Abstract
ROPs or RACs are plant Rho-related GTPases implicated in the regulation of a multitude of signaling pathways that function at the plasma membrane by virtue of posttranslational lipid modifications. The relationship between ROP activation status and membrane localization has not been established. Here we demonstrate that endogenous ROPs, as well as a transgenic His6-green fluorescent protein (GFP)-AtROP6 fusion protein, were partitioned between Triton X-100-soluble and -insoluble membranes. In contrast, an activated His6-GFP-Atrop6CA mutant protein accumulated exclusively in detergent-resistant membranes. GDP induced accumulation of ROPs in Triton-soluble membranes, whereas GTPγS induced accumulation of ROPs in detergent-resistant membranes. Recombinant wild-type and constitutively active AtROP6 isoforms were purified from Arabidopsis plants, and their lipids were cleaved and analyzed by gas chromatography-coupled mass spectrometry. In Triton-soluble membranes, wild-type AtROP6 was only prenylated, primarily by geranylgeranyl. The activated AtROP6 that accumulated in detergent-resistant membranes was modified by prenyl and acyl lipids. The acyl lipids were identified as palmitic and stearic acids. In agreement, activated His6-GFP-Atrop6CAmS156 in which cysteine156 was mutated into serine accumulated in Triton-soluble membranes. These findings show that upon GTP binding and activation, AtROP6 and possibly other ROPs are transiently S acylated, which induces their partitioning into detergent-resistant membranes.
Membrane attachment of almost all members of the Ras superfamily of small GTP-binding proteins depends on isoprenylation of C-terminal cysteine residues and concomitant proteolysis and carboxy methylation (6-9, 15, 26). Prenylation involves the covalent attachment via a thioether linkage of either farnesylpyrophosphate or geranylgeranylpyrophosphate isoprenoid intermediates. The prenylated cysteines are part of a conserved C-terminal sequence motif designated the CaaX box or double-cysteine motifs in Rab proteins (24). H-Ras, N-Ras, and several Rho proteins are S acylated, as well as prenylated (15). S acylation, more commonly referred to as palmitoylation, involves the attachment of palmitate (C16:0) or other saturated lipids to cysteine residues through a reversible thioester linkage (37).
Because of its reversibility, S acylation has attracted much attention as a mechanism modulating signaling by regulation of plasma membrane (PM) localization, attraction to lipid rafts, and protein-protein interactions. Acylated (palmitoylated) proteins tend to partition into anionic detergent-resistant membrane (DRM) domains, suggesting their localization in lipid rafts, while prenylated proteins show little affinity for DRMs (23, 25, 38). Thus, transient S acylation could provide a means to spatially separate proteins. Acylation-deacylation cycles promote transport of H-Ras and N-Ras proteins from the Golgi apparatus to the PM and vice versa (34). Activated H-Ras and K-RasB are excluded from lipid rafts, while the inactive form of H-Ras, but not of K-RasB, accumulates in lipid rafts (30-32).
Plant ROPs or RACS are Rac-related GTPases (5) and the only known plant Ras superfamily proteins involved in signaling. ROPs regulate multiple signaling pathways involved in growth, development, and response to pathogens, organization of the actin cytoskeleton, Ca2+, phosphoinositide, and reactive oxygen species signaling (29). In Arabidopsis and other plants, ROPs constitute multimember protein families. On the basis of differences in gene structure and sequences of their C-terminal hypervariable domains, the plant ROPs were subdivided into two major subgroups designated type I and type II (40). Type I ROPs terminate with a conserved CaaL box motif and a proximal polybasic domain, and it was suggested that they likely undergo prenylation. Type II ROPs undergo S acylation but not prenylation (19), depending on a unique C-terminal sequence motif (20). An activated type II ROP, AtROP11 (AtRAC10), was localized in DRM (3). It is not known what governs the membrane association of type I ROPs, whether they undergo acylation as well as prenylation, or whether they are localized in or excluded from DRM. The multiplicity of ROP homologues, which are highly identical outside their hypervariable domains, makes them an attractive model to study spatial and function regulation of GTPases by differential lipidation.
In comparison to human Ras proteins, less is known about the membrane distribution of Rho superfamily members. In addition, direct chemical analysis of the composition of lipid moieties on small GTPases in correlation to their activation status has not been carried out. In this work, we analyzed the membrane distribution of AtROP6 (also designated AtRAC3 or AtRAC1) (21, 39), a type I ROP from Arabidopsis, in relation to its activation status and identified the corresponding type of lipid moieties on active and inactive protein isoforms by using gas chromatography (GC)-coupled mass spectrometry (MS).
MATERIALS AND METHODS
Molecular cloning.
The AtROP6 cDNA was amplified from a flower cDNA library with primers SYP179 and SYP198. The amplified fragment was subcloned into pGEM (Promega) to create plasmid pSY175. In turn, pSY175 was digested with SacI and subcloned into pET28a (Novagen) to create plasmid pSY804, in which AtROP6 was expressed fused to an N-terminal His6 tag.
Plasmid pGFP-MRC (35) was modified by the addition of a His6 tag upstream of green fluorescent protein (GFP) to create plasmid pSY245. AtROP6 cDNA was digested by SacI from pSY175 and subcloned into pSY245 to create plasmid pSY811. The His6-GFP-Atrop6CA mutant protein was created by changing AtROP6 Gly15 to valine. Mutagenesis was carried out with a QuikChange mutagenesis kit (Stratagene) with pSY811 as the template and primers SYP189 and SYP190. The resulting plasmid was designated pSY812. pSY812 was used as the template for site-directed mutagenesis changing the CaaX box Cys195 into serine to create His6-GFP-Atrop6CAmS195 with a QuikChange mutagenesis kit (Stratagene) and primers SYP610 and SYP611. The new plasmid was designated pSY817. pSY812 was used as the template for site-directed mutagenesis changing Cys156 into serine to create His6-GFP-Atrop6CAmS156 with a QuikChange mutagenesis kit (Stratagene) and primers SYP859 and SY860. The new plasmid was designated pSY1800. pSY811, pSY812, pSY817, and pSY1800 were digested with HindIII to isolate cassettes containing 35S promoter::TL (translational enhancer)-His6-GFP-AtROP6/Atrop6CA/Atrop6CAmS195/Atrop6CAmS156-nopaline synthase terminator. The cassettes were ligated into HindIII-digested plant binary plasmid pCambia3300 to obtain pSY815 (AtROP6), pSY814 (Atrop6CA), pSY806 (Atrop6CAmS195), and pSY1803 (Atrop6CAmS156). All clones were fully sequenced to verify the relevant mutations and that no PCR-generated errors were introduced. All of the plasmids and oligonucleotide primers used in this study are listed in Tables 1 and 2, respectively.
TABLE 1.
Plasmids used in this study
| Plasmid name | Description | Source or reference |
|---|---|---|
| pGEM | PCR product TA cloning vector | Promega |
| pSY175 | pGEM-AtROP6 | This study |
| pSY804 | pET28a-AtROP6 | This study |
| pSY245 | pHis6GFP | This study |
| pGFP-MRC | 35S::GFP-nopaline synthase 3′ end | 35 |
| pSY811 | pHis6-GFP-AtROP6 | This study |
| pSY812 | pHis6-GFP-Atrop6CA | This study |
| pSY817 | pHis6-GFP-Atrop6CAmS195 | This study |
| pCAMBIA3300 | Plant T-DNA-based binary vector | CAMBIA (Canberra, Australia) |
| pSY814 | pCAMBIA3300-His6-GFP-Atrop6CA | This study |
| pSY806 | pCAMBIA3300-His6-GFP-Atrop6CAmS195 | This study |
| pSY815 | pCAMBIA3300-His6-GFP-AtROP6 | This study |
| pSY1800 | pHis6-GFP-Atrop6CAmS156 | This study |
| pSY1803 | pCAMBIA3300-His6-GFP-Atrop6CAmS156 | This study |
TABLE 2.
Primers used in this study
| Primer name | Sequence | Product of target genea |
|---|---|---|
| SYP179 | GGAAGAGCTCAGTGCTTCAAGGTTTATCAAG | AtROP6-F |
| SYP198 | GTTGAATTCGAGCTCTCACTTTTGCCCTTTCTTCCT | AtROP6-R |
| SYP189 | CACTGTCGGCGACGTTGCTGTTGGAAAGAC | AtROP6CA-F |
| SYP190 | GTCTTTCCAACAGCAACGTCGCCGACAGTG | AtROP6CA-R |
| SYP610 | GAGAAAATCTCAGAAAGGTTCTTCTATTACTCTGACTCGGAG | AtROP6CAmS195-F |
| SYP611 | CTCGAGTCAGAGTATAGAAGAACCTTTCTGAGATTTTCTC | AtROP6CAmS195-R |
| SYP859 | GGGGCGCCTGCTATATCGAATCCAGTGCAAAAACTCAACAGAATGTG | AtROP6CAmS156-F |
| SYP860 | CACATTCTGTTGAGTTTTTGCACTGGATTCGATATAAGCAGGCGCCCC | AtROP6CAmS156-R |
F, forward; R, reverse.
Protein expression in Escherichia coli and Arabidopsis.
