Abstract
The tumor suppressor function of the retinoblastoma protein pRB is largely dependent upon its capacity to inhibit the E2F transcription factors and thereby cell proliferation. Attempts to study the interplay between pRB and the E2Fs have been hampered by the prenatal death of Rb; E2f nullizygous mice. In this study, we isolated Rb; E2f3 mutant embryonic stem cells and generated Rb−/−; E2f3−/− chimeric mice, thus bypassing the lethality of the Rb−/−; E2f3−/− germ line mutant mice. We show that loss of E2F3 has opposing effects on two of the known developmental defects arising in Rb−/− chimeras; it suppresses the formation of cataracts while aggravating the retinal dysplasia. This model system also allows us to assess how E2f3 status influences tumor formation in Rb−/− tissues. We find that E2f3 is dispensable for the development of pRB-deficient pituitary and thyroid tumors. In contrast, E2f3 inactivation completely suppresses the pulmonary neuroendocrine hyperplasia arising in Rb−/− chimeric mice. This hyperproliferative state is thought to represent the preneoplastic lesion of small-cell lung carcinoma. Therefore, our observation highlights a potential role for E2F3 in the early stages of this tumor type.
Mutations of the retinoblastoma gene (RB) or components of the RB pathway are common to nearly all human tumors (25-27, 33, 54). pRB belongs to the family of pocket proteins which includes p107 and p130. While these proteins have partially redundant functions, Rb is the sole gene in the family that when mutated alone predisposes to cancer (12, 47). pRB exerts its tumor suppressive function mostly through its capacity to inhibit proliferation. In its hypophosphorylated state, pRB binds to the E2F transcription factors and prevents the activation of genes necessary for DNA replication and cell cycle regulation. This inhibition is released when mitogenic stimuli cause the phosphorylation of pRB, allowing E2F-dependent transcription. Thus, this cascade of events positions the E2Fs as key downstream effectors of pRB's antiproliferative activity (22, 62).
The E2F transcription factors are part of a family that to date comprises eight genes (E2f1 through E2f8), several of which encode multiple protein products (5, 10, 38, 41). In addition to their role in proliferation, E2Fs also influence other biological processes, such as differentiation, the DNA damage response, and apoptosis (20, 45, 49). E2Fs bind the members of the pRB family with different specificities; E2F1, E2F2, and E2F3 bind exclusively to pRB, E2F5 preferentially binds p130, and E2F4 is unique in its capacity to bind all the pocket proteins. On the contrary, E2F6, E2F7, and E2F8 do not contain a binding region for the pocket proteins, and very little is known about their mechanisms of action (6, 10, 18, 21, 38, 41, 56, 58).
The pocket protein-binding E2Fs can be divided into two groups based on their roles in transcription and cell cycle regulation. In studies conducted in vitro and in vivo, E2F1 and E2F2 have been shown to act preferentially as transcriptional activators and positive regulators of cell division. These E2Fs are locked in an inactive state when bound to pRB and activate transcription when freed (46, 59). In contrast, E2F4 and E2F5 mostly act in quiescent cells and have been shown to play a key role as repressors (59). When bound to the pocket proteins, these E2Fs actively repress E2F-responsive genes by recruiting histone deacetylases and chromatin remodeling complexes to E2F-regulated promoters (7, 57). In the subdivision of activators and repressors, E2f3 occupies a unique position. The E2f3 locus encodes two distinct proteins, E2F3a and E2F3b, which are translated from transcripts driven by two different promoters (1, 36). These two proteins are both regulated specifically by pRB, but they are thought to have opposing transcriptional activities. E2F3a acts as a classic activator; its expression is regulated during the cell cycle, and when overexpressed it is able to drive quiescent cells in S phase (19, 34, 35). In contrast, E2F3b is expressed in both dividing and quiescent cells (1, 36). Based on this observation and on recent studies showing that E2F3b contributes to the transcriptional repression of the Arf tumor suppressor (4), E2F3b has been proposed to function as a repressor of E2F-responsive genes.
E2f3 is essential for viability (28). Mice lacking both E2F3a and E2F3b die mainly during embryogenesis between embryonic day 13.5 (E13.5) and E18.5, possibly due to heart defects (13, J. Cloud-King, I. Moskowitz, P. G. Burgon, F. Ahmad, J. R. Stone, J. G. Seidman, and J. A. Lees, submitted for publication). The rare animal that reaches adulthood dies of heart failure without any signs of tumor. Combined deletion of Rb and E2f3 has highlighted the relevance of the interaction between these proteins in development. Rb null embryos die in mid-gestation with defects in fetal liver hematopoiesis, ectopic S phase, and extensive apoptosis in the central nervous system, peripheral nervous system, and lens (11, 29). Many of these defects have recently been demonstrated to be secondary to a primary placental defect (18, 66). Simultaneous loss of E2f3 and Rb extends the life span of the embryos and dramatically reduces the severity of the Rb-dependent defects, implicating E2F3 in extraembryonic tissue development. Still, Rb−/−; E2f3−/− embryos die before birth, displaying a combination of defects in skeletal muscle, lungs, peripheral blood cells, and muscle fiber density in the heart (70).
Preliminary studies have been performed in an attempt to clarify the roles of specific E2Fs in tumors induced by loss of pRB. Rb+/− mice survive as adults but die from intermediate-lobe pituitary tumors, with a vast percentage of them also displaying C-cell thyroid tumors; importantly, both tumor types show loss of heterozygosity (LOH) for the wild-type allele (11, 29). Deletion of E2F1 or E2F4 in these animals suppresses both thyroid and pituitary tumor development (32, 67). Obversely, E2f3 loss accelerates the onset of thyroid tumors in Rb+/− mice and increases their incidence and metastatic potential. At the same time, however, it delays the onset of pituitary tumors (69). Thus, E2F3 appears to play a dual role as promoter and suppressor of the tumors induced by Rb loss. While these studies have begun to elucidate the role that specific E2Fs play in Rb-dependent tumorigenesis, interpretation of the results is complicated by intrinsic limitations of the models used. First, as the tumors require Rb LOH, one cannot distinguish between a direct role for E2F in the rate of Rb loss and a specific transcriptional function of E2F required for Rb-dependent tumorigenesis. Second, the pituitary and the thyroid are endocrine glands that control each other through hormonal secretion; thus, in Rb+/−; E2f3−/− mutant mice, tumor onset may result from altered hormonal signaling.
To overcome the limitations of the Rb+/−; E2f3−/− mutant mouse model, we isolated embryonic stem (ES) cell lines in which Rb and E2f3 losses are combined and generated chimeric mice. As previously reported, Rb−/− chimeras are fully viable but develop pituitary tumors as early as 3 months of age (39, 65). Here we compare the phenotypes of chimeric mutant mice in which Rb, E2f3, or both Rb and E2f3 have been inactivated. This allowed us to overcome the embryonic lethality of the E2f3−/− and Rb−/−; E2f3−/− germ line mutants and to directly compare the tumorigenicity of Rb−/−; E2f3−/− versus Rb−/− cells when loss of the wild-type Rb allele is not a rate-limiting event. Furthermore, due to its stochastic nature, this system facilitates the study of mutation in a single tissue (i.e., pituitary) or multiple ones (i.e., pituitary and thyroid), which enables the analysis of cross talk between different tissues. By this approach we show that E2f3 is dispensable for the development of Rb null thyroid and pituitary tumors. Moreover, we show that lack of E2F3 partially corrects the lens defects characteristic of Rb−/− chimeras while at the same time aggravates retinal dysplasia. Finally, we report the presence of neuroendocrine hyperplasia within the lungs of pRB-deficient chimeras. This lesion is thought to represent the preneoplastic stage for small-cell lung tumors, a tumor type that is strongly linked to Rb inactivation in both mice and humans. Strikingly, we find that E2f3 loss completely ablates these lesions, indicating that E2f3 plays a potential role in the early steps of lung tumorigenesis.
MATERIALS AND METHODS
Generation of ES cells and chimeric animals.
