Abstract
The molecular mechanisms underlying epidermal growth factor (EGF) receptor tyrosine kinase down-regulation in response to growth factor binding are coming into focus and involve cbl-mediated receptor ubiquitination followed by lysosomal degradation. However, mechanisms underlying the ligand-stimulated degradation of the related receptor tyrosine kinases of the ErbB family do not involve cbl and remain unexplored. Previous studies have demonstrated that the E3 ubiquitin ligase Nrdp1 contributes to the maintenance of steady-state ErbB3 levels by mediating its growth factor-independent degradation. Here we demonstrate that treatment of cells with the ErbB3 ligand neuregulin-1 (NRG1) stabilizes the deubiquitinating enzyme USP8, which in turn stabilizes Nrdp1. The catalytic activity of USP8 is required for NRG1-induced Nrdp1 stabilization. We provide evidence that Akt-mediated phosphorylation of USP8 threonine residue T907 contributes to USP8 stability. Finally, we demonstrate that Nrdp1 or USP8 knockdown suppresses NRG1-induced ErbB3 ubiquitination and degradation in MCF7 breast cancer cells. We conclude that an NRG1-induced protein stability cascade involving USP8 and Nrdp1 mediates the down-regulation of ErbB3. Our observations raise the possibility that the ligand-induced augmentation of pathways involved in the maintenance of basal levels of receptor tyrosine kinases can contribute to ligand-stimulated down-regulation.
The ErbB family of receptor tyrosine kinases (RTKs) consists of four members (epidermal growth factor [EGF] receptor, ErbB2, ErbB3, and ErbB4) that play essential roles in a variety of developmental processes (7, 8, 15, 49). Years of accumulating evidence also implicate the aberrant activation of ErbB receptors in the malignancy of various human tumors. The overexpression of EGF receptor, ErbB2, and ErbB3 has been observed in numerous solid tumor types and correlates with a high degree of receptor activation (21). For example, amplification of the erbB2 gene is observed in 25 to 30% of breast cancer patients, and overexpression of the product correlates with an earlier relapse and poor prognosis (46, 47). ErbB2 is a validated target for therapeutic intervention, and a number of antibody and small-molecule agents are either already in clinical use or under development for the treatment of patients whose tumors overexpress ErbB2 (35).
The members of the ErbB receptor family undergo a network of homo- and heterodimerization events as part of their signaling mechanism. Particularly noteworthy is a strong propensity of ErbB2 to heterodimerize with and activate ErbB3 (3, 9, 37, 42). Since ErbB3 lacks intrinsic tyrosine kinase activity (16) and no diffusible ligand that binds to ErbB2 has been described, these two receptors must necessarily collaborate in propagating signals in response to growth factors such as the ErbB3 ligand neuregulin-1 (NRG1) ( 9, 48). In vitro, ErbB2 and ErbB3 synergize in promoting the growth and transformation of cultured fibroblasts (2, 10) and the proliferation of breast tumor cells (20). Several studies suggest that the two receptors synergize in mediating increased invasiveness induced by NRG1 in breast tumor cell lines (1, 19, 56), and numerous studies have also established a strong link between the coordinate overexpression and activation of ErbB2 and ErbB3 in breast tumor cell lines and in patient samples (26, 36, 40, 45). Moreover, ErbB3 overexpression and activation is also observed in mammary tumors from transgenic mice generated by overexpressing Neu/ErbB2 (45). Interestingly, ErbB3 overexpression in these tumors appears to be at the protein level; ErbB3 message remains constant in normal and tumor tissue (45). On the basis of such expression studies, it has been suggested that the ErbB3 protein may also be used as a marker for patient prognosis (36) and that ErbB3 may contribute to the progression of ErbB2-overexpressing breast tumor cells from noninvasive to invasive states.
Growth factor receptors are subject to a number of negative regulatory mechanisms that prevent the hyper-signaling associated with disease, and it is likely that tumor cells must suppress these mechanisms as part of their receptor-dependent growth program (51). One of the primary mechanisms by which cells negatively regulate receptor activity is through receptor degradation. Recent studies point to a key role for ubiquitination in the down-regulation and degradation of a variety of plasma membrane proteins (25), including RTKs (44). Upon growth factor binding, many RTKs localize to clathrin-coated pits, become internalized, and are delivered to endosomes. Receptors are sorted in endosomes according to whether they are to be recycled to the cell surface or degraded in lysosomes. Ligand binding stimulates the multiple monoubiquitination of EGF receptor (17), and it has been demonstrated that monoubiquitination is sufficient to drive EGF receptor internalization and degradation (17, 34). Moreover, growth factor-stimulated monoubiquitination of endosomal sorting accessory proteins may regulate their function as ubiquitin receptors (13, 18), underscoring the central role of protein ubiquitination in receptor trafficking and degradation. Very recent evidence suggests that EGF also stimulates the K63 polyubiquitination of the EGF receptor as well (23), although the function of this modification is unknown.
Ubiquitination of EGF receptor is mediated, at least in part, by the RING finger E3 ubiquitin ligase cbl. cbl is recruited to the receptor in an activation-dependent manner by the binding of its tyrosine kinase binding domain to phosphorylated tyrosine 1045 of the EGF receptor (52). Recruited cbl becomes tyrosine phosphorylated by the receptor, activating its ubiquitin ligase activity (28, 29). cbl is then thought to ubiquitinate the receptor on kinase domain lysine residues to mediate receptor trafficking to multivesicular bodies for ultimate degradation in the lysosome (17, 23). A number of studies point to the notion that the escape of RTKs from cbl-mediated down-regulation promotes cellular growth properties associated with oncogenesis (11, 28, 29, 38, 41). Interestingly, cbl has been reported not to be an efficient substrate of the other ErbB family receptors under physiological conditions (27). These observations are consistent with reports that ErbB2, ErbB3, and ErbB4 may not undergo significant ligand-induced down-regulation (4, 5) and underscore the importance of other proteins or mechanisms in keeping these receptors in check.
Our previous studies have implicated the RING finger E3 ubiquitin ligase Nrdp1 (neuregulin receptor degradation protein-1) as a key component in a pathway responsible for maintaining steady-state levels of ErbB3 and ErbB4 (12). Nrdp1 binds to these receptors independently of growth factor stimulation and promotes their degradation by mediating their ligand-independent ubiquitination (39). We have also observed that Nrdp1 is labile in cells and that its stability can be markedly and specifically enhanced by the deubiquitinating enzyme USP8 (55). Here we demonstrate that the stabilities of both USP8 and Nrdp1 are regulated by NRG1, and these components encompass a pathway that contributes to the ligand-induced down-regulation of ErbB3.
MATERIALS AND METHODS
Antibodies and cell culture.