E. coli DH5α was used for DNA propagation and protein expression. pSY804 was transformed into E. coli BL21 CodonPlus DE3 RIL (Stratagene) cells. Cells were grown to an optical density at 600 nm of 0.6, and protein expression was induced by adding 2 mM isopropyl-β-d-thiogalactopyranoside (IPTG). Protein extraction and purification on Ni-nitrilotriacetic acid (NTA) columns was performed according to the manufacturer's (QIAGEN) instructions. pSY815, pSY814, and pSY806 were transformed into Arabidopsis Col-0 plants by the floral-dip method (10). Several independent transgenic lines stably expressing His6-GFP-AtROP6, His6-GFP-Atrop6CA, His6-GFP-Atrop6CAmS195, or His6-GFP-Atrop6CAmS156 were selected. Following analysis of five independent transgenic lines of each genotype and verification that the plant phenotypes and protein localizations were the same, one line from each group was selected for further work.
Ab production, purification, and protein immunoblotting.
Anti-AtROP6 polyclonal antibodies (Abs) were raised in rabbits against E. coli-expressed protein. The Abs were affinity purified over an AtROP6-conjugated 1-ml HiTrap activated-N-hydroxysuccinimide column according to the manufacturer's (Amersham Biosciences, Little Chalfont, United Kingdom) instructions by Akta fast protein liquid chromatography (Amersham Biosciences, Little Chalfont, United Kingdom). Proteins were resolved by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and transferred onto nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany). For protein immunoblotting, the affinity-purified anti-AtROP6 Abs or anti-Arabidopsis AtRac3 Abs (Sigma R9529, lot 106K4831; Sigma, St. Louis, MO) were used at a dilution of 1:3,000 together with blotting grade, horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (Bio-Rad, Hercules, CA). Both the anti-AtROP6 and commercial anti-AtRac3 Abs recognized both type I and type II ROPs and were highly specific (Fig. 1; see Fig. S1 in the supplemental material). Anti-GFP monoclonal Abs (Covance MMS 118P; BAbCO, Berkeley, CA) were used at a dilution of 1:5,000, and anti-His6 monoclonal Abs (Sigma H1029; Sigma, St. Louis, MO) were used at a dilution of 1:3,000 together with blotting grade, horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (Bio-Rad, Hercules, CA). Detection was performed with EZ-ECL (Biological Industries, Bet Haemek, Israel). Quantification of bands on blots was carried out by densitometry on a gel-film scanner (Amersham/Pharmacia Biotech Little Chalfont, United Kingdom). Intensity values of the bands were normalized by dividing the values in the DRM bands by the values in Triton X-100-soluble membrane (TSM) bands. The data are presented as a percentage of the protein in DRM.
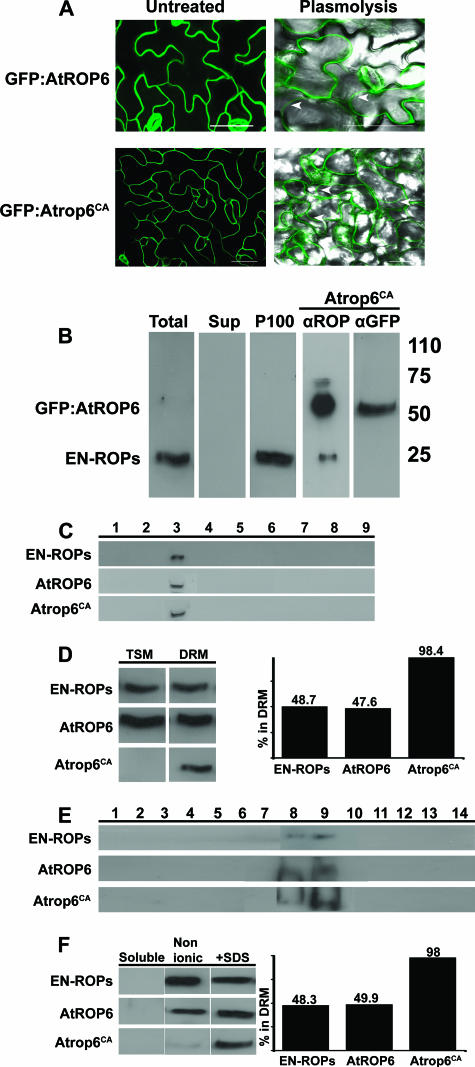
FIG. 1.
Membrane localization and partitioning of endogenous and transgenic ROPs. (A) Images of leaf epidermal cells stably expressing the His6-GFP-AtROP6 or His6-GFP-Atrop6CA fusion protein. Both GFP fusion proteins were localized in the PM, as evident in the thin fluorescent line circumventing the cells (left side) and confirmed by plasmolysis (right side). Images of plasmolyzed cells were produced by fluorescent differential interference contrast overlay. Arrowheads denote cell wall-detached PM. Bars are 50 μm. All images are projection stacks of multiple confocal sections that were produced with a confocal laser scanning microscope. (B to F) Protein immunoblotting with anti-ROP and anti-GFP Abs (B) or only anti-ROP Abs (C to F). (B) Endogenous ROPs (EN-ROPs) and transgenic His6-GFP-Atrop6CA (GFP:AtROP6) were only detected in the insoluble membrane pellet following centrifugation (P100). Note the reduced intensity of the endogenous ROP band in the His6-GFP-Atrop6CA transgenic protein extract (Atrop6CA) likely due to the large excess of the recombinant protein. Total, total protein extract; Sup, supernatant following centrifugation at 100,000 × g. Values on the right are molecular masses in kilodaltons. (C) Membrane floatation in sucrose gradients prior to solubilization by Triton X-100. Endogenous ROPs (EN-ROPs) and transgenic His6-GFP-AtROP6 and His6-GFP-Atrop6CA were only detected in fraction 3, where membranes float, and not in the bottom fractions, where the soluble proteins accumulate. (D) Triton X-100 solubilization of membranes separated by floatation as in panel C. The bar graph is a quantification of the bands presented as a percentage of the protein in the DRM. (E) Membrane floatation following solubilization with Triton X-100. ROPs floated in fractions 8 and 9. (F) Differential detergent extraction of membranes isolated by centrifugation as in panel B. ROP partitioning between DRM and TSM was identical to that achieved in membranes separated by membrane floatation as shown in panel D. Nonionic, either 1% Triton X-100 or 0.5% NP-40. +SDS, the same buffer with 0.1% SDS. The bar graph is a quantification of bands as in panel D.
Differential detergent protein extraction and purification.
To prepare protein extracts containing His6-GFP-AtROP6 and His6-GFP-Atrop6CA, 50 g of rosette leaves from 2-week-old transgenic plants was harvested and batch frozen in liquid nitrogen. Proteins were extracted from the frozen leaves by grinding the tissue with a pestle and mortar in 3× volumes (150 ml) of plant extraction buffer (50 mM NaH2PO4 [pH 7.6], 5 mM MgCl2, 300 mM NaCl, 10% glycerol, 2 mM β-methanol, plant protease inhibitor mixture [Sigma, St. Louis, MO]). To precipitate insoluble material, extracts were centrifuged at 75,000 × g for 30 min. The resulting supernatants were discarded, and the insoluble pellet was incubated on ice for 30 min in the same volume (150 ml) of plant extraction buffer containing 0.5% NP-40 or 1% Triton X-100. Solubilized extracts were centrifuged again to separate the NP-40-soluble, TSM, and insoluble fractions. The resulting supernatant was collected for further analysis, and the pellet was solubilized in 150 ml of plant extraction buffer containing 0.5% NP-40 or 1% Triton X-100 and 0.1% SDS. Extracts were centrifuged again at 75,000 × g for 30 min. Supernatants containing NP-40- and Triton X-100-insoluble, SDS-soluble fractions were collected for further analysis. Protein extracts containing His6-GFP-Atrop6CAmS195 were prepared in a similar manner, except for the following changes. Following extraction in a plant extraction buffer (without detergents) and centrifugation, the supernatants were collected and used as the source for protein purification.
His6-GFP-AtROP6/Atrop6CA/Atrop6CAmS195 was purified from total protein extracts in two steps. The first step was purification either by DEAE-cellulose ion-exchange chromatography or by differential ammonium sulfate precipitations. Purification over ready-to-use DEAE-cellulose was done according to the manufacturer's (Bio-Rad, Hercules, CA) instructions with column buffer containing Tricine-NaOH (pH 8.5), 0.1% NP-40, and 2 mM β-methanol. Protein extracts were loaded onto 5-ml-bed-volume columns. Fractions containing the recombinant proteins were eluted with 100, 200, and 300 mM NaCl. His6-GFP-AtROP6/Atrop6CA/Atrop6CAmS195-containing fractions were pulled together for the next purification step. Differential ammonium sulfate precipitations were carried out by standard protocols (1). His6-GFP-AtROP6/Atrop6CA/Atrop6CAmS195 was precipitated in the 30 to 45% fraction. Prior to final purification, samples were dialyzed against 50 mM NaH2PO4-(pH 6.0)-300 mM NaCl-2 mM β-methanol-0.5% NP-40. The dialyzed fractions were collected, and the His6-GFP-AtROP6 recombinant proteins were purified over a 0.5-ml-bed-volume Ni-NTA column according to the manufacturer's (QIAGEN) instructions. Typically, 400 μg of purified protein was obtained.
Membrane floatation on sucrose step gradients.
