Abstract
p120-catenin is an adherens junction-associated protein that controls E-cadherin function and stability. p120-catenin also binds intracellular proteins, such as the small GTPase RhoA. In this paper, we identify the p120-catenin N-terminal regulatory domain as the docking site for RhoA. Moreover, we demonstrate that the binding of RhoA to p120-catenin is tightly controlled by the Src family-dependent phosphorylation of p120-catenin on tyrosine residues. The phosphorylation induced by Src and Fyn tyrosine kinases on p120-catenin induces opposite effects on RhoA binding. Fyn, by phosphorylating a residue located in the regulatory domain of p120-catenin (Tyr112), inhibits the interaction of this protein with RhoA. By contrast, the phosphorylation of Tyr217 and Tyr228 by Src promotes a better affinity of p120-catenin towards RhoA. In agreement with these biochemical data, results obtained in cell lines support the important role of these phosphorylation sites in the regulation of RhoA activity by p120-catenin. Taken together, these observations uncover a new regulatory mechanism acting on p120-catenin that contributes to the fine-tuned regulation of the RhoA pathways during specific signaling events.
E-cadherin function is controlled posttranslationally by a family of proteins, named catenins, that bind to its cytosolic tail. Two members of this family, p120-catenin and β-catenin, interact at different sites of the E-cadherin molecule and are engaged in distinct functions. Whereas β-catenin is required for recruiting the actin cytoskeleton, p120-catenin is necessary for the stabilization of E-cadherin at the cell membrane (3). As a consequence, E-cadherin is rapidly internalized and degraded in the absence of p120-catenin (7, 13). Consequently, p120-catenin ablation in vivo causes E-cadherin deficiency, leading to severe defects in adhesion, cell polarity, and epithelial morphogenesis (7).
Besides this role in regulating E-cadherin stability, p120-catenin interacts with other proteins involved in the modulation of cell-to-cell contacts. For instance, p120-catenin associates with Fer and Fyn tyrosine kinases (16, 27, 36). These kinases specifically phosphorylate β-catenin in Tyr142 (27), a modification that promotes release of β-catenin from the adherens junctional complex and transport to the nucleus (2, 27). Moreover, p120-catenin can interact with the Yes tyrosine kinase (27) and with a number of phosphotyrosine (PTyr) phosphatases, such as PTPμ (39), DEP1 (12), and SHP-1 (14, 21). These multiple associations suggest a role for p120-catenin as a scaffold protein for enzymes regulating events such as the stability of the adherens junctional complex (29).
p120-catenin modulates the activity of other cellular factors. Similarly to β-catenin, it can be detected in the nucleus (34), where it interacts with the transcriptional factor Kaiso (6). Studies performed with Xenopus laevis have demonstrated that association of p120-catenin relieves the repression caused by Kaiso on Wnt signaling (17, 25).
Several results indicate that p120-catenin can also control the activity of small GTPases. For instance, overexpression of p120-catenin represses RhoA activity (1, 23) and activates Rac1 (10, 23). Effects on RhoA have been attributed to the ability of p120-catenin to behave as a Rho guanine nucleotide dissociation inhibitor (RhoGDI), a biological activity that inhibits RhoA activity by blocking its normal exchange of guanosine nucleotides (1). The direct interaction of p120-catenin and RhoA has also been detected in living cells (20). The activation of Rac1 seems to be more indirect, occurring through the interaction of p120-catenin with the guanosine nucleotide exchange factor (GEF) Vav2 (23). It has been shown recently that repression of Rho activity by p120-catenin affects the activation of NF-κB transcriptional factor, since epidermal cells from conditional p120-catenin null mice activate nuclear NF-κB (26).
p120-catenin contains a central armadillo domain with 10 tandem 42-amino-acid repeats that is responsible for binding E-cadherin and a 325-amino-acid-long N-terminal regulatory domain. The latter region has several tyrosine residues that can be phosphorylated in vivo by tyrosine kinases (see Fig. 1A). Despite this evidence, the role of tyrosine phosphorylation in the association of p120-catenin with the different cofactors remains unknown except in the case of E-cadherin: phosphorylation of p120-catenin by Src increases the in vitro association of these two proteins (30). In this article, we present new results demonstrating that Src and Fyn can phosphorylate the regulatory domain of p120-catenin with different functional outcomes. Moreover, we have identified Tyr112, a residue of p120-catenin specifically phosphorylated by Fyn, as a key regulator of the p120-catenin-RhoA interaction both in vitro and in vivo.
FIG. 1.
RhoA interacts with the N-terminal regulatory domain of p120-catenin. (A) Diagram of the different domains of p120-catenin. Alternative splicing in the N-terminal domain gives rise to isoforms 1, 2, 3, and 4, each initiating at distinct ATG start codons (1, 55, 102, and 340, respectively) (arrows). The regulatory domain contains nine tyrosines (Y96, Y112, Y217, Y228, Y257, Y280, Y291, Y296, and Y302) capable of being phosphorylated in vitro (15, 17). The length and composition of the protein fragments used in this work are shown. (B) GST fusion proteins (1.5 pmol) were incubated with 5 pmol of RhoA. Protein complexes were affinity purified with glutathione-Sepharose and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting (WB) with anti-RhoA MAb. RhoA (1.2 pmol) was included as an internal reference standard (St). When indicated, the assay was performed in the presence of a 100-fold molecular excess of cytoEcad. Blots were reanalyzed with anti-GST to ensure that similar amounts of fusion proteins were present in all cases. (C) GST-p120-catenin fusion proteins or GST-β-catenin (7 pmol) was incubated with 270 μg of whole-cell extracts from RWP-1 cells. Protein complexes were affinity purified as described above and analyzed with anti-E-cadherin and anti-GST MAbs. In the Input lane, 5% of the total cell extract used for the assay was loaded. (D) Either GST-cytoEcad or GST (1.2 pmol) was incubated with 30 pmol of RhoA in the presence of 3 pmol of p120-catenin-1 when indicated. Protein complexes were affinity purified with glutathione-Sepharose and analyzed with specific MAbs. p120-catenin (0.6 pmol) was included as an internal reference standard. In panels B, C, and D, the numbers below the lanes correspond to the amounts of bound RhoA, E-cadherin, or p120-catenin, respectively, determined after densitometry, compared to the standard or to the amount bound to full-length GST-p120-catenin. The results presented in this figure correspond to an experiment representative of three performed.
MATERIALS AND METHODS
Generation of p120-catenin mutants.
The full-length coding region of mouse p120Cas1B (obtained from Albert Reynolds, Vanderbilt University) was cloned into the EcoRV site of cDNA3.1 (Invitrogen). A DNA fragment encoding the entire open reading frame of mouse p120-catenin was released from pcDNA3.1-p120-catenin(1-911) (fragment comprising amino acids 1 to 911) by EcoRI and NotI digestion and cloned into EcoRI/NotI-linearized pGEX-6P3 (GE Healthcare). To generate the bacterial expression plasmid pGEX, encoding a glutathione S-transferase (GST) protein fused to the p120-catenin region encompassing amino acids 234 to 911, the cDNA fragment was released from pcDNA3-Cas1 plasmid by digestion with SmaI/NotI and cloned into SmaI/NotI-linearized pGEX-6P1. The armadillo repeat domain of p120-catenin (amino acids 350 to 824) was amplified by PCR using as a template the pcDNA3.1-p120-catenin plasmid and primers annealing to the nucleotide sequences 1050 to 1064 and 2457 to 2472. To allow the subsequent cloning step, these oligonucleotides were modified to include EcoRI and EcoRV sites at their respective 5′ ends. The 1.4-kbp-long cDNA fragment obtained was then digested with those enzymes and cloned into pGEX-6P1 previously digested with EcoRI/SmaI. The 0.8-kbp-long p120-catenin cDNA fragment encompassing amino acids 350 to 911 was generated by cutting the pGEX-p120-catenin(1-911) plasmid with XhoI and NotI and subsequently ligating it to the XhoI/NotI-digested p120-catenin(350-824) plasmid. A DNA fragment encoding amino acids 1 to 234 of p120-catenin was amplified by PCR from pcDNA3.1-p120-catenin by using appropriate primers containing either EcoRI or BamHI sites at their 5′ ends. The 0.7-kbp amplification fragment was then digested with EcoRI and BamHI and cloned into the same sites of pGEX-6P2. To generate p120-catenin(1-96), the pGEX-p120-catenin(1-911) plasmid was digested with NotI and BsrGI, filled in, and religated. The fragment of p120-catenin from amino acids 102 to 911 was obtained by PCR using oligonucleotides including restriction sites for EcoRI and SacII at their 5′ ends. The PCR product was digested with SacII, and overhang ends were filled with Klenow polymerase. The product was then digested with EcoRI and ligated in pGEX-6P1 that was digested with EcoRI and SmaI. The pGEX-6P3-RhoA plasmid was generated from pGEX-4T3-RhoA, containing the full-length human RhoA sequence, by standard cloning procedures using the flanking EcoRI and BamHI sites present in the plasmid polylinkers.