Mouse colonies were maintained in compliance with Institutional Animal Care and Use Committee guidelines. E2f3 and Rb mutant mice were previously described (28, 29). E2f3+/− Rb+/− 129/Sv mice were crossed to 129/Sv Rosa β-geo 26 (Rosa26) animals (24). The E2f3+/− Rb+/− Rosa26 mice so obtained were mated, and the females were sacrificed at 3.5 days postcoitum. E3.5 blastocysts were collected in M16 medium (Sigma-Aldrich) and seeded individually onto mitomycin-treated CD-1 murine embryonic fibroblasts plated on 0.1% gelatin-coated four-well dishes. PD98059 (50 μM MEK1 inhibitor; New England Biolabs) was added for the first two passages to a culture medium composed of the following: high-glucose Dulbecco's minimal essential medium (4,500 mg/liter glucose), 15% fetal bovine serum (ES-cell tested; HyClone), 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, l-glutammine, 0.1 mM β-mercaptoethanol, and leukemia inhibitory factor (1,000 U/ml; Chemicon). After an initial expansion the ES cells were genotyped and frozen at a low passage number. Chimeras were generated by injecting 10 to 12 mutant ES cells into C57Bl/6 blastocysts.
X-Gal staining.
Embryos and adult tissues were fixed in 1% formaldehyde, 0.2% glutaraldehyde, 2 mM MgCl2, 5 mM EGTA, and 0.02% NP-40 in phosphate-buffered saline (PBS) for 90 to 120 min (embryos) or 3 to 4 h (adult tissues). The samples were rinsed three times for 10 min each in PBS/0.02% NP-40 and stained overnight at 37°C in PBS containing 0.01% sodium deoxycholate, 0.02% NP-40, 2 mM MgCl2, 5 mM K3FeCN, 5 mM K4FeCN, and 1 mg/ml X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). Samples were then rinsed three times for 10 min each in PBS, fixed overnight in phosphate-buffered formalin, and processed for paraffin embedding. Five-micrometer-thick sections were stained with hematoxylin and eosin (H&E) or nuclear fast red. Alternatively, adult tissues were embedded in optimal-cutting-temperature compound and frozen on dry ice. Five-micrometer-thick sections were fixed and stained for X-Gal as described above and counterstained with nuclear fast red.
Histology and immunohistochemistry.
Tissues were processed for X-Gal staining or directly fixed in phosphate-buffered formalin. To visualize the pituitary glands, adult heads were fixed for a week in Bouin's fixative. Five-micrometer-thick sections of paraffin-embedded tissues were stained with H&E. For immunohistochemistry, sections were dehydrated, heated for 15 min in 10 mM sodium citrate (pH 6.0)-0.05% Tween 20 in a bath of boiling water to retrieve the antigens, and blocked in 10% serum at room temperature. Primary antibodies were diluted in blocking solution and incubated overnight. Rabbit antineuroendocrine cell marker calcitonin gene-related peptide (anti-CGRP; Sigma) was used at a dilution of 1:5,000, and the mouse monoclonal antibodies anti-PCNA (sc56; Santa Cruz) and antisynaptophysin (clone SY38; Chemicon) were used at dilutions of 1:2,000 and 1:500, respectively. After being washed in PBS, slides were incubated for 30 min with biotinylated secondary antibody and then incubated for 30 min with streptavidin-horseradish peroxidase (Vectastain ABC kits; Vector Laboratories). Antigen-antibody complexes were detected with diaminobenzidine.
Protein analysis.
Protein extracts were prepared with a buffer containing 50 mM Tris-Cl (pH 8), 150 mM NaCl, 0.5% NP-40, 5 mM EDTA (pH 8), and protease inhibitors, or with 1× sodium dodecyl sulfate loading buffer. Thirty to forty micrograms of proteins was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride membrane (Bio-Rad), and incubated with a mouse monoclonal antibody, LLF3#2G2, that recognizes both human and mouse E2F3a and E2F3b proteins. Alternatively, the membrane was incubated with rabbit polyclonal antibody anti-E2F1 (sc-193) or anti-E2F2 (sc-633) or with mouse monoclonal antibody anti-GAPDH for normalization. Detection was performed by chemiluminescence (ECL kit; Amersham).
RESULTS
Isolation and analysis of ES cells.
Analysis of Rb+/−; E2f3−/− mutant mice has shown that E2F3 acts as a promoter of pituitary tumors induced by Rb deficiency and as a suppressor of thyroid tumors (69). This differential modulation of tumorigenesis could arise from effects of E2F3 on the rate of Rb LOH and/or from cell-autonomous or non-cell-autonomous effects. Rb−/−; E2f3−/− animals die during embryogenesis; thus, to overcome the limitations of the Rb+/−; E2f3−/− mouse model and elucidate the role of E2f3 in the development of Rb-deficient tumors, we generated compound Rb; E2f3 mutant ES cell lines and chimeric mice. To obtain de novo marked wild-type, E2f3+/−, E2f3−/−, Rb−/−, and Rb−/−; E2f3−/− ES cells, we intercrossed 129/Sv Rb+/−; E2f3+/− mice to transgenic Rosa26 mice ubiquitously expressing the β-galactosidase gene (24). From subsequent intercrossing of the resulting animals we collected E3.5 embryos and isolated at least two independent Rosa26 marked wild-type, E2f3+/−, E2f3−/−, Rb−/−, and Rb−/−; E2f3−/− ES cell lines.
To establish the pluripotency of ES cell lines, and to determine whether they had specific defects in cell differentiation, we ectopically injected both mutant and wild-type ES cells into the flanks of nude mice (NC-Foxm1mu) and analyzed the teratomas formed after 4 to 8 weeks. In these tumors, early embryonic events occur in a random manner, giving rise to a mass containing derivatives of the three germ layers (3, 16, 43). Regardless of the genotype, no detectable differences in the size or composition of the teratomas formed were observed (data not shown). We therefore conclude that the ES cells were pluripotent and that E2f3−/−, Rb−/−, and Rb−/−; E2f3−/− mutant cells still possess an intrinsic capacity to form diverse tissues.
Contribution of mutant ES cells to embryonic and adult tissues.
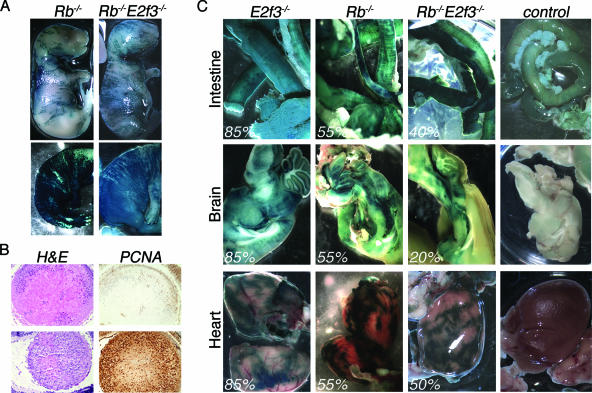
Having established that all the isolated ES cell lines were pluripotent, we analyzed their effectiveness in giving rise to diverse tissues within chimeric embryos. Two different clones for each ES cell line (mutant and wild type) were injected into E3.5 blastocysts. The resulting chimeric embryos were screened for β-galactosidase activity to determine whether, and to what extent, the ES cells participated in the formation of each tissue. With each cell line, we recovered highly chimeric embryos at multiple stages of development (i.e., E13.5 to E18.5) as evidenced by widespread β-galactosidase activity (Fig. 1A). Indeed, the contribution was so great, sometimes approaching 100%, that at E18.5 we observed sporadic lethality in Rb−/− and Rb−/−; E2f3−/− embryos, suggesting that a threshold exists, above which Rb−/− animals exhibit reduced viability. Moreover, histological analysis showed that these pRB-deficient chimeras displayed the same spectrum of defects previously observed in late-stage Rb−/− embryos (39, 65, 66), including disorganized muscle fibers and hyperproliferation of the lens (Fig. 1B and data not shown).
FIG. 1.
Mutant Rb−/− and Rb−/−; E2f3−/− ES cells contribute efficiently to embryonic and adult tissues. (A) Examples of low (top) and high (bottom) degrees of chimerism in E18.5 Rb−/− and Rb−/−; E2f3−/− embryos screened for β-galactosidase activity (blue). Note that the highly chimeric Rb−/−; E2f3−/− embryo (bottom right) was found dead. (B) H&E and α-PCNA staining on sections of Rb−/− chimeric embryos with different degrees of chimerism, showing only patched (top, low chimerism) or very extended (bottom, high chimerism) hyperproliferation of the lens. Magnification, ×40. (C) β-Galactosidase staining shows that E2f3−/−, Rb−/−, and Rb−/−; E2f3−/− mutant ES cells all contribute to numerous adult tissues such as intestines, brain, and heart. β-Galactosidase staining of nonchimeric tissues is included for comparison. The percentages indicate the degree of chimerism as judged by coat color.