For protein extraction, 100 mg of tissue was frozen in liquid N2 and ground to powder with a pestle and mortar. Proteins were extracted by adding 1 ml of extraction buffer (50 mM HEPES-KOH [pH 7.5], 10 mM KCl, 5 mM EDTA, 5 mM EGTA, 10% sucrose, 1 mg/ml phenylmethylsulfonyl fluoride, protease inhibitor cocktail [Roche Diagnostics, Indianapolis, IN]) and incubated for 20 min on ice. The extract was precipitated at 3,000 × g for 10 min. The pellet was discarded, and the supernatant was collected for further analysis. To create a sucrose density step gradient, 250 μl of the supernatant was mixed with 1.25 ml of 85% sucrose in TE buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA [pH 8.0]). This mixture was overlaid with 7 ml of 65% sucrose and then 3 ml of 10% sucrose in TE. Centrifugal separation was performed with an SW41 rotor (Beckman Coulter, Fullerton, CA) at 100,000 × g for 18 h. At the end of the centrifugation, 1.25-ml samples were collected from the top of the gradient. Proteins were then collected for analysis by either of two alternating protocols. For precipitating the total protein in each fraction, 0.5-ml samples were mixed with 125 μl of 50% trichloroacetic acid and 1 μg of bovine serum albumin and then incubated at −20°C for 10 min. Proteins were precipitated by centrifugation at 10,000 × g for 20 min, and then the precipitated protein pellets were dried in a SpeedVac. Dried protein pellets were dissolved in 50 μl of SDS-PAGE sample buffer and resolved by SDS-PAGE. The following protocol was used to analyze the membrane-attached proteins in each fraction. To dilute the sucrose, 3.75 ml of TE was added to each fraction and membranes were precipitated by centrifugation at 100,000 × g for 1 h. Membrane pellets were solubilized in 50 μl of 1% Triton X-100 in TE for 20 min on ice. To separate TSM from insoluble membranes, mixtures were centrifuged for 1 h at 100,000 × g. The insoluble pellet was solubilized by homogenization in 50 μl of 1% Triton X-100-0.1% SDS in TE. Equal volumes of protein extracts in the TSM and insoluble fractions were resolved by SDS-PAGE, and proteins were identified by immunoblotting. Protein immunoblotting was performed as described above. All centrifugation steps were carried out at 4°C.
Membrane floatation on continuous sucrose gradients with Triton X-100.
Protein extracts were prepared as described above. Soluble and insoluble fractions were them separated by centrifugation at 15,000 × g for 15 min. The supernatant was discarded, and the insoluble pellet fraction was then resuspended with extraction buffer (as specified above) containing 1% Triton X-100 and incubated for 20 min on ice. To remove gross insoluble material, the resulting extract was separated by centrifugation at 3,000 × g for 10 min. The pellet was discarded, and the supernatant was collected for further analysis. Samples in a volume of 0.5 ml were mixed with an equal volume of 90% sucrose in TE buffer. The 1-ml fractions of 45% sucrose-sample mixtures were overlaid with 1.2 ml of 35% sucrose, 1 ml of 30% sucrose, 1 ml of 25% sucrose, and 1 ml of 5% sucrose in TE. Membranes were separated by centrifugation in a Beckman SW55 rotor for 16 h at 48,000 rpm (218,300 × g). Fractions in 0.4-ml volumes were collected from the top of the gradients and diluted into 1 ml of TE. The diluted fractions were precipitated by centrifugation at 100,000 × g for 30 min. Equal volumes of protein extracts from the different fraction were resolved by SDS-PAGE and analyzed by immunoblotting as described above.
Raney nickel cleavage.
Raney nickel cleavage was performed as previously described in detail (12). Briefly, 40 μg of purified His6-GFP-AtROP6/Atrop6CA/Atrop6CAmS195 was dried in a vacuum concentrator inside sealable glass vials (2-ml reactive vial; Whatman catalog no. 986276) and resuspended in formic acid-ethanol (1:4, vol/vol). The samples were then washed three times with 0.5 ml of pentane-formic acid-ethanol (10:1:4, vol/vol) to wash away noncovalently bound lipids. A total of 6.5 mg of platinum (IV) oxide was added per 400-μl sample, and proteins were hydrogenated for 90 min. Following hydrogenation, approximately 70 mg of ethanol-washed Raney nickel (Fluka) was added (before use, 5 g of ready-to-use Raney nickel was washed with 50 ml of 100% ethanol 10 consecutive times). The Raney nickel cleavage was performed at 100°C for 16 h while continuously steering the reactions. Extraction of released lipids was done by adding 0.5 ml of pentane. This extraction was repeated three times, and each time the pentane was transferred into a new tube. The pentane washes were pulled and concentrated under nitrogen to a final volume of 15 μl. Concentrated samples were analyzed by GC-MS. A positive control with 1 μg of commercially purchased N-acetyl S-farnesyl (Sigma, St. Louis, Mo) was used in parallel with every experiment.
GC-MS analysis.
Samples for analysis were manually injected in 1-μl aliquots into an Agilent Technologies GC/MSD system (Agilent Technologies network 6890-N gas chromatography system and 5973-N mass selective detector; Agilent Technologies, Santa Clara, CA) equipped with an Rtx-5 SIL column (Restek, Bellefonte, PA). The column had the following dimensions: length, 30 m; inside diameter, 0.25 mm; film thickness, 0.25 μm. The column stationary phase was 95% dimethyl, 5% diphenyl polysiloxane. Helium was used as the carrier gas at a flow rate of 0.8 ml min−1. The injection temperature was set to 250°C (splitless mode), the interface was at 280°C, and the ion source was adjusted to 200°C. The analysis was performed under the following temperature program: 5 min of isothermal heating at 100°C, followed by a 5°C min−1 oven temperature ramp up to 280°C. The system was equilibrated for 1 min at 100°C before injection of the next sample. Mass spectra were recorded at 4.59 scans s−1 with a 41-to-350 mass-to-charge (m/z) ratio scanning range and an electron energy of 70 eV. Compounds were tentatively identified (>95% match) on the basis of the NIST98 Mass Spectral Library (data version NIST 05, software version 2.0d) with the Chemstation V.D.038 program (Agilent Technologies, Santa Clara, CA). Further identification of major compounds was based on comparisons of mass spectra and retention times with those of authentic standards (farnesol, geranylgeraniol, palmitic acid, and stearic acid) analyzed under similar conditions.
Plant material.
Wild-type (WT) Col-0 and His6-GFP-Atrop6/Atrop6CA/AtropCAmS195/AtropCAmS156 Arabidopsis plants were grown in 5-cm pots. Plants were grown on soil (Marom Golan mix) and irrigated from below. Plants were grown under long-day conditions (16-h light, 8-h dark cycle) at 21°C. The light intensity was 100 μE · m−2 · s−1.
Plasmolysis.
Plasmolysis was carried out as previously described (19, 20).
Imaging.
All images were produced with a Leica TCS-SL confocal laser scanning microscope (Leica Microsystems, Heidelberg, Germany). Imaging of GFP was carried out by excitation with an argon laser at 488 nm, a 500-nm beam splitter, and the spectral detector set at 505 to 550 nm. λ scans were carried out to examine the fluoresce emission spectra. Image analysis was carried out with a Leica TCS (Leica Microsystems, Heidelberg, Germany), a Zeiss LSM browser (Zeiss, Jena, Germany), and Adobe Photoshop 7 (Adobe, Mountain View, CA).
GDP or GTPγS infiltration and exchange.
Leaves were injected with 100 μM GDP or GTPγS diluted in water. Injections were carried out by pressing a syringe to the abaxial leaf side. Following nucleotide injections, plants were incubated for 2 h before leaves were harvested for protein extraction. For protein extraction, the injected tissue and a small part of the surrounding noninjected tissue (as determined by eye) were collected. Tissue samples were collected from several injected leaves to a total amount of 100 mg, and proteins were extracted by adding 300 μl (3:1 ratio) of extraction buffer and further extracted as described above. GDP-GTP exchange assays were carried out as follows. Five-day-old seedlings that were grown on plates containing 0.5× Murashige-Skoog growth medium were collected and infiltrated with double-distilled water under a vacuum for 30 min and then transferred to the growth room and further incubated for 90 min in double-distilled water. After 2 h, seedlings in a total amount of 100 mg were collected for protein extraction. Remaining seedlings were infiltrated with 100 μM GDP for 30 min under a vacuum and then transferred in the same solution to the growth room for additional incubation. After 1.5 h, seedlings in total amount of 100 mg were collected for protein extraction and the remaining seedlings were infiltrated under a vacuum with 10 mM EDTA for 30 min and then with 20 mM MgCl2 and 100 μM GTPγS for an additional 30 min. Seedlings were removed in the same solution to the growth room for further incubation. After 1.5 h, 100 mg of the remaining seedlings was collected for protein extraction.
RESULTS
Membrane localization of ROPs and their accumulation in DRM.
Both type I and type II ROPs localize in the PM and were not detected in the endomembrane system (3, 16, 18-20, 22, 27). Images of cells expressing either His6-GFP-AtROP6 or His6-GFP-Atrop6CA showed that both proteins were localized in the PM. PM localization was evident by the thin fluorescent line surrounding the cells and the absence of fluorescent patches following plasmolysis, which causes shrinkage and condensation of the cytoplasm and vacuole and detachment of the PM from the cell wall (Fig. 1A). Cell wall-detached thin lines of GFP fluorescence are accepted as an indication of PM localization (3, 16, 19, 20). Because plasmolysis was incomplete, some cell wall-attached fluorescence could still be visualized (Fig. 1A).