p120-catenin point mutants (Y96E, Y96F, Y112E, Y112F, Y217E, Y217F, Y228E, and Y228F mutants) were obtained using a QuikChange site-directed mutagenesis kit (Stratagene) according to the manufacturer's instructions and either phrGFP-p120-catenin wild type (where GFP is green fluorescent protein) or p120-catenin(1-234) as the template. The sense primers used for the generation of the above-described mutants were, respectively, 5′-AACCACCTTCTGGAAAGCACTATCCCC-3′, 5′-AACCACCTTCTGTTTAGCACTATCCCC-3′, 5′-ATTGTGGAAACCGAAACCGAG GAGGAC-3′, 5′-ATTGTGGAAACCTTTACCGAGGAGGAC-3′, 5′-GGGTATGGCC GACACGAAGAAGATGGTTATCC-3′ , 5′-GGGTATGGCCGACACTTTGAAGA TGGTTATCC-3′, 5′-TGGCAGTGACAACGAAGGCAGTCTGTCC-3′, and 5′-TGGCAGTGACAACTTTGGCAGTCTGTCC-3′. Nucleotides modified with respect to the p120-catenin sequence (GenBank accession number Z17804) are indicated in bold.
Generation of GFP-p120-catenin constructs.
A DNA fragment encoding amino acid 8 to the C terminus of p120-catenin was obtained from pGEX-p120-catenin(1-911) by using oligonucleotides corresponding to nucleotide sequences 22 to 36 and 2717 to 2733 that contained EcoRI and SacII sites at the respective 5′ ends. The 2.7-kbp amplification fragment was digested with EcoRI and SacII and cloned in the same sites of phrGFP-N1 (Stratagene). phrGFP-p120-catenin(234-911) was generated from pGEX-p120-catenin(1-911) by using oligonucleotides corresponding to nucleotide sequences 693 to 704 and 2717 to 2733 that contained EcoRI and SacII sites at the respective 5′ ends. The 2-kbp amplification fragment was digested with EcoRI and SacII and cloned in the same sites of phrGFP-N1.
Preparation of recombinant RhoA and loading with GDP or GTP.
GST fusion proteins containing full-length human RhoA were expressed in Escherichia coli and purified by affinity chromatography on glutathione-Sepharose. GST-RhoA was first incubated with 200 μl of binding buffer (20 mM Tris, pH 7.5, 100 mM EDTA, 100 mM NaCl, 0.1% Triton X-100, 1 mM dithiothreitol [DTT], 10 μg/ml aprotinin, and 1 mM phenylmethylsulfonyl fluoride) for 30 min at room temperature in order to release the bound nucleotides. For GDP or GTP binding, the same buffer was supplemented with 50 mM MgCl2 plus GDP or GTP-γ-S (450 μM) (Sigma) and proteins were incubated for 25 min at room temperature. Loaded RhoA bound to Sepharose beads was washed with binding buffer in order to eliminate unbound nucleotides. Beads containing the loaded GTPase were used for binding assays to p120-catenin as described below. When required, the fusion protein was cleaved with PreScission protease (GE Healthcare) to remove the GST tag.
Protein binding assays.
GST fusion proteins corresponding to the indicated forms of p120-catenin, the cytoplasmic domain of E-cadherin (cytoEcad), or β-catenin were expressed in E. coli and purified by affinity chromatography on glutathione-Sepharose (30). Binding assays were carried out by incubating the indicated protein with a constant amount of either GST fusion protein used as bait or the nonchimeric GST used as a control. Details of these assays have already been described (27, 30). Pull-down assays were performed by using purified recombinant proteins fused to GST and cell extracts from RWP-1 cells, NIH 3T3 cells, and IEC-18 cells overexpressing K-ras oncogene (IEC-K-Ras cells), as reported previously (30). Glutathione-Sepharose-bound proteins were analyzed by Western blotting with specific monoclonal antibodies (MAbs) against p120-catenin, RhoA, E-cadherin, Fyn, PTyr, p120-catenin pY96, p120-catenin pY228 (all from Transduction Laboratories), AU5 tag (Covance), and GFP (Clontech). The polyclonal antibody to the GST protein was from GE Healthcare. Serial immunoblot assays were performed after stripping the membranes. All binding assays were repeated three times. In order to quantify the amount of bound protein, the autoradiograms were scanned in a densitometer.
Cell fractionation and immunoprecipitation.
Cytosolic fractions were prepared by homogenizing cells in buffer C (25 mM Tris-HCl, pH 7.6, 200 mM NaCl, 1 mM EGTA, 1 mM DTT, 5 mM MgCl2) supplemented with protease inhibitors (0.3 μM aprotinin, 1 μM leupeptin, 1 μM pepstatin,1 mM Pefabloc) and phosphatase inhibitors (10 mM NaF, 0.1 mM sodium orthovanadate). Cell homogenates were centrifuged at 20,000 × g for 5 min at 4°C to obtain the cytosolic fractions. Pellets were resuspended in the same volume of radioimmunoprecipitation assay buffer (25 mM Tris-HCl, pH 7.6, 200 mM NaCl, 1 mM EGTA, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1% Nonidet P-40) supplemented with protease and phosphatase inhibitors. After passing cells 10 times by a 20-gauge syringe, extracts were left on ice for 20 min and centrifuged at 20,000 × g for 5 min at 4°C. Supernatants constituted the membrane-associated fraction. Alternatively, cell extracts were prepared by resuspending cells in buffer D (50 mM Tris-HCl, pH 7.6, 200 mM NaCl, 5 mM MgCl2, 0.1% Nonidet P-40, 1 mM DTT) supplemented with protease and phosphatase inhibitors. Lysates were centrifuged at 12,000 × g in a microfuge for 15 min at 4°C.
Proteins were immunoprecipitated from cell extracts (100 to 300 μg) by using 4 μg/ml of the appropriate antibody for 16 h at 4°C and then collected using 30 μl of protein A-agarose (Sigma). Immunoprecipitates were washed three times with lysis buffer and proteins bound to beads directly eluted with electrophoresis sample buffer and analyzed by Western blotting. When appropriate, mouse TrueBlot reagent (BD Biosciences) was used as a secondary antibody in order to eliminate interferences in the autoradiogram.
Fluorescence microscopy.
NIH 3T3 cells were transfected with GFP-p120-catenin wild type, GFP-p120-catenin Y112E, GFP-p120-catenin Y217E, and GFP-p120-catenin(234-end) by using Lipofectamine (Life Technologies) according to the manufacturer's instructions. Cells were plated on glass coverslips and fixed with 4% paraformaldehyde 2 days after transfection. After 20 min, coverslips were washed with phosphate-buffered saline for 5 min and with water for 3 min. Coverslips were mounted onto glass slides and cells visualized by confocal microscopy. Rhodamine-conjugated phalloidin (Sigma) was used to visualize F-actin distribution.
Kinase assays.
c-Src and recombinant Fyn kinase were purchased from Upstate Biotechnology. Fer kinase was cloned in pcDNA3-His(C) (Invitrogen) that labeled the protein with Xpress and poly-His tags, transfected to RWP-1 cells, and purified by nickel-agarose chromatography (27). Phosphorylation assays were performed in a final volume of 50 μl of kinase buffer (25 mM Tris-HCl, pH 6.8, 25 mM MgCl2, 5 mM MnCl2, 0.5 mM EGTA, 1 mM DTT, 0.25 mM sodium orthovanadate, 0.1 mM ATP) for either 2 h at 23°C (in the case of Src assays) or 1 h at 30°C (in the case of Fer and Fyn assays). pEF-Bos-active Fyn was a kind gift of A. Carrera (Centro Nacional de Biotecnología, Madrid, Spain).
