Abstract
Transcription factor ATF-2 is a nuclear target of stress-activated protein kinases, such as p38, which are activated by various extracellular stresses, including UV light. Here, we show that ATF-2 plays a critical role in hypoxia- and high-cell-density-induced apoptosis and the development of mammary tumors. Compared to wild-type cells, Atf-2−/− mouse embryonic fibroblasts (MEFs) were more resistant to hypoxia- and anisomycin-induced apoptosis but remained equally susceptible to other stresses, including UV. Atf-2−/− and Atf-2+/− MEFs could not express a group of genes, such as Gadd45α, whose overexpression can induce apoptosis, in response to hypoxia. Atf-2−/− MEFs also had a higher saturation density than wild-type cells and expressed lower levels of Maspin, the breast cancer tumor suppressor, which is also known to enhance cellular sensitivity to apoptotic stimuli. Atf-2−/− MEFs underwent a lower degree of apoptosis at high cell density than wild-type cells. Atf-2+/− mice were highly prone to mammary tumors that expressed reduced levels of Gadd45α and Maspin. The ATF-2 mRNA levels in human breast cancers were lower than those in normal breast tissue. Thus, ATF-2 acts as a tumor susceptibility gene of mammary tumors, at least partly, by activating a group of target genes, including Maspin and Gadd45α.
The ATF family of transcription factors (13), which have the b-ZIP-type DNA-binding domain, contains multiple members that play diverse roles (24, 27, 39, 53). ATF-2, one member of the ATF family (28), can form either homodimers or heterodimers with c-Jun and subsequently bind to the cyclic AMP response element (CRE) (5′-TGACGTCA-3′) (12, 29, 48). Heterodimerization of ATF-2 with Jun appears to be crucial for at least some of its functions; for instance, the oncogenic activity of ATF2 in chicken cells critically depends on its ability to dimerize with c-Jun (16). The c-Jun/ATF-2 heterodimers are more potent transcriptional activators for the minimal promoters containing CRE than ATF-2 homodimers (3, 16, 50). The target genes of c-Jun/ATF-2 heterodimers, which are implicated in growth control, include c-jun itself, cyclin D1, cyclin A, and beta interferon (2, 8, 31, 42).
The ATF-2 subfamily includes ATF-7 (originally called ATF-a) and CRE-BPa. All three of these members bear the trans-activation domain in their N-terminal regions (10, 35), which consists of two subdomains, namely, the N-terminal subdomain containing the well-known zinc finger motif and the C-terminal subdomain containing the SAPK phosphorylation sites targeted by stress-activated protein kinases (SAPKs) (33). The latter subdomain has a highly flexible and disordered structure. SAPKs, such as JNK (c-Jun N-terminal protein kinase) and p38, phosphorylate ATF-2 at Thr-69 and Thr-71 in the C-terminal subdomain and thereby enhance its trans-activating capacity (11, 26, 49). SAPKs are activated by various extracellular stresses, such as UV, osmotic stress, hypoxia, and inflammatory cytokines (6, 32). Activation of ATF-2 by p38/JNK is thought to play a role in apoptosis (11, 38).
Phosphorylation of ATF-2 by p38 is also induced by the transforming growth factor β-TAK1 pathway, while ATF-2 interacts with Smad3/4 to synergistically activate transcription (40). Thus, ATF-2 plays an important role in transforming growth factor β signaling by acting as a common nuclear target of both the Smad and TAK1 pathways. In fibroblasts, ATF-2 is also activated by insulin, epidermal growth factor (EGF), and serum via a two-step mechanism involving two distinct Ras effector pathways, the Raf-MEK-ERK and Ral-RalGDS-Src-p38 pathways (36). Serum stimulation leads to induction of ATF-3 gene transcription by activation of ATF-2 via these Ras effector pathways (47). Although CBP binds to ATF-2 and stimulates ATF-2 activity (41), the interaction between CBP and ATF-2 is phosphorylation independent, and the mechanism whereby SAPK-induced phosphorylation stimulates ATF-2 activity remains unknown. Recently, the protein kinase ATM was shown to phosphorylate ATF-2 on Ser-490 and Ser-498 following ionizing radiation, which resulted in its rapid colocalization with γ-H2AX into ionizing radiation-induced foci (4). In this case, a role for ATF-2 in the DNA damage response appears to be uncoupled from its transcriptional activity.
ATF-2 is ubiquitously expressed, with the highest level of expression being observed in the brain (45). Hypomorphic Atf-2 mutant mice, which still express an alternatively spliced ATF-2 protein, exhibit lower postnatal viability and growth, defects in endochondrial ossification, and a reduced number of cerebellar Purkinje cells (39). Null Atf-2 mutant mice die shortly after birth (27). In the mutant embryos, hypoxia occurs and was suggested to lead to strong gasping respiration with consequent aspiration of the amniotic fluid containing meconium. Impaired development of cytotrophoblast cells and a decreased level of expression of the platelet-derived growth factor receptor α, which is one of the ATF-2 target genes, were observed in the mutant placenta, suggesting a possible linkage of these events. However, the precise normal physiological role of ATF-2 remains elusive.
MATERIALS AND METHODS
Detection of apoptotic cells.
Cells (3 × 105 per 60-mm dish) were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), and apoptotic cells were detected by the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) method using the Deadend Colorimetric Apoptosis Detection System (Promega). Hypoxic conditions were introduced using the oxygen absorber Anaero Pack (Mitsubishi Gas Chemical). Conditions of other stresses were introduced as follows: methyl methanesulfonate (MMS) (1 mM for 20 h), anisomycin (AM) (5 μg/ml for 12 h), and UV light (50 J/m2 20 h before TUNEL assays). The average of three independent experiments was obtained from three different mouse embryonic fibroblast (MEF) preparations. To examine GADD45α-induced apoptosis, p53−/− MEFs were transfected with 3 μg of pact-GADD45-IRES-EGFP or pact-IRES-EGFP plasmid using Lipofectamine Plus (Invitrogen). After 40 h, dead cells were detected by TUNEL using an ApopTagRed in situ apoptosis detection kit (Intergen). To investigate apoptosis at the saturated cell density, MEFs and SK-BR-3 cells were cultivated to confluence, and apoptotic cells were detected by the TUNEL method.
DNA microarrays.
MEFs were mixed with TRIzol reagent (Invitrogen), and biotin-labeled RNAs for GeneChip analysis were prepared according to the protocol available on the Affymetrix website. The Mouse Genome Array, containing more than 12,422 probe sets (Affymetrix; U74Av2 DNA Chip), was used for the analysis.
Northern analysis.
RNA was prepared from MEFs and MEF-derived immortalized cell lines using the NIH 3T3 protocol. Cells were seeded at 2 × 106 per 10-cm dish and stimulated with either hypoxic conditions (24 h), AM (5 μg/ml for 4 h), MMS (1 mM for 4 h), or UV light (50 J/m2 4 h before cells were harvested). Twenty micrograms of total RNA was then electrophoresed in a 1.2% agarose formaldehyde gel and then transferred onto a nitrocellulose membrane (S&S). The blot was hybridized with a 32P-labeled mouse Gadd45α 500-bp cDNA probe that covered the entire protein-coding region. The signals from Northern blotting were quantitated by using Image Analyzer (Fuji).
Reporter assays.
To investigate the responsiveness of the Gadd45α promoter to the transcriptional activator p53, p53−/− MEF-derived immortalized cells were transfected with the Gadd45α promoter-Luci reporter (0.2 μg), in which the human GADD45α promoter region (nucleotides −2256 to + 287) was linked to the luciferase gene, the p53 expression plasmid (pact-p53), or the control empty vector (5 μg), and the internal control pRL-SV40 (10 ng; Promega) using Lipofectamine Plus (Invitrogen). The transfected cells were irradiated by UV light (20 J/m2) 24 h before being harvested. Forty hours after transfection, luciferase activity was measured by using the Dual-luciferase reporter assay system (Promega). To examine the c-Jun-dependent trans-activation, p53−/− MEF-derived immortalized cells were transfected with the chloramphenicol acetyltransferase (CAT) reporter (pTRE-TK-CAT; 1 μg), in which the herpesvirus thymidine kinase gene promoter was linked to four tandem repeats of the TRE (tetradecanoyl phorbol acetate response element) from the collagenase gene promoter, the c-Jun expression plasmid (pRSV-c-Jun), or the control empty vector (4 μg), and the internal control pCMV-β-gal (2 μg) using the CaPO4 method. CAT activity was measured 40 h after transfection.
Western blotting.
To detect ATF-2 and c-Jun proteins in MEFs, nuclear extracts were prepared from MEFs as described by Dignam et al. (7) and used for Western blotting. To detect ATF-2 and Maspin proteins in tumors, whole-cell extracts were prepared from tumors by using RIPA buffer (50 mM Tris-HCl, pH 7.8, 150 mM NaCl, 1% Triton X-100, 1% deoxycholate, 0.1% sodium dodecyl sulfate, 1% Trasylol), and used for Western blotting. Anti-ATF-2 (Santa Cruz C-19), anti-c-Jun (Santa Cruz H-79), and anti-Maspin (Santa Cruz C-20) antibodies were used.
ChIP assays.
Chromatin immunoprecipitation (ChIP) assays were carried out essentially as described previously (18) with minor modifications. Cells were treated under hypoxic conditions for 30 h. Immunoprecipitations were performed with either rabbit anti-ATF2 (Santa Cruz C-19) or rabbit normal immunoglobulin G (IgG). The primers for the amplification of the Gadd45α promoter region were as follows: forward (5′-TCTGGGTGTCAACGTCTGCTAA-3′), reverse (5′-CAGGTCCAGCTTCATTTGCA-3′), and TaqMan probe (5′-6-carboxyfluorescein [FAM]-TTGCATAACCCAATGGCCTGACTGC-6-carboxytetramethylrhodamine [TAMRA]-3′).
RT-PCR.
