Abstract
Diffuse large B-cell lymphomas (DLBCLs) consist of clinically distinct subtypes: germinal center B-cell (GCB)-like and activated-B-cell (ABC)-like tumors, characterized by long and short survival, respectively. We reported distinct interleukin 4 (IL-4) responsiveness and STAT6 signaling in these DLBCL subtypes. Increased nuclear dephosphorylation of phospho-STAT6 (pSTAT6) was observed in ABC-like tumors, which exhibited a different expression profile of protein tyrosine phosphatases (PTPs). Among the differentially expressed PTPs, only T-cell PTP (TCPTP) localizes to the nucleus. Herein, we report that the elevated expression of TCPTP in ABC- versus GCB-like DLBCL tumors is not due to the distinct ontogeny of these neoplasms but rather may be an acquired feature of the tumors. Moreover, we report that STAT6 may serve as a physiological nuclear substrate for TCPTP. We demonstrate interactions between endogenous TCPTP and STAT6 and delineate the domains responsible for the interaction. Overexpression of TCPTP ameliorates IL-4-induced STAT6 phosphorylation and associated gene transcription, whereas knockdown of endogenous TCPTP results in increased IL-4-induced STAT6 signaling. Moreover, we report that TCPTP protein levels may be increased in response to IL-4 and that TCPTP may serve in a negative feedback loop for the suppression of IL-4-induced signaling. Taken together, these results identify TCPTP as a physiological regulator of STAT6 phosphorylation and suggest that specific increases in TCPTP expression in ABC-like DLBCLs may contribute to the different biological characteristics of these tumors.
Tyrosine phosphorylation is fundamental to the control of numerous important physiological processes, and its dysregulation can contribute to the pathogenesis of varied inherited and acquired human diseases, from immune deficiencies to cancer. Protein tyrosine phosphatases (PTPs) are a large family of enzymes that catalyze the dephosphorylation of tyrosyl-phosphorylated proteins, and their actions are integral to the maintenance of homeostasis and health (2, 3). T-cell PTP (TCPTP), also known as protein tyrosine phosphatase nonreceptor type 2 (PTPN2), is a classical tyrosine-specific PTP expressed predominantly in cells of hematopoietic origin. TCPTP mRNA can be alternatively spliced to generate 48-kDa and 45-kDa TCPTP variant proteins with distinct subcellular localizations: 48-kDa TCPTP is targeted to the endoplasmic reticulum by a hydrophobic C terminus (8, 16), whereas 45-kDa TCPTP (TCPTP-45) lacks the hydrophobic C terminus and is targeted to the nucleus by a bipartite nuclear localization sequence (39). Despite TCPTP-45 having an apparently exclusively nuclear localization in resting cells, specific stimuli can induce TCPTP-45 shuttling to the cytoplasm (20) where it can access cytoplasmic substrates that include the epidermal growth factor receptor (18), the insulin receptor (12), Src family protein tyrosine kinases (41), the adaptor protein p52Shc (38), and Janus family protein tyrosine kinases 1 and 3 (JAK1 and JAK3) (35), to regulate multiple intracellular signaling pathways. At this time, STAT1 (signal transducer and activator of transcription 1) is the only physiological nuclear TCPTP-45 substrate that is known, although studies utilizing overexpression approaches have reported that STAT3, STAT5A, and STAT5B may also serve as TCPTP substrates (6, 36, 43) The identification of physiological substrates is a crucial step for delineating the functional spectrum of TCPTP-45 in vivo.
Interleukin 4 (IL-4) is a multifunctional cytokine that plays several critical roles in the regulation of immune responses and in the pathogenesis of allergic disorders. We have recently demonstrated qualitatively different IL-4 effects on germinal center B-cell (GCB)-like and activated-B-cell (ABC)-like diffuse large B-cell lymphoma (DLBCL) cell lines derived from primary tumors (24). In GCB-like DLBCL cells, IL-4 induced the activation of STAT6 and expression of IL-4 target genes. In contrast, in ABC-like DLBCL cells, IL-4 neither induced the expression of IL-4 target genes nor did it induce sustained increases in nuclear phosphorylated STAT6. Defective JAK-STAT6 signaling in the ABC-like cell lines was attributed to increased cytoplasmic and nuclear STAT6 dephosphorylation (24). Dephosphorylation of activated STAT6 is fundamental to the control of IL-4 signaling and serves to prevent allergic responses and may underlie at least some changes in gene expression and the biology of DLBCL subtypes (24). However, only a few studies have focused on the mechanisms of STAT6 dephosphorylation, and currently STAT6 nuclear PTP is unknown.
Our initial study revealed distinct expression profiles for several PTP mRNAs in GCB-like and ABC-like cell lines and in primary DLBCL tumors (24). In particular, we reported that TCPTP expression was increased in ABC-like DLBCL cell lines compared to GCB-like DLBCL cell lines, and this correlated with elevated nuclear phosphatase activity (24). Herein, we report that STAT6 is a physiological nuclear substrate for TCPTP-45. We demonstrate an interaction between TCPTP-45 and STAT6 in cells and delineate the protein domains responsible for this interaction. We show that IL-4 increases TCPTP-45 protein levels, suggesting the presence of a new negative feedback loop for the inactivation of STAT6 activation. Furthermore, we report that the increased TCPTP expression in ABC-like DLBCLs does not stem from higher expression in their nonmalignant activated B-cell counterparts. Thus, our results suggest that TCPTP expression may be increased specifically during ABC-like DLBCL development and point towards TCPTP expression contributing to the unique characteristics of the ABC-like DLBCL subtype.
MATERIALS AND METHODS
Reagents.
Recombinant human IL-4 was purchased from R&D Systems, Inc. (Minneapolis, MN); recombinant mouse IL-4 was purchased from PeproTech (Rocky Hill, NJ); sodium orthovanadate, staurosporine, and recombinant TC-PTP were purchased from Sigma (St. Louis, MO). Pyridone compound 6 (P6) (37) was a generous gift of William Bornmann and Jacqueline Bromberg. Tyrosyl-phosphorylated STAT6 (pSTAT6) (phosphorylated on tyrosine 641) was detected with rabbit antibodies from Cell Signaling Technology (Beverly, MA). Monoclonal nucleolin (MS-3), JAK1, JAK3, and polyclonal rabbit STAT6 (M200 or S20) antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), and monoclonal green fluorescent protein (GFP) antibody (JL-8) was from BD Biosciences (San Diego, CA). Monoclonal TC-PTP CF4-1D antibody was from Sigma (St. Louis, MO), polyclonal 6288 antibodies directed against the TCPTP catalytic domain have been described previously (41), and monoclonal TCPTP 6F3 antibody and TCPTP−/+ mice (44) were kindly provided by M. L. Tremblay (McGill University, Quebec, Canada). Antibodies for mouse STAT6 and tubulin were from Sigma (Castle Hill, Australia). Protein G agarose was from Invitrogen Corporation (Carlsbad, CA).
Cells and cell culture.
HEK (human embryonic kidney) 293 and HeLa cervical adenocarcinoma cells were cultured at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) (Mediatech, Inc., Herndon, VA) supplemented with 10% fetal bovine serum (FBS) (HyClone, Logan, UT), 100 units/ml penicillin, and 100 μg/ml streptomycin (GIBCO BRL, Grand Island, NY). GCB-like SUDHL6, SUDHL4, and VAL cell lines were grown in RPMI 1640 medium (Mediatech, Inc., Herndon, VA), supplemented with 10% fetal bovine serum, 2 mM glutamine (GIBCO BRL, Grand Island, NY), and penicillin/streptomycin. OCILY10 cells were grown in Iscove's modified Dulbecco's essential medium (Mediatech, Inc., Herndon, VA), supplemented with 20% fresh human plasma and 50 μM β-mercaptoethanol (Gibco, Grand Island, NY).
TCPTP+/− mutant mice (44) were maintained at the Monash University Mouseworks animal facility and treated according to the National Health and Medical Research Council (NHMRC) Australian Code of Practice for the Care and Use of Animals (project number BAM/B/2003/10). TCPTP−/−, TCPTP−/+, and TCPTP+/+ primary mouse embryo fibroblasts were isolated from littermate embryos at 14.5 days of gestation. Genotypes were determined by Southern blot hybridization as described by You-Ten et al. (44). Embryo carcasses were minced using sterile scalpel blades and passed through an 18-gauge needle into DMEM containing 5% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml), and the resulting cells were cultured for up to eight passages at 37°C and 5% CO2. Cells were serum starved for 4 h in medium containing 0.1% (vol/vol) FBS prior to the stimulation with 10 ng/ml of recombinant mouse IL-4.
CD19-positive B cells were isolated from the spleens of 14- to 16-day-old TCPTP−/− mice and TCPTP+/+ littermates using a CD19-MicroBeads kit and the AutoMACS cell separation system (Miltenyi Biotech, Germany) according to the manufacturer's protocols. The purity of the isolated cells was typically more than 95%. Cells were allowed to rest for 4 h in complete RPMI 1640 medium with 10% heat-inactivated FBS at 37°C and 5% CO2 and then serum starved for 1 h in RPMI 1640 medium containing 0.1% FBS before stimulation with 40 ng/ml recombinant murine IL-4.
Human B cells were negatively selected from primary GCB-like (5) and ABC-like (2) DLBCL tumors by magnetic cell separation using the B-cell isolation kit II (Miltenyi Biotech, Germany) according to the manufacturer's instructions. The purity of the enriched tumor B cells was more than 95% as analyzed by flow cytometry analysis.