GDP dissociation assays.
The effect of purified GST-p120-catenin on the dissociation of [3H]GDP from RhoA was examined by preloading GST-RhoA (20 pmol) with [3H]GDP (1.5 μM, 1,000 cpm/pmol) for 10 min at 30°C. Incubations were performed in the presence of 25 mM Tris-HCl, pH 7.8, 120 mM NaCl, 1 mM EDTA, and 3 mM MgCl2 in a final volume of 100 μl. [3H]GDP-RhoA was incubated with equimolar amounts of p120-catenin at 30°C for 10 min. An excess of GTP (10 μM) was then added to allow the exchange of bound [3H]GDP-RhoA for GTP for 10 additional minutes. [3H]GDP-bound RhoA was separated from free nucleotide by filtration, and the amount of RhoA bound to [3H]GDP was determined and compared to the radionucleotide bound before the excess of unlabeled GTP was added.
RESULTS
RhoA binds to the regulatory domain of p120-catenin.
In order to study the binding of p120-catenin to RhoA and E-cadherin, we expressed several p120-catenin deletion mutants as GST fusion proteins (Fig. 1A). Binding assays demonstrated that RhoA interacts similarly with a p120-catenin fragment comprising amino acids 1 to 234 and the full-length protein (Fig. 1B, lanes 2 and 5). No association with a protein containing the sequence from amino acid 234 to the end or the first 96 amino acids of p120-catenin was observed (Fig. 1B, lanes 4 and 6). A p120-catenin form comprising amino acids 102 to the end, which corresponds to isoform 3, bound RhoA similarly to the full-length protein (Fig. 1B, right panel, lanes 2 and 3). Therefore, these results suggest that the binding site for RhoA in p120-catenin was mapped in the regulatory domain of p120-catenin, probably between amino acids 102 and 234. As has been proposed previously, E-cadherin bound to a protein fragment comprising amino acids 350 to 824, including the armadillo repeat domain (Fig. 1C, lane 5). The interaction of E-cadherin with this domain was twofold stronger than its association with the full-length p120-catenin (Fig. 1C, lanes 3 and 5), similarly to that shown for β-catenin (Fig. 1C, lanes 2 and 5). As expected, E-cadherin did not bind to p120-catenin(1-234) (data not shown). E-cadherin and RhoA could be detected interacting simultaneously with p120-catenin, further suggesting that those proteins bind to different regions of p120-catenin (Fig. 1B, lane 3). Additional binding experiments demonstrated that RhoA associated to GST-cytoEcad only when p120-catenin was present (Fig. 1D, lane 3), indicating that a complex of the three proteins can be formed.
Phosphorylation of the regulatory domain by different kinases affects binding of p120-catenin to RhoA and E-cadherin.
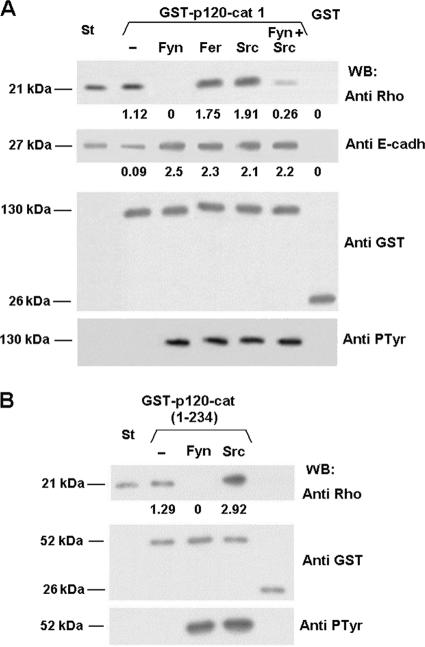
Our results indicate that RhoA associates to the regulatory domain of p120-catenin, a sequence known to be heavily phosphorylated by several tyrosine kinases (21, 22, 24). These observations led us to check whether this interaction might be modulated by tyrosine phosphorylation. To this end, we analyzed the effect of recombinant Src and Fyn tyrosine kinases and of immunopurified Fer tyrosine kinase on the p120-catenin-RhoA interaction. Surprisingly, whereas phosphorylation by Fer and Src kinase stimulated p120-catenin-RhoA binding (Fig. 2A, upper panel, compare lane 2 with lanes 4 and 5), Fyn totally prevented it (Fig. 2A, upper panel, lane 3). Fyn had a dominant effect on this regulation, since simultaneous phosphorylation of p120-catenin by Src and Fyn diminished the amount of RhoA associated to this protein with respect to the control, unphosphorylated protein (Fig. 2A, upper panel, compare lanes 2 and 6). Similar results were observed when the effects of Fyn and Src on the binding of RhoA to the N-terminal p120-catenin(1-234) fragment were analyzed (Fig. 2B, upper panel, lanes 2, 3, and 4), indicating that the relevant tyrosine residues under the control of this interaction were placed within this sequence. Effects of the kinases were dependent on the addition of ATP (data not shown), demonstrating that although Fyn interacts with this domain, repression of RhoA binding is not due to steric competition.
FIG. 2.
Tyrosine phosphorylation of p120-catenin modulates its interaction with RhoA. Full-length GST-p120-catenin (A) or GST-p120-catenin(1-234) (B) was phosphorylated with Fyn, Fer, Src, or Fyn and Src tyrosine kinases, as described in Materials and Methods. Control and phosphorylated GST-p120-catenin proteins or GST as a control (1.5 pmol) were incubated with 5 pmol of RhoA (upper panel) or with 2 pmol cytoEcad (middle panel) in a final volume of 200 μl. Protein complexes were affinity purified with glutathione-Sepharose and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting (WB) with specific MAbs. RhoA (1 pmol) (A and B) or cytoEcad (0.1 pmol) (A) were included as internal reference standards (St). The numbers below the lanes correspond to the amounts of RhoA or E-cadherin bound to GST-p120-catenin fusion proteins compared to the standard. The results presented in this figure correspond to an experiment representative of at least three performed.
The effect of the phosphorylation of p120-catenin by these kinases on E-cadherin binding was also determined. In the three cases studied, phosphorylation promoted an increased association between p120-catenin and E-cadherin (Fig. 2A, second panel from top, compare lane 2 with lanes 3, 4, and 5). This effect was not observed when binding of E-cadherin to the armadillo repeat domain of p120-catenin (amino acids 350 to 824) was analyzed, since this domain was not phosphorylated by any of these kinases under our experimental conditions (data not shown).
Fyn-mediated phosphorylation of Tyr112 of p120-catenin interferes with its binding to RhoA.
Since the effect of Fyn on p120-catenin-RhoA binding was dominant, we reasoned that this kinase was probably targeting a Tyr residue not recognized by Src. Therefore, we searched for a Tyr residue in the p120-catenin(1-234) amino acid sequence modified differently by Fyn and Src kinases. Four Tyr residues have previously been reported to be phosphorylated in this region: Tyr96, Tyr112, Tyr217, and Tyr228 (Fig. 3A) (21, 24). Other putative phosphorylation site tyrosines of the regulatory domain are located downstream of amino acid residue 234. Taking advantage of the availability of monoclonal antibodies specific for PTyr96 and PTyr228, we first checked whether either of these two residues was phosphorylated differently by Fyn and Src. These two residues were modified similarly by both kinases (see Fig. SA1 in the supplemental material), ruling out the possibility that modification of either of these two amino acids is responsible for the different effects of Fyn.
FIG. 3.