Affinity-purified anti-ROP polyclonal Abs were used to determine the subcellular localization of endogenous and transgenic ROPs. These purified Abs were highly specific for ROPs, as was evident from the absence of nonspecific bands on protein immunoblotting (Fig. 1B). The Abs identified a single band at a molecular mass of 20 to 25 kDa in the total (Fig. 1B, Total) and insoluble pellet (Fig. 1B, P100) fractions but not in the soluble supernatant (Fig. 1B, Sup). The anti-AtROP6/AtRac3 Abs showed cross-reactivity with type II ROPs (see Fig. S1A, B, D, and E in the supplemental material), indicating that the ROP band in plant extracts corresponded to different ROP proteins. Furthermore, depletion of the anti-ROP Abs by incubation with Ni-NTA resin-bound AtROP6 resulted in disappearance of the ROP band, confirming that the Abs indeed identified ROP proteins in the plant protein extracts (see Fig. S1C in the supplemental material). In protein extracts prepared from 35S::GFP-Atrop6CA plants, a major band with a molecular mass of approximately 50 kDa, corresponding to the His6-GFP-Atrop6CA fusion protein, has been identified in addition to the band of the endogenous ROPs (Fig. 1B, Atrop6CA αROP). When the same protein extract was decorated with anti-GFP Abs, only the His6-GFP-Atrop6CA fusion protein was identified (Fig. 1B, Atrop6CA αGFP). The data in Fig. 1A and B suggested that both endogenous and recombinant ROPs exist in the PM and could not be detected in the cytoplasm. Protein immunoblotting confirmed that the affinity-purified polyclonal anti-ROP Abs used in this study were highly specific for ROPs.
Membrane floatation on sucrose density gradients in the absence of detergents demonstrated that endogenous ROPs, as well as the transgenic His6-GFP-AtROP6 WT protein or an activated His6-GFP-Atrop6CA mutant protein, were exclusively localized in the membrane, as no traces were detected in soluble fractions (Fig. 1C; see Fig. S3 in the supplemental material). The results confirmed that both endogenous and transgenic His6-GFP-ROP fusion proteins were associated with membranes and that their accumulation in insoluble pellets did not result from aggregation.
Next, solubility of ROPs in detergents was determined (Fig. 1D to F). Membranes were separated by floatation on sucrose gradients. The isolated membrane fractions were precipitated by centrifugation, and proteins were solubilized with 1% Triton X-100. The TSM and insoluble fractions were separated by a second centrifugation step, and proteins were resolved by SDS-PAGE and identified by immunoblotting with anti-ROP Abs (Fig. 1D). While endogenous ROPs and recombinant His6-GFP-AtROP6 were distributed at approximately equal levels between the TSM and insoluble membrane, nearly 100% of the His6-GFP-Atrop6CA protein was found in the Triton X-100-insoluble fraction (DRM) (Fig. 1D). To verify that the ROP-containing Triton X-100-insoluble fractions indeed represented DRM, membranes were solubilized in 1% TritonX-100 and separated by floatation on sucrose density gradients (Fig. 1E). The membranes in each of the fractions were precipitated by an additional centrifugation step and resolved by SDS-PAGE, and ROPs were detected by immunoblotting with anti-ROP Abs. Endogenous ROPs, His6-GFP-AtROP6, and His6-GFP-Atrop6CA were detected in fractions 8 and 9 of the gradients, confirming the existence of the protein in DRM. Endogenous ROPs and GFP-AtROP6 that accumulated in the TSMs were not present because the blots show only the membrane pellet of each of the sucrose gradient fractions.
The ability to separate ROP-containing TSM and DRM fractions by differential detergent extraction was examined. Cells were fractionated into soluble and membrane fractions. Membrane fractions were incubated at 4°C with different nonionic detergent mixtures including 1% Triton X-100, 0.5% NP-40, 1.5% dodecyl-β-d-maltoside, 1%, 1% octyl-β-d-glucopyranoside 1% Triton X-100, and 1% Triton X-100 1% Na-deoxycholate. The results were the same following extraction with either detergent mixture. Therefore, throughout the work described here protein extractions were carried out with 1% Triton X-100 or 0.5% NP-40. Proteins in the NP-40- and Triton X-100-insoluble fractions were precipitated and further extracted with 0.1% SDS (Fig. 1F). As expected, neither the endogenous ROPs nor the transgenic His6-GFP-AtROP6 and His6-GFP-Atrop6CA mutant proteins were detected in the soluble fractions. Quantification of the blot by densitometry showed that the endogenous ROPs and WT His6-GFP- AtROP6 were equally distributed between the TritonX-100- and NP-40-soluble (Fig. 1F, Nonionic) and insoluble (Fig. 1F, +SDS) fractions. In contrast, the activated His6-GFP-Atrop6CA mutant protein was almost exclusively detected in the NP-40- and Triton X-100-insoluble, SDS-soluble fraction (Fig. 1F). Thus, identical results were obtained with the differential detergent extraction (Fig. 1F) and the membrane floatations (Fig. 1C and D). The experiments were repeated many times, and the results were same.
The results in Fig. 1 suggested that activation of ROPs induced their accumulation in DRMs. To further explore this possibility, leaves and seedlings were infiltrated with either the nonhydrolyzable GTP analogue GTPγS or GDP and the distribution of proteins in the membrane was examined (Fig. 2). Infiltration of GTPγS into WT untransformed plants induced accumulation of ROPs in the DRM. Quantification of the protein immunoblots showed that 94% of the ROPs accumulated in the DRM (Fig. 2A). GDP infiltration had the opposite effect, inducing accumulation of ROPs in the TSM. Blot quantification showed that only 7% of the protein remained in the DRM (Fig. 2A). Infiltration of water, used to solubilize GTPγS and GDP, had no effect on ROPs' distribution in the membrane, and proteins were equally partitioned between the DRM and TSM (Fig. 2A). Similarly, infiltration of GTPγS into His6-GFP-AtROP6 plants induced accumulation of 87% of the recombinant His6-GFP-AtROP6 protein in the DRM and infiltration of GDP had the opposite effect, inducing accumulation of His6-GFP-AtROP6 in TSMs, and only 13% of the protein was detected in the DRM (Fig. 2B). Following injection of water, His6-GFP-AtROP6 was equally distributed between the DRM and TSM, similar to endogenous ROPs. The GDP and GTP injections were repeated eight times with the exact same results. The quantifications represent one experiment. To examine whether the effects of GTPγS and GDP were reversible, WT untransformed seedlings were first infiltrated with water for 30 min (Fig. 2C, arrow 0′). After an additional 90 min, a sample was taken for preparation of protein extracts (Fig. 2C, arrow 120′) and seedlings were infiltrated for 30 min with GDP (Fig. 2C, arrow 120′). After an additional incubation of 90 min, samples were taken for preparation of protein extracts (Fig. 2C, arrow 240′) and seedlings were infiltrated with EDTA (Fig. 2C, dashed arrow at 240′) for 30 min to induce nucleotide release and then for 30 min with GTPγS and MgCl2 (Fig. 2C, arrow 270′). Protein extracts were then prepared after an additional 90-min incubation (Fig. 2C, arrow 390′). The results (Fig. 2C) show that while GDP induced accumulation of ROPs in the TSM, nucleotide exchange and incubation in GTPγS induced accumulation of ROPs in the DRM. The results strongly suggested that partitioning of ROP proteins between the TSM and DRM was dynamic and responded to their activation status. Endogenous ROP accumulation in the DRM and TSM in response to infiltration of GTPγs or GDP, respectively, had similar kinetics. Thirty minutes after infiltration of the respective nucleotide, there was a 50% change in the level of the protein in the DRM and after 90 min proteins were almost exclusively detected in either the DRM or the TSM (Fig. 2D).
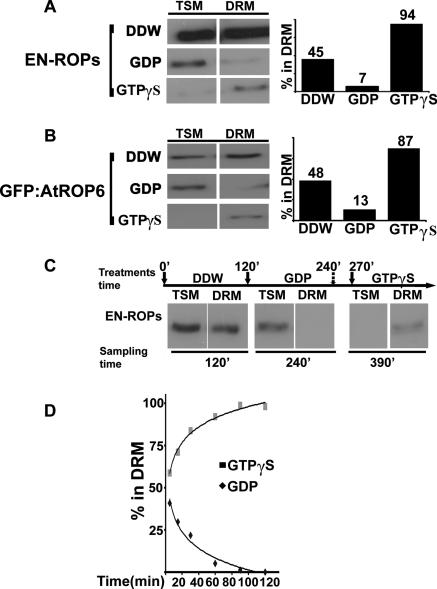
FIG. 2.
GDP-GTP exchange affects partitioning of ROPs in the PM. (A to C) Protein immunoblotting with anti-ROP Abs. TSMs and insoluble membranes (DRM) from WT nontransgenic plants (En-ROPs) (A) and His6-GFP-AtROP6 transgenic plants (GFP-AtROP6) (B) were injected with water, GDP, or GTPγS. Bar graphs are quantifications of the bands in the immunoblots. (C) Partitioning of endogenous ROP proteins between TSM and DRM in seedlings infiltrated successively with water, GDP, and GTPγS. Values above solid arrows denote times of water (0 min), GDP(120 min), and GTPγS (270 min) injection. The value above the dashed arrow (240 min) denotes the time of EDTA injection. Values below the panel denote the times when seedlings were collected for preparation of protein extracts. (D) Time course of accumulation in TSM or DRM of endogenous ROPs following injection of leaves with GDP or GTPγS. The graph was produced by quantification of bands on protein immunoblots.