To measure the levels of Arnt, DecxE1a (branched-chain α ketoacid decarboxylase E1a), and Cyt-β-actin mRNAs, cells were treated under hypoxic conditions for 24 h, and total RNA was prepared. Real-time reverse transcription (RT)-PCR was performed by using the ABI 9700 Real-Time PCR Instrument and the SYBR Green qRT-PCR Kit (QIAGEN) according to the supplier's protocol. The primers used were as follows: Arnt, 5′-CTTGGCTCTGTGAAGGAAGG-3′ and 5′-GTCATCATCTGGGAGGGAGA-3′; DecxE1a, 5′-AGAACCAGCCCTTCCTCATT-3′ and 5′-GGTCCTGCTTGTCCCAGTAA-3′; and Cyt-β-actin, 5′-GCGCAAGTACTCTGTGTGGA-3′ and 5′-ACATCTGCTGGAAGGTGGAC-3′.
Maspin and Gadd45α mRNA levels were determined by real-time RT-PCR by using the ABI 9700 Real-Time PCR Instrument and the QuantiTect Probe RT-PCR Kit (QIAGEN) according to the manufacturers' instructions. The PCR conditions were 50°C for 30 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min. The oligonucleotide primers and probe used were as follows: Maspin, 5′-GGAAGTACCATTGGCACAAACTG-3′, 5′-GAGCAGCACATTGGGAACCT-3′, and 5′-FAM-AGCCCACATCAGCTATCGTTCGTCCA-TAMRA-3′; mouse Gadd45α, 5′-GCTGGCTGCTGACGAAGAC-3′, 5′-CGGATGAGGGTGAAATGGAT-3′, and 5′-FAM-ACGACCGGGATGTGGCTCTG-TAMRA-3′; and human Gadd45α, 5′-GGATGCCCTGGAGGAAGTG-3′, 5′-TCGTACACCCCGACAGTGATC-3′, and 5′-FAM-TCAGCAAAGCCCTGAGTCAGCGC-TAMRA-3′.
To determine the mRNA levels in the mammary tumors of Atf-2+/− mice, total RNA was isolated from mouse tumor tissues and normal mammary glands using Isogen (Nippon Gene). To separate the mammary glands from surrounding fat cells, the mammary glands were minced and dissociated for 3 h at 37°C in M199 medium containing 420 μg/ml collagenase and 2.1 mg/ml hyaluronidase. The cells were washed twice with Waymouth's MB 752-1 medium and cultured overnight in the same medium supplemented with 10% FBS. Only cells from mammary glands that attached were collected and further analyzed. RT-PCR was performed using 0.5 μg of total RNA and the Superscript One-Step RT-PCR System (Invitrogen). After 30 min of incubation at 50°C, cDNAs were amplified with 22 cycles of PCR under the following conditions: 94°C for 15 s, 55°C for 30 s, and 72°C for 90 s. The following primers were used for PCR: Atf-2, 5′-ACCAATGGTGATACTGTAAAAGGCC-3′ and 5′-CAGAAAGGTCTTCTGAACTGTCATC-3′; p53, 5′-CCGAGGCCGGCTCTGAGTATACCACCATC-3′ and 5′-CTCATTCAGCTCCCGGAACATCTCGAAGCG-3′; Gadd45α, 5′-GAGGGACTCGCACTTGCAATATGAC-3′ and 5′-CAGGATGTTGATGTCGTTCTCGCAG-3′; Gapdh, 5′-TTCCAGTATGACTCCACTCA-3′ and 5′-ATCACGCCACAGCTTTCCAG-3′; and Maspin, 5′-TAAATAAATGGATGGTCAGCATTG-3′ and 5′-AGACCAAAATCCTTGTGGTTAATG-3′.
To determine the human ATF-2 mRNA levels, breast cancers and normal epithelial cells were obtained using the laser capture microdissection technique. The two samples contained comparable levels of epithelial cells. Total RNA was isolated from individual frozen tumors, from normal epithelial cells, and from cell lines using RNeasy (QIAGEN). One-tube real-time RT-PCR was performed by using the QuantiTect SYBR Green RT-PCR Kit (QIAGEN) according to the manufacturer's instructions. Expression of ATF2 in relation to the TFR gene was determined by real-time PCR by using the SYBR Green assay with an ABI Prism 7900 (PE Applied Biosystems). The following primers were used: ATF-2, 5′-CGACAAAAAAGGAAAGTCTGGGTT-3′ and 5′-GCTCTGTACTCTGGTCCGCCA-3′; TFR, 5′-GCAAAATGTGAAGCATCCGGT-3′ and 5′-GCCACTGTAAACTCAGGCCCA-3′.
Analysis of growth properties of MEFs and human mammary epithelial cells (HMEC).
MEFs were isolated from embryos at 14.5 days postcoitus. For growth curves, the cells were grown from a starting density of 2.5 × 104 cells per well in 24-well plates in DMEM supplemented with 10% FBS. To determine the focus-formation efficiency, cells were seeded onto 6-cm dishes at 100 cells/dish and incubated in DMEM plus 10% FBS. After 12 days, the samples were stained with Giemsa, and the number of visible foci was scored. For colony formation assays, MEFs were infected with murine leukemia virus carrying v-K-ras (multiplicity of infection, 1). After 48 h, 3 × 104 cells were suspended in 1.3% methylcellulose gel dissolved in culture medium and laid onto an agarose bed composed of 0.53% agarose in culture medium. Colonies were scored 3 weeks after being plated. For growth curves, focus formation assays, and colony formation assays, three independent experiments were carried out using three different MEF preparations. To reexpress ATF-2, the retrovirus expression vectors for ATF-2 were constructed by using the retroviral vector carrying the puromycin resistance marker and viruses were prepared as described previously (44).
To test the tumorigenicity of the cell clones, nude mice (BALB/c nu/nu; Clea Japan Inc.) were injected subcutaneously at 12 sites (2 sites per mouse) with 1 × 106 or 2 × 106 cells resuspended in 100 μl of phosphate-buffered saline. The sizes and weights of the resulting tumors were measured 10 days (for MEFs), 14 days (for MEFs, to examine the effect of Maspin), 16 days (for NIH 3T3 cells, to examine the effect of Maspin), 12 days (for MEFs, to examine the effect of Gadd45α), or 21 days (for MCF-12A cells) after injection. The retrovirus expression vectors for Maspin or Gadd45α were constructed by using the retroviral vector carrying the puromycin resistance marker, and viruses were prepared as described previously (44). Atf-2−/− MEF-derived cell clones expressing v-K-ras were infected with the virus, and the puromycin-resistant cell pool was used for injection into nude mice. To test the effect of Maspin on Ras-induced tumor formation, NIH 3T3-R21 cells that were transformed by activated K-ras were infected with the Maspin-expressing virus, and the puromycin-resistant cell pool was used for injection into nude mice.
To decrease ATF-2 mRNA levels by RNA interference, the pDECAP-ATF-2 construct containing the inverted sequence of the mouse Atf-2 protein-coding region that corresponded to amino acids 166 to 362 was constructed using the pDECAP vector (43). MCF12A cells were transfected with pDECAP-ATF-2 or the empty vector, and the tranformants were isolated. To overexpress ATF-2, SK-BR3 cells were transfected with the ATF-2 expression vector containing the β-actin promoter, insulator, and neomycin selection marker (pact-ATF-2-Ins) or the control empty vector, and clones were isolated. Their growth properties were analyzed as described above.
Mice, histological analysis, and immunohistochemistry.
All Atf-2+/− and control wild-type mice analyzed had a 75% BALB/c, 12.5% C57BL/6J, and 12.5% CBA genetic background. Tissues were fixed in 4% paraformaldehyde, dehydrated, and embedded in paraffin. Sections (5 μm) were stained with hematoxylin and eosin according to standard procedures. The histological assessment of tumors was performed according to the WHO classification. Frozen sections (10 μm) were used for immunohistochemistry. For indirect immunofluorescent staining, anti-ATF-2 and anti-GADD45α (N96 and H-165, respectively, from Santa Cruz) were used. The frozen sections were washed twice with Tris-buffered saline buffer (144 mM NaCl, 10 mM Tris-HCl, pH 7.6) and incubated overnight at 4°C with primary antibody. Biotin-conjugated anti-rabbit IgG antibody served as the secondary antibody and was incubated at room temperature for 2 h and further incubated with fluorescein isothiocyanate-streptavidin at room temperature for 1 h.
CGH.
To determine genomic changes, we performed comparative genomic hybridization (CGH) using fluorochrome-conjugated DNA as previously described (9). Briefly, tumor and normal DNAs were labeled by nick translation using Spectrum Green-dUTP and Spectrum Orange-dUTP (Vysis), respectively. Labeled tumor and normal DNAs (250 ng each), together with 10 μg mouse Cot-1 DNA (Gibco-BRL), were denatured and hybridized to normal mouse metaphase chromosome spreads that were prepared from fetal mouse skin fibroblasts. Slides were washed and counterstained with 4′,6′-diamidino-2-phenylindole (DAPI). Relative changes in the copy numbers of DNA sequences were analyzed in at least five cells per tumor by using a digital image analysis system (Quips-XL software; Vysis). Shifts in CGH profiles were rated as gains or losses if they reached at least the 1.2 and 0.8 thresholds, as described elsewhere (52).
Statistical analysis.
Some data are presented as means ± standard errors of the mean (SEM). The P values were obtained by Fisher's protected least significant difference PLSD test.
RESULTS
ATF-2 is critical for hypoxia-induced apoptosis.
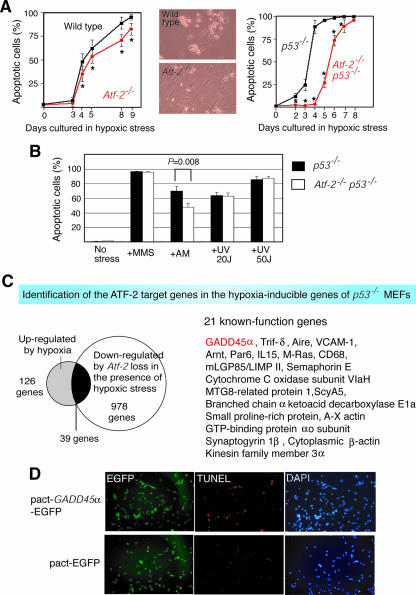
Since ATF-2 is a nuclear target of p38/JNK, which is involved in stress-induced apoptosis, we examined the rates of cell death induced by various treatments of wild-type and Atf-2−/− MEFs. Atf-2−/− MEFs were slightly but significantly more resistant to hypoxia-induced apoptosis than wild-type cells (statistical analysis indicated a P value of <0.01) (Fig. 1A, left). Moreover, although most of the wild-type cells that survived the hypoxia were rounded, the Atf-2−/− cells exhibited normal morphology (Fig. 1A, middle). The resistance of Atf-2−/− cells to hypoxia-induced apoptosis was more significant when Atf-2−/− p53−/− MEFs were compared to p53−/− MEFs (Fig. 1A, right). By the fourth day of hypoxic conditions, almost no apoptotic cells were detected in the Atf-2−/− p53−/− MEFs, whereas about 70% of p53−/− cells were apoptotic. These results suggest that some target genes of ATF-2 and p53 that are induced by hypoxic stress may overlap. In contrast to hypoxia, no differences were noted between the Atf-2−/− p53−/− and p53−/− cells in the degrees of apoptosis induced by MMS or UV light (Fig. 1B). Atf-2−/− p53−/− MEFs were slightly but significantly more resistant to AM-induced apoptosis than p53−/− cells (statistical analysis indicated a P value of <0.01). Thus, ATF-2 is critical for the apoptosis induced by specific stress, such as hypoxia and AM.