To analyze the interaction between Rb and E2F3 in adult mice, we generated a cohort of E2f3−/−, Rb−/−, and Rb−/−; E2f3−/− chimeric animals by injecting two different ES cell lines per genotype. We aged these animals together with their nonchimeric littermates and E2f3+/− chimeras. Consistent with what we observed in embryos, all ES cell lines generated chimeric animals that we scored for the degree of chimerism based on the agouti coat color. We obtained completely agouti chimeric E2f3−/− mice, suggesting that lack of E2F3 in chimeras allows full viability in adulthood. On the contrary, the highest degree of chimerism in the Rb−/− chimeric animals was 80% and was represented by 9 out of 18 animals. In the compound mutants the highest contribution was 50% and was found in 13 out of 44 animals, with the exception of one mouse that scored 80% for chimerism (Fig. 2 and data not shown). This was seen with two independent ES cell lines, suggesting that the combined loss of E2f3 and Rb does affect the viability of adult animals more than the loss of Rb alone. To confirm that the coat color mirrored the degree of chimerism in the other tissues of the animal, we assayed the contribution of the mutant ES cells to various organs. In the case of the blood, spleen, and thymus the staining was either nonquantifiable or unsuccessful, preventing the drawing of any conclusions about contribution. For most other tissues, such as brain, lung, liver, heart, intestine, skin, and kidneys, we found that regardless of the ES cell genotype, the amount of β-galactosidase staining correlated well with the percentage of agouti color in the coat (Fig. 1C and data not shown). The one exception to this was muscle. Although the Rb−/− and Rb−/−; E2f3−/− cells contributed to the muscles of the E18.5 embryos, to the extent that the muscle fibers appeared highly disorganized, we saw no evidence that these cells contributed to the muscles of adult chimeras. Taken together, these data suggest that there is negative selection against both Rb−/− and Rb−/−; E2f3−/− cells in the muscle, but not most other tissues and organs, of the adult animals.
FIG. 2.
Relationships between percentage of chimerism, time of death, and tumor type found in Rb−/−; E2f3−/− and Rb−/− chimeric animals. The onset of thyroid tumors does not strictly correlate with the age of the animal and/or the percentage of chimerism. Each Rb−/−; E2f3−/− (top) and Rb−/− (bottom) chimeric animal, ordered by time of death (gray line) from youngest to oldest, is represented by a bar reporting the percent chimerism. Animals presenting pituitary or thyroid tumors are indicated with P or T, respectively. In all the cases reported the pituitary tumors represented the primary cause of death.
E2F3 is dispensable for Rb loss-dependent tumorigenesis in both the pituitary and the thyroid.
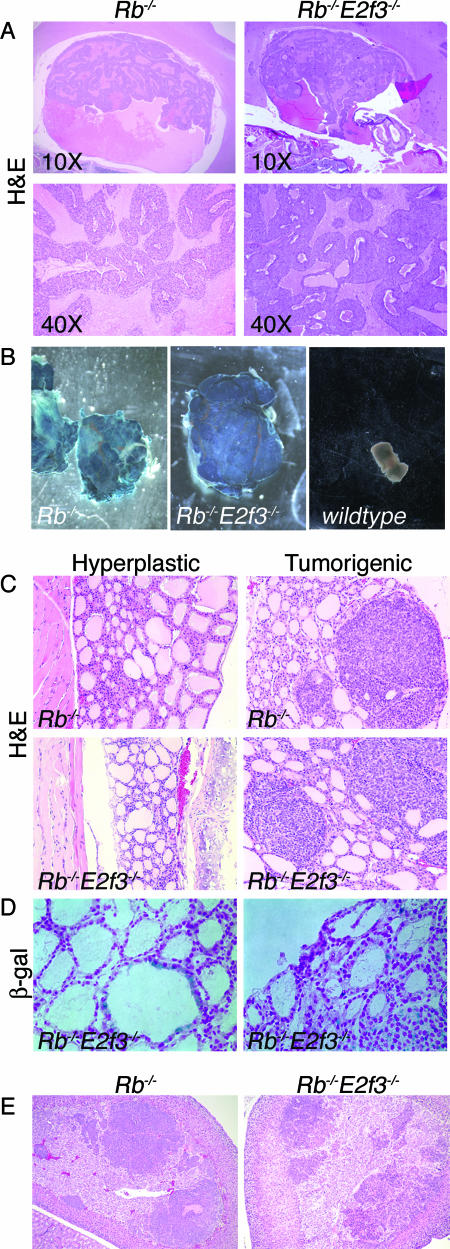
E2f3 loss has a complex effect on the tumor phenotype of Rb+/− mice. It delays and reduces the formation of pituitary tumors but simultaneously promotes the development and metastasis of medullary thyroid carcinomas and additional tumor lesions (69). We aged our cohort of chimeric animals and examined them for the presence of any of these tumors. E2f3+/− and E2f3−/− chimeras lived as long as their nonchimeric controls and did not develop any tumors. Interestingly, although germ line E2f3−/− animals die from heart failure, no signs of heart defects were detected even in high-percentage E2f3−/− chimeras that were sacrificed for histological analysis. The chimeras generated with Rb−/− ES cells started to die at 3 months of age due to pituitary tumors (69%, 9 out of 13 animals) (Table 1 and Fig. 2), confirming previous reports (39, 65). In a similar manner, the Rb−/−; E2f3−/− chimeras started to perish around 3 months of age, and by 9 months the majority of them had died, indicating that loss of E2f3 does not modify the life span of Rb-deficient animals. Consistent with this observation, we found that pituitary tumors were the primary cause of death in the double-mutant chimeras. The majority of Rb−/−; E2f3−/− mutant animals developed pituitary tumors (67%, 21 out of 31 animals) (Table 1) as early as 2 to 3 months. Even animals with as little as 5% chimerism showed this propensity to early-onset pituitary tumors. Histological analysis indicated that the tumors were indistinguishable with regard to both size and morphology from the tumors arising in the Rb−/− chimeras. They had the characteristics of adenocarcinomas and derived from the pars intermedia of the pituitary (Fig. 3A). Importantly, the tumors only derived from Rb−/−; E2f3−/− mutant cells, as they were all found positive for β-galactosidase staining (Fig. 3B). Thus, in the context of these chimeric mutant animals, E2f3 status has no detectable effect on the development of pRB-deficient pituitary tumors. This is in clear contrast to the dramatic suppression of pituitary tumors that is seen in the germ line Rb+/−; E2f3−/− mice (69).
TABLE 1.
Frequency of tumorigenic and developmental phenotypes in Rb−/− versus Rb−/−; E2f3−/− chimeric mice
| Phenotype | % Rb−/− (no. of animals) | % Rb−/−; E2f3−/− (no. of animals) |
|---|---|---|
| Pituitary tumors | 69 (13) | 67 (31) |
| Thyroid tumors | 31 (13) | 23 (31) |
| C-cell hyperplasia | 31 (13) | 26 (31) |
| Adrenal gland lesions | 58 (12) | 57 (30) |
| Neuroendocrine cell hyperplasia | 77 (13) | 0 (26) |
| Cataracts | 75 (12) | 35 (20) |
| Retinal defects | 42 (12) | 75 (20) |
FIG. 3.
E2f3 does not modify the predisposition of Rb−/− chimeras to develop pituitary, thyroid, or adrenal gland tumors. (A) Histological analysis of Rb−/− and Rb−/−; E2f3−/− pituitary tumors shows that these tumors arise from the intermediate lobe of the pituitary and that they have the properties of adenocarcinomas. (B). β-Galactosidase staining (blue) is detectable only in pituitary tumors derived from Rb−/− and Rb−/−; E2f3−/− animals and not in normal, nonchimeric pituitary, indicating that these tumors are derived specifically from mutant cells. (C) H&E staining on sections of Rb−/− and Rb−/−; E2f3−/− chimeric thyroids shows that similar lesions, either C-cell hyperplasias or carcinomas, arise in both genotypes. (D) Frozen sections of normal and tumorigenic thyroids of Rb−/−; E2f3−/− chimeric mice are positive for β-galactosidase activity (blue dots; nuclear fast red counterstaining), indicating that the mutant cells do contribute to the C-cell compartment. (E) H&E staining of chimeric adrenal glands shows that similar hyperplastic multifoci are present in both Rb−/− and Rb−/−; E2f3−/− mutant mice. (C, D, and E) Magnification, ×40.