Plasmid constructs and cell transfection.
pCG, TCPTP-pCG, TCPTP-D182A-pCG, TCPTP(1-349)-pCG, TCPTP-pEGFP-C1, TCPTP-pWZL, and TCPTP-D182A-pWZL plasmid constructs have been described previously (15, 18, 20). The full-length pVL1393-STAT6.myc plasmid was a generous gift of Paul Rothman (23). The C-terminus-truncated STAT6 (STAT6ΔC) pcDNA3 construct, designated TPU547, was generously provided by Tularik, Inc. (South San Francisco, CA; now Amgen Inc.) (25). The STAT6-driven luciferase reporter construct designated C/EBP-N4 (TPU474) was generously provided by Amgen Inc. (Thousand Oaks, CA) (25). The empty vectors pCG, pEGFP-C1 (Clontech, Mountain View, CA), pcDNA3 (Invitrogen, Carlsbad, CA), and pWZL were used as controls.
HEK 293 and HeLa cells were plated at 2.5 × 105 cells in 60-mm culture dishes (Nalgene Nunc International, Rochester, NY) in 4 ml of DMEM and grown overnight at 37°C and 5% CO2. Cells were transfected with FuGENE 6 reagent (Roche Applied Science, Indianapolis, IL) according to the manufacturer's instructions. Briefly, 6 μl of FuGENE 6 reagent was diluted in 194 μl of serum-free DMEM and mixed, and the mixture was incubated for 5 min at room temperature. Plasmid DNA (2 μg) was added to the mixture, which was incubated for 40 min at room temperature. The resulting mixture was added to the cells in a drop-wise manner, and the cells were incubated for 48 h before proceeding with further experiments.
VAL GCB-like DLBCL cells were transiently transfected with Amaxa Nucleofector methodology (Amaxa Inc., Gaithersburg, MD). Briefly, 1 × 106 to 5 × 106 VAL cells per nucleofection sample were centrifuged for 10 min at 200 × g, spun down, and resuspended in 100 μl of Nucleofector solution V at room temperature. Two micrograms of TCPTP-pEGFP-C1 or control pEGFP-C1 plasmid DNA was added to each cell suspension and transferred to an Amaxa-certified cuvette. Nucleofection was performed using the U-15 program. The cells were incubated in a humidified incubator at 37°C and 5% CO2 for 24 hours and sorted for GFP expression by flow cytometry before proceeding with further experiments.
Phoenix package cells (a generous gift of Garry Nolan, Stanford) were transfected transiently with pWZL-TC45 plasmid (18) (2 μg) using FuGENE 6 reagent as described above. After 24 h of incubation, the medium was changed to RMPI 1640 medium, and the cells were incubated for an additional 24 h. The cells were centrifuged at 400 × g for 5 min to pellet cells and debris, and the supernatant was filtered through a 0.45-μm filter. SUDHL4 cells were infected by the spin method as described previously (30). Briefly, SUDHL4 cells were resuspended in the viral supernatant with 10 μg/ml DOTAP {N-[1-(2,3-dioleoyloxy)propyl]-N,N,N,-trimethylammonium methylsulfonate} liposomal transfection reagent (Roche Applied Science, Indianapolis, IN) at a concentration of 1 × 106 cells/ml. Cells were placed in six-well culture plates and centrifuged at approximately 1,000 × g for 90 min at room temperature. Half of the supernatant was removed, complete RPMI 1640 medium was added, and the cells were grown at 37°C and 5% CO2 for an additional 2 or 3 days before experimentation. Infection efficiency was monitored by parallel infections of virus generated from packaging cells transfected with pBMN-I-GFP and was routinely 15 to 20% as assessed by immunofluorescence microscopy.
Forty-eight hours after transfection or transduction, cells were stimulated with human recombinant IL-4 (100 U/ml) for 30 min or 6 h for protein and RNA expression experiments, respectively.
For knocking down expression of TCPTP, HeLa cells, grown in 10% FBS in RPMI 1640 medium, were transfected with 100 nM TCPTP small interfering RNA (siRNA) (target sequence, AAG AGU UGG AUA CUC AGC GUC) or control scrambled siRNA by using the Transpass R1 reagent (New England Biolabs, Ipswich, MA) according to the manufacturer's protocol. At 72 h posttransfection, the expression of TCPTP and IL-4-induced pSTAT6 proteins was examined by immunoblotting.
For knocking down expression of STAT6, VAL cells were transfected with 1.5 μg of siRNA STAT6 or nontargeting siRNA (Dharmacon RNA Technologies, Lafayette, CO) using the Amaxa Nucleofector methodology (Amaxa Inc., Gaithersburg, MD). At 60 h posttransfection, IL-4 (100 U/ml) was added for 3, 6, and 12 h, and the expression of TCPTP, STAT6, and actin proteins was examined by immunoblotting.
Microscopy.
The subcellular localization of enhanced GFP (EGFP)-TCPTP fusion protein was assessed by immunofluorescence microscopy of HEK 293 cells transfected with pEGFP-TCPTP-C1. The cells, seeded on glass coverslips in six-well culture dishes, were transfected as described above. Forty-eight hours posttransfection, the cells were fixed in 4% formaldehyde for 8 min at room temperature. The cells were washed with phosphate-buffered saline (PBS), permeabilized in 0.2% Triton X-100 in PBS, washed in PBS, stained with 4′,6′-diamidino-2-phenylindole (DAPI) (Molecular Probes, Eugene, OR), and visualized with a Leica DMIRE microscope.
Whole-cell and nuclear extract preparation, Western blot analysis, and immunoprecipitation.
Human whole-cell extracts for Western blot analysis were prepared by lysing 5 × 106 cells in radioimmunoprecipitation assay (RIPA) buffer (1× phosphate-buffered saline, 1% Nonidet P-40 [NP-40], 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 10 mM phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, 100 mM sodium orthovanadate) on ice for 30 min. Whole-mouse lymphocyte extracts for Western blot analysis were prepared by washing the cells with ice-cold PBS, lysing the cells in modified RIPA lysis buffer (50 mM HEPES [pH 7.4], 1% [vol/vol] Triton X-100, 1% [vol/vol] sodium deoxycholate, 0.1% [vol/vol] SDS, 150 mM NaCl, 10% [vol/vol] glycerol, 1.5 mM MgCl2, 1 mM EGTA, 100 mM NaF, leupeptin [5 μg/ml], pepstatin A [1 μg/ml], 1 mM benzamadine, 2 mM phenylmethysulfonyl fluoride, 1 mM sodium vanadate, 0.1 μM okadaic acid), followed by centrifugation (16,000 × g for 5 min at 4°C) or otherwise collected directly in hot 3× Laemmli sample buffer.
Nuclear extracts were prepared according to a previously described protocol (7). Briefly, 1 × 107 cells, washed twice with cold PBS, were incubated for 15 min on ice in 400 μl of hypotonic buffer (buffer A, consisting of 10 mM HEPES, pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 1 mM dithiothreitol [DTT], 0.5 mM phenylmethylsulfonyl fluoride, and protease inhibitor cocktail [1 μg/ml leupeptin, 1 μg/ml aprotinin, 10 μM pepstatin]). Cells were lysed with 25 μl of 10% NP-40. After brief vortexing and centrifugation, the supernatant (cytoplasmic extract) was stored at −80°C until use, while the pellet was resuspended with hypertonic buffer (buffer B, consisting of 20 mM HEPES, pH 7.9, 0.4 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 1 mM DTT, 0.5 mM phenylmethylsulfonyl fluoride, 5% glycerol, and protease inhibitor cocktail [1 μg/ml leupeptin, 1 μg/ml aprotinin, 10 μM pepstatin]) and gently agitated for 45 min at 4°C. After centrifugation, the supernatant was assayed for protein concentration by the BCA assay kit (Pierce Biotechnology Inc., Rockford, IL) and either used immediately or stored at −80°C.
For Western blotting, 60 μg of nuclear extract or 20 μg of whole-cell lysate was separated on 10% SDS-polyacrylamide gels, transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories Inc., Hercules, CA), and immunoblotted with specific antibodies.
For immunoprecipitation, nuclear extracts, prepared from untransfected and transfected IL-4-stimulated cells, were mixed for 3 h with prewashed agarose-linked anti-STAT6 antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA) or mouse anti-TCPTP antibodies linked to anti-mouse immunoglobulin G (IgG) beads (eBioscience, San Diego, CA) and washed extensively with buffer (PBS with 0.1% NP-40 [Roche Applied Science]). The immunoprecipitates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with the indicated antibodies.
Luciferase reporter transactivation assay.
Cotransfection of the STAT6-driven luciferase reporter construct designated C/EBP-N4 (TPU474, 4 μg), the constitutively active Renilla reniformis luciferase-producing vector pRL (0.2 μg) (Promega, Madison, WI), and the expression plasmids TCPTP-pEGFP-C1 and pEGFP-C1 (4 μg, each) in HEK 293 cells was done with the Polyfect transfection reagent (QIAGEN, Valencia, CA) according to the manufacturer's instructions. Forty-eight hours after transfection, the cells were either left untreated or stimulated for 12 h with 100 U/ml of IL-4. Firefly (Photinus pyralis) and R. reniformis luciferase activities were detected with the Dual-Luciferase assay kit according to the manufacturer's instructions (Promega).
RNA isolation, RT reaction, and real-time PCR.
