
- 14.German, J. 1993. Bloom syndrome: a mendelian prototype of somatic mutational disease. Medicine (Baltimore) 72:393-406. [PubMed] [Google Scholar]
- 15.German, J. 1995. Bloom's syndrome. Dermatol. Clin. 13:7-18. [PubMed] [Google Scholar]
- 16.Goldrath, A. W. 2002. Maintaining the status quo: T-cell homeostasis. Microbes Infect. 4:539-545. [DOI] [PubMed] [Google Scholar]
- 17.Gounari, F., I. Aifantis, C. Martin, H. J. Fehling, S. Hoeflinger, P. Leder, H. von Boehmer, and B. Reizis. 2002. Tracing lymphopoiesis with the aid of a pTalpha-controlled reporter gene. Nat. Immunol. 3:489-496. [DOI] [PubMed] [Google Scholar]
- 18.Reference deleted.
- 19.Guidos, C. J., C. J. Williams, I. Grandal, G. Knowles, M. T. Huang, and J. S. Danska. 1996. V(D)J recombination activates a p53-dependent DNA damage checkpoint in scid lymphocyte precursors. Genes Dev. 10:2038-2054. [DOI] [PubMed] [Google Scholar]
- 20.Haks, M. C., P. Krimpenfort, J. Borst, and A. M. Kruisbeek. 1998. The CD3gamma chain is essential for development of both the TCRalphabeta and TCRgammadelta lineages. EMBO J. 17:1871-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hand, R., and J. German. 1975. A retarded rate of DNA chain growth in Bloom's syndrome. Proc. Natl. Acad. Sci. USA 72:758-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hickson, I. D. 2003. RecQ helicases: caretakers of the genome. Nat. Rev. Cancer 3:169-178. [DOI] [PubMed] [Google Scholar]
- 23.Hoffman, E. S., L. Passoni, T. Crompton, T. M. Leu, D. G. Schatz, A. Koff, M. J. Owen, and A. C. Hayday. 1996. Productive T-cell receptor beta-chain gene rearrangement: coincident regulation of cell cycle and clonality during development in vivo. Genes Dev. 10:948-962. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh, C. L., C. F. Arlett, and M. R. Lieber. 1993. V(D)J recombination in ataxia telangiectasia, Bloom's syndrome, and a DNA ligase I-associated immunodeficiency disorder. J. Biol. Chem. 268:20105-20109. [PubMed] [Google Scholar]
- 25.Hu, P., S. F. Beresten, A. J. van Brabant, T. Z. Ye, P. P. Pandolfi, F. B. Johnson, L. Guarente, and N. A. Ellis. 2001. Evidence for BLM and topoisomerase IIIalpha interaction in genomic stability. Hum. Mol. Genet. 10:1287-1298. [DOI] [PubMed] [Google Scholar]
- 26.Hutteroth, T. H., S. D. Litwin, and J. German. 1975. Abnormal immune responses of Bloom's syndrome lymphocytes in vitro. J. Clin. Investig. 56:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang, D., M. J. Lenardo, and J. C. Zuniga-Pflucker. 1996. p53 prevents maturation to the CD4+CD8+ stage of thymocyte differentiation in the absence of T cell receptor rearrangement. J. Exp. Med. 183:1923-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaye, J., M. L. Hsu, M. E. Sauron, S. C. Jameson, N. R. Gascoigne, and S. M. Hedrick. 1989. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature 341:746-749. [DOI] [PubMed] [Google Scholar]
- 29.Lee, P. P., D. R. Fitzpatrick, C. Beard, H. K. Jessup, S. Lehar, K. W. Makar, M. Perez-Melgosa, M. T. Sweetser, M. S. Schlissel, S. Nguyen, S. R. Cherry, J. H. Tsai, S. M. Tucker, W. M. Weaver, A. Kelso, R. Jaenisch, and C. B. Wilson. 2001. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 15:763-774. [DOI] [PubMed] [Google Scholar]
- 30.Luo, G., I. M. Santoro, L. D. McDaniel, I. Nishijima, M. Mills, H. Youssoufian, H. Vogel, R. A. Schultz, and A. Bradley. 2000. Cancer predisposition caused by elevated mitotic recombination in Bloom mice. Nat. Genet. 26:424-429. [DOI] [PubMed] [Google Scholar]
- 31.Mak, T. W., A. Hakem, J. P. McPherson, A. Shehabeldin, E. Zablocki, E. Migon, G. S. Duncan, D. Bouchard, A. Wakeham, A. Cheung, J. Karaskova, I. Sarosi, J. Squire, J. Marth, and R. Hakem. 2000. Brcal required for T cell lineage development but not TCR loci rearrangement. Nat. Immunol. 1:77-82. [DOI] [PubMed] [Google Scholar]
- 32.Maraskovsky, E., L. A. O'Reilly, M. Teepe, L. M. Corcoran, J. J. Peschon, and A. Strasser. 1997. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor-deficient mice but not in mutant rag-1−/− mice. Cell 89:1011-1019. [DOI] [PubMed] [Google Scholar]
- 33.Marsden, V. S., and A. Strasser. 2003. Control of apoptosis in the immune system: Bcl-2, BH3-only proteins and more. Annu. Rev. Immunol. 21:71-105. [DOI] [PubMed] [Google Scholar]
- 34.McDaniel, L. D., N. Chester, M. Watson, A. D. Borowsky, P. Leder, and R. A. Schultz. 2003. Chromosome instability and tumor predisposition inversely correlate with BLM protein levels. DNA Repair (Amsterdam) 2:1387-1404. [DOI] [PubMed] [Google Scholar]
- 35.Mombaerts, P., A. R. Clarke, M. A. Rudnicki, J. Iacomini, S. Itohara, J. J. Lafaille, L. Wang, Y. Ichikawa, R. Jaenisch, M. L. Hooper, et al. 1992. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature 360:225-231. [DOI] [PubMed] [Google Scholar]
- 36.Reference deleted.