Mouse antibody to FLAG epitope (M2) and mouse anti-β-actin AC-15 were purchased from Sigma. Antibody to V5 epitope (immunoglobulin G2a [IgG2a]) was purchased from Invitrogen. Mouse anti-green fluorescent protein (GFP) and rabbit anti-ErbB3 antibody C-17 were from Santa Cruz Biotechnology. Rabbit anti-phospho-Akt (Ser 473) was from Cell Signaling Technology, and antihemagglutinin (anti-HA) epitope antibody (12CA5; IgG2b) was from Boehringer Mannheim. Antiubiquitin antibody was from Covance. Horseradish peroxidase-conjugated goat anti-mouse IgG and goat anti-rabbit IgG were from Zymed and Chemicon, respectively. Fluorescent secondary antibodies to mouse IgG2a and IgG2b were from Molecular Probes. Affinity-purified rabbit antibodies to Nrdp1 and USP8 were described previously (12, 55). 293T, COS7, and MCF7 cells from the ATCC were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and penicillin and streptomycin antibiotics in 10% CO2. Construction and purification of the recombinant bacterially expressed beta isoform of NRG1 was previously described (14).
Plasmids.
Plasmids encoding human ErbB2, human ErbB3, and human Nrdp1 C-terminally tagged with the FLAG epitope have been previously described (12). The pEGFP-C1 vector for GFP expression was from Clontech. Mouse wild-type and C748A USP8 tagged at the C terminus with the V5 epitope were previously described (55). A T907A point mutation of USP8 was created using a QuikChange site-directed mutagenesis kit (Stratagene) according to the directions of the manufacturer. HA-tagged rat dominant negative Akt1 (K179M, T308A, S473A [24]) and HA-tagged constitutively active (ΔPH-myr-Akt) human Akt1, both in the pAdTrack-CMV plasmid, were the kind gifts of Toshiyuki Obata.
Transfection, immunoblotting, and coimmunoprecipitation.
Transfections were carried out using Fugene6 (Roche) according to the directions of the manufacturer, and cells were allowed to express protein for 24 h following transfection. Dishes were treated with 50 nM NRG1 or control as indicated in the figure legends. For immunoblotting experiments, cells in six-well dishes were lysed in 350 μl sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer. Lysates were resolved by dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and blotted with the antibodies indicated in the figures. Detection was carried out using horseradish peroxidase-conjugated secondary antibodies. Chemiluminescent images were captured using an Alpha Innotech imaging station and quantified using FluorChem software. For coimmunoprecipitation experiments, 293T cells in 100-mm dishes were transfected as described above. Twenty-four hours posttransfection, cells were lysed with immunoprecipitation buffer (20 mM Tris, pH 7.4, 150 mM NaCl, 1 mM MgCl2, 1% NP-40, 10% glycerol, 1 mM Na3VO4, 1 mM NaF, 1 mM ZnCl2, and 4 μg/ml [each] of aprotinin, pepstatin, leupeptin, and aminoethyl benzenesulfonyl fluoride). Cleared lysates were immunoprecipitated with 1.5 μg anti-V5 antibody. Proteins in precipitates were blotted as described above.
Immunofluorescence.
Cells were seeded onto coverslips in six-well dishes, grown overnight, and transfected as described above. Cells were rinsed with phosphate-buffered saline, fixed with 4% paraformaldehyde for 20 min, and then immunostained using primary antibodies for 1 h at room temperature. After three washes with phosphate-buffered saline, Alexa Fluor 488- or Alexa Fluor 546-conjugated secondary antibodies (Molecular Probes) were applied for 1 h at room temperature. Receptors were visualized using an Olympus BX61 fluorescence microscope by capturing 12 z-plane images at 0.5-μm intervals encompassing the depth of the cell. Flat-field-corrected image stacks were deconvolved by the constrained iterative method to mathematically remove out-of-focus light from the fluorescent image set using SlideBook 4.1 software (Intelligent Imaging Innovations), resulting in confocal-quality images of protein localization in three dimensions. PS-Speck green and orange fluorescent beads (Molecular Probes) were used to generate the point spread functions used in deconvolutions.
RNAi.
For Nrdp1 short-hairpin RNA interference [shRNAi] oligonucleotide expression, annealed oligonucleotides 5′-gatccccGTACCTCGGATCATGCGGAACttcaagagaGTTCCGCATGATCCGAGGTACTtttta-3′ and 5′-agcttaaaaAGTACCTCGGATCATGCGGAACtctcttgaaGTTCCGCATGATCCGAGGTACggg (uppercase sequences are directed toward identical sequences in human, mouse, cow, and dog Nrdp1) were ligated into the BglII/HindIII sites of the pSuper.retro.neo+gfp vector (OligoEngine, Seattle, WA). 293GPG packaging cells were cotransfected with a retroviral vector and the pJ6Ωbleo plasmid (kind gift of Richard C. Mulligan) using Fugene 6, followed by selection with 100 μg/ml phleomycin (Zeocin; Invitrogen). Stably transfected packaging cell pools were sorted by fluorescence-activated cell sorting, and highly fluorescent cells were maintained in phleomycin. Virus production was initiated by tetracycline removal from the medium for 48 h. Collected medium was filtered using a 0.45-μm syringe filter and used in the infection of MCF7 cells. Forty-eight hours postinfection, MCF7 cells were selected in 800 μg/ml G418 and stable cell pools were kept for further analysis.
For shRNAi USP8 expression, the annealed oligonucleotides 5′-gatccccAGGTGAAGTGGCAGAAGAATTcaagagATTCTTCTGCCACTTCACCTTTtttggaaaa-3′ and 5′-ctagttttccaaaAAAGGTGAAGTGGCAGAAGAATctcttgAATTCTTCTGCCACTTCACCTggg (uppercase sequences are directed toward identical sequences in human, mouse, and rat USP8) were ligated into the linearized pShuttle-H1 vector (kind gift of Hongwu Chen [31]), and this was used in cotransfections. MCF7 cells stably transduced with USP8 shRNA were obtained as described above.
ErbB3 half-life.
MCF7 cells stably transduced with RNAi Nrdp1 or the pSuper vector were seeded into 35-mm dishes at a density of 4 × 105 cells/dish. Following overnight serum starvation, cells were treated with or without NRG1 for 5 min, followed by the treatment with 100 μg/ml cycloheximide for various lengths of time. Lysates were collected and immunoblotted for ErbB3 and Nrdp1. Bands were quantified using Alpha Innotech FluorChem software and plotted.
RESULTS
NRG1 stimulates Nrdp1 accumulation.