Isolation of RNA, its quantification, and the reverse transcription (RT) reactions were performed as reported previously (22). TCPTP mRNA expression in enriched B cells from primary DLBCL tumors and CD23 and TCPTP mRNA expression in transfected and untransfected VAL and SUDHL4 DLBCL cell lines cultured with and without 100 U/ml IL-4 and 10 μM P6 were measured by real-time PCR using the Applied Biosystems Assays-on-Demand gene expression product on an ABI PRISM 7900HT sequence detection system (Applied Biosystems, Foster City, CA). Expression of these genes was normalized to 18S expression that was used as an endogenous RNA/cDNA quantity control, as we reported previously (22).
TCPTP pulse and pulse-chase immunoprecipitation assays.
For assessing TCPTP-45 stability, chase experiments were performed. VAL cells (5 × 106) were rinsed with PBS and starved in methionine-free RPMI 1640 medium (Sigma, St. Louis, MO) for 2 h. Cells were pulsed with 10 μCi of [35S]methionine (SJ204; Amersham) for an additional 2 h. The cells were rinsed with PBS, incubated in complete RPMI 1640 medium containing 10% FBS, and either left unstimulated or stimulated with IL-4 (100 U/ml) for up to 24 h. To monitor TCPTP translation, pulse assays were performed as described previously (17). Briefly, 5 × 106 VAL cells were stimulated with IL-4 (100 U/ml) for 3, 6, and 12 h, then rinsed in PBS, and pulsed with 10 μCi of [35S]methionine and additional IL-4 for 15 min. Cells from both the chase and pulse assays were lysed in immunoprecipitation lysis buffer (see above), and TCPTP was immunoprecipitated with anti-TCPTP antibody and resolved by SDS-PAGE (12% polyacrylamide).
In vitro TCPTP dephosphorylation assay.
Nuclear extracts were prepared as described above from the SUDHL4 cells stimulated with IL-4 (100 U/ml) for 1 h and nonstimulated OCILY10 cells. One half of the OCILY10 nuclear extract was mixed with anti-TCPTP antibody linked to anti-mouse IgG beads (eBioscience) at 4°C for 2 h, and the supernatant was collected. The nuclear extracts from the stimulated SUDHL4 cells that served as a source of the tyrosyl-phosphorylated STAT6 as well as the intact or postimmunoprecipitation OCILY10 extracts were dialyzed in 1× reaction buffer (25 mM imidazole, pH 7.0, 0.1 mg/ml bovine serum albumin, 5 mM DTT, 50 mM NaCl, 2.5 mM EDTA) for 3 h. The dialyzed SUDHL4 nuclear extract (60 μg) was mixed with 3 μg of intact or postimmunoprecipitation OCILY10 nuclear extracts and incubated for 10, 30, and 60 min at 37°C. For positive controls, SUDHL4 nuclear extracts were mixed with 4 units of recombinant TCPTP. The reactions were terminated by adding 2× SDS-PAGE sample buffer (100 mM Tris-HCl, pH 6.8, 200 mM DTT, 4% SDS, 0.2% bromophenol blue, 20% glycerol). The nuclear extract proteins were resolved by SDS-PAGE (10% polyacrylamide), transferred to a polyvinylidene difluoride membrane (Bio-Rad Laboratories Inc.), and subjected to Western blotting with anti-pSTAT6 and anti-STAT6 antibodies.
TCPTP expression.
We previously reported the results of gene expression analyses using cDNA microarrays in 43 untreated patients with DLBCL as well as purified germinal center B cells, centroblasts, naïve peripheral B cells, memory B cells, and resting and activated human peripheral blood B cells (1). Within the Lymphochip microarrays employed in this study, we identified three cDNA clones of TCPTP (IMAGE 665903, IMAGE 740402, and IMAGE 1370148) derived from a GC B-cell cDNA library. Expression of these TCPTP clones in different normal B-cell compartments as well as in DLBCL tumors was reanalyzed. Expression of TCPTP in GCB-like and ABC-like tumors is presented as the mean expression in each DLBCL subtype, as reported previously by others (11).
Statistical analysis.
To test the differences in IL-4 inducibility of gene expression, TCPTP mRNA expression or phosphorylated JAK1 (pJAK1) and pJAK3 densitometry between the different experimental settings, we used the two-tailed Student t test.
RESULTS
TCPTP expression during B-cell differentiation and in DLBCL subtypes.
In our previous study, we demonstrated that TCPTP mRNA expression is higher in primary ABC-like DLBCL tumors than in GCB-like DLBCL tumors (24). These observations were based on reanalysis of global gene expression profiling of unmanipulated DLBCL tumors in two independent data sets reported by us (1) and Rosenwald et al. (32). We further demonstrated differences in TCPTP mRNA and protein expression in ABC-like versus GCB-like DLBCL cell lines (24); however, whether the observed differences pertain to isolated malignant lymphocytes from primary GCB-like and ABC-like tumors was not unequivocally demonstrated. To this end, we measured TCPTP mRNA expression in an independent cohort of five GCB-like and two ABC-like primary DLBCL malignant B-cells enriched to more than 95%. Enriched ABC-like tumor lymphocytes expressed significantly higher mRNA levels of TCPTP than GCB-like tumor lymphocytes did (Fig. 1A). Similarly, TCPTP-45 protein was expressed at higher levels in the ABC-like tumor lymphocytes (Fig. 1B).
FIG. 1.
TCPTP mRNA and protein expression in lymphocyte subsets and DLBCL subtypes. (A) Expression of TCPTP mRNA (relative expression of TCPTP normalized to 18S in relation to the TCPTP/18S ratio in the calibrator Raji cell line) in enriched B lymphocytes from five GCB-like and two ABC-like DLBCL specimens. TCPTP mRNA expression, measured by real-time RT-PCR as described in Materials and Methods, is significantly higher in the ABC-like DLBCLs (P < 0.0001). (B) TCPTP protein expression in GCB-like and ABC-like DLBCL specimens. (C) Expression of TCPTP mRNA (three separate clones) in different normal B-cell compartments as well as in DLBCL tumors assessed by cDNA microarrays, as reported previously (1). Expression of TCPTP in activated lymphocytes and in GCB-like and ABC-like DLBCL tumors is presented as a mean level of expression for multiple specimens. The relative expression of TCPTP in the specimens is depicted according to the color scale shown at the bottom. As indicated, the scale extends from fluorescence ratios of 0.25 to 4 (−2 to +2 in log base 2 units).
Whether the observed differences in TCPTP expression stem from the distinct ontogeny of these tumors or are an acquired feature of these tumors that is unrelated to their ontogeny is unknown. To address this question, we compared TCPTP mRNA expression at different differentiation stages of normal B lymphocytes and in DLBCL subtypes (Fig. 1C). Low TCPTP mRNA expression was observed in isolated GC lymphocytes and in GCB-like DLBCLs, thus suggesting that the low TCPTP expression in the latter may stem from its low expression in the normal counterparts from which these tumors originate. RNA expression of TCPTP was slightly higher in normal peripheral B cells stimulated with anti-B-cell receptor and/or anti-CD40 antibodies (activated B cells) compared to the GC lymphocytes, but it was markedly lower than TCPTP mRNA expression in ABC-like DLBCL tumors and cell lines. Taken together, these results suggest that TCPTP expression may be specifically increased in ABC-like DLBCL tumors.
TCPTP-45 may contribute to STAT6 dephosphorylation in ABC-like DLBCL cells.
In our previous experiments we observed markedly lower levels of IL-4-induced nuclear pSTAT6 in ABC-like DLBCL cell lines compared to those in GCB-like DLBCL cell lines (24). We have hypothesized that TCPTP-45, which is expressed at higher levels in the nuclei of the ABC-like DLBCLs (24), might contribute to the pSTAT6 dephosphorylation. However, differences in the JAK kinase activity in the GCB-like and ABC-like DLBCL cell lines might also contribute to the lower levels of IL-4-induced nuclear pSTAT6 observed in the latter. To exclude this possibility, we used staurosporine, a protein kinase inhibitor. Staurosporine was added to the GCB-like (SUDHL6) and ABC-like (OCILY10) cells pretreated with IL-4, blocking the continuous phosphorylation of STAT6 by JAKs (Fig. 2A). Residual levels of preactivated nuclear pSTAT6 were then determined at several time points. Markedly higher levels of nuclear pSTAT6 were detected in the GCB-like cells than in the ABC-like cells both before and after addition of staurosporine. Further, after the addition of staurosporine, nuclear pSTAT6 levels decreased faster in the ABC-like cell lines, thus suggesting increased STAT6 dephosphorylation in the latter. To examine this possibility, we have evaluated the effect of depleting TCPTP-45 on the in vitro pSTAT6 dephosphorylation capacity of ABC-like DLBCL cells' nuclear extracts. To this end, nuclear extracts from IL-4-stimulated SUDHL4 cells, containing large amounts of pSTAT6 and small amounts of TCPTP-45, were exposed to OCILY10 nonmanipulated nuclear extracts or to OCILY10 nuclear extracts from which TCPTP-45 was depleted by prior immunoprecipitation. Following in vitro incubation for 10, 30, and 60 min at 37°C, the nuclear extract proteins were resolved by SDS-PAGE and immunoblotted with anti-pSTAT6 and anti-STAT6 antibodies (Fig. 2B). Immunodepletion of TCPTP-45 from OCILY10 nuclear extracts markedly decreased the rate of in vitro pSTAT6 dephosphorylation, especially prominent after 30 min of incubation (Fig. 2B). These observations suggest that TCPTP-45, which is expressed at high levels in the nuclei of ABCL-like DLBCL cells, may contribute to the extensive STAT6 dephosphorylation observed in these cells.