We hypothesized that the cycling of ROPs to and out of DRM involves acylation-deacylation cycles. The next series of experiment was designed to explore the lipid protein modifications of ROPs purified from NP-40-soluble, TSM, and insoluble membrane fractions.
Purification of recombinant AtROP6 and Atrop6CA from transgenic Arabidopsis plants.
As shown in Fig. 1 and 2, the activity and subcellular localization of ROPs are unaltered when they are fused to GFP. Therefore, the His6-GFP-AtROP6 and His6-GFP-Atrop6CA transgenic lines were used in the analysis of lipid moieties.
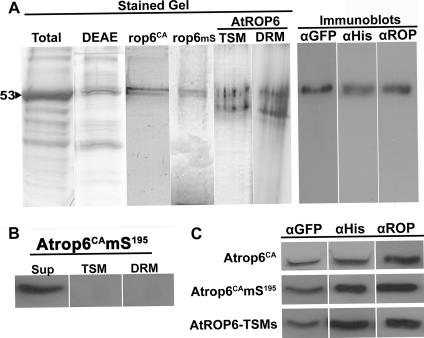
As a negative control, we created transgenic Arabidopsis plants expressing a nonprenylated His6-GFP-Atrop6CAmS195 mutant protein in which the prenyl acceptor cysteine was mutated to serine. Following centrifugation at 100,000 × g, all of the mS mutant protein resided in the soluble fraction (Fig. 3B). His6-GFP-AtROP6 was solubilized first with NP-40 to extract the detergent-soluble fraction of the protein. The NP-40-insluble fraction was further solubilized in a buffer containing 0.1% SDS to extract NP-40-insoluble, SDS-soluble His6-GFP-AtROP6. Because only a minute fraction of His6-GFP-Atrop6CA existed in the NP-40-soluble fraction, this fusion protein was purified only from the NP-40-insoluble, SDS-soluble fraction. Purification of His6-GFP-AtROP6, His6-GFP-Atrop6CA, and His6-GFP-Atrop6CAmS195 was achieved in two steps. Solubilized (or soluble in the case of the Atrop6CAmS195 mutant protein) protein mixtures containing the fusion proteins were first partially purified by either DEAE-cellulose ion-exchange chromatography or differential ammonium sulfate precipitations. These first stages were followed by purification over Ni-NTA resin. Fusion proteins were purified to almost complete homogeneity, with only one contaminating protein detected in silver-stained SDS-PAGE gels (Fig. 3A, Stained Gel). The purity of the fusion proteins after Ni-NTA purification was the same when either ion-exchange chromatography or differential ammonium sulfate precipitation had been used as the preceding step in the purification. However, the yields obtained following ammonium sulfate precipitations were higher and therefore this method was preferred.
FIG. 3.
Purification of recombinant ROPs from plants. (A) Stained Gel, silver-stained SDS-PAGE of purified recombinant ROP proteins. Total, total protein extract; DEAE, membrane proteins purified over a DEAE ion-exchange column; rop6CA, His6-GFP-AtROP6CA; rop6mS, His6-GFP-Atrop6CAmS195; AtROP6, His6-GFP-AtROP6 from TSM or DRM following purification over Ni-NTA columns. Immunoblots, identity and intactness of AtROP6 purified from the DRM verified by protein immunoblotting with anti-GFP, anti-His6, and anti-ROP Abs. (B) Protein immunoblotting with anti-ROP Abs showing that prenyl acceptor mutant protein GFP-Atrop6CAmS195 was found only in the soluble fraction (Sup) and neither in the TSM nor in the DRM. (C) Identities and intactness of Ni-NTA-purified ROP fusion proteins were verified by protein immunoblotting with anti-GFP, anti-His6, or anti-ROP Abs. Immunoblotting was done as shown in panel A, and only single bands were detected. Only the relevant bands are shown because of space considerations.
The identity and intactness of all fusion proteins were verified by immunoblotting with anti-GFP and -His6 monoclonal Abs and anti-AtROP6 polyclonal Abs (Fig. 3A and C). All three Abs recognized the upper 53-kDa band (Fig. 3A, Immunoblots), confirming the identities and intactness of the fusion proteins. The purified His6-GFP-AtROP6/Atrop6CA/Atrop6CAmS195fusion proteins were used for analysis of lipid modifications by GC-MS.
Analysis of lipid modifications by GC-MS.
Prior to lipid extraction, the purified proteins were subjected to three successive washes in large volumes of pentane to remove any noncovalently bound lipids. The lipids were then released from the purified proteins by hydrogenation and cleavage with Raney nickel.
Commercially purchased N-acetyl S-farnesyl was used as a positive control for the hydrogenation and Raney nickel cleavage. Hydrogenation and subsequent Raney nickel cleavage released 2,6,10-trimethyldodecane, a reduced form of farnesol, which eluted with a retention time of 10.00 min (see Fig. S2 in the supplemental material). This analysis confirmed that the hydrogenation and Raney nickel cleavage were yielding the expected results. Hydrogenation and subsequent Raney nickel cleavage reactions with His6-GFP-Atrop6CAmS195 and His6-AtROP6 produced in E. coli were used as negative controls. No lipids were extracted from either of these proteins (Fig. 4D and E). The minor peaks along the chromatogram are all recognized column contaminants.
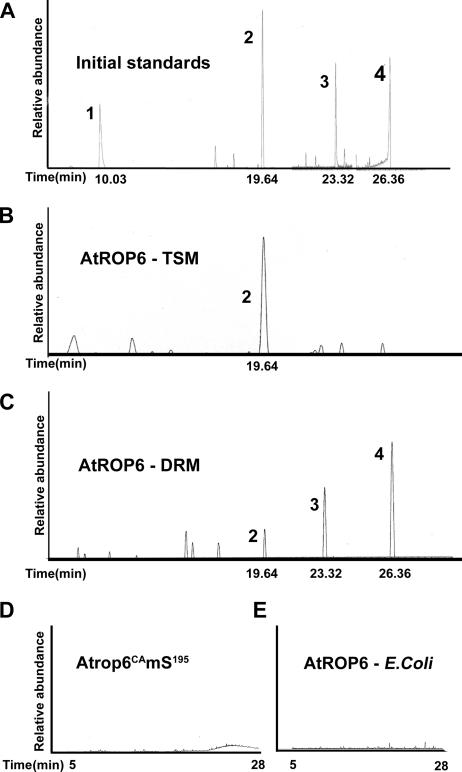
FIG. 4.
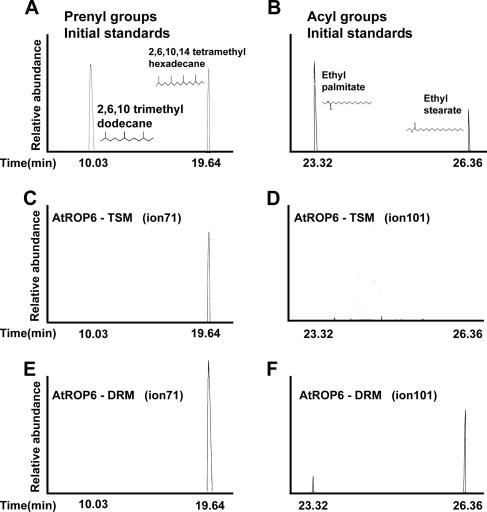
AtROP is prenylated in TSM and both prenylated and acylated in DRM. Total-ion chromatograms of initial standards are shown (A). Lipids were cleaved from NP-40-soluble purified His6-GFP-AtROP6 (AtROP6-TSM) (B) and NP-40-insoluble purified His6-GFP-AtROP6 (AtROP6-DRM) (C) by sequential hydrogenation and Raney nickel cleavage. In TSMs, AtROP6 was only prenylated by geranylgeranyl (B), and in DRM, it was both prenylated by geranylgeranyl and S acylated by palmitic and stearic acids (C). Peaks: 1, 2,6,10-trimethyldodecane, saturated derivative of farnesol; 2, 2,6,10,14-tetramethylhexadecane, saturated derivative of geranylgeraniol; 3, ethyl palmitate, derivative of palmitate formed following hydrogenation; 4, ethyl stearate, derivative of stearate formed following hydrogenation. No lipids were detected following lipid extraction of purified His6-GFP-Atrop6CAmS195 (Atrop6mS195) (D) or His6-AtROP6 purified from E. coli cells (E).
A single peak corresponding to reduced geranylgeraniol that eluted at a retention time of 19.64 min was observed in a full-ion chromatogram of the lipids extracted from TSM His6-GFP-AtROP6 (Fig. 4A and B). In contrast, three peaks that eluted at retention times of 19.64, 23.32, and 26.36 min were observed in the full-ion chromatograms of lipids extracted from His6-GFP-AtROP6 purified from the DRM. These peaks corresponded to the reduced derivatives of geranylgeraniol and palmitic and stearic acids (Fig. 4A and C).