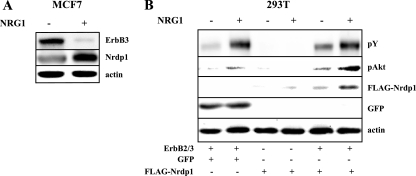
Our previous studies indicate that Nrdp1 is an intrinsically labile protein and that autoubiquitination may contribute to its efficient degradation (55). In further characterizing the stability of Nrdp1, we observed that its levels can be significantly enhanced by stimulation with the NRG1 growth factor. For example, NRG1 stimulated levels of endogenous Nrdp1 protein two- to fivefold in MCF7 human breast tumor cells (Fig. 1A) after 6 hours of growth factor treatment but did not alter Nrdp1 message levels in these cells (not shown). At the same time, ErbB3 levels were dramatically suppressed, indicating that growth factor treatment induced the down-regulation of this receptor. These observations indicate that the Nrdp1 E3 ubiquitin ligase is a posttranscriptional target of NRG1 signaling, raising the possibility that NRG1 stimulation of ErbB2/ErbB3 signaling augments Nrdp1 stability.
FIG. 1.
NRG1 stimulates Nrdp1 accumulation. (A) MCF7 cells were treated without or with NRG1 for 6 h, and lysates were immunoblotted with antibodies to ErbB3, Nrdp1, and actin. (B) 293T cells were cotransfected with both ErbB2 and ErbB3, GFP, or FLAG-Nrdp1, as indicated. Cells were treated without and with NRG1 for 6 h, and lysates were blotted with antibodies to phosphotyrosine (pY), phospho-Akt (pAkt), GFP, FLAG, and actin.
To explore this possibility further, we established a transient-expression system for examining the functions of ErbB signaling pathway components and their mutants (Fig. 1B). The expression of FLAG-tagged Nrdp1 in transiently transfected HEK 293T cells was very weak, and expression levels varied from one experiment to the next, consistent with the properties of a labile protein. NRG1 poorly stimulated levels of FLAG-Nrdp1 in cells not cotransfected with the ErbB2 and ErbB3 receptors, consistent with the low levels of endogenous ErbB2 and ErbB3 receptors in these cells. Cotransfection of ErbB2 and ErbB3 markedly augmented Nrdp1 accumulation, consistent with the significant degree of constitutive activity of these receptors when overexpressed. Treatment of ErbB2/ErbB3-expressing cells with NRG1 caused a further increase in ErbB receptor tyrosine phosphorylation and accumulation of Nrdp1, indicating that Nrdp1 accumulation is downstream of the ErbB2/ErbB3 signaling cascade. The expression of transfected GFP was not affected by NRG1 treatment, consistent with the interpretation that the growth factor does not affect transcription from the cytomegalovirus promoter.
USP8 mediates NRG1-induced Nrdp1 accumulation.
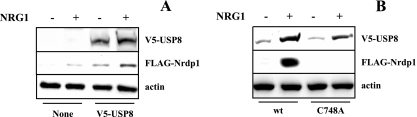
Our previous observations also indicated that the USP8-deubiquitinating enzyme markedly enhances Nrdp1 stability (55). To determine whether USP8 might contribute to NRG1-induced Nrdp1 accumulation, we coexpressed FLAG-Nrdp1 and ErbB2/3 receptors in 293T cells without and with V5-tagged USP8. As shown in Fig. 2A, the presence of USP8 resulted in an elevation in the basal levels of Nrdp1 and augmented NRG1-stimulated Nrdp1 accumulation. The increase in Nrdp1 levels correlated with an NRG1-induced increase in USP8 levels. Figure 2B indicates that although a catalytically inactive form of USP8 (C748A [55]) was similarly augmented by NRG1, this form was unable to mediate NRG1-mediated Nrdp1 accumulation. Taken together, these observations point to the existence of a growth factor-regulated protein stability cascade where NRG1-induced stabilization of USP8 leads to the stabilization of the Nrdp1 E3 ubiquitin ligase.
FIG. 2.
USP8-deubiquitinating activity mediates NRG1-induced Nrdp1 accumulation. (A) 293T cells were cotransfected with ErbB2/3 and FLAG-Nrdp1, without and with V5-USP8 as indicated. Cells were treated without and with NRG1, and lysates were blotted with the indicated antibodies. (B) 293T cells were cotransfected with ErbB2/ErbB3 and FLAG-Nrdp1 and V5-tagged versions of either wild-type (wt) or inactive mutant (C748A) USP8.
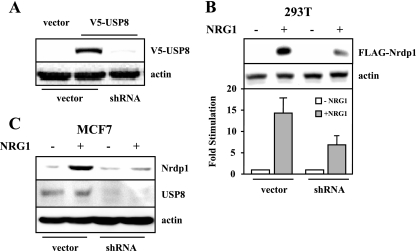
To determine whether USP8 expression is required for NRG1-induced Nrdp1 accumulation, we first developed a vector-delivered short-hairpin oligonucleotide directed toward human and mouse USP8 for use in RNAi knockdown experiments. Figure 3A shows that the oligonucleotide efficiently knocked down the expression of recombinant mouse USP8, strongly suggesting that it can function on endogenous USP8 in human and mouse cell lines. As shown in Fig. 3B, USP8 knockdown reproducibly suppressed NRG1-stimulated Nrdp1 accumulation in 293T cells. Moreover, stable expression of the USP8 shRNA oligonucleotide in MCF7 cells resulted in an ∼75% loss of endogenous USP8 protein and a suppression of the ability of NRG1 to induce Nrdp1 accumulation. These observations suggest that a physiological function of USP8 is to regulate ligand-stimulated Nrdp1 stability in cells.
FIG. 3.
USP8 knockdown suppresses NRG1-stimulated Nrdp1 accumulation. (A) 293T cells were cotransfected with V5-USP8 or its vector and USP8 shRNA or its vector, and lysates were blotted with antibodies to V5 epitope and actin. (B) 293T cells were cotransfected with ErbB2/ErbB3 and FLAG-Nrdp1 without and with USP8 shRNA plasmid, and lysates were blotted with antibodies to FLAG tag or actin. Blots were quantified, and the graph depicts the average (±standard error [SE]) stimulation of NRG1-induced Nrdp1 accumulation over three independent experiments. (C) MCF7 cells stably transduced with an empty vector or the USP8 shRNA vector were treated with NRG1, and lysates were blotted with the indicated antibodies.
NRG1-stimulated USP8 and Nrdp1 accumulation are dependent on phosphatidyl inositol 3-kinase (PI3K) pathway activity.