Phosphorylation of Tyr112 inhibits the p120-catenin-RhoA interaction. (A) Diagram of the N-terminal regulatory domain of p120-catenin(1-347). Tyrosine residues phosphorylated by tyrosine kinases are indicated (Y96, Y112, Y217, Y228, Y257, Y280, Y291, Y296, and Y302). (B) Mapping of the residues involved in Src and Fyn phosphorylation of p120-catenin. Various amounts (2 pmol in the case of Fyn and 4 pmol in the case of Src) of different GST-p120-cat(1-234) point mutants were phosphorylated with Fyn (left panel) or Src (right panel). Phosphorylation was analyzed by Western blotting (WB) with an anti-PTyr MAb. The membrane was stripped and reanalyzed against GST to check that similar levels of the different GST-p120-cat(1-234) point mutants were used. (C) RhoA-binding assays were performed with 5 pmol of RhoA and 5 pmol of GST or GST-p120-cat(1-234) fusion proteins corresponding to the wild-type (WT) form, the Y96F Y228F double mutant, or the Y96F Y228F Y112F triple mutant. When indicated, the fusion proteins were phosphorylated with recombinant Fyn or Src tyrosine kinases. Protein complexes were affinity purified with glutathione-Sepharose and analyzed by Western blotting with anti-RhoA. RhoA (1 pmol [left panel] or 0.4 pmol [right panel]) was included as an internal reference standard (St). (D) GST-p120-catenin and Y112E, Y217E, and Y228E point mutants (1.5 pmol) were incubated with 5 pmol of RhoA in a final volume of 200 μl, as indicated above. RhoA (1 pmol [left panel] or 2.5 pmol [right panel]) was included as an internal reference standard. (E) Pull-down assays were performed by incubating 6 pmol of the different GST-p120-catenin point mutants with 300 μg of whole-cell extracts from RWP-1 cells; protein complexes were affinity purified with glutathione-Sepharose and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting with anti-E-cadherin. Blots were reanalyzed with anti-GST antibodies to ensure equal loading of samples. In the Input lane, a sample containing 5% of the total cell extracts used for the assay was loaded. (F) RWP-1 cells were cotransfected with different GFP-p120-catenin fusion proteins and pEF-Bos-active Fyn or the empty plasmid. After 48 h, cell extracts were prepared, 300 μg of each cell extract was immunoprecipitated with anti-GFP, and the associated proteins were analyzed with specific MAbs against GFP, RhoA, and Fyn. The numbers below the lanes correspond to the amounts of RhoA or E-cadherin bound to GST-p120-catenin fusion proteins, compared to the standard or to the amount bound to wild-type GST-p120-catenin. The results presented in this figure correspond to an experiment representative of at least three performed.
To further characterize the phosphorylation sites involved in this regulatory step, we mutated tyrosines 112 and 217 to phenylalanine. We performed these mutations in a p120-catenin(1-234) fragment in which the tyrosine residues at positions 96 and 228 had been replaced previously by phenylalanine residues in order to eliminate the background phosphorylation induced by Fyn and Src in those amino acids. As shown in Fig. 3B, the Tyr112Phe and Tyr217Phe p120 mutations differently affected the phosphorylation of the p120-catenin(1-234) fragment catalyzed by Fyn (Fig. 3B, left panel) and Src (Fig. 3B, right panel). Thus, the Tyr112Phe mutation abolished almost completely the phosphorylation of this fusion protein by Fyn but it affected only slightly the phosphorylation promoted by Src (Fig. 3B, lane 2, left and right panels). By contrast, the behavior of the Tyr217Phe mutation was the opposite of that found with the Tyr112Phe replacement (Fig. 3B, lane 3, left and right panels). These results indicate that p120-catenin Tyr112 is phosphorylated more actively by Fyn than by Src, suggesting that the posttranslational modification of this residue is responsible for the inhibition of the interaction of p120-catenin with RhoA.
In order to demonstrate the relevance of Tyr112 in the inhibition of the RhoA-p120-catenin interaction by Fyn, we performed binding assays using the p120-catenin(1-234) form, where Tyr96 and Tyr228 had been replaced by Phe. As shown in Fig. 3C, this mutant protein interacted with RhoA with affinities similar to that of its wild-type counterpart (Fig. 3C, right panel, compare lanes 2 and 3). Phosphorylation by Fyn prevented the interaction, whereas Src enhanced it (Fig. 3C, right panel, lanes 3, 4, and 5), similarly to what happened with the wild-type form, further supporting the irrelevance of Tyr96 and Tyr228 in the effects of Fyn. When the same assays were performed with a mutant containing the additional replacement of Tyr112 by Phe, phosphorylation by Fyn increased its affinity towards RhoA (Fig. 3C, left panel). These results suggest that the Fyn-dependent phosphorylation of p120-catenin on Tyr112 prevents RhoA binding, whereas the Src/Fyn-dependent phosphorylation of the Try217 residue induces the opposite effect.
To further check the relevance of the phosphorylation of these residues, we replaced each of them with glutamate residues to mimic the effect of the negative charge introduced by the phosphate groups. As shown in Fig. 3D, binding of RhoA to the p120-catenin Tyr112Glu mutant was completely abolished. Instead, the p120-catenin Tyr217Glu mutant interacted with RhoA significantly better than the wild-type form (approximately 2.05-fold ± 0.14-fold [n = 3]). A Tyr228Glu mutation also upregulated the binding of p120-catenin to RhoA, although to a lower extent than the Tyr217Glu mutation (Fig. 3D). These mutant proteins were also tested for E-cadherin binding by use of a similar experimental approach. The Tyr112Glu and Tyr217Glu p120-catenin mutants presented a better association with E-cadherin than did the wild-type counterpart (Fig. 3E) (2.55-fold ± 0.24-fold and 2.85-fold ± 0.31-fold [n = 3], respectively, for Tyr112Glu and Tyr217Glu). A mutant protein harboring both mutations interacted with E-cadherin slightly better than each of the single mutants alone (data not shown).
The relevance of the p120-catenin Tyr112 phosphorylation by Fyn was also determined in RWP-1 epithelial cells. Ectopic expression of Fyn greatly downregulated the amount of RhoA capable of coimmunoprecipitating with cotransfected p120-catenin (Fig. 3F, lane 2). A similar block in the interaction was observed when the Tyr112Glu mutant was introduced in cells in the absence of ectopic Fyn (Fig. 3F, lane 3). However, when Tyr112Phe p120-catenin was used, Fyn was unable to inhibit the association between this p120-catenin form and RhoA (Fig. 3F, lanes 4 and 5). Actually, Fyn increased this binding event slightly, although significantly, a result probably derived from its ability to phosphorylate tyrosine residues in addition to Tyr112 (e.g., Tyr217).
Phosphorylation of Tyr112 blocks p120-catenin effects on RhoA activity.
It is known that p120-catenin binds better to the inactive than to the active form of RhoA. To test the behavior of our mutant proteins in this biochemical property, we performed binding assays with RhoA proteins that were loaded with either GDP or GTP-γ-S (see Materials and Methods). p120-catenin was pulled down with a higher efficiency by a GST-RhoA loaded with GDP, approximately 4.5-fold (±0.52-fold) (n = 3) better than by GST-RhoA loaded with GTP (Fig. 4A). Assays using different mutants of RhoA confirmed our conclusion: p120-catenin interacted better with the RhoA Thr19Asn mutant, an inactive RhoA mutant (4, 32), than with the RhoA Gln63Leu mutant, a RhoA mutant with impaired GTPase activity and, therefore, constitutively bound to GTP molecules (15) (Fig. 4B, lanes 3 and 4). Consistent with this result, we also observed that p120-catenin acts as a RhoGDI-like molecule (Fig. 5). The inhibition of the exchange of GDP with GTP was dependent on p120-catenin binding, since a deletion mutant lacking the RhoA-binding domain [p120-catenin(234-end)] was unable to retard the GDP release. As expected from the results described above, the p120-catenin Tyr112Glu mutant was totally inactive in this assay, confirming again that this residue is important for a stable interaction with RhoA. The binding domain by itself (amino acids 1 to 234) was not functional either, indicating that additional sequences in p120-catenin were required for stabilization. The use of additional controls demonstrated that the assay worked properly (Fig. 5).
FIG. 4.
p120-catenin interacts better with the RhoA-bound GDP form. (A) p120-catenin (1 pmol) was incubated with 2 pmol of GDP- or GTP-loaded GST-RhoA. Protein complexes were affinity purified with glutathione-Sepharose and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting (WB) with an anti-p120-catenin MAb. p120-catenin (0.25 pmol) was included as an internal reference standard (St). (B) NIH 3T3 cells were transfected with two RhoA mutants: AU5-RhoAQ63L (which is always active) or AU5-RhoAT19N (inactive). After 48 h, cell extracts were prepared and incubated (300 μg) with GST-p120-catenin. Protein complexes were purified with glutathione-Sepharose and associated proteins analyzed with a monoclonal antibody specific to the AU5 tag. Blots were reanalyzed with anti-GST to ensure that similar levels of fusion protein forms were present in all samples. In the Input lane, a sample containing 5% of the total cell extracts used for the assay was loaded. The results presented in this figure correspond to an experiment representative of three (A) or two (B) performed.