To monitor the prenyl and acyl lipids, the data were examined by GC-MS in the single-ion mode (Fig. 5). The typical ions for each lipid group are ion 71 for the prenyl lipids and ion 101 for the acyl lipids. In the case of prenyl lipids, ion 71 represents the basic five-carbon isoprenoid unit, in which the two double bonds were reduced, increasing the molecular mass from 69 to 71. The ion 71-monitored gel chromatogram shows that the compound eluting at a retention time of 19.64 min corresponded to the reduced geranylgeraniol derivative 2,6,10,14-tetramethylhexadecane (Fig. 5A, C, and E). For an MS chromatogram of 2,6,10,14-tetramethylhexadecane, see Fig. S4 in the supplemental material. Ion 71 and the molecular ions 282 (reduced geranylgeraniol) are shown. No eluting compounds were detected in the ion 101-monitored gel chromatogram of TSM-resident His6-GFP-AtROP6 (Fig. 5D), indicating that it was not acylated. In contrast, eluting compounds were detected in both ion 71- and ion 101-monitored gel chromatograms of DRM-resident His6-GFP-AtROP6 (Fig. 5E and F). A single compound corresponding to the reduced geranylgeraniol (2,6,10,14-tetramethylhexadecane) was detected in the ion 71-monitored gel chromatogram (Fig. 5A and E). For an MS chromatogram of 2,6,10,14-tetramethylhexadecane, see Fig. S4 in the supplemental material. When the gel chromatogram was monitored for ion 101, two compounds were detected (Fig. 5F), a compound eluting at a retention time of 23.32 min, corresponding to ethyl palmitate, and a bigger second peak at 26.36 min, corresponding to ethyl stearate (Fig. 5B and F). The ethyl derivatives of both compounds were formed during the hydrogenation procedure, which was carried out in the presence of formic acid and ethanol. Palmitic acid and stearic acid standards that underwent the same treatments were also modified by ethyl groups (Fig. 5B). For MS chromatograms of both ethyl palmitate and ethyl stearate, see Fig. S5 in the supplemental material. The chromatograms show the typical ion 101 and molecular ions 284, corresponding to ethyl palmitate, and 312, corresponding to ethyl stearate. Only ethyl stearate was detected following hydrogenation and Raney nickel treatment of a stearic acid standard (see Fig. S6 in the supplemental material), indicating that DRM-resident His6-GFP-AtROP6 was modified by both palmitic acid and stearic acid or either one and that the palmitic acid found was not a cleavage product of stearic acid.
FIG. 5.
Single-ion GC scans showing the modifying prenyl and acyl groups of lipid cleaved from His6-GFP-AtROP6 purified from the TSM and the DRM. (A, C, and E) Ion 71 gel chromatograms of farnesol (2,6,10-trimethyldodecane) and geranylgeraniol (2,6,10,14-tetramethylhexadecane) initial standards (A), lipids cleaved from NP-40-soluble purified His6-GFP-AtROP6 (AtROP6-TSM) (C), and NP-40-insoluble, SDS-soluble purified His6-GFP-AtROP6 (E) by sequential hydrogenation and Raney nickel cleavage. (B, D, and F) Ion 101 gel chromatograms showing palmitic acid (ethyl palmitate) and stearic acid (ethyl stearate) initial standards (B). Acyl lipid cleaved from NP-40-soluble purified His6-GFP-AtROP6 (AtROP6-TSM) (D) and NP-40-insoluble, SDS-soluble purified His6-GFP-AtROP6 (F). AtROP6 purified from DRM was modified by palmitic and stearic acids (F), but no cleaved acyl lipids were detected in AtROP6 purified from the TSM (D).
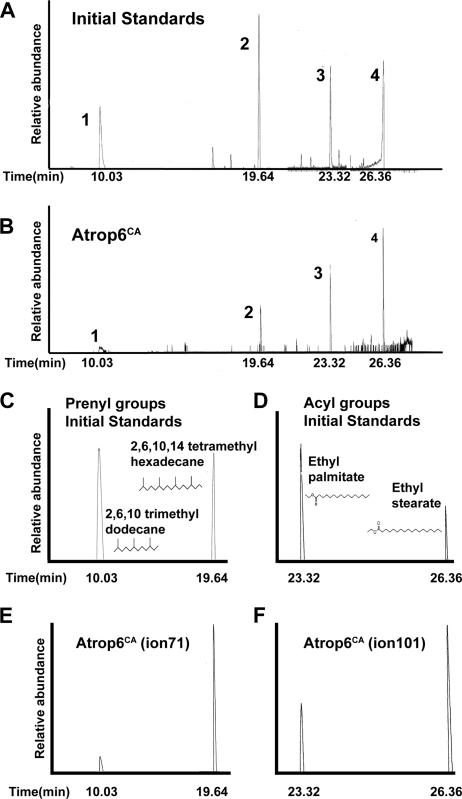
Together, the results in Fig. 1, 2, 4, and 5 suggested that upon activation, AtROP was S acylated, which induced its partitioning in DRM. To substantiate this assumption, the lipid groups attached to His6-GFP-Atrop6CA were analyzed (Fig. 6). His6-GFP-Atrop6CA fusion protein was purified from the DRM as described above. The results of the GC-MS analysis were similar to those of the DRM-resident His6-GFP-AtROP6 WT protein. The ion 71-monitored gel chromatograms show a compound eluting at a retention time of 19.64 min, corresponding to the reduced geranylgeraniol derivative 2,6,10,14-tetramethylhexadecane (Fig. 6A, B, C, and E). A lower peak corresponding to the reduced farnesol derivative 2,6,10-trimethyldodecane appears at a retention time of 10.00 min (Fig. 6A, B, C, and E). Because of the low abundance, an additional minor compound eluting at a retention time of 10.21 min, corresponding to dodecane, was detected. For the corresponding MS chromatograms of both 2,6,10,14-tetramethylhexadecane and 2,6,10-trimethyldodecane, geranylgeraniol, and farnesol derivatives, respectively, see Fig. S4 in the supplemental material. Ion 71 and the molecular ions 282 (reduced geranylgeraniol) and 212 (reduced farnesol) are evident.
FIG. 6.
Activated His6-GFP-Atrop6CA was modified by both prenyl and acyl lipids. Total-ion chromatograms of the initial standard (A) and lipids extracted from purified His6-GFP-Atrop6CA (Atrop6CA) by sequential hydrogenation and Raney nickel cleavage (B) are shown. AtROP6 was modified by both prenyl and acyl lipids. Peaks: 1, 2,6,10-trimethyldodecane, saturated derivative of farnesol; 2, 2,6,10,14-tetramethylhexadecane, saturated derivative of geranylgeraniol; 3, ethyl palmitate, derivative of palmitate formed following hydrogenation; 4, ethyl stearate, derivative of stearate formed following hydrogenation. (C to F) Single-ion GC scans corresponding to the total-ion chromatogram shown in panels A and B, showing the modifying prenyl and acyl groups. (C and E) Ion 71 gel chromatograms of farnesol and geranylgeraniol initial standards (C) and farnesol and geranylgeraniol cleaved from purified His6-GFP-Atrop6CA (Atrop6CA [ion 71]) (E). (D and F) Ion 101 gel chromatograms of palmitic and stearic acid initial standards (D) and lipids cleaved from purified His6-GFP-Atrop6CA (Atrop6CA [ion 101]) (F).
When the gel chromatogram was monitored for ion 101, two compounds were detected (Fig. 6D and F). A smaller peak at a retention time of 23.32 min corresponded to ethyl palmitate and a bigger second peak at 26.36 min corresponded to ethyl stearate (Fig. 6D and F). For the corresponding MS chromatograms of both ethyl palmitate and ethyl stearate, see Fig. S5 in the supplemental material.
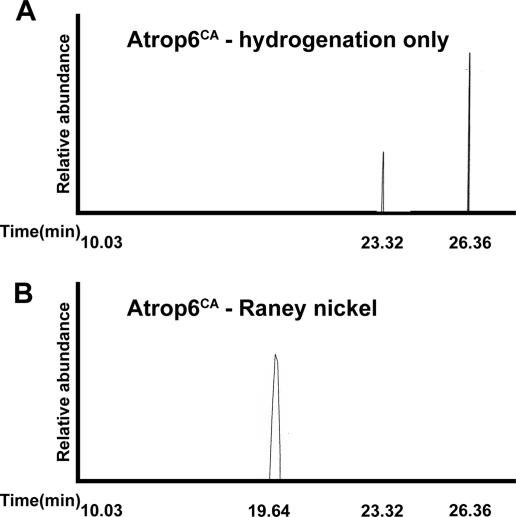
To further substantiate the finding that Atrop6CA was prenylated and S acylated, lipids were extracted from the 53-kDa band corresponding to His6-GFP-Atrop6CA (Fig. 3), which was purified by SDS-PAGE. The organic extraction was done twice, once after the hydrogenation step and then following the Raney nickel cleavage step (Fig. 7A and B). The hydrogenation step was sufficient to break the thioester bonds between the acyl groups and the protein releasing palmitic and stearic acids (Fig. 7A). Geranylgeraniol was released following the cleavage of the thioether bond between the prenyl group and the protein by boiling in Raney nickel (Fig. 7B). No farnesol was detected, most likely because of the smaller amounts of protein. The results confirmed that His6-GFP-Atrop6CA was both prenylated and S acylated.
FIG. 7.
Sequential release of acyl and prenyl lipids from gel-purified His6-GFP-Atrop6CA. (A) Ethyl palmitate and ethyl stearate released by hydrogenation of the gel-purified His6-GFP-Atrop6CA band. (B) 2,6,10,14-Tetramethylhexadecane (geranylgeraniol) released by Raney nickel treatment of the hydrogenated and organic extracted protein from panel A.
Identification of the acylation site.