FIG. 1.
Role of ATF-2 in hypoxia-induced apoptosis. (A) Deletion of ATF-2 in MEFs confers resistance to hypoxia-induced apoptosis (left), and this is enhanced in the p53−/− background (right). MEFs were prepared from the indicated genotypes, and apoptosis was induced by hypoxia. Apoptotic cells were detected by TUNEL labeling. The averages of three measurements are shown with their SEM. *, P < 0.01. The morphologies of the Atf-2−/− and wild-type cells on day 9 are shown (middle). (B) Effects of loss of Atf-2 on the apoptosis induced by various stresses. p53−/− and Atf-2−/− p53−/− MEFs were treated with various stresses, and apoptotic cells were detected by TUNEL labeling. The averages of three measurements are shown with their SEM. (C) Identification of Gadd45α as an ATF-2 target gene that is induced by hypoxia. RNAs from p53−/− and Atf-2−/− p53−/− MEFs that had been exposed to hypoxic stress for 2 days were subjected to DNA array analysis; 978 genes were down-regulated more than threefold by the loss of Atf-2. DNA array analysis was also performed on RNAs from p53−/− MEFs with or without hypoxia treatment, which revealed that 126 genes were up-regulated more than threefold by hypoxia. Comparison of the 978 down-regulated and 126 up-regulated genes indicated that 39 genes were common to both groups. Of these 39, the functions of 21 (including Gadd45α) are known. These genes are listed on the right. (D) Overexpression of GADD45α in MEFs induces apoptosis. p53−/− MEFs were transfected with a plasmid expressing both GADD45α and enhanced green fluorescent protein (EGFP) or EGFP alone, and apoptotic cells were detected by TUNEL labeling. All cells were detected by DAPI staining, while the transfected cells were marked by EGFP expression.
A group of genes, such as Gadd45α, are regulated by ATF-2 upon hypoxic stress.
To identify the ATF-2 target genes that participate in hypoxia-induced apoptosis, we performed DNA array analysis using RNAs from p53−/− and Atf-2−/− p53−/− MEFs after 2 days of hypoxic treatment. The loss of Atf-2 caused greater than threefold down-regulation of 978 genes (Fig. 1C). When RNAs from p53−/− MEFs treated with and without hypoxic stress were evaluated by DNA array analysis, 126 genes were up-regulated more than threefold by hypoxic stress (Fig. 1C). A comparison of the 978 down-regulated and the 126 up-regulated genes revealed 39 shared genes. Thus, of all the hypoxia-inducible genes, ATF-2 appears to regulate ∼30% (39/126) of them, suggesting that ATF-2 is a major inducer of gene expression during hypoxic stress. The remaining 939 genes were down-regulated by a loss of ATF-2 during hypoxic stress.
Of these ATF-2 target genes, the functions of 21 genes are known (Fig. 1C), and most of them have been reported to be involved in the regulation of apoptosis. To more precisely analyze the ATF-2-dependent transcriptional regulation of these genes, we focused on the Gadd45α gene, which has been clearly demonstrated to be a regulator of apoptosis (46). In fact, we found that GADD45α overexpression induced apoptosis in p53−/− MEFs (Fig. 1D). However, in earlier studies, Gadd45α mutant mice did not exhibit the defects in hypoxia-induced apoptosis (15), suggesting that multiple ATF-2 target genes cooperatively contribute to the hypoxia-induced apoptosis and that a loss of one gene among them, such as Gadd45α, is not sufficient to abrogate the hypoxia-induced apoptosis.
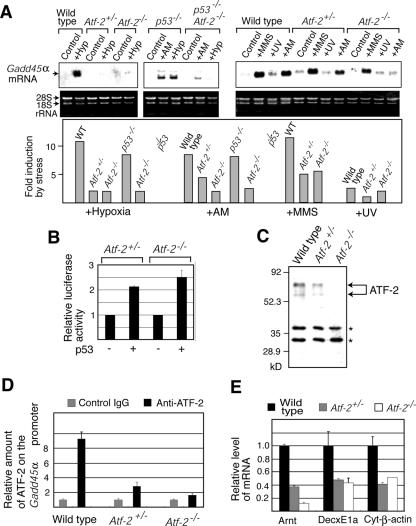
When we examined the Gadd45α expression levels induced by various stresses in wild-type, Atf-2+/−, and Atf-2−/− MEFs, there were almost no differences in the degrees of induction of Gadd45α expression among them (data not shown). This is consistent with our observation that similar numbers of apoptotic cells were detected in wild-type and Atf-2−/− MEFs after hypoxic stress (Fig. 1A, left). However, we found a clear difference between the degrees of Gadd45α induction in wild-type and Atf-2−/− cells immortalized from MEFs (Fig. 2A). The loss of p53 function in immortalized cells may be involved, because spontaneously immortalized cell lines derived from primary MEFs have been shown to exhibit either p53 or Arf loss of function with high frequency (20, 21). Indeed, the role of ATF-2 in hypoxia-induced apoptosis was more clear in p53−/− cells than in p53+/+ cells (Fig. 1A).
FIG. 2.
Loss of one allele of Atf-2 impairs the induction of Gadd45α upon hypoxic (Hyp) stress. (A) Northern blotting of Gadd45α mRNA. RNAs were isolated from wild-type (WT), Atf-2+/−, and Atf-2−/− cells immortalized from MEFs and p53−/− and p53−/− Atf-2−/− MEFs that were subjected to the stress indicated above each lane, and expression of Gadd45α mRNA was analyzed by Northern blotting. The degree of induction is shown below in the bar graphs. (B) p53 responsiveness of the Gadd45α gene promoter. The Gadd45α promoter-luciferase reporter was cotransfected into Atf-2+/− or Atf-2−/− cells immortalized from MEFs with or without a p53 expression vector. Transfected cells were irradiated by UV, and luciferase activity was measured. The averages of relative degrees of activation by p53 (n = 3) are shown with SEM. (C) Levels of ATF-2 protein. Whole-cell extracts from wild-type, Atf-2+/−, and Atf-2−/− cells immortalized from MEFs were used for Western blotting with the anti-ATF-2 antibody. The bands indicated by asterisks are nonspecific signals. (D) ChIP assays. Wild-type, Atf-2+/−, and Atf-2−/− cells immortalized from MEFs were subjected to hypoxic stress for 30 h. Soluble chromatin was prepared and immunoprecipitated with anti-ATF-2 or control IgG. The final DNA extraction was amplified by real-time PCR using the primers that cover the Gadd45α promoter region. The relative densities of bands are indicated. (E) Expression levels of ATF-2 target genes. Wild-type, Atf-2+/−, and Atf-2−/− cells immortalized from MEFs were subjected to hypoxic stress, and total RNA was prepared. The levels of three ATF-2 target genes (Arnt, DecxE1a, and Cyt-β-actin), which were identified using DNA array analysis in panel C, were measured by real-time RT-PCR. The levels (mean plus SEM; n = 3) are shown relative to the levels of wild-type cells.
In previous reports, p53 induced Gadd45α transcription (19), and our reporter assays using the Gadd45α promoter-luciferase reporter confirmed that p53 activates the Gadd45α promoter upon UV irradiation in Atf-2+/− and Atf-2−/− cells, suggesting that p53 can function in these cells (Fig. 2B). In wild-type MEFs, Gadd45α mRNA levels were increased 10.5- and 8-fold by hypoxia and AM, respectively, while a similar degree of induction was observed in p53−/− MEFs (Fig. 2A). However, this parameter was elevated only 2- to 2.5-fold in both Atf-2−/− and Atf-2−/− p53−/− cells, indicating that ATF-2 plays a role in the p53-independent induction of Gadd45α. This is consistent with our observation that ATF-2 is recruited to the sites in the GADD45α promoter, which are different from the p53-binding sites (unpublished results). The lack of an effect of p53 mutation on the hypoxia-dependent induction of Gadd45α suggests that ATF-2 can mediate the induction of Gadd45α upon hypoxic stress in p53−/− cells.
In wild-type cells, MMS and UV irradiation treatment increased the Gadd45α mRNA levels by 11- and 2.5-fold, respectively (Fig. 2A). In contrast to the effects of hypoxia or AM, MMS further increased Gadd45α mRNA levels by fivefold in Atf-2−/− cells. Deletion of Atf-2 had little effect on UV light-induced Gadd45α transcription. This is consistent with our observations that Atf-2−/− MEFs are not resistant to MMS- or UV light-induced apoptosis.
Down-regulation of hypoxia-induced Gadd45α expression by loss of one copy of Atf-2.