The six putative p85 binding sites in ErbB3 suggest that this receptor is uniquely suited to couple to the PI3K pathway. To determine whether this signaling pathway might connect activated receptors to USP8, we first examined the effect of a small-molecule PI3K inhibitor on NRG1-induced Nrdp1. We observed that the PI3K inhibitor LY294002 significantly suppressed NRG1-induced Nrdp1 accumulation in transfected 293T cells (Fig. 4A), suggesting that that the PI3K/Akt pathway contributes to USP8 stability and thus Nrdp1 stabilization. Likewise, a dominant negative form of Akt reproducibly suppressed the ability of NRG1 to promote Nrdp1 accumulation (Fig. 4B). Interestingly, the threonine residue at position 907 in USP8 is a predicted Akt phosphorylation site, suggesting that this residue may play an important role in growth factor-induced USP8 function. When cotransfected with ErbB receptors in 293T cells, wild-type USP8 was efficiently detected with an antibody to phosphothreonine only after NRG1 treatment (Fig. 4C), suggesting that NRG1 stimulates USP8 phosphorylation on a threonine residue(s). However, a point mutation form of USP8 with T907 changed to alanine (T907A) could not be detected following NRG1 treatment, suggesting that this residue is phosphorylated in response to NRG1 stimulation.
FIG. 4.
USP8 is phosphorylated on threonine 907 in response to NRG1 treatment. (A) 293T cells were transfected with ErbB2/ErbB3 and FLAG-Nrdp1 and simultaneously treated for 6 h without and with LY294001 and NRG1, as indicated. Lysates were blotted with the indicated antibodies. (Lower panel) Lysates from NRG1-stimulated cells treated without and with LY294001 were blotted for phosphorylated Akt. (B) 293T cells were cotransfected with FLAG-Nrdp1 without or with HA-tagged dominant negative Akt (HA-DN-Akt). The graph depicts the average (±SE) stimulation of NRG1-induced Nrdp1 accumulation from three independent experiments. (C) 293T cells were transfected with either wild type (wt) or T907A USP8, with or without ErbB2 and ErbB3, as indicated. Cells were treated with NRG1 for 6 h, and anti-V5 immunoprecipitates were blotted with phosphothreonine and V5 antibodies. Lysates were blotted for phosphotyrosine (pY) and actin.
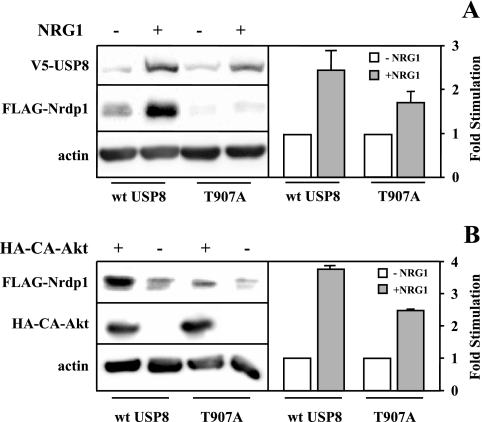
To determine whether T907 is necessary for USP8 function, we examined the ability of the T907A mutant to mediate NRG1-induced Nrdp1 accumulation. As illustrated in Fig. 5A, the T907A mutant less efficiently mediated Nrdp1 accumulation in response to NRG1. Likewise, this mutant exhibited a suppressed ability to mediate NRG1-induced Nrdp1 accumulation upon the expression of a constitutively active form of Akt (Fig. 5B). It should be noted that dominant negative Akt did not fully block NRG1-induced Nrdp1 accumulation and that the T907A mutant was not fully impaired in this regard. These observations suggest that Akt-mediated T907 phosphorylation may not be the only pathway that contributes to USP8-mediated Nrdp1 accumulation in response to growth factor.
FIG. 5.
NRG1-stimulated T907 phosphorylation augments Nrdp1 accumulation. (A) Cells were cotransfected with FLAG-Nrdp1 and either wild-type or T907A mutant USP8, without and with HA-tagged constitutively active Akt (HA-CA-Akt), as indicated. (B) Cells were cotransfected with FLAG-Nrdp1 and either wild-type or T907A mutant USP8, with or without constitutively active Akt (CA-HA-Akt), and blotted with the indicated antibodies. The graphs in each panel depict the average (±SE) stimulation of NRG1-induced Nrdp1 accumulation from three independent experiments.
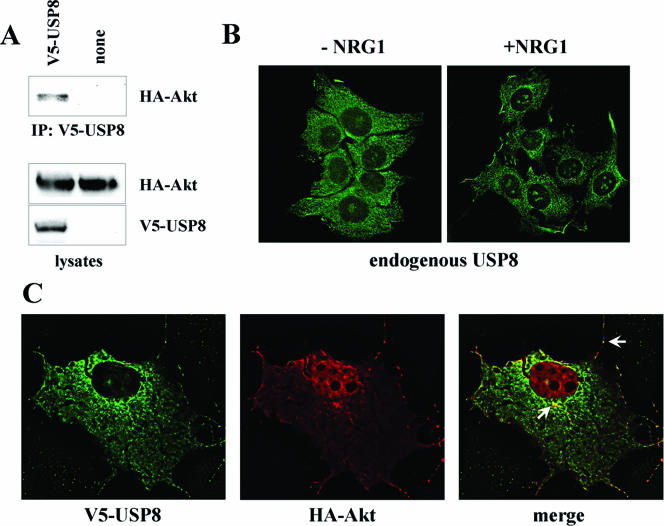
Epitope-tagged Akt could be coimmunoprecipitated with USP8 in cotransfected 293T cells (Fig. 6A), suggesting that the two proteins are capable of forming a stable complex. In addition, USP8 and Akt colocalized at the cell surface and at perinuclear structures when coexpressed in COS7 cells (Fig. 6C). Finally, NRG1 induced the recruitment of endogenous USP8 to the periphery of MCF7 cells, consistent with the recruitment of the Akt-USP8 complex to the plasma membrane (Fig. 6B). Taken together, the observations illustrated in Fig. 4, 5, and 6 point to a role for Akt in the regulation of USP8 function.
FIG. 6.
USP8 association with Akt. (A) Lysates from 293T cells cotransfected with HA-Akt and V5-USP8 were immunoprecipitated (IP) with anti-V5. Lysates and precipitates were blotted with the indicated antibodies. (B) Serum-starved MCF7 cells were treated without and with NRG1 for 15 min, and endogenous USP8 was visualized by immunofluorescence. (C) V5-USP8 (green) and HA-Akt (red) in cotransfected COS7 cells were visualized by deconvolving immunofluorescence microscopy. White arrows point to regions of colocalization at the cell surface and in a perinuclear structure.
USP8 regulates the ubiquitination of Nrdp1 and ErbB3.