FIG. 2.
Analysis of dephosphorylation of STAT6 in GCB-like and ABC-like DLBCL cells. (A) GCB-like (SUDHL6) and ABC-like (OCILY10) DLBCL cell lines were treated with IL-4 (100 U/ml) for 15 min, followed by a staurosporine chase for various time periods (in minutes) as indicated. Nuclear lysates were extracted and blotted for TCPTP, tyrosine-phosphorylated STAT6 (pSTAT6), STAT6, and nucleolin. The exposure time of every blot is indicated (5 seconds or 1 minute). (B) Nuclear extracts were prepared as described in Materials and Methods from the SUDHL4 cells stimulated with IL-4 (100 U/ml) for 1 h and from nonstimulated OCILY10 cells. Half of the OCILY10 nuclear extract was mixed with anti-TCPTP antibody linked to anti-mouse IgG beads at 4°C for 2 h, and the supernatant was collected. The dialyzed SUDHL4 nuclear extract (60 μg) was mixed with 3 μg of intact (+) or postimmunoprecipitation OCILY10 nuclear extract (post TCPTP IP) and incubated for 10, 30, and 60 min at 37°C. For a positive control, SUDHL4 nuclear extracts were also mixed with 4 units of recombinant TCPTP. Equal amounts of nuclear lysates were separated by SDS-PAGE and immunoblotted with the indicated antibodies (anti-pSTAT6 and anti-STAT6). The results shown are representative of the results of two independent experiments.
TCPTP-45 dephosphorylates STAT6.
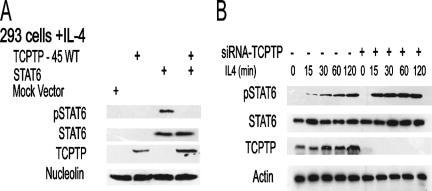
The increased TCPTP expression in ABC-like DLBCLs correlated with increased STAT6 dephosphorylation in these tumors (24). In addition, TCPTP-45 was capable of dephosphorylating STAT6 in vitro (24), and ex vivo TCPTP-45 depletion resulted in increased levels of pSTAT6 (see above). However, it is not known whether TCPTP-45 regulates IL-4-induced STAT6 phosphorylation in vivo. To explore this possibility, plasmid constructs for the expression of wild-type 45-kDa TCPTP and STAT6 were cotransfected in HEK 293 cells, which express low levels of endogenous STAT6 and TCPTP-45 (Fig. 3A). At 48 h posttransfection, cells were stimulated with IL-4 for 30 min, and the phosphorylation of nuclear STAT6 was assessed by immunoblot analysis using antibodies specific for the tyrosyl-phosphorylated STAT6. IL-4 induced the phosphorylation of ectopic STAT6 in cells cotransfected with vector control but not in cells overexpressing TCPTP-45 (Fig. 3A). Comparable expression of STAT6 was confirmed by immunoblotting with anti-STAT6 antibody, and equal loading was confirmed by immunoblotting with a nucleolin antibody (Fig. 3A). In contrast, a siRNA-induced decrease in TCPTP-45 protein expression in the HeLa cells was associated with an increase in IL-4-induced pSTAT6 (Fig. 3B). These results indicate that TCPTP-45 may regulate IL-4-induced STAT6 signaling in a cellular context.
FIG. 3.
TCPTP-45 dephosphorylates IL-4-induced STAT6 in the nucleus. (A) HEK 293 cells were transfected (+) with 45-kDa TCPTP plasmid (TCPTP-45 WT) and either STAT6 plasmid or empty vector (Mock Vector). Forty-eight hours after transfection, cells were stimulated with IL-4 (100 U/ml) for 30 min. Nuclear lysates were extracted and blotted for tyrosine-phosphorylated STAT6 (pSTAT6), STAT6, TCPTP, and nucleolin. (B) HeLa cells were transfected with either siRNA for TCPTP (siRNA-TCPTP) (+) or scrambled control (100 nM) (−). At 72 h posttransfection, the cells were stimulated with IL-4 (100 U/ml) for 15 to 120 min. Cellular lysates were extracted at the indicated time points and blotted for pSTAT6, STAT6, TCPTP, and actin. The blots shown are representative of the blots for three independent experiments.
The TCPTP-45-mediated suppression of IL-4-induced STAT6 phosphorylation in cells might be due to the direct dephosphorylation of STAT6 and/or due to the inactivation of upstream JAK tyrosine kinases. Notably, previous studies have demonstrated that TCPTP-45 can dephosphorylate JAK1 and JAK3 (35). To ascertain whether the observed effects of TCPTP-45 on STAT6 dephosphorylation are mediated by the inactivation of JAK tyrosine kinases in the cytoplasm (35) or can also be attributed to direct activity of TCPTP on STAT6 in the nucleus, we assessed whether a nucleus-restricted GFP-tagged TCPTP-45 fusion protein could suppress STAT6 phosphorylation. The molecular mass of TCPTP-45 allows it to readily diffuse across the nuclear pore complex, whereas the larger size of the GFP-tagged TCPTP-45 (∼72 kDa) prevents passive diffusion and restricts access to nuclear substrates (20). Microscopic analysis of HEK 293 cells transfected with pEGFP-TCPTP-45 plasmid confirmed the predominant nuclear localization for GFP-tagged TCPTP-45 (Fig. 4A). No pSTAT6 was detected in the nuclear extracts when GFP-tagged TCPTP-45-expressing cells were stimulated with IL-4, while nuclear pSTAT6 was readily detected in IL-4-stimulated control cells transfected with the mock pEFGP-C1 vector (Fig. 4B). Notably, IL-4-induced cytoplasmic JAK1 and JAK3 phosphorylation was not decreased by the nucleus-restricted GFP-tagged TCPTP-45 (Fig. 4C). Taken together, these results indicate that in addition to previously reported dephosphorylation of JAK1 and JAK3 in the cytoplasm, TCPTP-45 may also directly dephosphorylate STAT6 in the nucleus.
FIG. 4.
The nucleus-restricted GFP-tagged TCPTP-45 fusion protein dephosphorylates IL-4-induced STAT6 in the nucleus. (A) HEK 293 cells were transfected with pEGFP-TCPTP-C1 plasmid as described in Materials and Methods. Forty eight hours posttransfection, the cells were fixed in 4% formaldehyde, stained with DAPI, and visualized with a Leica DMIRE microscope. (B) HEK 293 cells were transfected (+) with STAT6 and pEGFP-TCPTP-C1 or pEGFP-C1 plasmids. Forty-eight hours posttransfection, the cells were stimulated with IL-4 (100 U/ml) for 30 min. Equal amounts of nuclear lysates were resolved by SDS-PAGE and immunoblotted as indicated. (C) HEK 293 cells were transfected (+) with plasmids expressing STAT6 and either GFP-tagged TCPTP-45 (GFP-TCPTP-45) or GFP. Forty-eight hours posttransfection, the cells were stimulated with IL-4 (100 U/ml) for 30 min. Cell lysates were prepared and divided into two halves for either JAK immunoprecipitation (IP) or pSTAT6 and STAT6 immunoblotting (Western blotting [WB]). JAK1 or JAK3 immunoprecipitates were resolved by SDS-PAGE and immunoblotted with phosphotyrosine (pTyr) or JAK1- and JAK3-specific antibodies, respectively. The results of densitometry analysis (mean plus standard deviation [error bar] of three independent experiments) of JAK1 or JAK3 tyrosine phosphorylation normalized against the respective JAK1 or JAK3 protein are presented. In panels B and C, the blots shown representative of those for three independent experiments are shown.
To ascertain whether TCPTP-45 might regulate IL-4-induced STAT6 signaling in vivo, we examined IL-4-induced STAT6 tyrosine phosphorylation in primary mouse embryonic fibroblasts and splenic B cells isolated from TCPTP-deficient mice versus wild-type mice (44). IL-4-induced STAT6 phosphorylation was elevated and prolonged in both TCPTP-deficient fibroblasts and B cells compared to their wild-type counterparts (Fig. 5A and B). To discern whether the elevated STAT6 phosphorylation in TCPTP-deficient cells was at least in part attributable to TCPTP effects on STAT6 as opposed to the JAK protein tyrosine kinases, we determined whether STAT6 phosphorylation remained elevated after JAK protein tyrosine kinases were inhibited with staurosporine. Although inhibition of JAK protein tyrosine kinases with staurosporine suppressed STAT6 phosphorylation in both TCPTP-deficient (−/−) and wild-type (+/+) cells, nuclear pSTAT6 remained elevated and sustained in the −/− versus +/+ mouse embryo fibroblasts. Therefore, these results are consistent with TCPTP-45 being a physiological negative regulator of IL-4-induced STAT6 phosphorylation.
FIG. 5.
IL-4 induces enhanced STAT6 phosphorylation in TCPTP-deficient cells. (A) Mouse embryo fibroblasts from TCPTP-deficient (−/−), wild-type (+/+), and heterozygous (−/+) mice were serum starved for 4 h and then stimulated with 10 ng/ml IL-4 for the indicated times. Activation of STAT6 was assessed with phosphotyrosine-specific STAT6 antibodies. The genotypes of cells were confirmed by immunoblotting for TCPTP. The results shown are representative of the results of three independent experiments. (B) Purified splenic B cells from TCPTP−/− (−/−) and TCPTP+/+ (+/+) mice were serum starved for 1 h and then stimulated with 40 ng/ml IL-4 and processed for immunoblot analysis with the indicated antibodies. (C) Serum-starved mouse embryo fibroblasts from TCPTP-deficient (−/−) and wild-type (+/+) mice were stimulated with 10 ng/ml IL-4 for 15 min, followed by a staurosporine (0.5 μM) chase for various periods, as indicated. Nuclear lysates were extracted and blotted for pSTAT6 and STAT6. The results shown are representative of the results of two independent experiments.