Hypoxia increased Gadd45α mRNA expression in Atf-2−/− cells and in Atf-2+/− cells only twofold (Fig. 2A). Western blotting analysis indicated that the two forms of ATF-2 protein, consisting of 70- and 62-kDa polypeptides, were expressed in wild-type MEFs (Fig. 2C). These two forms had been detected previously in brain extracts (27) and may be generated by alternative splicing, as reported previously (17). In Atf-2+/− cells, the amounts of both forms of ATF-2 were about half of that in wild-type cells, while no ATF-2 was detected in Atf-2−/− cells. ChIP assays showed an ∼80% reduction in the amount of ATF-2 bound to the Gadd45α promoter in Atf-2+/− cells compared to wild-type cells (Fig. 2D). These results suggest that the loss of even one copy of Atf-2 reduces the amount of ATF-2 bound to the Gadd45α promoter and impairs hypoxia-induced Gadd45α transcription. Perhaps the rapid induction of Gadd45α that occurs with hypoxic stress requires large amounts of ATF-2; a 50% reduction in ATF-2 levels may cause the inefficient induction of Gadd45α. To further confirm whether loss of one copy of Atf-2 reduces the expression levels of other ATF-2 target genes upon hypoxic stress, we examined the levels of three ATF-2 target genes identified above. The level of these genes, encoding Arnt, DecxE1a, and cytoplasmic β-actin, were significantly reduced in Atf-2+/− cells compared to wild-type cells (Fig. 2E). Thus, loss of one copy of Atf-2 reduces the levels of multiple hypoxia-inducible genes that are regulated by ATF-2.
Growth properties of Atf-2-deficient cells.
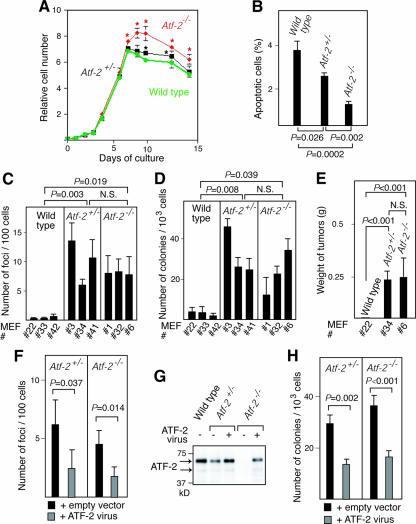
When grown at low densities, wild-type, Atf-2+/−, and Atf-2−/− MEFs were morphologically indistinguishable and had no significant differences in their growth rates (Fig. 3A) and similar numbers of cells in each phase of the cell cycle (data not shown). However, Atf-2+/− and Atf-2−/− MEF monolayers reached 5% and 20% higher cell densities, respectively, than wild-type MEFs (statistical analysis indicated a P value of <0.01). Since ATF-2 is involved in stress-induced apoptosis, the higher cell densities achieved by Atf-2+/− and Atf-2−/− MEFs may indicate that the apoptosis normally induced by high cell density has been abrogated. In fact, when cells reached saturation density, 3.7% and 2.8% of wild-type and Atf-2+/− MEFs were apoptotic, respectively, whereas apoptotic Atf-2−/− MEFs were only 1.2% (Fig. 3B).
FIG. 3.
The loss of one copy of Atf-2 predisposes MEFs to tumorigenic transformation. (A) Growth curves of MEFs. The averages of three measurements with their SEM are shown. *, P < 0.01 compared to wild-type cells. (B) Decreased apoptosis in Atf-2+/− and Atf-2−/− cells at saturated cell density. Apoptotic cells at saturated cell density were detected by TUNEL labeling, and the percentages of apoptotic cells (mean plus SEM; n = 3) are shown. The P values obtained by Fisher's PLSD test are indicated (B to F and H). (C) The number of foci appearing after low-density seeding. The three independent MEFs used are indicated below each bar graph (C and D). The number of foci formed per 102 cells (mean plus SEM; n = 3) is indicated. N.S., no significant difference. (D) The number of colonies generated in methylcellulose after infection with v-K-ras virus. The number of colonies formed per 103 cells (mean plus SEM; n = 3) is indicated. (E) Weights of tumors formed 10 days after injection of the indicated MEFs generated in panel D into nude mice (12 sites of six mice). The average weights and SEM of the resulting 12 tumors from Atf-2+/− and Atf-2−/− cells are shown. No tumors were generated from wild-type cells. (F) Effects of reexpression of ATF-2 on the focus-forming capacities of Atf-2 mutant cells. Atf-2+/− and Atf-2−/− MEFs were infected with the ATF-2-expressing virus and seeded, and the number of foci was measured as described in the legend to panel C. (G) Reexpression of ATF-2 in Atf-2+/− and Atf-2−/− cells. Atf-2+/− and Atf-2−/− cells expressing v-K-ras were infected with the virus encoding ATF-2 and the puromycin resistance marker, and the puromycin-resistant cell pools were selected. Whole-cell lysates were prepared and used for Western blotting with anti-ATF-2 antibody. (H) Effects of reexpression of ATF-2 on the colony-forming capacities of Atf-2 mutant cells. Cells isolated in panel G were used for colony formation assays in methylcellulose, and the number of colonies was measured as described in the legend to panel D.
An examination of the transformation capacity showed that Atf-2+/− and Atf-2−/− MEFs generated 24 to 46 and 12 to 34 colonies, respectively, per 103 cells in methylcellulose after infection with retrovirus carrying v-K-ras (Fig. 3C), whereas wild-type MEFS formed only 1 to 4 colonies (Fig. 3D). Moreover, when colonies were injected into nude mice, both Atf-2+/− and Atf-2−/− MEFs expressing v-K-ras formed tumors, whereas wild-type MEFs did not (Fig. 3E). To further confirm that the growth properties of Atf-2+/− and Atf-2−/− MEFs were due to decreased levels of ATF-2, we reexpressed ATF-2 in these cells using the ATF-2-expressing retrovirus. Statistical analyses of these results (Fig. 3C to E) indicated that both Atf-2+/− and Atf-2−/− MEFs apparently have capacities different from those of wild-type cells (P < 0.05), whereas there is no clear difference between Atf-2+/− and Atf-2−/− MEFs (P = 0.20 to 0.66). These results indicate that loss of one or both copies of Atf-2 facilitates transformation by activated ras in cell culture.
To further confirm the role of ATF-2 in the growth properties of MEFs, we reexpressed ATF-2 in Atf-2+/− and Atf-2−/− MEFs. Both types of MEFs infected with the ATF-2-expressing virus exhibited lower capacities for focus formation following low-density seeding than those infected with the control virus (Fig. 3F). The levels of ATF-2 in Atf-2+/− and Atf-2−/− cells infected with ATF-2 virus were about 80% and 60% of that in wild-type cells, respectively (Fig. 3G). Reexpression of ATF-2 significantly reduced the colony formation capacities of both types of cells in methylcellulose (Fig. 3H). Incomplete suppression of tumorigenicity by exogenous expression of ATF-2 could be related to some irreversible events occurring during cloning of cell lines, such as chromosome instability (see Fig. 7).
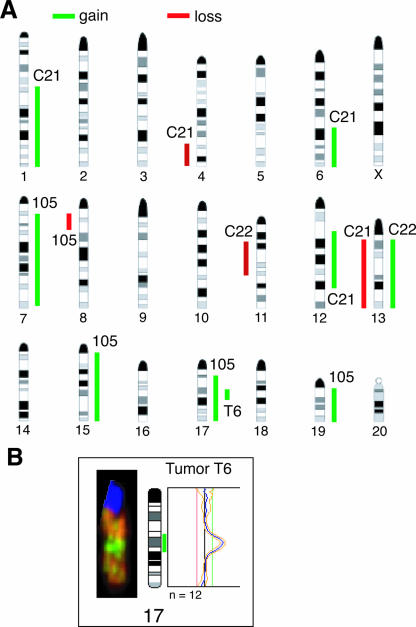
FIG. 7.
The mammary tumors of Atf-2+/− mice exhibit chromosome instability. (A) Summary of the CGH-detected genomic imbalances in 29 esophageal squamous cell carcinoma cell lines using four mammary tumors (C22, C21, T6, and 105, described in Table 1). The vertical lines on the left of each chromosome show losses of genomic material in cell lines, whereas those on the right correspond to copy number gains. (B) High-level gain indicative of gene amplification was detected in tumor T6 at chromosome 17q. Representative CGH images of chromosome 17 (left) and its profile (right) are shown.
Maspin is a target gene of ATF-2.
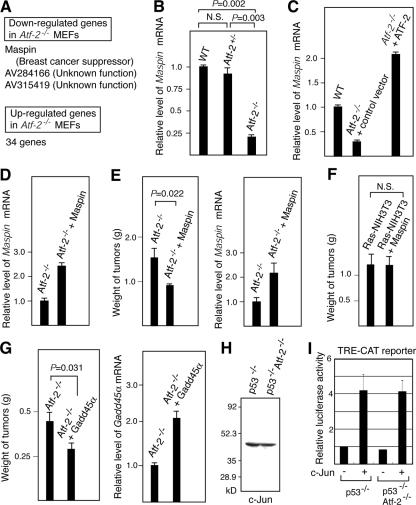
To identify the ATF-2 target genes involved in apoptosis in the absence of hypoxic stress, RNAs prepared from normally cultured wild-type and Atf-2−/− MEFs were subjected to DNA array analysis. The expression levels of only three genes, namely, Maspin and two other genes whose functions are unknown, were decreased by more than threefold in Atf-2−/− MEFs compared to wild-type cells (Fig. 4A). Maspin, a member of the serine protease inhibitor (serpin) family, was originally identified as a tumor suppressor in human breast cancers (54) and was recently shown to enhance cellular sensitivity to apoptotic stimuli (25). On the other hand, 34 genes, 20 of known function and 14 of unknown function, were up-regulated in Atf-2−/− MEFs (see the supplemental material). All of these genes, whose expression levels are down- or up-regulated by a loss of ATF-2, may contribute to the higher-saturation cell density of Atf-2−/− MEFs compared to wild-type cells. However, we focused on Maspin, since, of these genes, only Maspin appeared to be correlated with transformation. Maspin mRNA levels in Atf-2−/− MEFs were about one-eighth of that in wild-type cells, although no decrease in Maspin mRNA was observed in Atf-2+/− MEFs (Fig. 4B). Furthermore, reexpression of ATF-2 in Atf-2−/− MEFs restored the expression levels of Maspin (Fig. 4C). We found that ATF-2 directly binds to its specific site in the Maspin promoter and activates transcription (unpublished results).
FIG. 4.