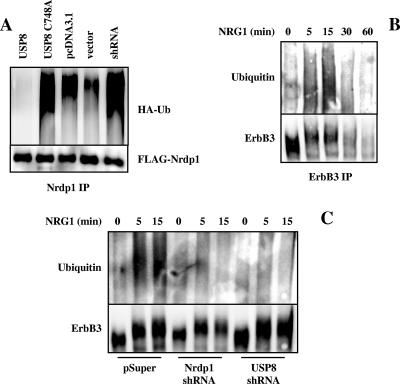
A model that emerges from our observations is that growth factor augmentation of USP8 function results in the deubiquitination and stabilization of Nrdp1, which in turn mediates ErbB3 ubiquitination and down-regulation. To test this model, we first examined the role of USP8 in Nrdp1 ubiquitination. Indeed, USP8 was able to suppress Nrdp1 ubiquitination (Fig. 7A). On the other hand, the catalytically inactive mutant augmented Nrdp1 ubiquitination relative to that of the pcDNA3.1 vector control, suggesting that this form acts in a dominant negative capacity. Likewise, expression of USP8 knockdown oligonucleotides elevated Nrdp1 ubiquitination relative to that of its vector control, suggesting that this is a physiological function of the enzyme. Moreover, we have observed that in MCF7 cells, NRG1 stimulated the transient ubiquitination of endogenous ErbB3, which precedes its loss from the cell (Fig. 7B). NRG1-stimulated ErbB3 ubiquitination is suppressed in MCF7 cells stably transduced with viruses encoding Nrdp1 (see reference 57) or USP8 (Fig. 3C) short-hairpin RNAi oligonucleotides. These observations indicate that Nrdp1 and USP8 are involved in growth factor-induced ErbB3 ubiquitination and hence may play a role in NRG1-induced ErbB3 down-regulation.
FIG. 7.
Regulation of Nrdp1 and ErbB3 ubiquitination by USP8. (A) 293T cells were cotransfected with Nrdp1 and HA-ubiquitin (HA-Ub) along with wild-type USP8, the C748A mutant, or their vector pcDNA3.1 or with shRNA USP8 or its vector. Cells were treated with MG132 to stabilize Nrdp1, and Nrdp1 immunoprecipitates (IP) were blotted with antibodies to the HA epitope and ubiquitin. (B) MCF7 cells were treated for various times with NRG1, and ErbB3 immunoprecipitates were blotted with antibodies to ubiquitin and ErbB3. (C) MCF7 cells stably transduced with the pSuper vector, Nrdp1 shRNA, or USP8 shRNA were treated for the indicated times with NRG1, and ErbB3 immunoprecipitates were blotted for ubiquitin and ErbB3.
It has been reported that ErbB3 is unable to bind to the cbl E3 ligase (27) or to become down-regulated in response to growth factor (4). However, in MCF7 cells we find that ErbB3 protein levels are reduced with NRG1 treatment and that reduction is inhibited by the lysosome inhibitor concanamycin (Fig. 8A). In fact, ErbB3 levels increased with time in the presence of concanamycin, consistent with a model where lysosome-mediated ErbB3 degradation counteracts ErbB3 synthesis to maintain modest steady-state ErbB3 levels in these cells. The half-life of ErbB3 was significantly reduced upon NRG1 treatment (Fig. 8B), indicating that NRG1 treatment augments ErbB3 protein degradation pathways. Interestingly, ErbB2 was not efficiently down-regulated in response to NRG1 in these cells, suggesting that its trafficking is independent of ErbB3 despite the dimerization of the two receptors upon growth factor treatment.
FIG. 8.
NRG1 induces ErbB3 but not ErbB2 down-regulation in MCF7 cells. (A) MCF7 cells were treated without and with 0.1 μM concanamycin (Con), as indicated. Cells were then treated with NRG1 for the indicated times, and lysates were blotted with antibodies to ErbB2 and ErbB3. (B) MCF7 cells were treated with cycloheximide to inhibit protein synthesis and then treated without and with NRG1 for various times. Lysates were blotted with anti-ErbB3 (upper panel), and bands were quantified and plotted (lower panel). ErbB3 half-life was calculated by fitting the data to a single exponential.
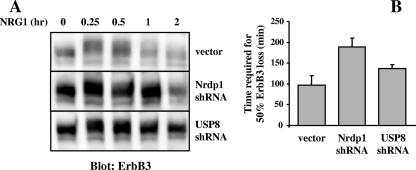
To determine whether the USP8/Nrdp1 pathway is involved in ErbB3 down-regulation, we analyzed NRG1-induced ErbB3 loss in MCF7 cells with Nrdp1 or USP8 knocked down. We observed that loss of expression of these proteins reproducibly elevated steady-state levels of ErbB3 protein (Fig. 9A) and suppressed the kinetics of NRG1-induced ErbB3 loss (Fig. 9B), indicating that both proteins contribute to the efficiency of ErbB3 down-regulation. Collectively, our observations are consistent with a model whereby NRG1 stimulation of Akt (and possibly other pathways) augments USP8 stability and activity, leading to Nrdp1 deubiquitination and stabilization. Elevated Nrdp1 protein levels in turn contribute to ErbB3 ubiquitination and degradation (Fig. 10).
FIG. 9.
Nrdp1 and USP8 augment NRG1-induced ErbB3 down-regulation. (A) MCF7 cells stably transduced with the vector, Nrdp1 shRNA, or USP8 shRNA were treated with NRG1 for the indicated times and blotted with antibodies to ErbB3. (B) Bands were quantified, ErbB3 half-lives for the three cell lines were calculated as described for Fig. 8, and the averages (±SEs) from three independent experiments were plotted.
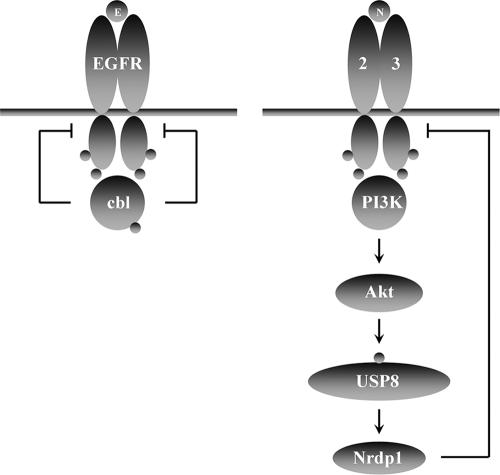
FIG. 10.
Comparison of known EGF receptor and ErbB3 down-regulation pathways. EGF stimulation causes recruitment of cbl to the EGF receptor. Tyrosine phosphorylation of cbl then promotes receptor ubiquitination and trafficking to the lysosome. In contrast, NRG1 stimulates the PI3K/Akt pathway, resulting in USP8 stabilization through the phosphorylation of T907. USP8 in turn stabilizes Nrdp1, which ubiquitinates ErbB3 to promote its degradation.
DISCUSSION
Our previous studies have established that Nrdp1 is an E3 ubiquitin ligase involved in regulating steady-state ErbB3 levels by mediating its ligand-independent ubiquitination and degradation (12, 39). Those studies demonstrated that overexpression of wild-type Nrdp1 suppresses cellular ErbB3 levels but that overexpression of a dominant negative form potently augments receptor levels. In the absence of ligand binding, growth factor receptors undergo constant internalization and trafficking through endosomes. While most internalized receptors are returned to the cell surface, a fraction of unoccupied receptors is targeted for degradation. The competing processes of recycling and degradation establish an equilibrium that defines receptor half-life and hence steady-state levels of cell surface receptors (54). We envision that Nrdp1 is a key component of the mechanism that acts on constitutively internalizing ErbB3 receptors to divert them from the recycling pathway to the lysosomal degradation pathway.