We also screened for the presence of thyroid tumors within our chimeric mutant animals. In the Rb−/− chimeras, we observed a relatively low frequency of either neoplastic thyroid lesions (31%; 4 out of 13 animals (Table 1 and Fig. 2) or C-cell hyperplasia (31%, 4 out of 13 animals) (Table 1). Notably, E2f3 did not alter the extent or frequency of neoplastic thyroid lesions or of C-cell hyperplasia (23%, 7 out of 31 animals; or 26%, 8 out of 31 animals, respectively) (Table 1 and Fig. 2 and 3C) and there was also no evidence of metastases in the lung and/or liver of these animals. Curiously, the presence and nature of the lesions were not dependent on the percentage of agouti color in the coat or the age of the animals, although we did not find thyroid tumors in animals younger than 5 months (Fig. 2). To verify that the low frequency of thyroid tumors was not simply due to an impaired ability of the Rb−/−; E2f3−/− ES cells to contribute to the C cells of the thyroid, we assayed for the presence of sections of normal and hyperplastic thyroid with β-galactosidase activity from Rb−/−; E2f3−/− chimeras. We found that Rb−/−; E2f3−/− ES cells accounted for the tumorigenic C cells and they also made significant contributions to the normal thyroid tissue (Fig. 3D). Thus, we can exclude the possibility that the lack of aggressive thyroid tumors in the Rb−/−; E2f3−/− chimeras reflects a poor ability of Rb−/−; E2f3−/− cells to contribute or survive in this compartment. Thus, in a manner similar to that of the pituitary tumor phenotype, we conclude that E2f3 status does not influence the development of thyroid tumors in Rb−/− chimeras, despite its enhancing effect upon the development of tumors in the Rb+/− germ line mouse model.
Multifoci resembling pheochromocytomas were also detected at the same frequency in the adrenal medulla of the Rb−/− and Rb−/−; E2f3−/− chimeras (58% versus 57%) (Table 1). These lesions were either unilateral or bilateral and always positive for β-galactosidase activity, indicating that they originated from mutant cells (Fig. 3E and data not shown). They were never present in the E2f3−/− or E2f3+/− chimeras. From this observation, we conclude that E2F3 is also not required for the onset of this tumor type in Rb−/− chimeras.
A new role for E2F3 in lung tumorigenesis.
The ability to generate adult tissues simultaneously lacking Rb and E2f3 gave us the opportunity to search for new tumor types that might be modulated by these proteins. We performed necropsy and whole histology on 26 Rb−/−; E2f3−/− and 13 Rb−/− chimeras and found a striking difference in their lungs. We observed that 10 out of 13 Rb−/− chimeras showed hyperplastic lesions in the lung; numerous small groups of epithelial cells (typically between 5 and 20 per section), whose morphology closely resembled neuroendocrine cells, were facing the lumen of the bronchi and bronchioli in these animals (Fig. 4A, left panels). Neuroendocrine cell hyperplasia has not previously been reported in Rb−/− chimeras, but it is well documented in other Rb mutant models (17, 64). Thus, we performed immunohistochemical analyses on Rb−/− chimeric lungs with the neuroendocrine cell markers calcitonin-related peptide, CGRP, and synaptophysin and confirmed that the hyperplastic foci are of neuroendocrine origin (Fig. 4A, left panels). Moreover, we established that these lesions derive from Rb−/− mutant cells, as we found that they colocalized with blue staining in sections of lungs assayed for β-galactosidase activity (Fig. 4A, middle panels). Interestingly, in these lungs we also found nonhyperplastic mutant neuroendocrine cells, indicating that loss of Rb alone may need additional events to lead to hyperplasia (data not shown). We then conducted a thorough screen for this defect in the Rb−/−; E2f3−/− chimeric animals. Remarkably, there was no evidence of hyperplastic lesions in the lungs of any of the 26 Rb−/−; E2f3−/− chimeric animals (Table 1). Importantly, we readily detected β-galactosidase activity in Rb−/−; E2f3−/− chimeric lungs, confirming the presence of mutant cells in this organ (Fig. 4A, right panels). However, since neuroendocrine cells represent only a small population in the lung epithelium, we could not exclude that lack of hyperplasia in Rb−/−; E2f3−/− lungs resulted from the absence of this specific cell type. To address this issue, we also performed immunohistochemical analyses with anti-CGRP and antisynaptophysin antibodies on Rb−/−; E2f3−/− chimeric lungs and found cells positive for activity of the markers CGRP, synaptophysin, and β-galactosidase, confirming the presence of Rb−/−; E2f3−/− mutant neuroendocrine cells (Fig. 4A). Thus, we conclude that E2f3 inactivation is sufficient to completely suppress the hyperproliferation of pRB-deficient neuroendocrine cells.
FIG. 4.
In Rb−/− chimeric mice E2f3 loss completely suppresses the pulmonary neuroendocrine hyperplasia and also plays a dual role in the eyes: inhibition of the cataracts and aggravation of the retinal defects. (A) (Left and middle panels) H&E staining on section of Rb−/− chimeric lung shows hyperplastic (darker) epithelial cells facing the lumen of the bronchi (right) and bronchioli (left). Immunohistochemistry with α-CGRP and α-synaptophysin antibodies on sections of unstained (left) or stained (right) Rb−/− chimeric lungs shows that the hyperplastic foci are of neuroendocrine origin. (Right panels) Mutant Rb−/−; E2f3−/−, β-galactosidase-positive cells costain with the markers CGRP and synaptophysin, indicating that the absence of hyperplastic foci does not derive from the inability of mutant cells to contribute to the neuroendocrine compartment. (B) H&E staining of normal, Rb−/−, and Rb−/−; E2f3−/− chimeric eyes shows a cataract in the Rb−/− but not the Rb−/−; E2f3−/− lens. Magnification, ×4. In the lower panel a comparison of normal, Rb−/−, and Rb−/−; E2f3−/− chimeric eyes shows thinning of the outer layer (O) in the Rb−/− chimeric retinas and aggravation of this retinal defect in Rb−/−; E2f3−/− animals where the outer layer is partially missing and the inner cell layer (i) is thinner. Magnification, ×40.
Role of E2F3 in the Rb−/− eye.
The examination of the eyes of Rb−/− versus Rb−/−; E2f3−/− chimeras also revealed differences in defects of both the lens and the retina. The adult Rb−/− chimeras have previously been shown to have cataracts (39, 65). We found this defect in 75% (9 out of 12) of the Rb−/− animals, as opposed to only 35% (7 out of 20) of the Rb−/−; E2f3−/− animals, indicating that E2F3 participates in forming the cataracts induced by loss of Rb (Fig. 4B, upper panels). An opposite phenomenon was observed in the retinas of the Rb−/− and Rb−/−; E2f3−/− chimeras. The Rb−/− chimeric retinas appear to have local dysplasia and thinning of the outer layer (39, 65). We confirmed this phenotype in about 42% (5 out of 12) of the Rb−/− animals, but we found it at a much higher percentage (75%; 15 out of 20 animals) in the Rb−/−; E2f3−/− chimeras, indicating that E2F3 acts to restrain the dysplasia of Rb-deficient retinal cells (Fig. 4B and Table 1). Moreover, in Rb−/−; E2f3−/− chimeras, we observed aggravation of the retinal defect to the point that not only the outer layer, but also the inner cell layer, was missing (Fig. 4B). To understand whether abnormal proliferation and/or apoptosis was the underlying cause of these defects, we analyzed mutant Rb−/− and Rb−/−; E2f3−/− chimeric retinas, respectively, for the proliferation marker Ki67 and by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling assay but were unable to detect any aberrant cell division or apoptotic figure (data not shown). This is actually consistent with the reported observation that the retinal morphogenetic processes that induce defects in the Rb−/− chimeric eyes take place at the perinatal stage (40, 61). Remarkably, the lesions found in Rb−/−; E2f3−/− chimeras never progressed to retinoblastoma, indicating that while E2F3 is required for the morphogenesis of the normal eye, its loss is not sufficient to induce tumors in Rb−/− retinas.