FIG. 5.
Phosphorylation of p120-catenin on Tyr112 blocks its RhoGDI activity. [3H]GDP-bound RhoA (20 pmol) was incubated with equimolar amounts of the different GST-p120-catenin fusion proteins for 10 min. An excess of GTP (10 μM) was then added to allow the exchange of bound [3H]GDP-RhoA for GTP, and samples were incubated for an additional 10 min. [3H]GDP-bound RhoA was separated from free nucleotide by filtration, and the amount of [3H]GDP bound to RhoA was determined and compared to the radionucleotide bound at time zero. The figure shows the averages ± standard deviations of three experiments performed in duplicate.
As an additional way of corroborating our biochemical observations, we analyzed the role of Tyr112 phosphorylation in p120-catenin activity in vivo. To this end, we tested the effects of Src and Fyn on the levels of GTP-bound RhoA by using transient transfections in RWP-1 cells coupled to pull-down experiments with GST-rhotekin, which specifically retains the activated form of this GTPase. These experiments indicated that the overexpression of Src decreased the total levels of activated, GTP-bound RhoA (Fig. 6A, upper panel, compare lanes 4 and 5). In contrast, Fyn promoted RhoA stimulation due to the probable elimination of the RhoGDI activity of p120-catenin through phosphorylation of Tyr112 and the disruption of the p120-catenin-RhoA interaction (Fig. 6A, upper panel, lane 6). To further test this hypothesis, Fyn and p120-catenin were overexpressed in these cells. In the presence of Fyn, wild-type p120-catenin only slightly decreased the levels of active RhoA; however, the Tyr112Phe mutant decreased by 90% the amount of active RhoA in the presence of Fyn (Fig. 6B). The ectopic expression of p120-catenin mutants also confirmed our conclusions, since the transfection of wild-type p120-catenin markedly downregulated the levels of active RhoA whereas its Tyr112Glu mutant was totally ineffective in such action (Fig. 6C).
FIG. 6.
Phosphorylation of p120-catenin on Tyr112 by Fyn prevents repression of RhoA activity by p120-catenin. SW480 cells were cotransfected with 7.5 μg of pcDNA3.1-Myc-His-RhoA wild type (WT) and (A) with 7.5 μg of pEF-Bos-active Fyn, pEF-Bos-active v-Src, or pEF-Bos alone, (B) with pEF-Bos-active Fyn and 7.5 μg of GFP, GFP-p120-catenin wild type, or GFP-p120-catenin Y112F, or (C) with the same amounts of GFP, GFP-p120-catenin wild type, or GFP-p120-catenin Y112E. After 48 h, cell extracts were prepared and added to a suspension of GST-rhotekin-glutathione-Sepharose. The amount of active RhoA was analyzed by Western blotting (WB) with a specific MAb against RhoA. Blots were reanalyzed with anti-Fyn and -Src (A), with anti-Fyn and anti-GFP (B), or with anti-GFP (C). In the Input lane, a sample of 5% of the total cell extract used for the assay was loaded. The right panels show the representation of active Rho with respect to the total amount of this protein. The averages ± standard deviations of the three different experiments performed in each case are presented.
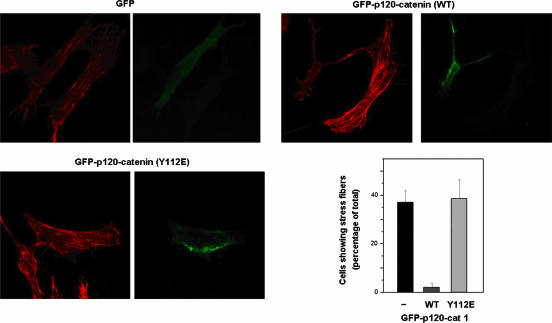
Next, we checked the relevance of the phosphorylation regarding two effects of p120-catenin dependent on the inhibition of RhoA. RhoA controls formation of stress fibers through the activation of Rho-kinase and the inhibition of myosin phosphatase (18). Therefore, we checked the effect of the transfection of the wild type or Tyr112Glu p120-catenin on the formation of stress fibers in fibroblasts. As shown in Fig. 7, cells transfected with GFP presented abundant stress fibers, detected by staining with rhodamine-phalloidin. Cells overexpressing GFP-p120-catenin displayed very few stress fibers: compare in Fig. 7 (right upper panels) the transfected (GFP-positive) and untransfected cells. On the other hand, expression of the Tyr112Glu mutant did not significantly affect the number of cells with these structures, although the fibers seemed to be slightly shorter.
FIG. 7.
Tyr112 controls formation of stress fibers. NIH 3T3 cells were transfected with 5 μg of GFP vector alone, the GFP-p120-catenin wild type (WT), or the GFP-p120-catenin Y112E mutant. The presence of stress fibers was determined after staining F-actin with phalloidin-rhodamine and visualizing cells by fluorescence microscopy. Note that transfection of GFP or the GFP-p120-catenin Y112E mutant did not substantially affect the presence of stress fibers with respect to control untransfected cells not positive for GFP, whereas the p120-catenin wild type almost totally prevented it. The averages (±standard deviations) of the results obtained in two experiments in which at least 40 cells were examined are also included.
We also measured the activities of our mutant proteins in the previously described negative action of p120-catenin on NF-κB activity, dependent on the inhibition of RhoA (26). As expected, wild-type p120-catenin or Tyr112F reproducibly affected the activity of a promoter sensitive to the levels of NF-κB (see Fig. SA2 in the supplemental material). By contrast, the p120-catenin Tyr112Glu mutant did not inhibit the activity of this promoter. Additional controls confirmed that all p120-catenin versions were expressed at similar levels in cells (not shown).
Phosphorylation of Tyr112 prevents induction of the “branching” phenotype after p120-catenin overexpression in fibroblasts.
Overexpression of p120-catenin in fibroblasts induces a dendritic “branching” phenotype (1, 23, 28). The acquisition of this phenotype has been reported to be at least partially dependent on the repression of RhoA activity (1, 23). We observed this morphology in NIH 3T3 fibroblasts expressing GFP-p120-catenin. Approximately 50% of the transfected cells acquired a phenotype characterized by the presence of three or more long extensions (Fig. 8). A representative cell is shown in Fig. 8B and the quantification of the morphologies observed after transfecting this construction in Fig. 8K. A smaller percentage displayed an intermediate morphology, with one or two long extensions (Fig. 8C and K). Simultaneous overexpression of E-cadherin and p120-catenin prevented the induction of this phenotype (Fig. 8D and K), as previously reported (10, 23). The branching phenotype was not observed when the two deletion mutants [p120-catenin(1-234) and p120-catenin(234-end)] were expressed (Fig. 8G, H, and K). Curiously, these two fragments presented a cellular distribution different from those of the rest of the GFP-p120-catenin proteins, which were associated mostly to the cell periphery; p120-catenin was mainly cytosolic, whereas p120-catenin(234-end) was located also in the nucleus (Fig. 8G). The presence of the characteristic dendritic morphology was also observed after transfection of the p120-catenin Tyr217Glu mutant (Fig. 8E and K), which shows an enhanced interaction with RhoA. However, the Tyr112Glu mutant did not promote the acquisition of this phenotype (Fig. 8F): only 10% of the cells presented a morphology similar to that shown in Fig. 8B or E (Fig. 8K). When Fyn was cotransfected with GFP-p120-catenin, the acquisition of this phenotype was also partially prevented, further indicating that phosphorylation of Tyr112 in this protein prevents branching (Fig. 8J). Curiously, Fyn by itself promoted significant morphological changes, with a higher percentage of elongated cells (Fig. 8I and K). Therefore, these results suggest that interaction of p120-catenin with RhoA is necessary for the acquisition of the dendritic phenotype in fibroblasts.
FIG. 8.