Proteins involved in targeting receptors for ligand-independent degradation may play a significant role in suppressing tumor growth properties by suppressing receptor levels. In a transgenic mouse model of ErbB2-induced mammary tumorigenesis, the ErbB2 transgene product is highly expressed in tumors but is scarcely detectable in uninvolved or nontumor tissue (45, 57). Likewise, endogenous ErbB3 protein is markedly overexpressed only in tumors and not in uninvolved mammary tissues in these animals, and this cannot be attributed to differences in message levels (45, 57). These observations suggest that normal tissue harbors very potent posttranscriptional mechanisms that suppress ErbB receptor levels and that these mechanisms are inactivated during transformation and tumor initiation. Interestingly, we have observed that Nrdp1 protein is present in healthy mammary tissue from ErbB2-transgenic animals but is completely lost in tumors (57), suggesting that Nrdp1 may play a key role in suppressing tumor initiation and progression by maintaining ErbB3 at modest levels.
Our previous studies also demonstrate that Nrdp1 is intrinsically labile and that its stability may be regulated by USP8 (55). Together, our observations point to a model whereby cellular ErbB3 levels are inversely related to Nrdp1 levels (50, 51), suggesting that factors that contribute to Nrdp1 stabilization could suppress cellular ErbB3 levels. Here we demonstrate that one such factor is NRG1 and that USP8 and Nrdp1 contribute to ErbB3 down-regulation upon growth factor stimulation. Hence, these studies characterize for the first time a pathway involved in ligand-induced ErbB3 down-regulation.
Figure 10 contrasts the known E3 ubiquitin ligase pathways contributing to EGF receptor and ErbB3 down-regulation. Upon EGF binding, Y1045 of the EGF receptor becomes phosphorylated, which then acts as a docking site for the tyrosine kinase binding domain of cbl. cbl-mediated EGF receptor ubiquitination then traffics receptors to multivesicular bodies for lysosomal degradation. This pathway provides a very rapid mechanism for mediating the acute degradation of activated receptors and is independent of mechanisms that are involved in maintaining steady-state EGF receptor levels in the absence of ligand. In contrast, NRG1 binding hyperactivates a pathway responsible for the maintenance of basal ErbB3 levels. The successive stabilization of USP8 and Nrdp1 as an outcome of PI3K/Akt activation results in the suppression of ErbB3 levels and a concomitant suppression of NRG1-mediated cellular growth properties (57). In vivo, this pathway could play a role in growth factor-mediated tissue maintenance; such a mechanism may fine-tune receptor number in response to signaling intensity in cells that are chronically exposed to growth factor to provide an overall modest signaling output.
A number of studies have suggested that in contrast to EGF receptor, the other members of the ErbB family do not undergo significant ligand-induced receptor down-regulation (4, 5, 22, 30, 53). A common feature of these studies is that they employed human tumor cells that overexpress ErbB receptors. In contrast, we find that NRG1 stimulates ErbB3 loss in MCF7 human breast tumor cells, which express modest levels of ErbB3 and harbor an intact USP8/Nrdp1 pathway. Interestingly, we have observed that ErbB3 protein overexpression is a relatively common event in breast cancer, occurring in 63% of patient breast tumors. Moreover, Nrdp1 protein levels were potently suppressed in almost 70% of the tumors that overexpressed ErbB3, indicating that Nrdp1 loss often accompanies ErbB3 overexpression in tumors (57). These observations raise the possibility that the ErbB-overexpressing tumor cell lines employed in previous receptor trafficking and degradation studies lack intact negative regulatory pathways and are incapable of mediating efficient receptor down-regulation in response to ligand.
USP8 (also called Ubpy) may play a much broader role in growth factor receptor trafficking and degradation than in simply stabilizing Nrdp1. A series of four recent articles suggest that USP8 may regulate EGF receptor down-regulation by mediating the deubiquitination of endosomal trafficking components (6, 32, 33, 43). These reports suggest that USP8 function is required for efficient ligand-induced down-regulation of EGF receptor (6) by contributing to the efficiency of multivesicular body delivery to lysosomes (33, 43), perhaps by mediating the stability of the endosomal protein STAM (43) or by inhibiting the monoubiquitination of eps15 (33). Hence, NRG1-induced USP8 stabilization may augment global membrane protein degradative processes as part of the cellular growth or differentiation program.
Acknowledgments
We thank Sylvie Urbe for providing the USP8 antibody.
This research was supported by NIH grants GM068994 (K.L.C.), CA123541 (K.L.C.), and CA118384 (C.S.). L.Y. is the recipient of a DoD Breast Cancer Research Program postdoctoral fellowship.
Footnotes
Published ahead of print on 8 January 2007.