A down-regulation of Maspin is correlated with the tumorigenicity of of Atf-2−/− cells. (A) Identification of Maspin as an ATF-2 target gene in the absence of hypoxic stress. RNAs prepared from normally cultured Atf-2−/− and wild-type MEFs were subjected to DNA array analysis. Three and 34 genes were down- and up-regulated more than threefold by the loss of Atf-2, respectively. (B) Decreased levels of Maspin mRNA in Atf-2−/− cells. The Maspin mRNA levels in the indicated MEFs were analyzed by real-time RT-PCR, and the relative degree (mean plus SEM; n = 3) is indicated. (C) Ectopic expression of ATF-2 leads to up-regulation of Maspin expression. Atf-2−/− cells were infected with the retrovirus encoding ATF-2 and the puromycin resistance marker or control empty virus, and the puromycin-resistant cells were selected. Maspin mRNA levels were determined by real-time RT-PCR. (D) Reexpression of Maspin in Atf-2−/− cells. Cells were infected with the retrovirus encoding Maspin and the puromycin resistance marker, and the puromycin-resistant cells were selected. Maspin mRNA levels were determined by real-time RT-PCR. (E) Maspin overexpression suppresses the tumorigenicity of Atf-2−/− MEFs. Atf-2−/− MEFs expressing v-K-ras were infected with the Maspin-expressing virus or control empty virus and injected into 12 sites of six nude mice. The average weights and SEM of the 12 tumors that formed 14 days after the injection are shown (left). Note that double the number of cells were injected in these experiments as in Fig. 3E. Maspin mRNA levels in the tumors were determined by real-time RT-PCR (right). (F) Maspin does not suppress Ras-induced tumor formation. NIH 3T3 cells expressing activated K-ras were infected with the Maspin-expressing virus or control virus. Cells were injected into nude mice, and the weights of tumors formed were analyzed as described above. (G) Gadd45α overexpression suppresses the tumorigenicity of Atf-2−/− MEFs. Atf-2−/− MEFs expressing v-K-ras were infected with the Gadd45α-expressing virus or control empty virus and injected into nude mice. The weights of tumors formed were analyzed as described above (left). Gadd45α mRNA levels in the tumors were determined by real-time RT-PCR (right). (H) Loss of Atf-2 does not affect c-Jun levels. Whole-cell lysates were prepared from p53−/− and p53−/− Atf-2−/− cells and used for Western blotting with anti-c-Jun antibody. (I) Loss of Atf-2 does not affect c-Jun-dependent trans-activation. The CAT reporter, in which the TRE-containing promoter was linked to the CAT gene, was cotransfected into p53−/− and p53−/− Atf-2−/− cells with or without a c-Jun expression vector. CAT activity was measured, and the average of relative CAT activity (n = 3) is shown with SEM.
Maspin and Gadd45α are involved in the tumorigenicity of Atf-2−/− MEFs.
Atf-2−/− MEFs expressing v-K-ras were infected with a Maspin-expressing retrovirus vector and then injected into nude mice. The Maspin mRNA levels in the Maspin virus-infected cells were about 2.3-fold higher than that in Atf-2−/− MEFs but were still only about one-fourth of that in wild-type cells (Fig. 4D). Overexpression of Maspin partially but significantly suppressed the size of tumors generated by v-K-ras-expressing Atf-2−/− MEFs (Fig. 4E). This incomplete suppression of tumorigenicity could be due to the low levels of Maspin expression. In related studies, activated Ras-expressing NIH 3T3 cells were infected with the Maspin-expressing virus or the control virus and injected into nude mice. Overexpression of Maspin did not suppress the size of tumors (Fig. 4F), indicating that Maspin does not affect Ras-induced transformation. We also examined the effect of overexpression of Gadd45α on the tumorigenicity of Atf-2−/− MEFs expressing v-K-ras. Overexpression of Gadd45α partially but significantly suppressed the size of tumors generated by v-K-ras-expressing Atf-2−/− MEFs (Fig. 4G). Thus, both Maspin and Gadd45α suppressed the tumorigenicity of Atf-2−/− MEFs, indicating that down-regulation of these two genes contributes at least partly to transformation by a loss of Atf-2.
The c-Jun/ATF-2 heterodimers are more potent transcriptional activators than ATF-2 homodimers and regulate various genes that are implicated in growth control (3, 16, 50). In Atf-2−/− p53−/− and p53−/− MEFs, the levels of c-Jun alone, as well as the levels of CAT expression from the promoter containing the TRE, which may be mediated by endogenous c-Jun, were similar in both types of cells (Fig. 4H and I). Further, ectopically expressed c-Jun also similarly enhanced the CAT expression from the TRE-containing promoter in these cells. These results suggest that c-Jun function is not affected by a loss of Atf-2 and that the tumorigenicity of Atf-2−/− MEFs is not due to the changes in c-Jun activity.
Mammary tumors in Atf-2 heterozygous mice.
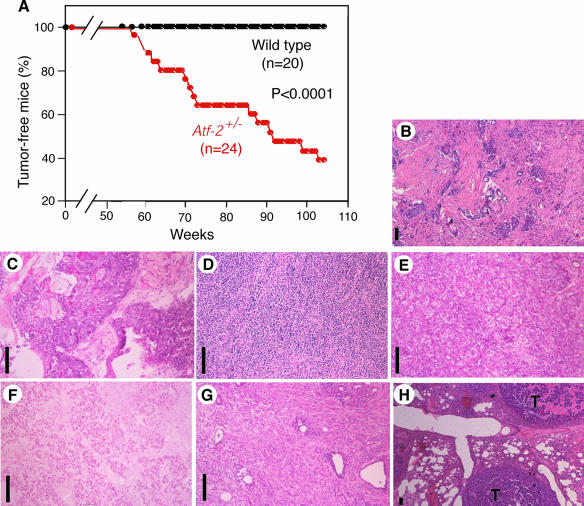
The fact that Atf-2+/− MEFs exhibited a decrease in apoptosis and impaired Gadd45α induction compared to wild-type cells suggested that Atf-2+/− mice could be prone to tumor formation. Indeed, we found that, after a long latency period of about 60 weeks, female Atf-2+/− mice developed mammary tumors (Fig. 5A). These tumors occurred in 15 of 24 female Atf-2+/− mice, whereas all 20 age-matched, wild-type mice remained tumor free. Atf-2+/− male mice did not develop any tumors during a period of 105 weeks. The median time (T50) for tumor-free survival of Atf-2+/− female mice was about 90 weeks. Histological analyses indicated that these tumors included invasive ductal carcinomas with scirrhous, squamous, anaplastic, solid-tubular, and papillary invasion and a sarcomatoid carcinoma with spindle cell invasion (Fig. 5B to G and Table 1). In one case (tumor T2), metastasis of a carcinoma to the lung was also observed (Fig. 5H). Invasive ductal carcinoma with scirrhous invasion, which was most frequently found in Atf-2+/− mice, is also a common human breast cancer (37).
FIG. 5.
Female Atf-2+/− mice spontaneously develop mammary tumors. (A) Kaplan-Meier tumor-free survival curves of Atf-2+/− and wild-type mice. The P value obtained by Fisher's PLSD test is also shown. The mammary tumors of 15/24 Atf-2+/− mice are described in Table 1. (B to H) Histology of the mammary tumors of Atf-2+/− mice, showing invasive ductal carcinomas with scirrhous (B), squamous (C), anaplastic (D), solid-tubular (E), and papillary (F) invasion; a sarcomatoid carcinoma with spindle cell invasion (G); and a carcinoma that had metastasized to the lung (H). Bar, 100 μm. T, tumor.
TABLE 1.
Tumors in Atf-2+/− mice
| Mouse | Sexa | Age (wk) | Site | Histologyb | Expressiond
|
|||
|---|---|---|---|---|---|---|---|---|
| Atf-2 | p53 | Maspin | Gadd45α | |||||
| T2 | F | 57 | Mammary gland | Idc. scirrhous | ND | ND | ND | ND |
| T1 | F | 70 | Mammary gland | Idc. scirrhous | ND | ND | ND | ND |
| T8 | F | 72 | Mammary gland | Idc. scirrhous | + | + | Red | Red |
| T5 | F | 73 | Mammary gland | Idc. scirrhous | − | − | − | − |
| T7 | F | 91 | Mammary gland | Idc. scirrhous | − | + | − | − |
| C21 | F | 86 | Mammary gland | Idc. squamous | − | + | − | − |
| T4 | F | 88 | Mammary gland | Idc. squamous | Red | + | − | − |
| C22 | F | 92 | Mammary gland | Idc. squamous | Red | + | − | Red |
| 104 | F | 60 | Mammary gland | Idc. anaplastic | Red | + | − | − |
| T6 | F | 62 | Mammary gland | Idc. solid-tubular | Red | + | Red | Red |
| T13 | F | 99 | Mammary gland | Idc. Papillary | − | Red | − | − |
| 105 | F | 64 | Mammary gland | Sarc. ca. spindle cell | − | − | − | − |
| C25 | F | 59 | Mammary gland | Unknown | + | + | Red | Red |
| T3 | F | 71 | Mammary gland | NDc | ND | ND | ND | ND |
| T16 | F | 103 | Mammary gland | ND | ND | ND | ND | ND |
F, female.
Idc. scirrhous, invasive ductal carcinoma with scirrhous invasion; Idc. squamous, invasive ductal carcinoma with squamous invasion; Idc. anaplastic, invasive ductal carcinoma with anaplastic invasion; Idc. solid-tubular, invasive ductal carcinoma with solid-tubular invasion; Idc. papillary, invasive ductal carcinoma with papillary invasion; Sarc. ca. spindle cell, sarcomatoid carcinoma with spindle cell invasion.
ND, not determined due to degradation of tumor tissues and mRNA.
+, the expression level was almost the same as that in the wild-type mammary gland; −, almost no expression; Red, the expression level was reduced to 10% to 60% of that in the wild-type mammary gland.