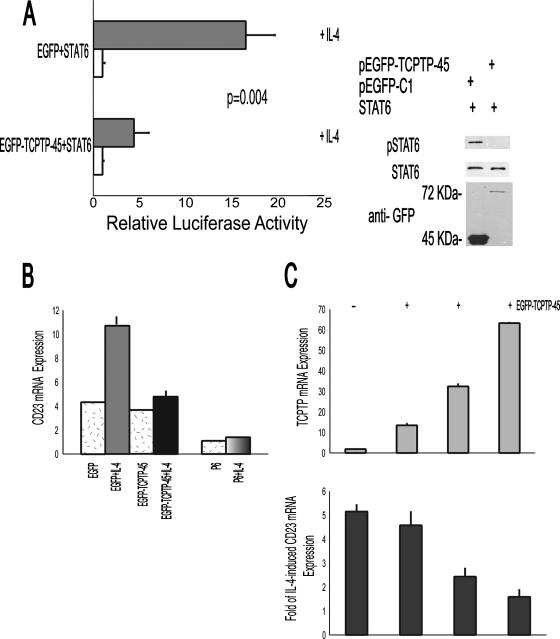
We next examined the functional significance of pSTAT6 dephosphorylation by TCPTP-45 on IL-4-induced gene expression. To this end, GFP-tagged TCPTP-45 expression markedly reduced IL-4-induced STAT6 reporter luciferase activity (Fig. 6A). Furthermore, overexpression of GFP-tagged TCPTP-45 in the GCB-like VAL cells reduced the IL-4-induced mRNA expression of CD23, a classic IL-4 target gene (Fig. 6B). Similar effects on IL-4-induced CD23 expression were demonstrated in GCB-like SUDHL4 cells that were transduced with TCPTP-45-expressing retroviruses (data not shown). The inability of TCPTP-45 to completely abrogate IL-4-induced CD23 expression might be related to the level of TCPTP-45 overexpression, since an inverse correlation was observed between the expression of TCPTP-45 and IL-4-induced CD23 expression (Fig. 6C).
FIG. 6.
TCPTP-45 inhibits IL-4-induced gene transcription. (A) STAT6-driven luciferase reporter construct C/EBP-N4, STAT6, pRL, and either the EGFP-TCPTP-45 or EGFP plasmid were coexpressed in the HEK 293 cells. For each transfection, 4 μg of each expression plasmid, 4 μg of C/EBP-N4, and 0.2 μg of pRL plasmids were used. Luciferase activity was determined 48 h posttransfection in either unstimulated cells or cells that had been stimulated with IL-4 (100 U/ml) for 12 h. The values are relative luciferase activities, with the average value obtained for the reporter without activator set at 1. Values are means plus standard deviations (error bars) of three independent experiments, each performed in three replicate samples. (B) VAL cells were transiently transfected with either control EGFP vector or EGFP-TCPTP-45 vectors, as described in Materials and Methods. Forty-eight hours after transfection, cells were sorted for EGFP expression and incubated with IL-4 (100 U/ml) or without IL-4 for 6 h. In control experiments, VAL cells were preincubated for 30 min with JAK inhibitor P6 (10 μM) before the addition of IL-4. RNA was extracted, and CD23 RNA expression was measured by real-time RT-PCR, in triplicate samples, as described in Materials and Methods. The results shown are representative of the results of five independent experiments. (C) Correlation between the levels of TCPTP-45 mRNA overexpression and IL-4-induced CD23 mRNA expression. CD23 and TCPTP mRNA expression represents relative expression of CD23 and TCPTP, each normalized to 18S in relation to the CD23/18S or TCPTP/18S ratio, respectively, in the calibrator Raji cell line.
TCPTP-45 interacts with STAT6.
Direct dephosphorylation of STAT6 by TCPTP-45 would require a physical interaction between the two proteins. Notably, previous studies have reported that TCPTP-45 might interact directly with other STAT family members and in the case of STAT3, this may occur via the STAT3 C terminus (43). To ascertain whether STAT6 and TCPTP-45 might interact directly, STAT6 and TCPTP-45 coimmunoprecipitation experiments were performed. Constructs for the expression of either STAT6 or a STAT6 mutant missing the C terminus (STAT6ΔC [Fig. 7A ]) and either TCPTP-45 or a substrate-trapping mutant form of TCPTP-45 (TCPTP-45D182A) that can form stable complexes with substrates in a cellular context were transfected transiently into HEK 293 cells (Fig. 7B and C). Cells were stimulated with IL-4 and lysed, nuclear fractions were isolated, and STAT6 (Fig. 7B) or TCPTP-45 (Fig. 7C) was immunoprecipitated with specific antibodies. Immunoprecipitates were resolved by SDS-PAGE and immunoblotted with anti-TCPTP (Fig. 7B) or anti-STAT6 (Fig. 7C) antibodies. These experiments demonstrated that TCPTP-45 can interact with STAT6 (Fig. 7B and C). Cotransfection of STAT6 and TCPTP-45D182A substrate-trapping mutant further increased the amount of STAT6 interacting with TCPTP-45 (Fig. 7B). However, the C-terminus-deleted STAT6ΔC mutant was not able to interact with TCPTP-45 (Fig. 7B and C), indicating that the C-terminal domain of STAT6 may be required for the interaction with TCPTP-45. Furthermore, endogenous STAT6 interacted with endogenous TCPTP-45 in IL-4-stimulated HeLa cells (Fig. 7D) and GCB-like SUDHL6 and ABC-like OCILY10 DLBCL cells (Fig. 7E). In comparison to the GCB-like SUDHL6 cells, larger quantities of TCPTP-45 were immunoprecipitated from the nuclear extracts of ABC-like OCILY10 DLBCL cells, consistent with our observations of higher TCPTP-45 expression levels in the ABC-like DLBCL cell lines and primary tumors. Notably, the interaction between TCPTP-45 and STAT6 was not detected in the absence of IL-4 stimulation in either HeLa cells (not shown) or lymphoma cells (Fig. 7E), which is consistent with this interaction having a regulatory nature.
FIG. 7.
TCPTP-45 interacts with the transactivation domain of STAT6. (A) Schematic representations of STAT6 and its mutant missing the C-terminal transactivation domain (STAT6ΔC). (B and C) HEK 293 cells were transfected (+) with STAT6 or STAT6 ΔC plasmids and either vector control or TCPTP-45 or TCPTP-45D182A plasmids. At 48 h posttransfection, cells were stimulated with IL-4 (100 U/ml) for 30 min. Nuclear lysates were extracted and subjected to immunoprecipitation (IP) with anti-STAT6 (B) or anti-TCPTP (C), followed by Western blotting (Blot) with anti-TCPTP and anti-STAT6 antibodies. The results shown are representative of the results of three independent experiments. (D) HeLa cells were stimulated with IL-4 (100 U/ml) for 30 min. Nuclear lysates were extracted and subjected to immunoprecipitation with anti-STAT6 followed by anti-TCPTP or anti-STAT6 blotting. Unconjugated beads served as a control. (E) GCB-like SUDHL6 and ABC-like OCILY10 DLBCL cells were either not stimulated (−) or stimulated with IL-4 (100 U/ml) for 30 min (+). Nuclear lysates were extracted and subjected to immunoprecipitation with anti-STAT6, followed by anti-TCPTP and STAT6 blotting.
Previous studies have shown that the noncatalytic C-terminal segment of TCPTP-45 dictates its subcellular localization and modulates TCPTP-45 catalytic activity. Moreover, previous studies have shown that the noncatalytic C terminus interacts with proteins that may regulate TCPTP-45 localization and activity (15, 39), whereas other studies have reported that the TCPTP-45 C terminus might interact with chromatin (31). We therefore examined whether the TCPTP-45 noncatalytic C terminus may be required for the dephosphorylation of STAT6 and whether it may mediate the interaction with STAT6. We assessed whether a TCPTP-45 C-terminal truncation mutant [TCPTP-45(1-349)] that readily diffuses throughout the nucleus and cytoplasm (39) could interact with STAT6 and suppress IL-4-induced STAT6 phosphorylation in HEK 293 cells. Deletion of the C-terminal domain of the TCPTP-45 did not abrogate its ability to dephosphorylate STAT6 (Fig. 8A). Furthermore, coimmunoprecipitation experiments demonstrated a physical interaction between STAT6 and the C-terminus-truncated TCPTP-45(1-349) (Fig. 8B). Therefore, these results suggest that the catalytic domain of TCPTP-45 may be both required and sufficient for the interaction and dephosphorylation of STAT6. To examine further whether the interaction with STAT6 was mediated by the TCPTP-45 catalytic domain, we assessed whether the PTP active site inhibitor sodium orthovanadate could abrogate the interaction. TCPTP-45 and STAT6 were coimmunoprecipitated in the presence or absence of sodium orthovanadate, and its effects on association were monitored by immunoblot analysis. In the presence of sodium orthovanadate, the binding of STAT6 to TCPTP-45 was markedly diminished (Fig. 8C). These results are consistent with the catalytic domain of TCPTP-45 being required for the interaction with STAT6.