REFERENCES
- 1.Adam, L., R. Vadlamudi, S. B. Kondapaka, J. Chernoff, J. Mendelsohn, and R. Kumar. 1998. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J. Biol. Chem. 273:28238-28246. [DOI] [PubMed] [Google Scholar]
- 2.Alimandi, M., A. Romano, M. C. Curia, R. Muraro, P. Fedi, S. A. Aaronson, P. P. Di Fiore, and M. H. Kraus. 1995. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene 10:1813-1821. [PubMed] [Google Scholar]
- 3.Alroy, I., and Y. Yarden. 1997. The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 410:83-86. [DOI] [PubMed] [Google Scholar]
- 4.Baulida, J., M. H. Kraus, M. Alimandi, P. P. Di Fiore, and G. Carpenter. 1996. All ErbB receptors other than the epidermal growth factor receptor are endocytosis impaired. J. Biol. Chem. 271:5251-5257. [DOI] [PubMed] [Google Scholar]
- 5.Baulida, J., and G. Carpenter. 1997. Heregulin degradation in the absence of rapid receptor-mediated internalization. Exp. Cell Res. 232:167-172. [DOI] [PubMed] [Google Scholar]
- 6.Bowers, K., S. C. Piper, M. A. Edeling, S. R. Gray, D. J. Owen, P. J. Lehner, and J. P. Luzio. 2006. Degradation of endocytosed epidermal growth factor and virally ubiquitinated major histocompatibility complex class I is independent of mammalian ESCRTII. J. Biol. Chem. 281:5094-5105. [DOI] [PubMed] [Google Scholar]
- 7.Buonanno, A., and G. D. Fischbach. 2001. Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr. Opin. Neurobiol. 11:287-296. [DOI] [PubMed] [Google Scholar]
- 8.Burden, S., and Y. Yarden. 1997. Neuregulins and their receptors: a versatile signaling module in organogenesis and oncogenesis. Neuron 18:847-855. [DOI] [PubMed] [Google Scholar]
- 9.Carraway, K. L., III, and L. C. Cantley. 1994. A neu acquaintance for erbB3 and erbB4: a role for receptor heterodimerization in growth signaling. Cell 78:5-8. [DOI] [PubMed] [Google Scholar]
- 10.Carraway, K. L., III, S. P. Soltoff, A. J. Diamonti, and L. C. Cantley. 1995. Heregulin stimulates mitogenesis and phosphatidylinositol 3-kinase in mouse fibroblasts transfected with erbB2/neu and erbB3. J. Biol. Chem. 270:7111-7116. [DOI] [PubMed] [Google Scholar]
- 11.de Melker, A. A., G. van der Horst, J. Calafat, H. Jansen, and J. Borst. 2001. c-Cbl ubiquitinates the EGF receptor at the plasma membrane and remains receptor associated throughout the endocytic route. J. Cell Sci. 114:2167-2178. [DOI] [PubMed] [Google Scholar]
- 12.Diamonti, A. J., P. M. Guy, C. Ivanof, K. Wong, C. Sweeney, and K. L. Carraway, III. 2002. An RBCC protein implicated in maintenance of steady-state neuregulin receptor levels. Proc. Natl. Acad. Sci. USA 99:2866-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Fiore, P. P., S. Polo, and K. Hofmann. 2003. When ubiquitin meets ubiquitin receptors: a signalling connection. Nat. Rev. Mol. Cell Biol. 4:491-497. [DOI] [PubMed] [Google Scholar]
- 14.Funes, M., J. K. Miller, C. Lai, K. L. Carraway III, and C. Sweeney. 2006. The mucin Muc4 potentiates neuregulin signaling by increasing the cell surface populations of ErbB2 and ErbB3. J. Biol. Chem. 281:19310-19319. [DOI] [PubMed] [Google Scholar]
- 15.Garratt, A. N., C. Ozcelik, and C. Birchmeier. 2003. ErbB2 pathways in heart and neural diseases. Trends Cardiovasc. Med. 13:80-86. [DOI] [PubMed] [Google Scholar]
- 16.Guy, P. M., J. V. Platko, L. C. Cantley, R. A. Cerione, and K. L. Carraway III. 1994. Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proc. Natl. Acad. Sci. USA 91:8132-8136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haglund, K., S. Sigismund, S. Polo, I. Szymkiewicz, P. P. Di Fiore, and I. Dikic. 2003. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat. Cell Biol. 5:461-466. [DOI] [PubMed] [Google Scholar]
- 18.Haglund, K., P. P. Di Fiore, and I. Dikic. 2003. Distinct monoubiquitin signals in receptor endocytosis. Trends Biochem. Sci. 28:598-603. [DOI] [PubMed] [Google Scholar]
- 19.Hijazi, M. M., E. W. Thompson, C. Tang, P. Coopman, J. A. Torri, D. Yang, S. C. Mueller, and R. Lupu. 2000. Heregulin regulates the actin cytoskeleton and promotes invasive properties in breast cancer cell lines. Int. J. Oncol. 17:629-641. [DOI] [PubMed] [Google Scholar]
- 20.Holbro, T., R. R. Beerli, F. Maurer, M. Koziczak, C. F. Barbas III, and N. E. Hynes. 2003. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc. Natl. Acad. Sci. USA 100:8933-8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holbro, T., G. Civenni, and N. E. Hynes. 2003. The ErbB receptors and their role in cancer progression. Exp. Cell Res. 284:99-110. [DOI] [PubMed] [Google Scholar]
- 22.Hommelgaard, A. M., M. Lerdrup, and B. van Deurs. 2004. Association with membrane protrusions makes ErbB2 an internalization-resistant receptor. Mol. Biol. Cell 15:1557-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang, F., D. Kirkpatrick, X. Jiang, S. Gygi, and A. Sorkin. 2006. Differential regulation of EGF receptor internalization and degradation by multiubiquitination within the kinase domain. Mol. Cell 21:737-748. [DOI] [PubMed] [Google Scholar]
- 24.Katome, T., T. Obata, R. Matsushima, N. Masuyama, L. C. Cantley, Y. Gotoh, K. Kishi, H. Shiota, and Y. Ebina. 2003. Use of RNA interference-mediated gene silencing and adenoviral overexpression to elucidate the roles of AKT/protein kinase B isoforms in insulin actions. J. Biol. Chem. 278:28312-28323. [DOI] [PubMed] [Google Scholar]
- 25.Katzmann, D. J., G. Odorizzi, and S. D. Emr. 2002. Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell Biol. 3:893-905. [DOI] [PubMed] [Google Scholar]
- 26.Lemoine, N. R., D. M. Barnes, D. P. Hollywood, C. M. Hughes, P. Smith, E. Dublin, S. A. Prigent, W. J. Gullick, and H. C. Hurst. 1992. Expression of the ERBB3 gene product in breast cancer. Br. J. Cancer 66:1116-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levkowitz, G., L. N. Klapper, E. Tzahar, A. Freywald, M. Sela, and Y. Yarden. 1996. Coupling of the c-Cbl protooncogene product to ErbB-1/EGF-receptor but not to other ErbB proteins. Oncogene 12:1117-1125. [PubMed] [Google Scholar]
- 28.Levkowitz, G., H. Waterman, S. A. Ettenberg, M. Katz, A. Y. Tsygankov, I. Alroy, S. Lavi, K. Iwai, Y. Reiss, A. Ciechanover, S. Lipkowitz, and Y. Yarden. 1999. Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol. Cell 4:1029-1040. [DOI] [PubMed] [Google Scholar]
- 29.Lill, N. L., P. Douillard, R. A. Awwad, S. Ota, M. L. Lupher, Jr., S. Miyake, N. Meissner-Lula, V. W. Hsu, and H. Band. 2000. The evolutionarily conserved N-terminal region of Cbl is sufficient to enhance down-regulation of the epidermal growth factor receptor. J. Biol. Chem. 275:367-377. [DOI] [PubMed] [Google Scholar]