Tissue-specific expression of E2F3a and E2F3b.
Our data show that E2F3 loss causes suppression of some Rb mutant phenotypes (lung hyperplasia) while it enhances others (retinal dysplasia). It is well established that E2f3 encodes two proteins, E2F3a and E2F3b, that are thought to have opposing biological activities (1, 4, 36). This raises the possibility that the differential effects on the Rb mutant phenotypes could reflect the action of these different proteins. To address this possibility, we examined the expression pattern of E2F3 in various tissues. Unfortunately, we could not conduct this analysis on the neuroendocrine cells because even when hyperplastic they represent such a small proportion of the lung tissue. We had similar problems with the lens, which yielded insufficient protein to allow E2F3 analysis. However, we were able to harvest pituitaries, thyroids, and retinas from wild-type animals and examine the expression of the E2F3 protein. For these experiments, we have used a monoclonal antibody previously generated in the lab that recognizes sequences that are present in both E2F3a and E2F3b. Strikingly, this analysis revealed strong tissue-specific differences in the expression of the two E2F3 isoforms: in the normal pituitary and thyroid tissue we detected only E2F3a, while the retina appeared to specifically express E2F3b (Fig. 5A). pRB-deficient pituitary and thyroid tumors arise from specific compartments—the intermediate lobe and the C cells, respectively—and thus these cell types represent the majority of the tumor tissue. We performed a Western blot analysis on pituitary and thyroid tumors excised from germ line Rb+/− and Rb+/−; E2f3+/− mutant animals and confirmed that they also specifically expressed E2F3a but not E2F3b (Fig. 5B). Since E2F1, -2, and -3a share a similar ability to activate E2F-responsive genes, it is possible that the development of pituitary and thyroid tumors in the Rb−/−; E2f3−/− chimeras reflects an ability of E2F1 and -2 to compensate for E2F3 loss. To explore this possibility, we analyzed the expression of E2F1 and E2F2 in pituitary tumors derived from Rb−/− and Rb−/−; E2f3−/− chimeras and in normal, nonchimeric pituitary. We found that although E2F1 was highly expressed in these tumors compared to the normal pituitary, its levels were not significantly different between Rb−/− and Rb−/−; E2f3−/− chimeric tissues (Fig. 5C). Moreover, when we conducted the same analysis on wild-type and tumorigenic pituitaries and thyroids from germ line Rb+/− and Rb+/−; E2f3−/− mutant animals, we found a similar result. E2F1 and E2F2 were both overexpressed in the tumors compared to wild-type tissues, independently of the genotype (Fig. 5D). These observations do not rule out the compensation model, but they make it seem less likely.
FIG. 5.
E2F3a and E2F3b are expressed differentially in the retina (Ret) versus the pituitary (Pit) and thyroid (Thy), and E2f3 loss does not significantly alter E2F1 and E2F2 levels in Rb-deficient tumors. (A) Western blot analysis performed with α-E2F3 antibodies on normal pituitary and thyroid shows that E2F3a is specifically expressed in these tissues, while in the retina only E2F3b is present. (B) E2F3a is also specifically detected in Rb+/− and Rb+/−; E2f3+/− tumorigenic (T-) thyroids and pituitaries that are enriched for C cells or intermediate lobe cells, respectively. (C) Comparison of expression levels of E2F1 and E2F2 in normal, nonchimeric pituitary and pituitary tumors from Rb−/− and Rb−/−; E2f3−/− chimeric mice shows upregulation of E2F1 exclusively in the tumor. (D) Both E2F1 and E2F2 are highly expressed in tumorigenic pituitary and thyroid of Rb+/− and Rb+/−; E2f3−/− germ line mice but not in normal wild-type tissue.
DISCUSSION
pRB's central role in tumor development stems, at least in part, from its capacity to control cell division through interaction with the E2F proteins. In these studies, we use Rb−/−; E2f3−/− chimeric mice as a model system to dissect the interplay between Rb and E2f3 in vivo. This approach has allowed us to bypass the lethality of Rb−/−; E2f3−/− germ line mutant mice and the requirement for Rb LOH in tumors arising in Rb+/−; E2f3−/− germ line mice. Our findings yield insight into how E2f3 contributes to both normal development and the aberrant consequences of pRB deficiency.
E2f3−/− chimeric mice are fully viable.
Through the analysis of germ line E2f3−/− mice, we previously showed that E2f3 is essential for full viability (28). This reflects a key role for E2f3 in cardiac development and function (28; Cloud-King et al., submitted). In the pure 129/Sv background, germ line E2f3−/− mice die in utero or perinatally with hypoplastic ventricular walls and/or severe septal defects. In the mixed C57BL6 ×129/Sv genetic background, a small fraction of the E2f3−/− animals survive to adulthood but die prematurely of congestive heart failure. In the present study, we have used two independent 129/Sv E2f3−/− ES cell lines to generate chimeric mutant mice. Remarkably, although we see near-complete contribution of the cells, as judged by analysis of their lacZ marker, these chimeras are fully viable and show no signs of heart defects. We cannot rule out the possibility that the presence of a very low level of wild-type cells is sufficient to suppress cardiac defects within these highly chimeric animals. However, we favor the notion that the differential phenotypes of the germ line and chimeric E2f3−/− mice reflect an essential role for E2f3 in placental development. This hypothesis is based on several observations. First, E2f3 inactivation suppresses defects within the Rb−/− embryos, including midgestational lethality, which have been shown to arise as a secondary consequence of the inappropriate expansion of the labyrinth layer of the Rb−/− placenta (66, 70). Second, there is evidence that cardiac defects often arise as a secondary consequence of placental insufficiency (2, 8). Finally, during the generation of chimeras, ES cell contribution is largely restricted to the embryo proper, and these cells rarely participate in the trophoblastic component of extraembryonic tissues (50). Combining these and our own observations, we speculate that E2f3 is required for the appropriate development of placental tissues and this contributes to the cardiac defects and reduced viability that are specifically observed in the germ line, but not the chimeric, E2f3−/− mice.
E2F3 role in Rb tumorigenesis.
Our primary goal in conducting these chimeric studies was to better understand the interplay between E2f3 and Rb in tumorigenesis. Through the analysis of germ line Rb+/−; E2f3−/− mutant mice, we have previously shown that E2f3 inactivation differentially modulates the spectrum of the Rb-deficient tumors by simultaneously suppressing pituitary tumors and promoting thyroid tumors (69). These differential effects could arise through a variety of mechanisms. At one extreme, this could be cell autonomous. For example, E2f3 deficiency could have a differential effect on either the rate of Rb LOH in pituitary versus thyroid cells or the viability of the resulting Rb−/−; E2f3−/− cells. It could also reflect distinct biological roles for the E2F3 isoform(s) expressed in the pituitary versus the thyroid. At the other extreme, non-cell-autonomous models have been proposed, including the possibility that suppression of the pituitary tumor phenotype could promote the development of thyroid tumors by altering hormonal signaling (68). We generated Rb−/−; E2f3−/− chimeric mice in order to distinguish between these models. This approach avoided the embryonic lethality of the Rb; E2f3 compound mutants and eliminated the requirement for LOH in the germ line Rb+/−; E2f3−/− mice. Remarkably, in complete contrast to the germ line Rb+/−; E2f3−/− model, E2f3 status had no effect on either the onset or nature of pituitary and thyroid Rb-null tumors in these chimeric mutant mice.