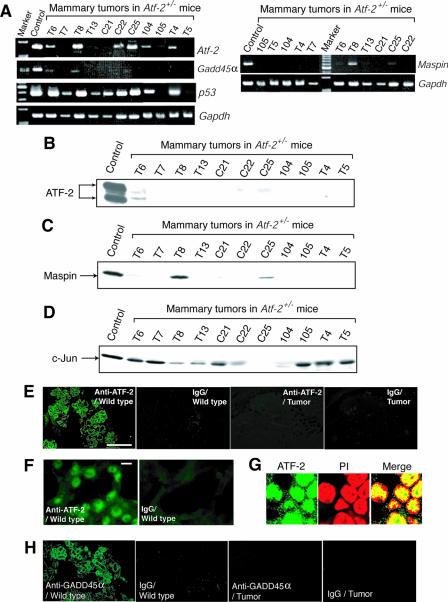
Most of the tumor suppressor genes identified to date fulfill Knudson's “two-mutation” criterion, in which tumors arise if both alleles have mutations or expression from both alleles is suppressed (23). DNA sequence analysis of the wild-type Atf-2 allele in 11 mammary tumors revealed no mutations in the ATF-2 protein-coding region (data not shown). Results of an RT-PCR analysis indicated that of these 11 tumors, Atf-2 mRNA was undetectable in five, significantly reduced in four, and not affected in two (Fig. 6A and Table 1). Western blotting using whole-cell lysates from mammary tumors indicated that the ATF-2 proteins were almost undetectable in all the tumors examined (Fig. 6B). These results suggest that ATF-2 is also regulated at the posttranscriptional level. Consistent with these observations, no ATF-2 immunostaining signals were observed in the tumors that did not express Atf-2 mRNA, whereas ATF-2 was localized in the nuclei of normal mammary epithelial cells (Fig. 6E, F, and G). These results indicate that Atf-2 heterozygosity predisposes mice to develop mammary tumors and that severe reduction of ATF-2 expression may enhance tumor development. Although the c-Jun level dramatically decreased in only 1 tumor, the significant levels of c-Jun were detected in 10 tumors (Fig. 6D). These results suggest that the c-Jun levels are not dramatically affected in most of the mammary tumors of Atf-2+/− mice. Aberrant DNA methylation of CpG islands around promoter regions can suppress transcription (1). However, the results of a methylation-specific PCR analysis using the bisulfite method did not reveal a significant increase in the degree of methylation of the Atf-2 promoter region in mammary tumors (data not shown). Thus, the wild-type Atf-2 allele in the mammary tumors may be silenced by unknown mechanisms, such as the mutation of positive regulators of Atf-2 gene transcription.
FIG. 6.
Down-regulation of Atf-2, Gadd45α, and Maspin in mammary tumors of Atf-2+/− mice. (A) RT-PCR analysis of Atf-2, Gadd45α, Maspin, p53, and Gapdh mRNAs in mammary tumors of Atf-2+/− mice. In the control lane, RNA from normal mammary tissue of Atf-2+/− mice served as the template. (B, C, and D) Levels of ATF-2, Maspin, and c-Jun proteins. Whole-cell lysates were prepared from mammary tumors of Atf-2+/− mice and used for Western blotting with anti-ATF-2 (B), anti-Maspin (C), or anti-c-Jun (D) antibodies. (E to H) Immunostaining of ATF-2 and Gadd45α. Sections of normal mammary gland or the T7 mammary tumors from Atf-2+/− mice (Table 1) were stained with anti-ATF-2 (E, F, and G) or anti-Gadd45α (H) antibody or control IgG. Lower (E) and higher (F) magnifications are indicated. Bars: D, 500 μm; E, 10 μm. In panel G, the nuclear localization of ATF-2 is indicated by costaining with propidium iodide (PI).
Decreased levels of Maspin and GADD45α mRNA and chromosome instability in mammary tumors of Atf-2+/− mice.
Since ATF-2 is involved in some types of apoptosis to which most cell types are probably susceptible, some of the ATF-2 target genes that we identified in MEFs could be shared by mammary epithelial cells. To test this notion, we analyzed the Maspin and Gadd45α expression in 11 mammary tumors from Atf-2+/− mice (Fig. 6A and Table 1). Maspin expression was lost in eight tumors and reduced in the remaining three, while Gadd45α expression was lost in seven tumors and reduced in four. In particular, in all five tumors that did not express detectable Atf-2 mRNA, neither Maspin nor Gadd45α mRNA was detected. With regard to protein expression, Maspin proteins were undetectable in eight tumors and significantly reduced in two tumors (Fig. 6C). Furthermore, no GADD45α immunostaining signals were observed in the tumors that did not express Atf-2 mRNA, whereas the wild-type mammary gland appeared to express GADD45α (Fig. 6H). p53 mRNA expression was lost in two tumors and reduced in one tumor but was not reduced in the remaining eight tumors (Fig. 6A and Table 1). Neither the estrogen receptor nor c-ErbB-2, two well-known markers of certain types of breast cancers, was expressed in tumors from the Atf-2+/− mice (data not shown).
Breast cancer cells often exhibit genomic instability (51), which has also been observed in Gadd45α-deficient mice (15). We performed CGH to assess genomic changes in four independent breast tumors from Atf-2+/− mice. An overview of the changes that we detected is shown in Fig. 7A. All four tumors analyzed showed copy number aberrations, with gains predominating over losses at a ratio of 2.25:1. The numbers of aberrations per tumor were five (tumors C21 and 105), two (tumor C22), and one (tumor T6). High-level amplification, indicative of gene amplification, was detected only at chromosome 17q in tumor T6 (Fig. 7B). Thus, mammary tumors from Atf-2+/− mice exhibit genomic instability.
Decreased levels of ATF-2 mRNA in human breast cancers.
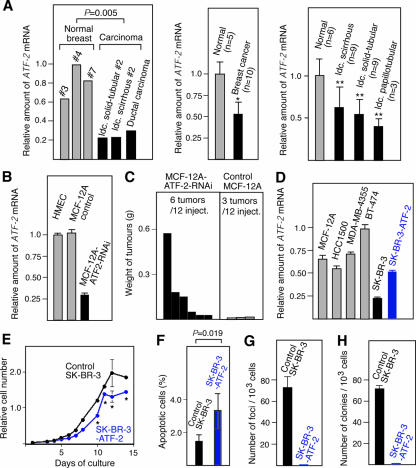
To investigate whether ATF-2 acts as a tumor susceptibility gene of human breast cancers, we determined the ATF-2 mRNA levels in human breast cancer cells isolated by the laser capture microdissection technique, which allowed us to selectively isolate epithelial cells. The ATF-2 expression levels in invasive ductal carcinomas with solid-tubular and scirrhous invasion and in ductal carcinoma in situ were about one-fifth to one-half of those found in normal breast epithelial cells (statistical analysis indicated a P value of 0.005) (Fig. 8A, left). On average, the ATF-2 mRNA level in 10 breast cancers of various types was about half of that in normal mammary epithelial cells (Fig. 8A, middle). Furthermore, analysis of specific types of breast cancers indicated that the ATF-2 mRNA levels in invasive ductal carcinomas with scirrhous, solid-tubular, and papillotubular invasion were 45%, 50%, and 60% lower, respectively, than the levels in normal mammary epithelial cells (Fig. 8A, right). Statistical analysis of these results indicated that the decreased levels of ATF-2 mRNA in human breast cancers were significant (P < 0.01). These findings suggest that ATF-2 may act as a tumor susceptibility gene for various types of breast cancers in humans.
FIG. 8.
ATF-2 expression in human breast cancer tissues and role of ATF-2 in the growth regulation of HMEC. (A) ATF-2 mRNA levels in human breast cancer cells and normal mammary epithelial cells isolated by the laser capture microdissection technique. It was confirmed that there were comparable levels of epithelial cells in both samples. The ATF-2 mRNA levels were measured by quantitative real-time RT-PCR using RNAs prepared from individual breast cancers (left), 10 breast cancers of various types (middle) (*, P = 0.01), specific types of breast cancers (right) (**, P < 0.01), or normal mammary glands. (B) RNA interference decreases the ATF-2 mRNA levels in HMEC. ATF-2 mRNA levels in HMEC, control MCF12A cells harboring empty vector, and MCF12A cells expressing ATF-2 double-stranded RNA (MCF12A-ATF2-RNAi) were measured by real-time RT-PCR. (C) Decreased ATF-2 expression elevates the susceptibility of HMEC to tumorigenesis. MCF12A and MCF12A-ATF2-RNAi cells were infected with the v-K-ras-bearing retrovirus and injected into 12 sites of six nude mice. The tumors and their weights are indicated. (D) ATF-2 mRNA levels in several human breast cancer cell lines and SK-BR3 cells transformed with an ATF-2-expressing vector were measured by real-time RT-PCR. (E to G) Effects of ATF-2 overexpression on the growth of SK-BR3 cells. Growth curves of ATF-2-overexpressing SK-BR3 cells and control SK-BR3 cells harboring the empty vector are shown (E) (*, P < 0.04). Apoptotic cells at saturated cell density were counted, and the percentages of apoptotic cells (mean plus SEM; n = 3) are shown (F). The number of foci appearing after low-density seeding (G) and the number of colonies that formed in soft agarose after infection with v-K-ras retrovirus (H) are also indicated. The averages of triplicate measurements and their SEM are shown.
A decrease in ATF-2 correlates with tumorigenicity of HMEC.
We next examined the tumorigenic effect of knocking down ATF-2 levels in the normal HMEC-derived cell line MCF12A by using RNA interference. MCF12A cells express levels of ATF-2 mRNA similar to those of HMEC (Fig. 8B). The ATF-2 double-stranded RNA expression plasmid was constructed using the pDECAP vector, which we recently developed (43), and introduced into MCF12A cells to generate MCF12A-ATF2-RNAi cells. In these cells, ATF-2 mRNA levels were about one-fourth of the level in control MCF12A cells harboring the empty vector (Fig. 8B). We found that when MCF12A and MCF12A-ATF2-RNAi cells were infected with a v-K-ras retrovirus and injected into nude mice, MCF12A-ATF2-RNAi cells were significantly more tumorigenic than MCF12A cells (Fig. 8C).
We also examined the ATF-2 mRNA levels of several cell lines established from human breast cancers and found that the SK-BR3 cell line expressed lower levels of ATF-2 mRNA than the others (Fig. 8D). To assess the effect of elevating ATF-2 expression in SK-BR3 cells, they were transfected with the ATF-2 expression vector. These transfectants expressed 60% higher levels of ATF-2 mRNA (Fig. 8D) and reached 25% lower cell densities than the control SK-BR3 cells harboring the empty vector (Fig. 8E). In addition, the population of apoptotic cells at saturated cell density was also increased from 1.5% to 3.4% by overexpression of ATF-2 (Fig. 8F). After infection with a v-K-ras retrovirus, SK-BR3 cells overexpressing ATF-2 did not produce either visible foci after low-density seeding or colonies in soft agarose, unlike the control SK-BR3 cells (Fig. 8G and H).