- 30.Longva, K. E., N. M. Pedersen, C. Haslekas, E. Stang, and I. H. Madshus. 2005. Herceptin-induced inhibition of ErbB2 signaling involves reduced phosphorylation of Akt but not endocytic down-regulation of ErbB2. Int. J. Cancer 116:359-367. [DOI] [PubMed] [Google Scholar]
- 31.Louie, M. C., J. X. Zou, A. Rabinovich, and H. W. Chen. 2004. ACTR/AIB1 functions as an E2F1 coactivator to promote breast cancer cell proliferation and antiestrogen resistance. Mol. Cell. Biol. 24:5157-5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizuno, E., T. Iura, A. Mukai, T. Yoshimori, N. Kitamura, and M. Komada. 2005. Regulation of epidermal growth factor receptor down-regulation by UBPY-mediated deubiquitination at endosomes. Mol. Biol. Cell 16:5163-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizuno, E., K. Kobayashi, A. Yamamoto, N. Kitamura, and M. Komada. 2006. A deubiquitinating enzyme UBPY regulates the level of protein ubiquitination on endosomes. Traffic 12:12. [DOI] [PubMed] [Google Scholar]
- 34.Mosesson, Y., K. Shtiegman, M. Katz, Y. Zwang, G. Vereb, J. Szollosi, and Y. Yarden. 2003. Endocytosis of receptor tyrosine kinases is driven by monoubiquitylation, not polyubiquitylation. J. Biol. Chem. 278:21323-21326. [DOI] [PubMed] [Google Scholar]
- 35.Mosesson, Y., and Y. Yarden. 2004. Oncogenic growth factor receptors: implications for signal transduction therapy. Semin. Cancer Biol. 14:262-270. [DOI] [PubMed] [Google Scholar]
- 36.Naidu, R., M. Yadav, S. Nair, and M. K. Kutty. 1998. Expression of c-erbB3 protein in primary breast carcinomas. Br. J. Cancer 78:1385-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olayioye, M. A., R. M. Neve, H. A. Lane, and N. E. Hynes. 2000. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 19:3159-3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peschard, P., and M. Park. 2003. Escape from Cbl-mediated downregulation: a recurrent theme for oncogenic deregulation of receptor tyrosine kinases. Cancer Cell 3:519-523. [DOI] [PubMed] [Google Scholar]
- 39.Qiu, X. B., and A. L. Goldberg. 2002. Nrdp1/FLRF is a ubiquitin ligase promoting ubiquitination and degradation of the epidermal growth factor receptor family member, ErbB3. Proc. Natl. Acad. Sci. USA 99:14843-14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajkumar, T., and W. J. Gullick. 1994. The type I growth factor receptors in human breast cancer. Breast Cancer Res. Treat. 29:3-9. [DOI] [PubMed] [Google Scholar]
- 41.Ravid, T., C. Sweeney, P. Gee, K. L. Carraway III, and T. Goldkorn. 2002. Epidermal growth factor receptor activation under oxidative stress fails to promote c-Cbl mediated down-regulation. J. Biol. Chem. 277:31214-31219. [DOI] [PubMed] [Google Scholar]
- 42.Riese, D. J., II, and D. F. Stern. 1998. Specificity within the EGF family/ErbB receptor family signaling network. Bioessays 20:41-48. [DOI] [PubMed] [Google Scholar]
- 43.Row, P. E., I. A. Prior, J. McCullough, M. J. Clague, and S. Urbe. 2006. The ubiquitin isopeptidase UBPY regulates endosomal ubiquitin dynamics and is essential for receptor down-regulation. J. Biol. Chem. 281:12618-12624. [DOI] [PubMed] [Google Scholar]
- 44.Shtiegman, K., and Y. Yarden. 2003. The role of ubiquitylation in signaling by growth factors: implications to cancer. Semin. Cancer Biol. 13:29-40. [DOI] [PubMed] [Google Scholar]
- 45.Siegel, P. M., E. D. Ryan, R. D. Cardiff, and W. J. Muller. 1999. Elevated expression of activated forms of Neu/ErbB-2 and ErbB-3 are involved in the induction of mammary tumors in transgenic mice: implications for human breast cancer. EMBO J. 18:2149-2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slamon, D. J., G. M. Clark, S. G. Wong, W. J. Levin, A. Ullrich, and W. L. McGuire. 1987. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177-182. [DOI] [PubMed] [Google Scholar]
- 47.Slamon, D. J., W. Godolphin, L. A. Jones, J. A. Holt, S. G. Wong, D. E. Keith, W. J. Levin, S. G. Stuart, J. Udove, A. Ullrich, et al. 1989. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244:707-712. [DOI] [PubMed] [Google Scholar]
- 48.Sliwkowski, M. X., G. Schaefer, R. W. Akita, J. A. Lofgren, V. D. Fitzpatrick, A. Nuijens, B. M. Fendly, R. A. Cerione, R. L. Vandlen, and K. L. Carraway III. 1994. Coexpression of erbB2 and erbB3 proteins reconstitutes a high affinity receptor for heregulin. J. Biol. Chem. 269:14661-14665. [PubMed] [Google Scholar]
- 49.Stern, D. F. 2003. ErbBs in mammary development. Exp. Cell Res. 284:89-98. [DOI] [PubMed] [Google Scholar]
- 50.Sweeney, C., and K. L. Carraway III. 2004. Negative regulation of ErbB family receptor tyrosine kinases. Br. J. Cancer 90:289-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sweeney, C., J. K. Miller, D. L. Shattuck, and K. L. Carraway III. 2006. ErbB receptor negative regulatory mechanisms: implications in cancer. J. Mammary Gland Biol. Neoplasia. 11:89-99. [DOI] [PubMed] [Google Scholar]
- 52.Thien, C. B., and W. Y. Langdon. 2001. Cbl: many adaptations to regulate protein tyrosine kinases. Nat. Rev. Mol. Cell Biol. 2:294-307. [DOI] [PubMed] [Google Scholar]
- 53.Wang, Z., L. Zhang, T. K. Yeung, and X. Chen. 1999. Endocytosis deficiency of epidermal growth factor (EGF) receptor-ErbB2 heterodimers in response to EGF stimulation. Mol. Biol. Cell 10:1621-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiley, H. S. 2003. Trafficking of the ErbB receptors and its influence on signaling. Exp. Cell Res. 284:78-88. [DOI] [PubMed] [Google Scholar]
- 55.Wu, X., L. Yen, L. Irwin, C. Sweeney, and K. L. Carraway III. 2004. Stabilization of the E3 ubiquitin ligase Nrdp1 by the deubiquitinating enzyme USP8. Mol. Cell. Biol. 24:7748-7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu, F. J., S. Stack, C. Boyer, K. O'Briant, R. Whitaker, G. B. Mills, Y. H. Yu, and R. C. Bast, Jr. 1997. Heregulin and agonistic anti-p185(c-erbB2) antibodies inhibit proliferation but increase invasiveness of breast cancer cells that overexpress p185(c-erbB2): increased invasiveness may contribute to poor prognosis. Clin. Cancer Res. 3:1629-1634. [PubMed] [Google Scholar]
- 57.Yen, L., Z. Cao, X. Wu, E. R. Q. Ingalla, C. Baron, L. J. T. Young, J. P. Gregg, R. D. Cardiff, A. D. Borowsky, C. Sweeney, and K. L. Carraway III. 2006. Loss of Nrdp1 enhances ErbB2/ErbB3-dependent breast tumor cell growth. Cancer Res. 66:11279-11286. [DOI] [PubMed] [Google Scholar]