FIG. 8.
TCPTP-45 interacts with STAT6 via its catalytic domain. (A) STAT6 and TCPTP(1-349) or GFP-tagged TCPTP-45 were transiently expressed in the HEK 293 cells. Forty-eight hours posttransfection, the cells were stimulated with IL-4 (100 U/ml) for 30 min. Nuclear lysates were prepared and blotted with the indicated antibodies. (B) STAT6 and TCPTP(1-349) or GFP-tagged TCPTP-45 were transiently expressed in the HEK 293 cells as described above for panel A and at 48 h posttransfection stimulated with IL-4 (100 U/ml) for 30 min. Nuclear lysates were extracted and subjected to immunoprecipitation (IP) with anti-STAT6 followed by blotting with either anti-TCPTP(6288) or anti-STAT6 antibodies. Unconjugated beads served as a control. (C) STAT6 and TCPTP-45 were transiently expressed in HEK 293 cells as described above. At 48 h posttransfection, the cells were left untreated or incubated for 30 min with sodium orthovanadate (Na3VO4) (2 mM). The cells were then stimulated with IL-4 (100 U/ml) for 30 min, and nuclear lysates were extracted and subjected to immunoprecipitation with anti-STAT6 followed by blotting with either anti-TCPTP or anti-STAT6 antibodies. The results shown are representative of the results of three independent experiments.
TCPTP-45 protein expression is induced by IL-4 stimulation.
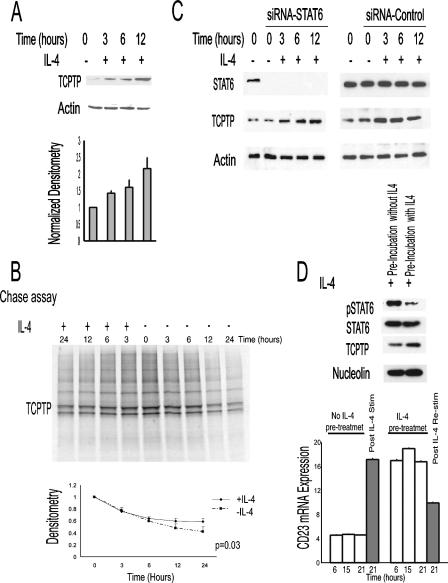
The mechanism(s) regulating TCPTP-45 expression is unknown. Cytokine-induced JAK/STAT signaling pathways are exquisitely sensitive to negative feedback loops, such as those mediated by the expression of suppressor of cytokine signaling (SOCS) proteins (34). Consequently, we examined the effects of IL-4 on TCPTP-45 mRNA and protein expression. GCB-like (VAL and SUDHL6) and ABC-like (OCILY10) DLBCL cell lines were stimulated with IL-4 for 3 to 48 h, and TCPTP-45 mRNA expression and protein expression were measured by real-time RT-PCR and immunoblotting, respectively. Consistent with our previous studies (24), IL-4 did not alter TCPTP-45 mRNA expression (data not shown) but resulted in increased levels of TCPTP protein in VAL cells (Fig. 9A) as well as in SUDHL6 and OCILY10 DLBCL cells (data not shown). These results demonstrate that TCPTP-45 protein levels may be induced by IL-4 and are consistent with TCPTP-45 serving in a negative feedback loop for the suppression of STAT6 signaling. To determine whether IL-4-induced increase in TCPTP-45 is due to enhanced protein translation or decreased turnover, pulse and pulse-chase immunoprecipitation studies were performed, respectively. Pulse studies, wherein IL-4-stimulated cells were incubated briefly in the presence of [35S]methionine to monitor incorporation into newly translated protein, demonstrated that IL-4 does not enhance TCPTP-45 translation (data not shown). In contrast, pulse-chase studies monitoring the degradation of [35S]methionine-labeled protein demonstrated that IL-4 decreased protein degradation (Fig. 9B). The IL-4-induced increase in TCPTP-45 protein levels was STAT6 independent, since siRNA-induced silencing of STAT6 protein expression in VAL cells did not ameliorate the induction of TCPTP-45 by IL-4 (Fig. 9C).
FIG. 9.
IL-4 stimulation increases TCPTP protein levels. (A) VAL DLBCL cells were stimulated with IL-4 (100 U/ml) (+) for 3, 6, and 12 h. Cell lysates were prepared, and TCPTP and actin were immunoblotted. Both the blots and results of densitometry analysis (mean plus standard deviation [error bar]) of TCPTP normalized against actin shown are representative of those of three independent experiments. (B) To monitor the effect of IL-4 on protein stability, pulse-chase experiments were performed as described in Materials and Methods. Briefly, VAL cells were starved of methionine and then labeled with [35S]methionine for 2 h after which they were either left unstimulated (−) or stimulated with IL-4 (100 U/ml) (+) for up to 24 h, and incorporated [35S]methionine was monitored in TCPTP immunoprecipitates by autoradiography. The blot shown is representative, and the densitometric analysis (mean ± standard deviation [error bar]) shows the results of three independent experiments. (C) VAL DLBCL cells were transfected with control or STAT6 siRNA. At 48 h after siRNA transfection, the cells were stimulated with IL-4 (+) for 3, 6, and 12 h. Cell lysates were immunoblotted for STAT6, TCPTP, and actin. Representative blots from two independent experiments are shown. (D) VAL DLBCL cells were grown in complete medium either without IL-4 or with IL-4 (100 U/ml) for 6 to 21 h. (Top) Cells that had been pretreated for 15 h were stimulated with IL-4 (100 U/ml) for 30 min, and nuclear lysates were extracted and blotted for pSTAT6, STAT6, TCPTP, and nucleolin. (Bottom) RNA was extracted from nonpretreated and IL-4-pretreated cells and those cells stimulated with IL-4 (Post IL-4 Stim) or restimulated with IL-4 (Post IL-4 Re-stim), respectively, and CD23 RNA expression was measured by real-time RT-PCR. The results shown are representative of two independent experiments performed in triplicate. CD23 mRNA expression is a relative expression of CD23 normalized to 18S in relation to the CD23/18S ratio in the calibrator Raji cell line.
To examine the potential physiological consequences of the IL-4-TCPTP-45 negative feedback loop, we examined STAT6 phosphorylation and CD23 mRNA expression upon restimulation with IL-4 (Fig. 9D). Prior exposure to IL-4 resulted in increased TCPTP-45 protein levels that were associated with reduced pSTAT6 nuclear levels (Fig. 9D, top panel) and abrogation of CD23 mRNA induction (Fig. 9D, bottom panel) upon IL-4 restimulation at 15 h. Overall, these observations suggest that the IL-4-TCPTP-45 negative feedback loop might control the magnitude and duration of IL-4-induced intracellular signaling.
DISCUSSION
IL-4 is a multifunctional cytokine that plays a critical role in the regulation of immune responses. It regulates the differentiation of antigen-stimulated naïve T cells, controls immunoglobulin class switching, induces gene expression, and is involved in the costimulation of B and T cells acting to promote the proliferation and protection of cells from apoptosis (27, 29). In addition to its physiological roles, IL-4 is also implicated in pathological conditions, such as asthma and allergy. IL-4 may affect malignant cells and can elicit potent antitumor activity against carcinoma and lymphoma cell lines in vitro and in animal models (13, 28, 33, 40).
Although IL-4 intracellular signaling pathways and its pleiotropic effects have been studied and characterized extensively, the mechanisms controlling the transient nature of intracellular signaling induced by IL-4 and other cytokines are less well understood. JAK-STAT signaling pathways are negatively regulated at many levels (34). JAKs can be suppressed or inactivated by suppressor of cytokine signaling (SOCS) proteins, PTPs, and ubiquitin-mediated protein degradation. STAT proteins can be regulated by protein inhibitor of activated STAT (PIAS) proteins (PIAS1, PIAS3, PIASX, and PIASY) that inhibit transcriptional activity of STATs, but to date, no PIAS proteins have been shown to affect the transcriptional activity of STAT6. SHP2 and PTPN1 were reported to directly dephosphorylate cytoplasmic STAT5 (5, 9), while SHP1 was reported to dephosphorylate cytoplasmic STAT6 (14). However, for efficient nuclear export to the cytoplasm and repeated cycles of activation and signaling, STAT proteins need to be dephosphorylated in the nucleus before returning to the cytoplasm (4). Although TCPTP-45 has been identified as a nuclear PTP for STAT1 and possibly STAT3 and STAT5 (6, 36, 43), PTPs dephosphorylating nuclear STAT6 have remained unknown.
In the present study, we have demonstrated that the nuclear 45-kDa form of the tyrosine phosphatase TCPTP dephosphorylates STAT6 to suppress the IL-4-mediated induction of STAT6 target genes. Moreover, we have shown that TCPTP-45 and STAT6 interact via the catalytic domain of TCPTP-45 and the C-terminal transactivation domain of STAT6, similarly to what was previously reported for the interaction between TCPTP-45 and STAT3 (43). Of particular note is that tyrosine 641 at the C terminus of STAT6 that is dephosphorylated by TCPTP-45 was not sufficient for the interaction, consistent with residues distal to tyrosine 641 contributing to binding.
Furthermore, we have demonstrated the existence of a new negative feedback loop which may restrict the time course of IL-4-induced STAT6 phosphorylation. Herein, we show that IL-4 not only induces STAT6 phosphorylation but also increases the protein levels of the phosphatase-TCPTP at least in part due to increased TCPTP stability. Although the mechanism for this remains unclear, the IL-4-induced increase in TCPTP-45 protein levels was independent of STAT6. The elucidation of this new negative regulatory feedback loop, coupled with previously reported TCPTP-mediated JAK dephosphorylation and cytokine-induced SOCS pathways, highlights the complexity of the intracellular regulation of cytokine signaling.