DISCUSSION
The present study indicates that a decrease in ATF-2 expression leads to development of breast cancers. This is correlated with higher cell density in Atf-2−/− MEFs, which may be related to a decrease in Maspin expression, resulting in decreased apoptosis at saturated cell density. Perturbation in p38 signaling is known to increase susceptibility to mammary tumors by a variety of downstream pathways (5, 22, 34), and our results are consistent with these previous studies. It is known that Gadd45α, an ATF-2 target, contributes to p38 activation (30). Thus, there is another potential positive feedback loop involving p38, ATF-2, and Gadd45α whose disturbance can be tumorigenic in breast epithelial cells. Although we have focused on the two ATF-2 target genes, Gadd45α and Mapin, in the present study, it is unlikely that the down-regulation of only these two genes leads to mammary tumors. We have identified 39 genes which are regulated by ATF-2 in response to hypoxia, and probably the changed expression of multiple ATF-2 target genes, including these, cooperatively contributes to the generation of mammary tumors in Atf-2+/− mice. Although the ectopic expression of Maspin or Gadd45α partially suppressed the tumorigenicity of Ras-expressing Atf-2−/− MEFs (Fig. 4E and G), the ectopic expression of Maspin or Gadd45α alone may not be enough to suppress the tumorigenicity of v-K-ras-expressing Atf-2−/− MEFs. Since defects in hypoxia-induced apoptosis have not been reported in Gadd45α mutant mice (15), multiple genes may be involved in the hypoxia-induced apoptosis. Therefore, the ectopic expression of multiple ATF-2 target genes in addition to Gadd45α may be necessary to rescue the defects in the hypoxia-induced apoptosis of Atf-2−/− cells.
The 978 genes were down-regulated by a loss of ATF-2 in the presence of hypoxic stress, while only 3 genes, including Maspin, were down-regulated in the absence of hypoxic stress. This big difference in the number of target genes may be explained by multiple mechanisms. The p53−/− cells were used to identify 978 genes, whereas p53+/+ cells were used to identify 3 genes. Induction of some hypoxia-induced ATF-2 target genes might be detected in p53−/− cells but not in p53+/+ cells. For instance, hypoxic stress induces a group of the repair-related genes via activation of ATM, which directly phosphorylates ATF-2 (4). Induction of these genes may not be detected in p53+/+ cells, because apoptosis and cessation of the cell cycle mediated by p53 occur in response to hypoxic stress in p53+/+ cells. Alternatively, some of the 978 genes might be synergistically up-regulated by both ATF-2 and other transcription factors that are activated or induced by hypoxic stress. Hypoxia induces the expression of many genes, including transcription factors, and also activates some transcription factors, such as the HIF family of proteins (20).
Notably, while ATF-2 is ubiquitously expressed in all cell types (45), Atf-2+/− mice developed only mammary tumors. This suggests that there may be some mammary epithelial cell-specific target genes of ATF-2 that are critical for mammary-tumor development. While the identification of these target genes is a subject for future investigation, down-regulation of Maspin and Gadd45α in mammary tumors of Atf-2+/− mice suggests that these two genes may contribute, at least partly, to the development of mammary tumors. Maspin was recently shown to enhance cellular sensitivity to apoptotic stimuli (25). Thus, decreased Maspin expression could contribute, at least partly, to immortalization by abrogating the apoptosis induced by cell-cell interactions or various signals. Once immortalized, Atf-2+/− and Atf-2−/− cells have a lower capacity than wild-type cells to induce apoptosis in response to hypoxia via the down-regulation of a group of genes that includes GADD45α. It is believed that solid tumors, such as mammary tumors, are exposed to hypoxic stress. GADD45α is involved in the control of apoptosis and cell cycle arrest at G2/M, as well as in DNA damage repair and genomic stability. Similar to what occurs in Gadd45α-deficient cells (15), we also observed chromosome instability in mammary tumors from Atf-2+/− mice. It is known that a hypoxic microenvironment of solid tumors correlates with their increased malignancy and potential for metastasis (14). Therefore, the lower susceptibility of Atf-2-deficient cells to hypoxia-induced apoptosis may promote the development of a malignant phenotype.
Various types of mammary tumors developed in Atf-2+/− mice, including invasive ductal carcinomas with scirrhous and squamous invasion, common types of human breast cancers. Consistent with this, a decrease in the ATF-2 mRNA levels was also observed in various types of human breast cancers, including invasive ductal carcinomas with scirrhous, solid-tubular, and papillotubular invasion. We tried to examine the relationship between a decrease in the ATF-2 mRNA in human breast cancers and the change in expression levels of the putative target genes using expression-profiling data from human breast cancer patients. However, we could not obtain a clear answer, because the level of ATF-2 mRNA in the healthy human breast is too low to be accurately measured by using the DNA array method, although it can be precisely measured by using the real-time RT-PCR method. Identification of the ATF-2 gene as a breast cancer susceptibility gene may be useful for individualized breast cancer risk assessment and could potentially lead to a reduction in breast cancer incidence. Since it is likely that hypoxia and defective apoptosis lead to genomic instability and the development of solid tumors, ATF-2 may contribute not only to breast cancers, but also to other types of cancers in humans.
Supplementary Material
Acknowledgments
We are grateful to H. Kanda for histological analysis, K. Fukumoto for DNA array analysis, M. Noda for murine leukemia virus, H. Saito for the GADD45α plasmid, K. Fukumoto for DNA array analysis, and members of the Experimental Animal Division of RIKEN Tsukuba Institute for maintenance of the mice.
This work was supported in part by Grants-in-Aid for Scientific Research and by grants from the Genome Network Project of the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org.
Published ahead of print on 22 December 2006.
REFERENCES
- 1.Baylin, S., and T. H. Bestor. 2002. Altered methylation patterns in cancer cell genomes: cause or consequence? Cancer Cell 1:299-305. [DOI] [PubMed] [Google Scholar]
- 2.Beier, F., R. J. Lee, A. C. Taylor, R. G. Pestell, and P. LuValle. 1999. Identification of the cyclin D1 gene as a target of activating transcription factor 2 in chondrocytes. Proc. Natl. Acad. Sci. USA 96:1433-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benbrook, D. M., and N. C. Jones. 1990. Heterodimer formation between CREB and JUN proteins. Oncogene 5:295-302. [PubMed] [Google Scholar]
- 4.Bhoumik, A., S. Takahashi, W. Breitweiser, Y. Shiloh, N. Jones, and Z. Ronai. 2005. ATM-dependent phosphorylation of ATF2 is required for the DNA damage response. Mol. Cell 18:577-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bulavin, D. V., C. Phillips, B. Nannenga, O. Timofeev, L. A. Donehower, C. W. Anderson, E. Appella, and A. J. Fornace, Jr. 2004. Inactivation of the Wip1 phosphatase inhibits mammary tumorigenesis through p38 MAPK-mediated activation of the p16Ink4a-p19Arf pathway. Nat. Genet. 36:343-350. [DOI] [PubMed] [Google Scholar]
- 6.Chang, L., and M. Karin. 2001. Mammalian MAP kinase signalling cascades. Nature 410:37-40. [DOI] [PubMed] [Google Scholar]
- 7.Dignam, J. D., R. M. Lebovitz, and R. G. Roeder. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11:1475-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falvo, J. V., B. S. Parekh, C. H. Lin, E. Fraenkel, and T. Maniatis. 2000. Assembly of a functional beta-interferon enhanceosome is dependent on ATF-2-c-jun heterodimer orientation. Mol. Cell. Biol. 20:4814-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukuda, Y., N. Kurihara, I. Imoto, K. Yasui, M. Yoshida, K. Yanagihara, J. G. Park, Y. Nakamura, and J. Inazawa. 2000. CD44 is a potential target of amplification within the 11p13 amplicon detected in four of 25 gastric cancer cell lines. Genes Chromosome Cancer 29:315-324. [DOI] [PubMed] [Google Scholar]
- 10.Gaire, M., B. Chatton, and C. Kedinger. 1990. Isolation and characterization of two novel, closely related ATF cDNA clones from HeLa cells. Nucleic Acids Res. 18:3467-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta, S., D. Campbell, B. Dérijard, and R. J. Davis. 1995. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science 267:389-393. [DOI] [PubMed] [Google Scholar]
- 12.Hai, T., and T. Curran. 1991. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc. Natl. Acad. Sci. USA 88:3720-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hai, T. W., F. Liu, E. A. Allegretto, M. Karin, and M. R. Green. 1988. A family of immunologically related transcription factors that includes multiple forms of ATF and AP-1. Genes Dev. 2:1216-1226. [DOI] [PubMed] [Google Scholar]
- 14.Hockel, M., and P. Vaupel. 2001. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J. Natl. Cancer Inst. 93:266-276. [DOI] [PubMed] [Google Scholar]
- 15.Hollander, M. C., M. S. Sheikh, D. V. Bulavin, K. Lundgren, L. Augeri-Henmueller, R. Shehee, T. A. Molinaro, K. E. Kim, E. Tolosa, J. D. Ashwell, M. P. Rosenberg, Q. Zhan, P. M. Fernandez-Salguero, W. F. Morgan, C. X. Deng, and A. J. Fornace, Jr. 1999. Genomic instability in Gadd45α-deficient mice. Nat. Genet. 23:176-184. [DOI] [PubMed] [Google Scholar]