- 37.Myung, K., A. Datta, C. Chen, and R. D. Kolodner. 2001. SGS1, the Saccharomyces cerevisiae homologue of BLM and WRN, suppresses genome instability and homeologous recombination. Nat. Genet. 27:113-116. [DOI] [PubMed] [Google Scholar]
- 38.Nacht, M., A. Strasser, Y. R. Chan, A. W. Harris, M. Schlissel, R. T. Bronson, and T. Jacks. 1996. Mutations in the p53 and SCID genes cooperate in tumorigenesis. Genes Dev. 10:2055-2066. [DOI] [PubMed] [Google Scholar]
- 39.Newton, K., C. Kurts, A. W. Harris, and A. Strasser. 2001. Effects of a dominant interfering mutant of FADD on signal transduction in activated T cells. Curr. Biol. 11:273-276. [DOI] [PubMed] [Google Scholar]
- 40.Penit, C., B. Lucas, and F. Vasseur. 1995. Cell expansion and growth arrest phases during the transition from precursor (CD4−8−) to immature (CD4+8+) thymocytes in normal and genetically modified mice. J. Immunol. 154:5103-5113. [PubMed] [Google Scholar]
- 41.Petrini, J. H., J. W. Donovan, C. Dimare, and D. T. Weaver. 1994. Normal V(D)J coding junction formation in DNA ligase I deficiency syndromes. J. Immunol. 152:176-183. [PubMed] [Google Scholar]
- 42.Reizis, B., and P. Leder. 2001. The upstream enhancer is necessary and sufficient for the expression of the pre-T cell receptor alpha gene in immature T lymphocytes. J. Exp. Med. 194:979-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saint-Ruf, C., K. Ungewiss, M. Groettrup, L. Bruno, H. J. Fehling, and H. von Boehmer. 1994. Analysis and expression of a cloned pre-T cell receptor gene. Science 266:1208-1212. [DOI] [PubMed] [Google Scholar]
- 44.Sentman, C. L., J. R. Shutter, D. Hockenbery, O. Kanagawa, and S. J. Korsmeyer. 1991. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell 67:879-888. [DOI] [PubMed] [Google Scholar]
- 45.Shinkai, Y., G. Rathbun, K. P. Lam, E. M. Oltz, V. Stewart, M. Mendelsohn, J. Charron, M. Datta, F. Young, A. M. Stall, et al. 1992. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68:855-867. [DOI] [PubMed] [Google Scholar]
- 46.Taghon, T., M. A. Yui, R. Pant, R. A. Diamond, and E. V. Rothenberg. 2006. Developmental and molecular characterization of emerging beta- and gammadelta-selected pre-T cells in the adult mouse thymus. Immunity 24:53-64. [DOI] [PubMed] [Google Scholar]
- 47.Taniguchi, N., M. Mukai, T. Nagaoki, T. Miyawaki, N. Moriya, H. Takahashi, and N. Kondo. 1982. Impaired B-cell differentiation and T-cell regulatory function in four patients with Bloom's syndrome. Clin. Immunol. Immunopathol. 22:247-258. [DOI] [PubMed] [Google Scholar]
- 48.Turley, H., L. Wu, M. Canamero, K. C. Gatter, and I. D. Hickson. 2001. The distribution and expression of the Bloom's syndrome gene product in normal and neoplastic human cells. Br. J. Cancer 85:261-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Gent, D. C., J. H. Hoeijmakers, and R. Kanaar. 2001. Chromosomal stability and the DNA double-stranded break connection. Nat. Rev. Genet. 2:196-206. [DOI] [PubMed] [Google Scholar]
- 50.Van Kerckhove, C. W., J. L. Ceuppens, M. Vanderschueren-Lodeweyckx, E. Eggermont, S. Vertessen, and E. A. Stevens. 1988. Bloom's syndrome. Clinical features and immunologic abnormalities of four patients. Am. J. Dis. Child. 142:1089-1093. [DOI] [PubMed] [Google Scholar]
- 51.von Boehmer, H., and H. J. Fehling. 1997. Structure and function of the pre-T cell receptor. Annu. Rev. Immunol. 15:433-452. [DOI] [PubMed] [Google Scholar]
- 52.Wang, X. W., A. Tseng, N. A. Ellis, E. A. Spillare, S. P. Linke, A. I. Robles, H. Seker, Q. Yang, P. Hu, S. Beresten, N. A. Bemmels, S. Garfield, and C. C. Harris. 2001. Functional interaction of p53 and BLM DNA helicase in apoptosis. J. Biol. Chem. 276:32948-32955. [DOI] [PubMed] [Google Scholar]
- 53.Wang, Y., D. Cortez, P. Yazdi, N. Neff, S. J. Elledge, and J. Qin. 2000. BASC, a super complex of BRCA1-associated proteins involved in the recognition and repair of aberrant DNA structures. Genes Dev. 14:927-939. [PMC free article] [PubMed] [Google Scholar]
- 54.Wolfer, A., A. Wilson, M. Nemir, H. R. MacDonald, and F. Radtke. 2002. Inactivation of notch1 impairs VDJbeta rearrangement and allows pre-TCR-independent survival of early alpha beta lineage thymocytes. Immunity 16:869-879. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.