In this study we have shown that STAT6 dephosphorylation by TCPTP can occur in HeLa, HEK 293, and primary human DLBCL cell lines and that IL-4-induced STAT6 phosphorylation is elevated in primary fibroblasts and splenic B-cells isolated from TCPTP knockout mice versus wild-type mice. In contrast to our findings, ten Hoeve et al. reported recently that STAT6 phosphorylation is not altered in an immortalized TCPTP-null fibroblast cell line (36). To assess the effect of TCPTP on STAT6 phosphorylation, ten Hoeve et al. used whole-cell lysates. In contrast, since TCPTP-45 is mainly localized in the nucleus, we evaluated its effects on pSTAT6 in the nuclear extracts. One possible explanation for the apparent discrepancy between our study and that of ten Hoeve et al. may be the existence of another cytoplasmic PTP that obscured TCPTP-45 effects on nuclear pSTAT6. Alternatively, the development of compensatory mechanisms during the process of fibroblast immortalization could contribute to the observed discrepancy.
In the DLBCL cell lines and tumors, dissimilar expression levels of TCPTP most probably underlie the different intracellular signaling and expression profiles of some of the STAT6 target genes that we have previously observed in the GCB-like and ABC-like DLBCL subtypes (24). The reason for the different expression levels of TCPTP in DLBCL subtypes is unknown. Examination of TCPTP expression at different B-cell ontogeny stages also demonstrated different TCPTP mRNA expression patterns, suggesting tight regulation of this gene. GC lymphocytes expressed lower TCPTP RNA than naïve B cells did. IL-4 is one of the cytokines stimulating GC lymphocytes and has been implicated in immunoglobulin class switching (21) and differentiation of GC lymphocytes to memory cells (10). Consequently, down-regulation of TCPTP expression at this ontogeny stage may be necessary for more efficient IL-4 signaling. Similarly, low TCPTP expression levels in GCB-like DLBCLs may reflect the ontogenic origin of these tumors. Stimulation of peripheral B lymphocytes with anti-immunoglobulin or anti-CD40 resulted in higher expression of the TCPTP mRNA. However, TCPTP RNA expression was even higher in primary ABC-like DLBCL tumors and cell lines than in nonmalignant activated B lymphocytes, thus suggesting specific increases in TCPTP expression in these tumors. The mechanisms regulating TCPTP expression are unknown. Interestingly, both mouse (42) and human TCPTP promoter regions (unpublished observations) contain potential NF-κB binding sites, and TCPTP expression might be regulated by this transcription factor. Notably, recent studies have demonstrated constitutive activation of the NF-κB signaling pathway in ABC-like DLBCLs (11) and a vital role for NF-κB in ABC-like DLBCL cell survival (19). Constitutive NF-κB signaling may contribute to the elevated TCPTP expression in ABC-like DLBCLs, but this remains to be evaluated. In addition to its effects on STAT6 signaling, high expression of TCPTP in these tumors might regulate intracellular signaling of multiple other cytokines secreted by surrounding reactive lymphocytes and stroma cells, thus affecting the cross talk between the ABC-like lymphoma cells and their microenvironment. The latter might affect tumor aggressiveness and response to chemotherapy, as suggested by our preliminary observations (26).
This study, in conjunction with our previous work, demonstrates that gene expression profiling studies may elucidate new functions for previously characterized proteins. Herein, we have demonstrated for the first time that the nuclear variant of TCPTP is an important regulator of STAT6 function downstream of IL-4 signaling. Identification of additional TCPTP targets, examination of TCPTP effects on additional signaling pathways in GCB-like and ABC-like tumors, and investigation of the consequences of these effects on lymphoma biology will further advance our understanding of the heterogeneous nature of DLBCL tumors.
Acknowledgments
This work was supported by grant RO1 CA109335 from the U.S. Public Health Service National Institutes of Health (I.S.L.) and funds from the Dwoskin Family Foundation (I.S.L.) and the NHMRC of Australia (T.T.).
Footnotes
Published ahead of print on 8 January 2007.
REFERENCES
- 1.Alizadeh, A. A., M. B. Eisen, R. E. Davis, C. Ma, I. S. Lossos, A. Rosenwald, J. C. Boldrick, H. Sabet, T. Tran, X. Yu, J. I. Powell, L. Yang, G. E. Marti, T. Moore, J. Hudson, Jr., L. Lu, D. B. Lewis, R. Tibshirani, G. Sherlock, W. C. Chan, T. C. Greiner, D. D. Weisenburger, J. O. Armitage, R. Warnke, L. M. Staudt, et al. 2000. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403:503-511. [DOI] [PubMed] [Google Scholar]
- 2.Alonso, A., J. Sasin, N. Bottini, I. Friedberg, A. Osterman, A. Godzik, T. Hunter, J. Dixon, and T. Mustelin. 2004. Protein tyrosine phosphatases in the human genome. Cell 117:699-711. [DOI] [PubMed] [Google Scholar]
- 3.Andersen, J. N., P. G. Jansen, S. M. Echwald, O. H. Mortensen, T. Fukada, R. Del Vecchio, N. K. Tonks, and N. P. Moller. 2004. A genomic perspective on protein tyrosine phosphatases: gene structure, pseudogenes, and genetic disease linkage. FASEB J. 18:8-30. [DOI] [PubMed] [Google Scholar]
- 4.Andrews, R. P., M. B. Ericksen, C. M. Cunningham, M. O. Daines, and G. K. Hershey. 2002. Analysis of the life cycle of stat6. Continuous cycling of STAT6 is required for IL-4 signaling. J. Biol. Chem. 277:36563-36569. [DOI] [PubMed] [Google Scholar]
- 5.Aoki, N., and T. Matsuda. 2000. A cytosolic protein-tyrosine phosphatase PTP1B specifically dephosphorylates and deactivates prolactin-activated STAT5a and STAT5b. J. Biol. Chem. 275:39718-39726. [DOI] [PubMed] [Google Scholar]
- 6.Aoki, N., and T. Matsuda. 2002. A nuclear protein tyrosine phosphatase TC-PTP is a potential negative regulator of the PRL-mediated signaling pathway: dephosphorylation and deactivation of signal transducer and activator of transcription 5a and 5b by TC-PTP in nucleus. Mol. Endocrinol. 16:58-69. [DOI] [PubMed] [Google Scholar]
- 7.Barbeau, B., R. Bernier, N. Dumais, G. Briand, M. Olivier, R. Faure, B. I. Posner, and M. Tremblay. 1997. Activation of HIV-1 long terminal repeat transcription and virus replication via NF-kappaB-dependent and -independent pathways by potent phosphotyrosine phosphatase inhibitors, the peroxovanadium compounds. J. Biol. Chem. 272:12968-12977. [DOI] [PubMed] [Google Scholar]
- 8.Bourdeau, A., N. Dube, and M. L. Tremblay. 2005. Cytoplasmic protein tyrosine phosphatases, regulation and function: the roles of PTP1B and TC-PTP. Curr. Opin. Cell Biol. 17:203-209. [DOI] [PubMed] [Google Scholar]
- 9.Chen, Y., R. Wen, S. Yang, J. Schuman, E. E. Zhang, T. Yi, G. S. Feng, and D. Wang. 2003. Identification of Shp-2 as a Stat5A phosphatase. J. Biol. Chem. 278:16520-16527.12615921 [Google Scholar]
- 10.Choe, J., H. S. Kim, R. J. Armitage, and Y. S. Choi. 1997. The functional role of B cell antigen receptor stimulation and IL-4 in the generation of human memory B cells from germinal center B cells. J. Immunol. 159:3757-3766. [PubMed] [Google Scholar]
- 11.Davis, R. E., K. D. Brown, U. Siebenlist, and L. M. Staudt. 2001. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J. Exp. Med. 194:1861-1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galic, S., M. Klingler-Hoffmann, M. T. Fodero-Tavoletti, M. A. Puryer, T. C. Meng, N. K. Tonks, and T. Tiganis. 2003. Regulation of insulin receptor signaling by the protein tyrosine phosphatase TCPTP. Mol. Cell. Biol. 23:2096-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golumbek, P. T., A. J. Lazenby, H. I. Levitsky, L. M. Jaffee, H. Karasuyama, M. Baker, and D. M. Pardoll. 1991. Treatment of established renal cancer by tumor cells engineered to secrete interleukin-4. Science 254:713-716. [DOI] [PubMed] [Google Scholar]
- 14.Hanson, E. M., H. Dickensheets, C. K. Qu, R. P. Donnelly, and A. D. Keegan. 2003. Regulation of the dephosphorylation of Stat6. Participation of Tyr-713 in the interleukin-4 receptor alpha, the tyrosine phosphatase SHP-1, and the proteasome. J. Biol. Chem. 278:3903-3911. [DOI] [PubMed] [Google Scholar]