Phosphorylation of p120-catenin on Tyr112 prevents its RhoA-dependent effects in vivo. NIH 3T3 cells were transfected with 1 μg of the different phrGFP vectors in order to express the indicated GFP fusion proteins. When appropriate, 5 μg of pCFP-E-cadherin (D) or pEF-Fyn (I and J) was also transfected. After 24 h, cells were plated on glass coverslips, fixed with 4% paraformaldehyde, and washed with phosphate-buffered saline. Coverslips were mounted on glass slides and the morphology of transfected cells visualized by confocal microscopy. Arrows indicate the membrane protrusions typically induced by the expression of wild-type (WT) p120-catenin. The most predominant morphology observed in each case is shown; other relevant phenotypes are also displayed in the case of cells transfected with GFP-p120-catenin (wild type). The percentages of cells showing the different phenotypes are included (K); this diagram corresponds to the result of one experiment representative of the three performed. Fifty transfected cells were observed under each condition. A cell was scored as having a branched phenotype (examples in panels B and E) if it presented three or more extensions longer than the cell body. Cells presenting one or two extensions at least twice the cell body were scored as having a partial branched phenotype (intermediate phenotype, examples in panels C and I).
Phosphorylation of Tyr112 contributes to the regulation of p120-catenin-RhoA binding and RhoA GTPase activity by K-ras in IEC-18 cells.
Cellular effects of p120-catenin on RhoA activity and branching phenotype have been attributed to the cytosolic fraction of this protein (23). However, activity of Fyn kinase is normally restricted to the membrane. We approached this problem by analyzing the cytosolic or membrane distribution of p120-catenin, RhoA, and Fyn in intestinal epithelial IEC-18 cells and in IEC-18 cells overexpressing K-ras oncogene (IEC-K-ras cells). In this cell system, K-ras oncogene expression has been shown to induce the phosphorylation of p120-catenin (19, 27) and the activation of Fyn kinase (27). Compared to control IEC cells, IEC-K-ras cells present low levels of active RhoA, as determined by GST-rhotekin pull-down assays (Fig. 9A). Cellular fractionation of IEC cells indicated that most of the endogenous p120-catenin was associated to the membrane fraction, although a significant pool was also detected in the cytosol (Fig. 9B). The distributions of p120-catenin were not significantly different in IEC and IEC-K-ras cells. Ectopically expressed p120-catenin was distributed similarly to the endogenous protein (Fig. 9C). K-Ras overexpression increased the extent of tyrosine phosphorylation on p120-catenin, as observed by analyzing with an anti-PTyr MAb p120-catenin immunoprecipitates. This increase was detected to occur exclusively in the membrane-associated fraction, probably because Fyn was present only in this fraction (Fig. 9B).
FIG. 9.
Effect of K-Ras overexpression in IEC-18 cells on RhoA distribution and activity. (A) Activity of RhoA in IEC or IEC-K-ras cells was determined using chromatography with GST-rhotekin-glutathione-Sepharose as indicated in Materials and Methods. Bound proteins were analyzed with a RhoA-specific MAb. The lower panel shows the representation of active Rho with respect to the total amount of this protein. The averages ± standard deviations of the three different experiments performed in each case are presented. (B) Cytosolic and membrane-associated fractions from IEC and IEC-K-ras cells were prepared as detailed in Materials and Methods. Fractions were precipitated with an anti-p120-catenin MAb and analyzed by Western blotting (WB) with the indicated MAbs. In parallel, an aliquot of the four different inputs was also analyzed. (C) IEC and IEC-K-ras cells were cotransfected with inactive AU5-RhoA(T19N) and GFP-p120-catenin, either the wild type or the Y112F mutant, or with GFP as a control. Cytosolic and membrane fractions were prepared as described above, and the exogenous p120-catenin was immunoprecipitated (IP) with an anti-GFP antibody. The immunoprecipitates and inputs were analyzed by Western blotting with the indicated antibodies. The results presented in panels B and C correspond to an experiment representative of three performed.
RhoA cellular distribution was sensitive to K-Ras overexpression. In IEC cells, similar levels of RhoA were found in the membrane and cytosolic fractions (Fig. 9B). K-ras transfectants presented lower levels of RhoA in the membrane fraction and higher levels in the cytosol, indicative of a release of RhoA from the membrane. Association of RhoA to p120-catenin was detected only in the cytosol by coimmunoprecipitation experiments (Fig. 9B). The amount of RhoA associated to p120-catenin in this fraction was increased in IEC-K-ras cells with respect to IEC, probably reflecting the fact that higher levels of RhoA are found in the cytosol in these cells.
We reasoned that the inability to detect RhoA associated to p120-catenin in the membrane might be due to the rapid activation of this GTPase and the low affinity of GTP-bound RhoA to p120-catenin. Therefore, we transfected an inactive (Thr19Asn) mutant of RhoA to IEC and IEC-K-ras cells and analyzed its interaction with wild-type p120-catenin or with a mutant where phosphorylation by Fyn was prevented (Tyr112Phe). As shown in Fig. 9C, binding of p120-catenin to RhoA was detected in the membrane fraction only when phosphorylation of Tyr112 was prevented.
Therefore, Fyn activation and p120-catenin phosphorylation in Tyr112 correlate with a diminished ability of RhoA to bind to the membrane-associated p120-catenin, promoting the subsequent release of this GTPase to the cytosol and its binding and inhibition by the unphosphorylated form of this p120-catenin present in this fraction.
DISCUSSION
In this work we have shown that the N-terminal regulatory domain of p120-catenin is the region responsible for the interaction with RhoA. Perhaps more importantly, we have demonstrated that the association between p120-catenin and RhoA or E-cadherin, two proteins binding to different elements, is modulated by Fyn, Src, and Fer tyrosine kinases. As we have described previously for Src (30), phosphorylation of p120-catenin increased the association of p120-catenin to E-cadherin. It is remarkable that the armadillo domain of p120-catenin, the region responsible for E-cadherin association, is not phosphorylated by these kinases. This result suggests that the armadillo and regulatory domains are in close spatial proximity and that, similarly to what happens in other proteins containing these repeats (5, 33), the binding capability of the armadillo domain is regulated by distal elements. Experiments performed with p120-catenin deletion mutants further support this conclusion: a fragment comprising only the armadillo repeats binds E-cadherin better than the full-length protein (Fig. 1C).
Other results suggest that both domains are functionally interdependent. As shown in our in vitro nucleotide exchange assays, p120-catenin acts as a GDI for RhoA. This activity requires the binding sequence in p120-catenin but also additional elements, as inferred from the observation that the fragment comprising amino acids 1 to 234 interacts with RhoA but is not sufficient to induce the inhibition of nucleotide exchange. This result suggests that the armadillo domain is necessary to protect the binding site for GDP, precluding the release of this nucleotide (Fig. 10). Our data also indicate that binding of E-cadherin and RhoA to p120-catenin can occur simultaneously. These results are not contradictory to observations by several labs indicating that the inhibition by p120-catenin of RhoA activity is blocked by E-cadherin overexpression (1, 10, 23). According to our model, binding to E-cadherin would not prevent p120-catenin association to RhoA but it might either induce a conformational change that would prevent the GDI activity (Fig. 10) or, alternatively, recruit a GEF responsible for the activation of RhoA, as it has been proposed previously (1).
FIG. 10.
Proposed model for the regulation of RhoA activity by p120-catenin. p120-catenin protein is composed by the regulatory and the armadillo domains (1). After binding of RhoA to the regulatory domain, the armadillo repeats are disposed in the close vicinity of the nucleotide binding site of RhoA, hindering the release of GDP and therefore contributing to the GDI effect of p120-catenin (3). In cells, most p120-catenin is located at the membrane due to its interaction with the juxtamembrane domain (JMD) of E-cadherin (2). This membrane-associated p120-catenin is unable to work as a GDI due to either a conformational change induced in the armadillo domain by E-cadherin or the recruitment of a RhoA GEF (not depicted) by this protein. Therefore, GDP bound to RhoA is exchanged by GTP, and the affinity of the GTPase by p120-catenin decreases and is rapidly released (4). This effect of E-cadherin or E-cadherin-associated factor is not present in the cytosol; therefore, only cytosolic p120-catenin can work as a GDI (3). Activation of Fyn tyrosine kinase leads to phosphorylation of tyrosines 112, 228, and others in the regulatory domain of the p120-catenin located in the membrane fraction, where Fyn is normally present (5), and not in the cytosol (7). Phosphorylation of Tyr112 prevents interaction of GDP-bound RhoA with p120-catenin (6), therefore precluding the activation of this protein and increasing the levels of this GTPase at the cytosol, where it can be bound and inhibited by unphosphorylated p120-catenin (8).