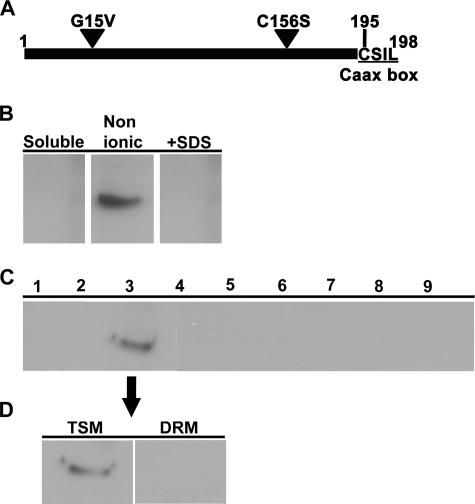
There are three additional cysteine residues in AtROP6, at positions 9, 17, and 156, in addition to the CaaX box cysteine. Cysteine156 is predicted to have a free thiol group, which is relatively exposed to the medium and close to the CaaX box cysteine, making it a potential acylation site. On the basis of the data presented in Fig. 1 to 7, our prediction was that an AtROP6 mutant protein bearing a mutation at cysteine156 would only be found in TSM. To test this prediction, we created a new activated AtROP6 mutant protein in which cysteine156 was mutated into serine. This mutant protein was designated Atrop6CAmS156 (Fig. 8A). Transgenic Arabidopsis plants expressing the His6-GFP-Atrop6CAmS156 mutant protein were created, and the localization of the fusion protein was examined. Following differential detergent extraction, His6-GFP-Atrop6CAmS156 was only found in the TSM (Fig. 8B, Nonionic) and not in the DRM (Fig. 8B, +SDS). Membrane floatation on sucrose density gradients confirmed that the His6-GFP-Atrop6CAmS156 mutant protein resided in the membrane (Fig. 8C), similar to His6-GFP-AtROP6 and His6-GFP-Atrop6CA. Differential detergent extraction of membranes isolated by membrane floatation further verified that the His6-GFP-Atrop6CAmS156 mutant protein resided in the TSM and not in the DRM (Fig. 8D). The results presented in Fig. 8, together with the data presented in Fig. 1 to 7, demonstrate that prenylation drives AtROP6 to nonionic-detergent-soluble membrane and that activation and consequent acylation at cysteine156 result in partitioning of the protein into the DRM.
FIG. 8.
The AtropCAmS156 mutant protein was localized in TSM membranes. (A) Schematic representation of the point mutations of Atrop6CAmS156. Triangles denote the Gly15-to-Val and Cys156-to-Ser mutations. The CaaX box is underlined, and the numbers of the first and last amino acids and of the CaaX box cysteine are noted. (B to D) Protein immunoblotting with anti-ROP Abs showing the localization of His6-GFP-Atrop6CAmS156. (B) Centrifugation and differential detergent extraction. Soluble, supernatant following centrifugal separation. Nonionic, TSM. +SDS, Triton X-100-insoluble, SDS-soluble fraction. (C) Membrane floatation on a sucrose density step gradient. Fractions are numbered from top to bottom. Membranes partitioned into fraction 3. (D) Differential detergent extraction of the membranes floating in fraction 3 of the sucrose gradient shown in panel C.
DISCUSSION
Here we demonstrated that ROPs are localized in the PM partitioned between the DRM and TSM. Partitioning in the membrane depended on the activation status of the protein and coupled S acylation. Inactive, GDP-bound ROPs were only prenylated and accumulated in TSM, while the active GTP-bound ROPs were prenylated and S acylated and accumulated in the DRM. AtROP6 was acylated by both palmitic and stearic acids. These findings directly link ROP activation, transient S acylation, and localization in membrane microdomains.
Membrane fractionation.
ROP membrane partitioning was examined by membrane floatation on sucrose gradients with or without Triton X-100 or by differential detergent extraction. The same results were obtained by all three methodologies. The Triton X-100-insoluble, SDS-soluble fraction represented the same membrane fractions that were insoluble in Triton X-100 following membrane floatation or the membrane fractions that floated in fractions 8 and 9 of Triton X-100-containing sucrose gradients. Importantly, the same results were obtained with endogenous and recombinant His6-GFP-AtROP6 fusion proteins, indicating that the insolubility in nonionic detergents was not a result of protein aggregation and indeed occurred because of partitioning of ROPs into different membrane domains.
ROP partitioning in DRM and cell polarity.
The anti-AtROP6/AtRac3 polyclonal Abs used in this study also recognized Arabidopsis type II ROPs (see Fig. S1 in the supplemental material), which are relatively divergent in sequence compared to type I ROPs. This strongly suggests that the anti-ROP Abs recognized most, if not all, type I ROPs. It is likely, therefore, that GTP binding-dependent transient S acylation and accumulation in DRM are common to some, if not all, type I ROPs. Likely, the activation-dependent DRM accumulation of ROPs plays an essential role in their function. Our results are in agreement with a recent proteomic analysis of tobacco reporting that a ROP protein was detected in a DRM preparation (28). Acylated cysteine156 is not part of the hypervariable domain and is conserved in all plant ROPs and in human Rac1 as well. Thus, activation-dependent acylation and consequent accumulation in DRM may be common to RAC proteins in plants and animals.
The importance of lipid rafts or DRM in the establishment of cell polarity has been established in several experimental systems including yeast (2, 33) and axonal growth (17). During the movement of animal cells, lipid rafts capture actin, microtubules, and phosphatidylinositol-4,5-diphosphate (14). In mammalian cells, Rac is found in membrane rich in low-density cholesterol via interaction with integrin (11). It could be that activation-dependent acylation of an internal cysteine residue(s) is an additional mechanism that drives partitioning of Racs into DRM.
The significance of ROPs' partitioning in DRM has yet to be determined. Accumulating data indicate that DRM (lipid rafts) may play an important role during polarity establishment in plants. Chemical analysis of the lipid content of Arabidopsis DRMs revealed that they are enriched in sterols and sphingolipids (4). Sterol-deficient Arabidopsis mutants display severely compromised polarity (13, 36). ROPs have been reported to compartmentalize with phosphatidylinositol-4,5-diphosphate during polar growth of pollen tubes (18) and to disrupt membrane cycling at the PM (3). The activation-dependent acylation of ROPs and their consequent partitioning in DRM could play a central role during the establishment of polarity in plants.
The mechanisms responsible for partitioning of ROPs in DRM.
Our data demonstrate that a mutation that changes cysteine156 to serine prevented the partitioning of activated AtROP6 in DRM, indicating that this residue is S acylated. Similarly, activation-inactivation cycles have been shown to induce partitioning of H-Ras and N-Ras out of and into DRM (31). Cysteine156 resides in the conserved N-terminal domain of AtROP6. Homology modeling of GTP- and GDP-bound AtROP6 suggested that activation status did not induce the conformational changes that caused displacement of Cys156 (N. Sorek and S. Yalovsky, unpublished data). Activation-dependent transient acylation may occur via interaction of ROPs with additional components. Alternatively, the conformational changes around cysteine156 might be below the resolution limit and therefore could not be observed. Type II ROPs are acylated on two or three cysteine residues in the hypervariable domain that are part of a conserved C-terminal GC-CG box domain (19, 20). All three Arabidopsis type II ROPs and their homologues in other plant species have a cysteine residue homologous to cysteine156 of AtROP6. Mutations changing the cysteines or other residues of the GC-CG box compromised attachment of type II ROPs to the PM (19, 20). It remains to be determined whether the type II ROPs undergo cycles of activation-dependent transient acylation on internal cysteine residues. The correlation between activation and distribution in the membrane could be further used as a tool to indicate the activation status of ROP following different triggers.
Analysis of protein S acylation and prenylation.
The method we describe for analysis of protein acylation allows direct identification of the modifying acyl groups. The formic acid-ethanol washes that preceded the hydrogenation step may have facilitated separation of acyl lipids by GC-MS. Through the direct analysis of modifying lipids, it was possible to determine that stearylation is at least as common as palmitoylation in Arabidopsis (Fig. 4 to 7). The methodology used for analysis of AtROP6-modifying lipids did not induce breakdown of stearic acid to palmitic acid (see Fig. S6 in the supplemental material), indicating that AtROP6 was indeed modified by both acyl lipids. Considering that TSM-localized AtROP6 was only prenylated (Fig. 4 and 5) and that a mutation in a single cysteine prevented the partitioning of activated Atrop6CAmS156 into the DRM (Fig. 8), it is likely that acylation occurs by either palmitic or stearic acid. This suggests that the AtROP6 proteins purified from the DRM represented mixed populations of palmitoylated and stearylated molecules.
The low level of farnesol cleaved from Atrop6CA indicated that the proteins were farnesylated as well as geranylgeranylated although at a lower efficiency. The absence of farnesol in the lipids cleaved from WT AtROP6 could have several causes. First, there was a lower level of starting protein material because proteins were partitioned between TSM and DRM and the analysis was carried out separately with proteins purified from either fraction. Second, the Raney nickel reaction is relatively inefficient, with only about 60% of the lipids cleaved. Third, sesquiterpenes are relatively volatile and could have been lost during the 16 h of incubation at 100°C with Raney nickel. It remains to be determined to what extent protein farnesyltransferase contributes to the prenylation and membrane association of type I ROPs.
Supplementary Material
Acknowledgments
We thank Doron Pappo, Shiri Shimoni, and Tali Yahalom for technical assistance and Aliza Finkler for materials.
This research was supported by grants from the Israel Academy of Sciences-Revson Foundation (ISF-399/03) and the German-Israel Science Foundation (GIF 834/2005) to S.Y.