The fundamental difference between the chimeric and germ line models is that the former contains cells that have been Rb deficient through development while the latter contains cells that are initially Rb+/− and are dependent upon LOH for tumor development. This suggests two possible explanations for the different effects of E2f3 in the two models. First, we hypothesized that, in the chimeric model, the sustained absence of pRB could induce compensatory changes during embryogenesis that somehow override E2f3's contribution to the development of pituitary tumors and suppression of thyroid tumors in the Rb+/−; E2f3−/− mice. Specifically, we envisaged that they might be compensatory changes in the levels of the other activating E2Fs. However, while we did observe a dramatic upregulation of E2F1 in Rb−/−; E2f3−/− chimeric pituitary tumors, this was comparable to the upregulation of E2F1 that occurred in Rb−/− chimeric pituitary tumors. Moreover, we observed a similar effect in the pituitary and thyroid tumors derived from both Rb+/−; E2f3−/− and Rb+/− germ line mice. Thus, the induction of E2F1 correlates with tumorigenicity, consistent with the known activation of E2f1 in proliferating cells, and not E2f3 status. Clearly, these observations do not rule the compensation model but they make it seem less likely. The second hypothesis to explain the differential phenotypes of the germ line and chimeric mutant mice is that E2f3 status influences the rate of LOH. Previous studies have shown that Rb LOH is the rate-limiting step for development of pituitary but not thyroid tumors; these clearly require additional mutational events (39, 48, 65). Notably, E2f3 inactivation has been reported to disrupt centrosome regulation and therefore ploidy (51). Thus, perhaps E2f3 inactivation promotes genetic instability in a manner that facilitates both the LOH and genetic changes that could account for the observed acceleration of thyroid tumors in the Rb+/−; E2f3−/− mice.
Our analysis of chimeric E2f3; Rb mutant mice has allowed to address some of the potential hypotheses for E2f3's role in the development of germ line tumors outlined previously. First, Rb−/−; E2f3−/− cells contribute at high frequency to the pituitary, excluding the hypothesis in which E2f3 inactivation suppresses the formation of pituitary tumors in germ line Rb+/− mice because Rb−/−; E2f3−/− pituitary cells are inviable. Second, it seems highly unlikely that aggravation of thyroid tumors in germ line Rb+/−; E2f3−/− mice is caused by loss of the repressor E2F3b, since the adult thyroid specifically expresses E2F3a and not E2F3b. Finally, we find that a fraction of the Rb−/−; E2f3−/− chimeras have late-onset pituitary tumors, but they do not develop aggressive thyroid tumors despite the clear presence of compound mutant C cells. This argues against the hypothesis that acceleration of thyroid tumor development in germ line Rb+/−; E2f3−/− mutant mice results indirectly from the suppression of pituitary tumors and a consequent change in hormonal signaling.
Functions of E2F3 in the eye.
The loss of pRB is known to disrupt development of two distinct regions of the eye, the retina and the lens, and our analysis of the Rb−/−; E2f3−/− chimeras highlights a role for E2f3 in both. In the case of the lens, it has previously been shown that Rb−/− chimeras develop cataracts that are defined by the posterior migration of epithelial cells and by disorganized lens fibers (39, 65). Lens development is known to require a series of coordinated processes that include proliferation and exit from the cell cycle followed by migration and differentiation (23). Notably, pRB loss is known to induce ectopic proliferation in the developing lens, and this is suppressed by the inactivation of either E2f1 or E2f3 (52, 60, 70). However, careful examination showed that the Rb−/−; E2f1−/− lens still displays disorganized fibers and vacuoles that reflect the deregulated expression of lens-specific crystallin and filensin genes associated with the differentiation process (37). This raised the possibility that pRB has two roles in lens development; it is required for cells to exit the cell cycle, at least in part via suppression of the E2Fs, and might also contribute more directly to the differentiation process. Because the Rb−/−; E2f1−/− and Rb−/−; E2f3−/− embryos are inviable, it was impossible to assess whether the E2f genes, and therefore ectopic proliferation, contribute to the formation of cataracts. As previously reported, we detected cataracts in a large proportion of our Rb−/− chimeras. The incidence of cataracts was greatly reduced (from 82% to 37%) in the Rb−/−; E2f3−/− chimeras. Thus, we conclude that the development of pRB-deficient cataracts results, at least in part, from the inappropriate action of E2F3. Since E2f3 contributes to both ectopic proliferation in the developing lens and cataract development, it is tempting to speculate that it could represent the link between these two processes. However, we cannot rule out the possibility that E2F3 acts downstream of pRB in both the proliferation and differentiation processes.
We also found that E2F3 deficiency modulated the retinal defects of Rb−/− chimeras. However, in contrast to the cataract phenotype, it actually enhanced the defect. Prior analysis of both chimeras and tissue-specific knockout animals has shown that Rb loss causes thinning of the photoreceptor layer, while in order for retinoblastomas to develop, simultaneous loss of p107 or p130 needs to occur (17, 39, 40, 65). Although the Rb−/−; E2f3−/− chimeras did not develop retinoblastomas, the Rb mutant retinal defect was aggravated to the point that not only the outer layer but also the inner cell layer was missing, and the degree of dysplasia was greatly increased. The increased severity of the retinal lesions shows that E2F3 is acting as a repressor in those tissues. Notably, our expression studies specifically detected E2F3b, and not E2F3a, in the retinal tissue. E2F3b is believed to be a repressor of classic E2F-responsive genes, although this has not been directly demonstrated, and it also contributes to the transcriptional repression of the Arf tumor suppressor (1, 4, 36). Our data provide evidence that E2F3b acts a repressor in vivo, indicating that it is required for the morphogenesis but not tumorigenesis of the retina. In this case, it is likely that this E2F3b function is distinct from its role in the regulation of the ARF pathway, since the Rb mutant retinal defect is unaffected by either Arf or p53 mutation (40, 61).
Requirements for E2F3 in lung tumorigenesis.
Our analysis of the single and compound Rb; E2f3 chimeric mice also showed that E2f3 loss completely suppresses the onset of neuroendocrine hyperplasia in the lungs of Rb−/− chimeric mice. These lung lesions have been studied in several single and compound Rb mutant mouse models, including Rb; p107 and Rb; p130 chimeric mice, although they have not been previously reported in Rb−/− chimeras (17, 44, 63, 64). Lung-specific Rb inactivation during development causes neuroendocrine cells, and not other pulmonary cells, to proliferate abnormally, indicating that Rb plays a critical role in this cell type (63). Importantly, in conditional RbF19/F19; Trp53F2-10/F2-10 mice, hyperplastic pulmonary neuroendocrine cells correlate with the appearance of aggressive tumors (44). In this model, where p53 and Rb are specifically inactivated in the adult lung, mice developed metastatic tumors resembling small-cell lung carcinoma (murine SCLC)-like tumors in addition to non-small-cell lung carcinomas (murine NSCLC). Rb loss is strictly required only for the development of neuroendocrine-derived tumors and SCLC, not for the NSCLC in this setting, suggesting that Rb loss plays a key role in the transformation of neuroendocrine cells. Although we observe neuroendocrine lung hyperplasia in a significant fraction of Rb−/− chimeric mice, this is completely absent in the Rb−/−; E2f3−/− chimeric mice despite the presence of mutant neuroendocrine cells within the lungs. This suggests that E2F3 plays a role in the early stages of murine SCLC.
Loss of E2F3 could abolish the hyperproliferation of the pulmonary neuroendocrine cells through a variety of mechanisms. E2F3a induces cell division through its role as a transcriptional activator of classic E2F-responsive genes, and thus its loss could counterbalance the effects of Rb deficiency. Alternatively, since p53 loss has been shown to promote the hyperplastic stage of SCLC (64), and E2F3 deficiency induces p53 expression (4), E2F3 loss could suppress the hyperplasia of the pulmonary neuroendocrine cells through upregulation of p53. Clearly, we would like to establish which form(s) of E2F3 is expressed in the neuroendocrine lung compartment, something that is beyond current technical capabilities due to the lack of E2F3 antibodies that work in immunohistochemistry. In addition, while our studies uncover a potential role for E2f3 in the early stages of SCLC, they do not rule out the possibility that E2F3 is also required at later stages of tumor development, such as maintenance or metastasis.
Lung tumors represent the most common cancer-related cause of death in the United States, and SCLCs account for about 20% of them (71). Importantly, a recent report demonstrates the presence of high levels of nuclear E2F3 in 15 out of 16 samples of human SCLC (15). Moreover, this study shows that nuclear E2F3 is also elevated in nonneuroendocrine lung tumors, such as adenocarcinomas (79%) and squamous cell carcinomas (55%). By Western blot analysis, we have found that total lung lysates contain the E2F3a but not the E2f3b isoform (T. Parisi and J. A. Lees, unpublished observations), consistent with a role of E2F3a as a promoter of proliferation (not shown). This raises the possibility that E2f3 may play a more general role in lung tumorigenesis, affecting tumors that are of both neuroendocrine and nonneuroendocrine origin. Although additional studies need to be done to assess the role of E2f3 in lung tumorigenesis, it is tempting to speculate that E2F3 may represent a good therapeutic target for SCLC and, quite possibly, a much broader spectrum of lung tumors.