- 15.Hao, L., T. Tiganis, N. K. Tonks, and H. Charbonneau. 1997. The noncatalytic C-terminal segment of the T cell protein tyrosine phosphatase regulates activity via an intramolecular mechanism. J. Biol. Chem. 272:29322-29329. [DOI] [PubMed] [Google Scholar]
- 16.Ibarra-Sanchez, M. J., P. D. Simoncic, F. R. Nestel, P. Duplay, W. S. Lapp, and M. L. Tremblay. 2000. The T-cell protein tyrosine phosphatase. Semin. Immunol. 12:379-386. [DOI] [PubMed] [Google Scholar]
- 17.Jin, L., B. W. Guzik, Y. C. Bor, D. Rekosh, and M. L. Hammarskjold. 2003. Tap and NXT promote translation of unspliced mRNA. Genes Dev. 17:3075-3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klingler-Hoffmann, M., M. T. Fodero-Tavoletti, K. Mishima, Y. Narita, W. K. Cavenee, F. B. Furnari, H. J. Huang, and T. Tiganis. 2001. The protein tyrosine phosphatase TCPTP suppresses the tumorigenicity of glioblastoma cells expressing a mutant epidermal growth factor receptor. J. Biol. Chem. 276:46313-46318. [DOI] [PubMed] [Google Scholar]
- 19.Lam, L. T., R. E. Davis, J. Pierce, M. Hepperle, Y. Xu, M. Hottelet, Y. Nong, D. Wen, J. Adams, L. Dang, and L. M. Staudt. 2005. Small molecule inhibitors of IkappaB kinase are selectively toxic for subgroups of diffuse large B-cell lymphoma defined by gene expression profiling. Clin. Cancer Res. 11:28-40. [PubMed] [Google Scholar]
- 20.Lam, M. H., B. J. Michell, M. T. Fodero-Tavoletti, B. E. Kemp, N. K. Tonks, and T. Tiganis. 2001. Cellular stress regulates the nucleocytoplasmic distribution of the protein-tyrosine phosphatase TCPTP. J. Biol. Chem. 276:37700-37707. [DOI] [PubMed] [Google Scholar]
- 21.Linehan, L. A., W. D. Warren, P. A. Thompson, M. J. Grusby, and M. T. Berton. 1998. STAT6 is required for IL-4-induced germline Ig gene transcription and switch recombination. J. Immunol. 161:302-310. [PubMed] [Google Scholar]
- 22.Lossos, I. S., D. K. Czerwinski, M. A. Wechser, and R. Levy. 2003. Optimization of quantitative real-time RT-PCR parameters for the study of lymphoid malignancies. Leukemia 17:789-795. [DOI] [PubMed] [Google Scholar]
- 23.Lu, B., M. Reichel, D. A. Fisher, J. F. Smith, and P. Rothman. 1997. Identification of a STAT6 domain required for IL-4-induced activation of transcription. J. Immunol. 159:1255-1264. [PubMed] [Google Scholar]
- 24.Lu, X., H. Nechushtan, F. Ding, M. F. Rosado, R. Singal, A. A. Alizadeh, and I. S. Lossos. 2005. Distinct IL-4-induced gene expression, proliferation, and intracellular signaling in germinal center B-cell-like and activated B-cell-like diffuse large cell lymphomas. Blood 105:2924-2932. [DOI] [PubMed] [Google Scholar]
- 25.Mikita, T., D. Campbell, P. Wu, K. Williamson, and U. Schindler. 1996. Requirements for interleukin-4-induced gene expression and functional characterization of Stat6. Mol. Cell. Biol. 16:5811-5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nechushtan, H., J. D. Rosenblatt, and I. S. Lossos. 2004. IL-4 affects proliferation, chemosensitivity, and rituximab sensitivity of germinal center B-cell-like (GCB) and activated B-cell-like (ABC) diffuse large B-cell lymphoma differently. Blood 104:73a-73a.15026317 [Google Scholar]
- 27.Nelms, K., H. Huang, J. Ryan, A. Keegan, and W. E. Paul. 1998. Interleukin-4 receptor signalling mechanisms and their biological significance. Adv. Exp. Med. Biol. 452:37-43. [DOI] [PubMed] [Google Scholar]
- 28.Obiri, N. I., G. G. Hillman, G. P. Haas, S. Sud, and R. K. Puri. 1993. Expression of high affinity interleukin-4 receptors on human renal cell carcinoma cells and inhibition of tumor cell growth in vitro by interleukin-4. J. Clin. Investig. 91:88-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paul, W. E. 1991. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood 77:1859-1870. [PubMed] [Google Scholar]
- 30.Quong, M. W., D. P. Harris, S. L. Swain, and C. Murre. 1999. E2A activity is induced during B-cell activation to promote immunoglobulin class switch recombination. EMBO J. 18:6307-6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radha, V., S. Kamatkar, and G. Swarup. 1993. Binding of a protein-tyrosine phosphatase to DNA through its carboxy-terminal noncatalytic domain. Biochemistry 32:2194-2201. [DOI] [PubMed] [Google Scholar]
- 32.Rosenwald, A., G. Wright, W. C. Chan, J. M. Connors, E. Campo, R. I. Fisher, R. D. Gascoyne, H. K. Muller-Hermelink, E. B. Smeland, J. M. Giltnane, E. M. Hurt, H. Zhao, L. Averett, L. Yang, W. H. Wilson, E. S. Jaffe, R. Simon, R. D. Klausner, J. Powell, P. L. Duffey, D. L. Longo, T. C. Greiner, D. D. Weisenburger, W. G. Sanger, B. J. Dave, J. C. Lynch, J. Vose, J. O. Armitage, E. Montserrat, A. Lopez-Guillermo, T. M. Grogan, T. P. Miller, M. LeBlanc, G. Ott, S. Kvaloy, J. Delabie, H. Holte, P. Krajci, T. Stokke, and L. M. Staudt. 2002. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N. Engl. J. Med. 346:1937-1947. [DOI] [PubMed] [Google Scholar]
- 33.Schwarz, M. A., L. Tardelli, H. D. Macosko, L. M. Sullivan, S. K. Narula, and J. S. Fine. 1995. Interleukin 4 retards dissemination of a human B-cell lymphoma in severe combined immunodeficient mice. Cancer Res. 55:3692-3696. [PubMed] [Google Scholar]
- 34.Shuai, K., and B. Liu. 2003. Regulation of JAK-STAT signalling in the immune system. Nat. Rev. Immunol. 3:900-911. [DOI] [PubMed] [Google Scholar]
- 35.Simoncic, P. D., A. Lee-Loy, D. L. Barber, M. L. Tremblay, and C. J. McGlade. 2002. The T cell protein tyrosine phosphatase is a negative regulator of janus family kinases 1 and 3. Curr. Biol. 12:446-453. [DOI] [PubMed] [Google Scholar]
- 36.ten Hoeve, J., M. de Jesus Ibarra-Sanchez, Y. Fu, W. Zhu, M. Tremblay, M. David, and K. Shuai. 2002. Identification of a nuclear Stat1 protein tyrosine phosphatase. Mol. Cell. Biol. 22:5662-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson, J. E., R. M. Cubbon, R. T. Cummings, L. S. Wicker, R. Frankshun, B. R. Cunningham, P. M. Cameron, P. T. Meinke, N. Liverton, Y. Weng, and J. A. DeMartino. 2002. Photochemical preparation of a pyridone containing tetracycle: a Jak protein kinase inhibitor. Bioorg. Med. Chem. Lett. 12:1219-1223. [DOI] [PubMed] [Google Scholar]
- 38.Tiganis, T., A. M. Bennett, K. S. Ravichandran, and N. K. Tonks. 1998. Epidermal growth factor receptor and the adaptor protein p52Shc are specific substrates of T-cell protein tyrosine phosphatase. Mol. Cell. Biol. 18:1622-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tiganis, T., A. J. Flint, S. A. Adam, and N. K. Tonks. 1997. Association of the T-cell protein tyrosine phosphatase with nuclear import factor p97. J. Biol. Chem. 272:21548-21557. [DOI] [PubMed] [Google Scholar]
- 40.Topp, M. S., C. A. Papadimitriou, F. Eitelbach, M. Koenigsmann, E. Oelmann, B. Koehler, D. Oberberg, B. Reufi, H. Stein, E. Thiel, et al. 1995. Recombinant human interleukin 4 has antiproliferative activity on human tumor cell lines derived from epithelial and nonepithelial histologies. Cancer Res. 55:2173-2176. [PubMed] [Google Scholar]
- 41.van Vliet, C., P. E. Bukczynska, M. A. Puryer, C. M. Sadek, B. J. Shields, M. L. Tremblay, and T. Tiganis. 2005. Selective regulation of tumor necrosis factor-induced Erk signaling by Src family kinases and the T cell protein tyrosine phosphatase. Nat. Immunol. 6:253-260. [DOI] [PubMed] [Google Scholar]
- 42.Wee, C., E. S. Muise, O. Coquelet, M. Ennis, J. Wagner, N. Lemieux, P. E. Branton, A. Nepveu, and M. L. Tremblay. 1999. Promoter analysis of the murine T-cell protein tyrosine phosphatase gene. Gene 237:351-360. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto, T., Y. Sekine, K. Kashima, A. Kubota, N. Sato, N. Aoki, and T. Matsuda. 2002. The nuclear isoform of protein-tyrosine phosphatase TC-PTP regulates interleukin-6-mediated signaling pathway through STAT3 dephosphorylation. Biochem. Biophys. Res. Commun. 297:811-817. [DOI] [PubMed] [Google Scholar]
- 44.You-Ten, K. E., E. S. Muise, A. Itie, E. Michaliszyn, J. Wagner, S. Jothy, W. S. Lapp, and M. L. Tremblay. 1997. Impaired bone marrow microenvironment and immune function in T cell protein tyrosine phosphatase-deficient mice. J. Exp. Med. 186:683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]