Our work has also demonstrated that the phosphorylation of different tyrosine residues of p120-catenin promotes distinctive effects on RhoA binding. Thus, whereas phosphorylation of Tyr112 totally blocks this association, the similar modification of Tyr217 (or Tyr228 to a lower extent) increases it. We consider it likely that the negative effect of phospho-Tyr112 is due to the interference in the association of RhoA. However, the positive effect might be more indirect (i.e., caused by the partial unfolding of the regulatory domain) and present analogies with the stimulation of the association of p120-catenin to E-cadherin. In this case, we have evidence indicating that the phosphorylation of several tyrosine residues promotes an accumulative effect because (i) the Tyr112,217Glu double mutant binds E-cadherin better than any of the single mutants and (ii) phosphorylation of this double mutant by Src can still increase affinity for E-cadherin (data not shown). It is likely, therefore, that the phosphorylation of several tyrosines cooperates in the modification of the structure of p120-catenin.
Phosphorylation by Fyn kinase exerts an effect opposite than that of Src or Fer kinases on RhoA-p120-catenin binding. Both Fer and Fyn are detected constitutively bound to p120-catenin through its regulatory domain (16, 27). It has to be determined whether both kinases can associate simultaneously to the same complex, but if this is not the case, as seems likely, the balance between the levels of these active protein kinases bound to p120-catenin would control the phosphorylation status of Tyr112 and, consequently, the association of RhoA to p120-catenin. The identification of the molecular mechanisms controlling the interactions of p120-catenin with these kinases is an interesting topic of research, as is the characterization of the signaling pathways regulating them.
We have also demonstrated that the interaction of RhoA with p120-catenin can be detected in vivo, since in intestinal IEC-18 cells, cytosolic p120-catenin coimmunoprecipitated with RhoA. Most of the cellular effects observed after transfection of p120-catenin are promoted by the cytosolic fraction of this protein, as has been demonstrated previously for the inhibition of RhoA activity (23). However, we have also shown that the interaction of RhoA with p120-catenin could be detected in the membrane fraction when we transfected an inactive form of this GTPase (Fig. 9). We have explained this result on the basis of the different affinities of p120-catenin by GDP- and GTP-bound RhoA and the lack of effect of membrane-associated p120-catenin on RhoA activity. Therefore, although GDP-RhoA also interacts with membrane-bound p120-catenin, it would be activated to GTP-RhoA by a local RhoA GEF and therefore it would be rapidly released. A model presenting this hypothesis is depicted in Fig. 10.
We have reported previously the activation of Fyn tyrosine kinase in IEC-18 cells transfected with K-ras oncogene (27). This tyrosine kinase is located in the membrane fraction; therefore, only the p120-catenin present in this fraction is phosphorylated under these conditions (Fig. 9). Phosphorylation prevents association of inactive RhoA to membrane p120-catenin, enhancing the levels of RhoA in the cytosol, where it can interact and be inhibited by the active form of p120-catenin. Therefore, phosphorylation of Tyr112 in membrane-associated p120-catenin would exert an inhibitory effect over RhoA activity, contributing to the diminished levels of active RhoA observed to occur in these cells after transfection of K-ras (Fig. 10). The different levels of stimulation of Fer, Fyn, or Src tyrosine kinases by this oncogene might help to explain the divergent results observed for RhoA activity in cells transfected with K-ras (9, 31, 38).
A recent report has described the involvement of Src tyrosine kinase in the regulation of Rho by another GDI, a 25-kDa RhoGDI (8). Phosphorylation of this protein by Src promotes its translocation to the plasma membrane and decreases its interaction with RhoA. On the contrary, on p120-catenin Src stimulates its binding to RhoA and enhances the interaction of this catenin with the membrane, probably through its binding to E-cadherin. It is possible that this low-molecular-mass GDI also participates in the inactivation of RhoA in the cytosol, playing the same role we proposed for p120-catenin in this compartment (Fig. 10). The relative affinities of RhoA for these two GDIs are an interesting matter of study, either when both GDIs are not modified or after the action of Src or Fyn tyrosine kinases.
Furthermore, different tyrosine kinases can also phosphorylate and activate another effector of RhoA, p190RhoGAP (11). This protein can be coupled to the adherens junctional complex through its association with p120-catenin (35). Although it has not been demonstrated formally, it has been proposed that tyrosine phosphorylation of p190RhoGAP facilitates its association with p120-catenin and the inactivation of RhoA in the membrane (35). The molecular details of this complex remain to be characterized, for instance, whether p120-catenin directly interacts with p190RhoGAP and whether this association can take place simultaneously with the binding of RhoA to both proteins. In any case, it is possible that phosphorylation of specific residues of p120-catenin may also affect its interaction with p190RhoGAP, adding a new element to the control of RhoA activity.
Different cellular roles for p120-catenin have been identified in past years. Some of these roles require inhibition of RhoA by p120-catenin. For instance, the induction of a dendritic, branched phenotype by p120-catenin has been attributed to the ability to block RhoA activity and activate Rac1 (1, 10, 23). The characterization of p120-catenin mutants unable to bind to RhoA described in this article provides new evidence supporting the relevance of the interaction of both proteins in the acquisition of the branched phenotype and introduces new tools to dissect the molecular pathways triggered by this pleiotropic protein.
Supplementary Material
Acknowledgments
We especially thank Neus Ontiveros for technical assistance.
This study was supported by grants awarded by the Spanish Ministerio de Ciencia y Tecnología (BMC2003-00410 and BFU2006-03203 to M.D. and SAF2003-02324 and SAF2006-00339 to A.G.H.). Funding by Generalitat de Catalunya (2005SGR00970) is also appreciated. G.S., I.R., J.C., D.C., and P.V. were recipients of predoctoral fellowships awarded by DGR-UAB (Generalitat de Catalunya) (to G.S.), Instituto de Salud Carlos III (Ministerio de Sanidad) (to I.R.), and Ministerio de Educación y Ciencia (to J.C., D.C., and P.V.).
Footnotes
Published ahead of print on 28 December 2006.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Anastasiadis, P. Z., S. Y. Moon, M. A. Thoreson, D. J. Mariner, H. C. Crawford, Y. Zheng, and A. B. Reynolds. 2000. Inhibition of RhoA by p120 catenin. Nat. Cell Biol. 2:637-644. [DOI] [PubMed] [Google Scholar]
- 2.Brembeck, F. H., T. Schwarz-Romond, J. Bakkers, S. Wilhelm, M. Hammerschmidt, and W. Birchmeier. 2004. Essential role of BCL9-2 in the switch between beta-catenin's adhesive and transcriptional functions. Genes Dev. 18:2225-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryant, D. M., and J. L. Stow. 2004. The ins and outs of E-cadherin trafficking. Trends Cell. Biol. 14:427-434. [DOI] [PubMed] [Google Scholar]
- 4.Carnac, G., M. Primig, M. Kitzmann, P. Chafey, D. Tuil, N. Lamb, and A. Fernandez. 1998. RhoA GTPase and serum response factor control selectively the expression of MyoD without affecting Myf5 in mouse myoblasts. Mol. Biol. Cell 9:1891-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castaño, J., I. Raurell, J. Piedra, S. Miravet, M. Duñach, and A. García de Herreros. 2002. β-Catenin N-and C-tails modulate the coordinated binding of adherens junction proteins to β-catenin. J. Biol. Chem. 277:31541-31550. [DOI] [PubMed] [Google Scholar]