Acknowledgments
We are grateful to the CCR Transgenic Facility and in particular to John M. Mkandawire and Peimin Qu for technical help. We are also thankful to C. Bender Kim for reagents and suggestions and to S. R. Frank, J. Shepard, A. Amsterdam, C. Sansam, P. Danielian, and L. Friesenhahn for critical reading of the manuscript and helpful discussion.
This project was supported by grants to J.A.L. from the National Cancer Institute (GM53204 and CA121921. J.A.L. is a Ludwig Scholar.
Footnotes
Published ahead of print on 8 January 2007.
REFERENCES
- 1.Adams, M. R., R. Sears, F. Nuckolls, G. Leone, and J. R. Nevins. 2000. Complex transcriptional regulatory mechanisms control expression of the E2F3 locus. Mol. Cell. Biol. 20:3633-3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams, R. H., A. Porras, G. Alonso, M. Jones, K. Vintersten, S. Panelli, A. Valladares, L. Perez, R. Klein, and A. R. Nebreda. 2000. Essential role of p38alpha MAP kinase in placental but not embryonic cardiovascular development. Mol. Cell 6:109-116. [PubMed] [Google Scholar]
- 3.Andrews, P. W. 2002. From teratocarcinomas to embryonic stem cells. Philos. Trans. R. Soc. Lond B Biol. Sci. 357:405-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aslanian, A., P. J. Iaquinta, R. Verona, and J. A. Lees. 2004. Repression of the Arf tumor suppressor by E2F3 is required for normal cell cycle kinetics. Genes Dev. 18:1413-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Attwooll, C., E. Lazzerini Denchi, and K. Helin. 2004. The E2F family: specific functions and overlapping interests. EMBO J. 23:4709-4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attwooll, C., S. Oddi, P. Cartwright, E. Prosperini, K. Agger, P. Steensgaard, C. Wagener, C. Sardet, M. C. Moroni, and K. Helin. 2005. A novel repressive E2F6 complex containing the polycomb group protein, EPC1, that interacts with EZH2 in a proliferation-specific manner. J. Biol. Chem. 280:1199-1208. [DOI] [PubMed] [Google Scholar]
- 7.Balciunaite, E., A. Spektor, N. H. Lents, H. Cam, H. Te Riele, A. Scime, M. A. Rudnicki, R. Young, and B. D. Dynlacht. 2005. Pocket protein complexes are recruited to distinct targets in quiescent and proliferating cells. Mol. Cell. Biol. 25:8166-8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barak, Y., M. C. Nelson, E. S. Ong, Y. Z. Jones, P. Ruiz-Lozano, K. R. Chien, A. Koder, and R. M. Evans. 1999. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol. Cell 4:585-595. [DOI] [PubMed] [Google Scholar]
- 9.Reference deleted.
- 10.Christensen, J., P. Cloos, U. Toftegaard, D. Klinkenberg, A. P. Bracken, E. Trinh, M. Heeran, L. Di Stefano, and K. Helin. 2005. Characterization of E2F8, a novel E2F-like cell-cycle regulated repressor of E2F-activated transcription. Nucleic Acids Res. 33:5458-5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clarke, A. R., E. R. Maandag, M. van Roon, N. M. van der Lugt, M. van der Valk, M. L. Hooper, A. Berns, and H. te Riele. 1992. Requirement for a functional Rb-1 gene in murine development. Nature 359:328-330. [DOI] [PubMed] [Google Scholar]
- 12.Classon, M., and N. Dyson. 2001. p107 and p130: versatile proteins with interesting pockets. Exp. Cell Res. 264:135-147. [DOI] [PubMed] [Google Scholar]
- 13.Cloud, J. E., C. Rogers, T. L. Reza, U. Ziebold, J. R. Stone, M. H. Picard, A. M. Caron, R. T. Bronson, and J. A. Lees. 2002. Mutant mouse models reveal the relative roles of E2F1 and E2F3 in vivo. Mol. Cell. Biol. 22:2663-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reference deleted.
- 15.Cooper, C. S., A. G. Nicholson, C. Foster, A. Dodson, S. Edwards, A. Fletcher, T. Roe, J. Clark, A. Joshi, A. Norman, A. Feber, D. Lin, Y. Gao, J. Shipley, and S. J. Cheng. 2006. Nuclear overexpression of the E2F3 transcription factor in human lung cancer. Lung Cancer 54:155-162. [DOI] [PubMed] [Google Scholar]
- 16.Damjanov, I. 1993. Teratocarcinoma: neoplastic lessons about normal embryogenesis. Int. J. Dev. Biol. 37:39-46. [PubMed] [Google Scholar]
- 17.Dannenberg, J. H., L. Schuijff, M. Dekker, M. van der Valk, and H. te Riele. 2004. Tissue-specific tumor suppressor activity of retinoblastoma gene homologs p107 and p130. Genes Dev. 18:2952-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Bruin, A., L. Wu, H. I. Saavedra, P. Wilson, Y. Yang, T. J. Rosol, M. Weinstein, M. L. Robinson, and G. Leone. 2003. Rb function in extraembryonic lineages suppresses apoptosis in the CNS of Rb-deficient mice. Proc. Natl. Acad. Sci. USA 100:6546-6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeGregori, J., G. Leone, A. Miron, L. Jakoi, and J. R. Nevins. 1997. Distinct roles for E2F proteins in cell growth control and apoptosis. Proc. Natl. Acad. Sci. USA 94:7245-7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimova, D. K., O. Stevaux, M. V. Frolov, and N. J. Dyson. 2003. Cell cycle-dependent and cell cycle-independent control of transcription by the Drosophila E2F/RB pathway. Genes Dev. 17:2308-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Stefano, L., M. R. Jensen, and K. Helin. 2003. E2F7, a novel E2F featuring DP-independent repression of a subset of E2F-regulated genes. EMBO J. 22:6289-6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dyson, N. 1998. The regulation of E2F by pRB-family proteins. Genes Dev. 12:2245-2262. [DOI] [PubMed] [Google Scholar]
- 23.Francis, P. J., V. Berry, A. T. Moore, and S. Bhattacharya. 1999. Lens biology: development and human cataractogenesis. Trends Genet. 15:191-196. [DOI] [PubMed] [Google Scholar]
- 24.Friedrich, G., and P. Soriano. 1991. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 5:1513-1523. [DOI] [PubMed] [Google Scholar]
- 25.Friend, S. H., R. Bernards, S. Rogelj, R. A. Weinberg, J. M. Rapaport, D. M. Albert, and T. P. Dryja. 1986. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature 323:643-646. [DOI] [PubMed] [Google Scholar]
- 26.Friend, S. H., J. M. Horowitz, M. R. Gerber, X. F. Wang, E. Bogenmann, F. P. Li, and R. A. Weinberg. 1987. Deletions of a DNA sequence in retinoblastomas and mesenchymal tumors: organization of the sequence and its encoded protein. Proc. Natl. Acad. Sci. USA 84:9059-9063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harbour, J. W., S. L. Lai, J. Whang-Peng, A. F. Gazdar, J. D. Minna, and F. J. Kaye. 1988. Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science 241:353-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humbert, P. O., R. Verona, J. M. Trimarchi, C. Rogers, S. Dandapani, and J. A. Lees. 2000. E2f3 is critical for normal cellular proliferation. Genes Dev. 14:690-703. [PMC free article] [PubMed] [Google Scholar]
- 29.Jacks, T., A. Fazeli, E. M. Schmitt, R. T. Bronson, M. A. Goodell, and R. A. Weinberg. 1992. Effects of an Rb mutation in the mouse. Nature 359:295-300. [DOI] [PubMed] [Google Scholar]
- 30.Reference deleted.
- 31.Reference deleted.