Footnotes
Published ahead of print on 22 January 2007.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1995. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 2.Bagnat, M., and K. Simons. 2002. Cell surface polarization during yeast mating. Proc. Natl. Acad. Sci. USA 99:14183-14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bloch, D., M. Lavy, Y. Efrat, I. Efroni, K. Bracha-Drori, M. Abu-Abied, E. Sadot, and S. Yalovsky. 2005. Ectopic expression of an activated RAC in Arabidopsis disrupts membrane cycling. Mol. Biol. Cell 16:1913-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borner, G. H., D. J. Sherrier, T. Weimar, L. V. Michaelson, N. D. Hawkins, A. Macaskill, J. A. Napier, M. H. Beale, K. S. Lilley, and P. Dupree. 2005. Analysis of detergent-resistant membranes in Arabidopsis. Evidence for plasma membrane lipid rafts. Plant Physiol. 137:104-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boureux, A., E. Vignal, S. Faure, and P. Fort. 2007. Evolution of the Rho family of Ras-like GTPases in eukaryotes. Mol. Biol. Evol. 24:203-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyartchuk, V. L., M. N. Ashby, and J. Rine. 1997. Modulation of ras and a-factor function by carboxyl-terminal proteolysis. Science 275:1796-1800. [DOI] [PubMed] [Google Scholar]
- 7.Bracha, K., M. Lavy, and S. Yalovsky. 2002. The Arabidopsis AtSTE24 is a CAAX protease with broad substrate specificity. J. Biol. Chem. 277:29856-29864. [DOI] [PubMed] [Google Scholar]
- 8.Choy, E., V. K. Chiu, J. Silletti, M. Feoktistov, T. Morimoto, D. Michaelson, I. E. Ivanov, and M. R. Philips. 1999. Endomembrane trafficking of Ras: the CaaX motif targets proteins to the ER and Golgi. Cell 98:69-80. [DOI] [PubMed] [Google Scholar]
- 9.Clarke, S. 1992. Protein isoprenylation and methylation at carboxyl-terminal cysteine residues. Annu. Rev. Biochem. 61:355-386. [DOI] [PubMed] [Google Scholar]
- 10.Clough, S. J., and A. F. Bent. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16:735-743. [DOI] [PubMed] [Google Scholar]
- 11.del Pozo, M. A., N. B. Alderson, W. B. Kiosses, H. H. Chiang, R. G. Anderson,and M. A. Schwartz. 2004. Integrins regulate Rac targeting by internalization of membrane domains. Science 303:839-842. [DOI] [PubMed] [Google Scholar]
- 12.Farnsworth, C. C., P. J. Casey, W. N. Howald, J. A. Glomset, and M. H. Gelb. 1990. Structural characterization of prenyl groups attached to proteins. Methods 1:231-240. [Google Scholar]
- 13.Fischer, U., S. Men, and M. Grebe. 2004. Lipid function in plant cell polarity. Curr. Opin. Plant Biol. 7:670-676. [DOI] [PubMed] [Google Scholar]
- 14.Golub, T., and P. Caroni. 2005. PI(4,5)P2-dependent microdomain assemblies capture microtubules to promote and control leading edge motility. J. Cell Biol. 169:151-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hancock, J. F., H. Paterson, and C. J. Marshall. 1990. A polybasic domain or palmitoylation is required in addition to the CAAX motif to localize p21ras to the plasma membrane. Cell 63:133-139. [DOI] [PubMed] [Google Scholar]
- 16.Ivanchenko, M., Z. Vejlupkova, R. S. Quatrano, and J. E. Fowler. 2000. Maize ROP7 GTPase contains a unique, CaaX box-independent plasma membrane targeting signal. Plant J. 24:79-90. [DOI] [PubMed] [Google Scholar]
- 17.Kamiguchi, H. 2006. The region-specific activities of lipid rafts during axon growth and guidance. J. Neurochem. 98:330-335. [DOI] [PubMed] [Google Scholar]
- 18.Kost, B., E. Lemichez, P. Spielhofer, Y. Hong, K. Tolias, C. Carpenter, and N. H. Chua. 1999. Rac homologues and compartmentalized phosphatidylinositol 4,5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J. Cell Biol. 145:317-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lavy, M., K. Bracha-Drori, H. Sternberg, and S. Yalovsky. 2002. A cell-specific, prenylation-independent mechanism regulates targeting of type II RACs. Plant Cell 14:2431-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavy, M., and S. Yalovsky. 2006. Association of Arabidopsis type-II ROPs with the plasma membrane requires a conserved C-terminal sequence motif and a proximal polybasic domain. Plant J. 46:934-947. [DOI] [PubMed] [Google Scholar]
- 21.Lemichez, E., Y. Wu, J. P. Sanchez, A. Mettouchi, J. Mathur, and N. H. Chua. 2001. Inactivation of AtRac1 by abscisic acid is essential for stomatal closure. Genes Dev. 15:1808-1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin, Y., Y. Wang, J.-K. Zhu, and Z. Yang. 1996. Localization of a Rho GTPase implies a role in tip growth and movement of the generative cell in pollen tubes. Plant Cell 8:293-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.London, E., and D. A. Brown. 2000. Insolubility of lipids in Triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts). Biochim. Biophys. Acta 1508:182-195. [DOI] [PubMed] [Google Scholar]
- 24.Maurer-Stroh, S., S. Washietl, and F. Eisenhaber. 2003. Protein prenyltransferases. Genome Biol. 4:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melkonian, K. A., A. G. Ostermeyer, J. Z. Chen, M. G. Roth, and D. A. Brown. 1999. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J. Biol. Chem. 274:3910-3917. [DOI] [PubMed] [Google Scholar]
- 26.Michaelson, D., J. Silletti, G. Murphy, P. D'Eustachio, M. Rush, and M. R. Philips. 2001. Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and RhoGDI binding. J. Cell Biol. 152:111-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molendijk, A. J., F. Bischoff, C. S. Rajendrakumar, J. Friml, M. Braun, S. Gilroy, and K. Palme. 2001. Arabidopsis thaliana Rop GTPases are localized to tips of root hairs and control polar growth. EMBO J. 20:2779-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morel, J., S. Claverol, S. Mongrand, F. Furt, J. Fromentin, J. J. Bessoule, J. P. Blein, and F. Simon-Plas. 2006. Proteomics of plant detergent resistant membranes. Mol. Cell. Proteomics 5:1396-1411. [DOI] [PubMed] [Google Scholar]
- 29.Nibau, C., H. M. Wu, and A. Y. Cheung. 2006. RAC/ROP GTPases: ‘hubs’ for signal integration and diversification in plants. Trends Plant Sci. 11:309-315. [DOI] [PubMed] [Google Scholar]
- 30.Niv, H., O. Gutman, Y. Kloog, and Y. I. Henis. 2002. Activated K-Ras and H-Ras display different interactions with saturable nonraft sites at the surface of live cells. J. Cell Biol. 157:865-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prior, I. A., A. Harding, J. Yan, J. Sluimer, R. G. Parton, and J. F. Hancock. 2001. GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nat. Cell Biol. 3:368-375. [DOI] [PubMed] [Google Scholar]
- 32.Prior, I. A., C. Muncke, R. G. Parton, and J. F. Hancock. 2003. Direct visualization of Ras proteins in spatially distinct cell surface microdomains. J. Cell Biol. 160:165-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Proszynski, T. J., R. Klemm, M. Bagnat, K. Gaus, and K. Simons. 2006. Plasma membrane polarization during mating in yeast cells. J. Cell Biol. 173:861-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rocks, O., A. Peyker, M. Kahms, P. J. Verveer, C. Koerner, M. Lumbierres, J. Kuhlmann, H. Waldmann, A. Wittinghofer, and P. I. Bastiaens. 2005. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science 307:1746-1752. [DOI] [PubMed] [Google Scholar]
- 35.Rodríguez-Concepcion, M., S. Yalovsky, M. Zik, H. Fromm, and W. Gruissem.1999. The prenylation status of a novel plant calmodulin directs plasma membrane or nuclear localization of the protein. EMBO J. 18:1996-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schrick, K., U. Mayer, A. Horrichs, C. Kuhnt, C. Bellini, J. Dangl, J. Schmidt, and G. Jurgens. 2000. FACKEL is a sterol C-14 reductase required for organized cell division and expansion in Arabidopsis embryogenesis. Genes Dev. 14:1471-1484. [PMC free article] [PubMed] [Google Scholar]
- 37.Smotrys, J. E., and M. E. Linder. 2004. Palmitoylation of intracellular signaling proteins: regulation and function. Annu. Rev. Biochem. 73:559-587. [DOI] [PubMed] [Google Scholar]
- 38.Wang, T. Y., R. Leventis, and J. R. Silvius. 2001. Partitioning of lipidated peptide sequences into liquid-ordered lipid domains in model and biological membranes. Biochemistry 40:13031-13040. [DOI] [PubMed] [Google Scholar]
- 39.Winge, P., T. Brembu, and A. M. Bones. 1997. Cloning and characterization of rac-like cDNAs from Arabidopsis thaliana. Plant Mol. Biol. 35:483-495. [DOI] [PubMed] [Google Scholar]
- 40.Winge, P., T. Brembu, R. Kristensen, and A. M. Bones. 2000. Genetic structure and evolution of RAC-GTPases in Arabidopsis thaliana. Genetics 156:1959-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.