- 6.Daniel, J. M., and A. B. Reynolds. 1999. The catenin p120ctn interacts with Kaiso, a novel BTB/POZ domain zinc finger transcription factor. Mol. Cell. Biol. 19:3614-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis, M. A., and A. B. Reynolds. 2006. Blocked acinar development, E-cadherin reduction, and intraepithelial neoplasia upon ablation of p120-catenin in the mouse salivary gland. Dev. Cell 10:21-31. [DOI] [PubMed] [Google Scholar]
- 8.DerMardirossian, C., G. Rocklin, J. Y. Seo, and G. Bokoch. 2006. Phosphorylation of RhoGDI by Src regulates RhoGTPase binding and cytosol-membrane cycling. Mol. Biol. Cell 17:4760-4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dreissigacker, U., M. M. Mueller, M. Unger, P. Siegert, F. Genze, P. Gierschik, and K. Giehl. 2006. Oncogenic K-ras down-regulates Rac1 and RhoA activity and enhances migration and invasion of pancreatic carcinoma cells through activation of p38. Cell. Signal. 18:1156-1168. [DOI] [PubMed] [Google Scholar]
- 10.Grosheva, I., M. Shtutman, M. Elbaum, and A. D. Bershadsky. 2001. p120 catenin affects cell motility via modulation of activity of Rho-family GTPases: a link between cell-cell contact formation and regulation of cell locomotion. J. Cell Sci. 114:695-707. [DOI] [PubMed] [Google Scholar]
- 11.Hernández, S. E., J. Settlemen, and A. J. Koleske. 2004. Adhesion-dependent regulation of p190RhoGAP in the developing brain by the Abl-related gene tyrosine kinase. Curr. Biol. 14:691-696. [DOI] [PubMed] [Google Scholar]
- 12.Holsinger, L. J., K. Ward, B. Duffield, J. Zachwieja, and B. Jallal. 2002. The transmembrane receptor protein tyrosine phosphatase DEP1 interacts with p120(ctn). Oncogene 21:7067-7076. [DOI] [PubMed] [Google Scholar]
- 13.Ireton, R. C., M. A. Davis, J. van Hengel, D. J. Mariner, K. Barnes, M. A. Thoreson, P. Z. Anastasiadis, L. Matrisian, L. M. Bundy, L. Sealy, B. Gilbert, F. van Roy, and A. B. Reynolds. 2002. A novel role for p120 catenin in E-cadherin function. J. Cell Biol. 159:465-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keilhack, H., U. Hellman, J. van Hengel, F. van Roy, J. Godovac-Zimmermann, and F. D. Bohmer. 2000. The protein-tyrosine phosphatase SHP-1 binds to and dephosphorylates p120 catenin. J. Biol. Chem. 275:26376-26384. [DOI] [PubMed] [Google Scholar]
- 15.Khoshravi-Far, R., P. A. Solski, G. J. Clark, M. S. Kinch, and C. J. Der. 1995. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol. Cell. Biol. 15:6443-6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim, L., and T. W. Wong. 1995. The cytoplasmic tyrosine kinase FER is associated with the catenin-like substrate pp120 and is activated by growth factors. Mol. Cell. Biol. 15:4553-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim, S. W., J. I. Park, C. M. Spring, A. K. Sater, H. Ji, A. A. Otchere, J. M. Daniel, and P. D. McCrea. 2004. Non-canonical Wnt signals are modulated by the Kaiso transcriptional repressor and p120-catenin. Nat. Cell Biol. 6:1212-1220. [DOI] [PubMed] [Google Scholar]
- 18.Kimura, K., M. Ito, M. Amano, K. Chihara, Y. Fukata, M. Nakafuku, B. Yamamori, J. H. Feng, T. Nakano, K. Okawa, A. Iwamatsu, and K. Kaibuchi. 1996. Regulation of myosin phosphates by Rho and Rho-associated kinase (Rho-kinase). Science 273:245-248. [DOI] [PubMed] [Google Scholar]
- 19.Kinch, M. S., G. J. Clark, C. J. Der, and K. Burridge. 1995. Tyrosine phosphorylation regulates the adhesion of ras-transformed breast epithelia. J. Cell Biol. 130:461-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magie, C. R., D. Pinto-Santini, and S. M. Parkhurst. 2002. Rho1 interacts with p120ctn and alpha-catenin, and regulates cadherin-based adherens junction components in Drosophila. Development 129:3771-3782. [DOI] [PubMed] [Google Scholar]
- 21.Mariner, D. J., P. Anastasiadis, H. Keilhack, F. D. Bohmer, J. Wang, and A. B. Reynolds. 2001. Identification of Src phosphorylation sites in the catenin p120ctn. J. Biol. Chem. 276:28006-28013. [DOI] [PubMed] [Google Scholar]
- 22.Mariner, D. J., M. A. Davis, and A. B. Reynolds. 2004. EGFR signaling to p120-catenin through phosphorylation at Y228. J. Cell Sci. 117:1339-1350. [DOI] [PubMed] [Google Scholar]
- 23.Noren, N. K., B. P. Liu, K. Burridge, and B. Kreft. 2000. p120 catenin regulates the actin cytoskeleton via Rho family GTPases. J. Cell Biol. 150:567-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ozawa, M., and T. Ohkubo. 2001. Tyrosine phosphorylation of p120(ctn) in v-Src transfected L cells depends on its association with E-cadherin and reduces adhesion activity. J. Cell Sci. 114:503-512. [DOI] [PubMed] [Google Scholar]
- 25.Park, J. I., S. W. Kim, J. P. Lyons, H. Ji, T. T. Nguyen, K. Cho, M. C. Barton, T. Deroo, K. Vleminckx, R. T. Moon, and P. D. McCrea. 2005. Kaiso/p120-catenin and TCF/beta-catenin complexes coordinately regulate canonical Wnt gene targets. Dev. Cell 8:843-854. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Moreno, M., M. A. Davis, E. Wong, H. A. Pasolli, A. B. Reynolds, and E. Fuchs. 2006. p120-catenin mediates inflammatory responses in the skin. Cell 124:631-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piedra, J., S. Miravet, J. Castaño, H. G. Palmer, N. Heisterkamp, A. Garcia de Herreros, and M. Duñach. 2003. p120 catenin-associated Fer and Fyn tyrosine kinases regulate beta-catenin Tyr-142 phosphorylation and beta-catenin-alpha-catenin interaction. Mol. Cell. Biol. 23:2287-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds, A. B., J. M. Daniel, Y. Y. Mo, and Z. Zhang. 1996. The novel catenin p120cas binds classical cadherins and induces an unusual morphological phenotype in NIH-3T3 fibroblasts. Exp. Cell Res. 225:328-337. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds, A. B., and A. Roczniak-Ferguson. 2004. Emerging roles for p120-catenin in cell adhesion and cancer. Oncogene 23:7947-7956. [DOI] [PubMed] [Google Scholar]
- 30.Roura, S., S. Miravet, J. Piedra, A. Garcia de Herreros, and M. Duñach. 1999. Regulation of E-cadherin/catenin association by tyrosine phosphorylation. J. Biol. Chem. 274:36734-36740. [DOI] [PubMed] [Google Scholar]
- 31.Sahai, E., M. Olson, and C. J. Marshall. 2001. Cross-talk between Ras and Rho signalling pathways in transformation favours proliferation and increased motility. EMBO J. 20:755-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw, R. J., M. Henry, F. Solomon, and T. Jacks. 1998. RhoA-dependent phosphorylation and relocalization of ERM proteins into apical membrane actin protrusions in fibroblasts. Mol. Biol. Cell 9:403-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solanas, G., S. Miravet, D. Casagolda, J. Castaño, I. Raurell, A. Corrionero, A. G. de Herreros, and M. Duñach. 2004. Beta-catenin and plakoglobin N- and C-tails determine ligand specificity. J. Biol. Chem. 279:49849-49856. [DOI] [PubMed] [Google Scholar]
- 34.van Hengel, J., P. Vanhoenacker, K. Staes, and F. van Roy. 1999. Nuclear localization of the p120(ctn) armadillo-like catenin is counteracted by a nuclear export signal and by E-cadherin expression. Proc. Natl. Acad. Sci. USA 96:7980-7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wildenberg, G. A., M. R. Dohn, R. Carnahan, M. A. Davis, N. A. Lobdell, J. Settleman, and A. B. Reynolds. 2006. p120-catenin and p190RhoGAP regulate cell-cell adhesion by coordinating antagonism between Rac and Rho. Cell 127:1027-1039. [DOI] [PubMed] [Google Scholar]
- 36.Xu, G., A. W. Craig, P. Greer, M. Miller, P. Z. Anastasiadis, J. Lilien, and J. Balsamo. 2004. Continuous association of cadherin with beta-catenin requires the non-receptor tyrosine-kinase Fer. J. Cell Sci. 117:3207-3219. [DOI] [PubMed] [Google Scholar]
- 37.Reference deleted.
- 38.Zondag, G. C., E. E. Evers, J. P. ten Klooster, L. Janssen, R. A. van der Kammen, and J. G. Collard. 2000. Oncogenic ras downregulates Rac activity, which leads to increased RhoA activity and epithelial-mesenchymal transition. J. Cell Biol. 149:775-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zondag, G. C., A. B. Reynolds, and W. H. Moolenaar. 2000. Receptor protein-tyrosine phosphatase RPTPμ binds to and dephosphorylates the catenin p120(ctn). J. Biol. Chem. 275:11264-11269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.