- 16.Huguier, S., J. Baguet, S. Perez, H. van Dam, and M. Castellazzi. 1998. Transcription factor ATF2 cooperates with v-Jun to promote growth factor-independent proliferation in vitro and tumor formation in vivo. Mol. Cell. Biol. 18:7020-7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ivashkiv, L. B., H. C. Liou, C. J. Kara, W. W. Lamph, I. M. Verma, and L. H. Glimcher. 1990. mXBP/CRE-BP2 and c-Jun form a complex which binds to the cyclic AMP, but not to the 12-O-tetradecanoylphorbol-13-acetate, response element. Mol. Cell. Biol. 10:1609-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin, W., T. Takagi, S. N. Kanesashi, T. Kurahashi, T. Nomura, J. Harada, and S. Ishii. 2006. Schnurri-2 controls BMP-dependent adipogenesis via interaction with Smad proteins. Dev. Cell. 10:461-471. [DOI] [PubMed] [Google Scholar]
- 19.Kaelin, W. G. 2005. Proline hydroxylation and gene expression. Annu. Rev. Biochem. 74:115-128. [DOI] [PubMed] [Google Scholar]
- 20.Kamijo, T., F. Zindy, M. F. Roussel, D. E. Quelle, J. R. Downing, R. A. Ashmun, G. Grosveld, and C. J. Sherr. 1997. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 91:649-659. [DOI] [PubMed] [Google Scholar]
- 21.Kastan, M. B., Q. Zhan, W. S. el-Deiry, F. Carrier, T. Jacks, W. V. Walsh, B. S. Plunkett, B. Vogelstein, and A. J. Fornace, Jr. 1992. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell 71:587-597. [DOI] [PubMed] [Google Scholar]
- 22.Kim, M. S., E. J. Lee, H. R. Kim, and A. Moon. 2003. p38 kinase is a key signaling molecule for H-Ras-induced cell motility and invasive phenotype in human breast epithelial cells. Cancer Res. 63:5454-5461. [PubMed] [Google Scholar]
- 23.Knudson A. G., Jr. 1971. Mutation and cancer: statistical study of retinoblastoma. Proc. Natl. Acad. Sci. USA 68:820-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kondo, S., T. Murakami, K. Tatsumi, M. Ogata, S. Kanemoto, K. Otori, K. Iseki, A. Wanaka, and K. Imaizumi. 2005. OASIS, a CREB/ATF-family member, modulates UPR signalling in astrocytes. Nat. Cell Biol. 7:186-194. [DOI] [PubMed] [Google Scholar]
- 25.Liu, J., S. Yin, N. Reddy, C. Spencer, and S. Sheng. 2004. Bax mediates the apoptosis-sensitizing effect of maspin. Cancer Res. 64:1703-1711. [DOI] [PubMed] [Google Scholar]
- 26.Livingstone, C., G. Patel, and N. Jones. 1997. ATF-2 contains a phosphorylation-dependent transcriptional activation domain. EMBO J. 14:1785-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maekawa, T., F. Bernier, M. Sato, S. Nomura, M. Singh, Y. Inoue, T. Tokunaga, H. Imai, M. Yokoyama, A. Reimold, L. H. Glimcher, and S. Ishii. 1999. Mouse ATF-2 null mutants display features of a severe type of meconium aspiration syndrome. J. Biol. Chem. 274:17813-17819. [DOI] [PubMed] [Google Scholar]
- 28.Maekawa, T., H. Sakura, C. Kanei-Ishii, T. Sudo, T. Yoshimura, J. Fujisawa, M. Yoshida, and S. Ishii. 1989. Leucine zipper structure of the protein CRE-BP1 binding to the cyclic AMP response element in brain. EMBO J. 8:2023-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuda, S., T. Maekawa, and S. Ishii. 1991. Identification of the functional domains of the transcriptional regulator CRE-BP1. J. Biol. Chem. 266:18188-18193. [PubMed] [Google Scholar]
- 30.Mita, H., J. Tsutsui, M. Takekawa, E. A. Witten, and H. Saito. 2002. Regulation of MTK1/MEKK4 kinase activity by its N-terminal autoinhibitory domain and GADD45 binding. Mol. Cell. Biol. 22:4544-4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morooka, H., J. V. Bonventre, C. M. Pombo, J. M. Kyriakis, and T. Force. 1995. Ischemia and reperfusion enhance ATF-2 and c-Jun binding to cAMP response elements and to an AP-1 binding site from the c-jun promoter. J. Biol. Chem. 270:30084-30092. [DOI] [PubMed] [Google Scholar]
- 32.Morrison, D. K., and R. J. Davis. 2003. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu. Rev. Cell Dev. Biol. 19:91-118. [DOI] [PubMed] [Google Scholar]
- 33.Nagadoi, A., K. Nakazawa, H. Uda, K. Okuno, T. Maekawa, S. Ishii, and Y. Nishimura. 1999. Solution structure of the transactivation domain of ATF-2 comprising a zinc finger-like subdomain and a flexible subdomain. J. Mol. Biol. 287:593-607. [DOI] [PubMed] [Google Scholar]
- 34.Neve, R. M., T. Holbro, and N. E. Hynes. 2002. Distinct roles for phosphoinositide 3-kinase, mitogen-activated protein kinase and p38 MAPK in mediating cell cycle progression of breast cancer cells. Oncogene 21:4567-4576. [DOI] [PubMed] [Google Scholar]
- 35.Nomura, N., Y. L. Zu, T. Maekawa, S. Tabata, T. Akiyama, and S. Ishii. 1993. Isolation and characterization of a novel member of the gene family encoding the cAMP response element-binding protein CRE-BP1. J. Biol. Chem. 268:4259-4266. [PubMed] [Google Scholar]
- 36.Ouwens, D. M., N. D. de Ruiter, G. C. van der Zon, A. P. Carter, J. Schouten, C. van der Burgt, K. Kooistra, J. L. Bos, J. A. Maassen, and H. van Dam. 2002. Growth factors can activate ATF2 via a two-step mechanism: phosphorylation of Thr71 through the Ras-MEK-ERK pathway and of Thr69 through RalGDS-Src-p38. EMBO J. 21:3782-3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Page, D. L., and T. J. Anderson. 1987. Diagnostic histopathology of the breast. Churchill Livingstone, London, United Kingdom.
- 38.Persengiev, S. P., and M. R. Green. 2003. The role of ATF/CREB family members in cell growth, survival and apoptosis. Apoptosis 8:225-228. [DOI] [PubMed] [Google Scholar]
- 39.Reimold, A. M., M. J. Grusby, B. Kosaras, J. W. Fries, R. Mori, S. Maniwa, I. M. Clauss, T. Collins, R. L. Sidman, M. J. Glimcher, and L. H. Glimcher. 1996. Chondrodysplasia and neurological abnormalities in ATF-2-deficient mice. Nature 379:262-265. [DOI] [PubMed] [Google Scholar]
- 40.Sano, Y., J. Harada, S. Tashiro, R. Gotoh-Mandeville, T. Maekawa, and S. Ishii. 1999. ATF-2 is a common nuclear target of Smad and TAK1 pathways in transforming growth factor-β signaling. J. Biol. Chem. 274:8949-8957. [DOI] [PubMed] [Google Scholar]
- 41.Sano, Y., F. Tokitou, P. Dai, T. Maekawa, T. Yamamoto, and S. Ishii. 1998. CBP alleviates the intramolecular inhibition of ATF-2 function. J. Biol. Chem. 273:29098-29105. [DOI] [PubMed] [Google Scholar]
- 42.Shimizu, M., Y. Nomura, H. Suzuki, E. Ichikawa, A. Takeuchi, M. Suzuki, T. Nakamura, T. Nakajima, and K. Oda. 1998. Activation of the rat cyclin A promoter by ATF2 and Jun family members and its suppression by ATF4. Exp. Cell Res. 239:93-103. [DOI] [PubMed] [Google Scholar]
- 43.Shinagawa, T., and S. Ishii. 2003. Generation of Ski-knockdown mice by expressing a long double-strand RNA from an RNA polymerase II promoter. Genes Dev. 17:1340-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shinagawa, T., T. Nomura, C. Colmenares, M. Ohira, A. Nakagawara, and S. Ishii. 2001. Increased susceptibility to tumorigenesis of ski-deficient heterozygous mice. Oncogene 20:8100-8108. [DOI] [PubMed] [Google Scholar]
- 45.Takeda, J., T. Maekawa, T. Sudo, Y. Seino, H. Imura, N. Saito, C. Tanaka, and S. Ishii. 1991. Expression of the CRE-BP1 transcriptional regulator binding to the cyclic AMP response element in central nervous system, regenerating liver, and human tumors. Oncogene 6:1009-1114. [PubMed] [Google Scholar]
- 46.Takekawa, M., and H. Saito. 1998. A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell 95:521-530. [DOI] [PubMed] [Google Scholar]
- 47.Tamura, K., B. Hua, S. Adachi, I. Guney, J. Kawauchi, M. Morioka, M. Tamamori-Adachi, Y. Tanaka, Y. Nakabeppu, M. Sunamori, J. M. Sedivy, and S. Kitajima. 2005. Stress response gene ATF3 is a target of c-myc in serum-induced cell proliferation. EMBO J. 24:2590-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Dam, H., M. Duyndam, R. Rottier, A. Bosch, L. de Vries-Smits, P. Herrlich, A. Zantema, P. Angel, and A. J. van der Eb. 1993. Heterodimer formation of cJun and ATF-2 is responsible for induction of c-jun by the 243 amino acid adenovirus E1A protein. EMBO J. 12:479-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Dam, H., D. Wilhelm, I. Herr, A. Steffen, P. Herrlich, and P. Angel. 1997. ATF-2 is preferentially activated by stress-activated protein kinases to mediate c-jun induction in response to genotoxic agents. EMBO J. 14:31798-31811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Dam, H., S. Huguier, K. Kooistra, J. Baguet, E. Vial, A. J. van der Eb, P. Herrlich, P. Angel, and M. Castellazzi. 1998. Autocrine growth and anchorage independence: two complementing Jun-controlled genetic programs of cellular transformation. Genes Dev. 12:1227-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Venkitaraman, A. R. 2002. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell 108:171-182. [DOI] [PubMed] [Google Scholar]
- 52.Yasui, K., I. Imoto, Y. Fukuda, A. Pimkhaokham, Z. Q. Yang, T. Naruto, Y. Shimada, Y. Nakamura, and J. Inazawa. 2001. Identification of target genes within an amplicon at 14q12-q13 in esophageal squamous cell carcinoma. Genes Chromosomes Cancer 32:112-118. [DOI] [PubMed] [Google Scholar]
- 53.Yoshida, H., T. Matsui, A. Yamamoto, T. Okada, and K. Mori. 2001. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107:881-891. [DOI] [PubMed] [Google Scholar]
- 54.Zou, Z., A. Anisowicz, M. J. Hendrix, A. Thor, M. Neveu, S. Sheng, K. Rafidi, E. Seftor, and R. Sager. 1994. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science 263:526-529. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.