- 32.Lee, E. Y., H. Cam, U. Ziebold, J. B. Rayman, J. A. Lees, and B. D. Dynlacht. 2002. E2F4 loss suppresses tumorigenesis in Rb mutant mice. Cancer Cell 2:463-472. [DOI] [PubMed] [Google Scholar]
- 33.Lee, E. Y., H. To, J. Y. Shew, R. Bookstein, P. Scully, and W. H. Lee. 1988. Inactivation of the retinoblastoma susceptibility gene in human breast cancers. Science 241:218-221. [DOI] [PubMed] [Google Scholar]
- 34.Lees, J. A., M. Saito, M. Vidal, M. Valentine, T. Look, E. Harlow, N. Dyson, and K. Helin. 1993. The retinoblastoma protein binds to a family of E2F transcription factors. Mol. Cell. Biol. 13:7813-7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leone, G., J. DeGregori, Z. Yan, L. Jakoi, S. Ishida, R. S. Williams, and J. R. Nevins. 1998. E2F3 activity is regulated during the cell cycle and is required for the induction of S phase. Genes Dev. 12:2120-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leone, G., F. Nuckolls, S. Ishida, M. Adams, R. Sears, L. Jakoi, A. Miron, and J. R. Nevins. 2000. Identification of a novel E2F3 product suggests a mechanism for determining specificity of repression by Rb proteins. Mol. Cell. Biol. 20:3626-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu, Y., and E. Zacksenhaus. 2000. E2F1 mediates ectopic proliferation and stage-specific p53-dependent apoptosis but not aberrant differentiation in the ocular lens of Rb deficient fetuses. Oncogene 19:6065-6073. [DOI] [PubMed] [Google Scholar]
- 38.Logan, N., A. Graham, X. Zhao, R. Fisher, B. Maiti, G. Leone, and N. B. La Thangue. 2005. E2F-8: an E2F family member with a similar organization of DNA-binding domains to E2F-7. Oncogene 24:5000-5004. [DOI] [PubMed] [Google Scholar]
- 39.Maandag, E. C., M. van der Valk, M. Vlaar, C. Feltkamp, J. O'Brien, M. van Roon, N. van der Lugt, A. Berns, and H. te Riele. 1994. Developmental rescue of an embryonic-lethal mutation in the retinoblastoma gene in chimeric mice. EMBO J. 13:4260-4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacPherson, D., J. Sage, T. Kim, D. Ho, M. E. McLaughlin, and T. Jacks. 2004. Cell type-specific effects of Rb deletion in the murine retina. Genes Dev. 18:1681-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maiti, B., J. Li, A. de Bruin, F. Gordon, C. Timmers, R. Opavsky, K. Patil, J. Tuttle, W. Cleghorn, and G. Leone. 2005. Cloning and characterization of mouse E2F8, a novel mammalian E2F family member capable of blocking cellular proliferation. J. Biol. Chem. 280:18211-18220. [DOI] [PubMed] [Google Scholar]
- 42.Reference deleted.
- 43.Martin, G. R. 1980. Teratocarcinomas and mammalian embryogenesis. Science 209:768-776. [DOI] [PubMed] [Google Scholar]
- 44.Meuwissen, R., S. C. Linn, R. I. Linnoila, J. Zevenhoven, W. J. Mooi, and A. Berns. 2003. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell 4:181-189. [DOI] [PubMed] [Google Scholar]
- 45.Muller, H., A. P. Bracken, R. Vernell, M. C. Moroni, F. Christians, E. Grassilli, E. Prosperini, E. Vigo, J. D. Oliner, and K. Helin. 2001. E2Fs regulate the expression of genes involved in differentiation, development, proliferation, and apoptosis. Genes Dev. 15:267-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muller, H., and K. Helin. 2000. The E2F transcription factors: key regulators of cell proliferation. Biochim. Biophys. Acta 1470:M1-M12. [DOI] [PubMed] [Google Scholar]
- 47.Mulligan, G., and T. Jacks. 1998. The retinoblastoma gene family: cousins with overlapping interests. Trends Genet. 14:223-229. [DOI] [PubMed] [Google Scholar]
- 48.Nikitin, A. Y., D. J. Riley, and W. H. Lee. 1997. Earlier onset of melanotroph carcinogenesis in mice with inherited mutant paternal allele of the retinoblastoma gene. Cancer Res. 57:4274-4278. [PubMed] [Google Scholar]
- 49.Ren, B., H. Cam, Y. Takahashi, T. Volkert, J. Terragni, R. A. Young, and B. D. Dynlacht. 2002. E2F integrates cell cycle progression with DNA repair, replication, and G(2)/M checkpoints. Genes Dev. 16:245-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rossant, J. 2001. Stem cells from the mammalian blastocyst. Stem Cells 19:477-482. [DOI] [PubMed] [Google Scholar]
- 51.Saavedra, H. I., B. Maiti, C. Timmers, R. Altura, Y. Tokuyama, K. Fukasawa, and G. Leone. 2003. Inactivation of E2F3 results in centrosome amplification. Cancer Cell 3:333-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saavedra, H. I., L. Wu, A. de Bruin, C. Timmers, T. J. Rosol, M. Weinstein, M. L. Robinson, and G. Leone. 2002. Specificity of E2F1, E2F2, and E2F3 in mediating phenotypes induced by loss of Rb. Cell Growth Differ. 13:215-225. [PubMed] [Google Scholar]
- 53.Reference deleted.
- 54.Sherr, C. J. 1996. Cancer cell cycles. Science 274:1672-1677. [DOI] [PubMed] [Google Scholar]
- 55.Reference deleted.
- 56.Storre, J., H. P. Elsasser, M. Fuchs, D. Ullmann, D. M. Livingston, and S. Gaubatz. 2002. Homeotic transformations of the axial skeleton that accompany a targeted deletion of E2f6. EMBO Rep. 3:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takahashi, Y., J. B. Rayman, and B. D. Dynlacht. 2000. Analysis of promoter binding by the E2F and pRB families in vivo: distinct E2F proteins mediate activation and repression. Genes Dev. 14:804-816. [PMC free article] [PubMed] [Google Scholar]
- 58.Trimarchi, J. M., B. Fairchild, J. Wen, and J. A. Lees. 2001. The E2F6 transcription factor is a component of the mammalian Bmi1-containing polycomb complex. Proc. Natl. Acad. Sci. USA 98:1519-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trimarchi, J. M., and J. A. Lees. 2002. Sibling rivalry in the E2F family. Nat. Rev. Mol. Cell. Biol. 3:11-20. [DOI] [PubMed] [Google Scholar]
- 60.Tsai, K. Y., Y. Hu, K. F. Macleod, D. Crowley, L. Yamasaki, and T. Jacks. 1998. Mutation of E2f-1 suppresses apoptosis and inappropriate S phase entry and extends survival of Rb-deficient mouse embryos. Mol. Cell 2:293-304. [DOI] [PubMed] [Google Scholar]
- 61.Tsai, K. Y., D. MacPherson, D. A. Rubinson, D. Crowley, and T. Jacks. 2002. ARF is not required for apoptosis in Rb mutant mouse embryos. Curr. Biol. 12:159-163. [DOI] [PubMed] [Google Scholar]
- 62.Weinberg, R. A. 1995. The retinoblastoma protein and cell cycle control. Cell 81:323-330. [DOI] [PubMed] [Google Scholar]
- 63.Wikenheiser-Brokamp, K. A. 2004. Rb family proteins differentially regulate distinct cell lineages during epithelial development. Development 131:4299-4310. [DOI] [PubMed] [Google Scholar]
- 64.Williams, B. O., L. Remington, D. M. Albert, S. Mukai, R. T. Bronson, and T. Jacks. 1994. Cooperative tumorigenic effects of germline mutations in Rb and p53. Nat. Genet. 7:480-484. [DOI] [PubMed] [Google Scholar]
- 65.Williams, B. O., E. M. Schmitt, L. Remington, R. T. Bronson, D. M. Albert, R. A. Weinberg, and T. Jacks. 1994. Extensive contribution of Rb-deficient cells to adult chimeric mice with limited histopathological consequences. EMBO J. 13:4251-4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu, L., A. de Bruin, H. I. Saavedra, M. Starovic, A. Trimboli, Y. Yang, J. Opavska, P. Wilson, J. C. Thompson, M. C. Ostrowski, T. J. Rosol, L. A. Woollett, M. Weinstein, J. C. Cross, M. L. Robinson, and G. Leone. 2003. Extra-embryonic function of Rb is essential for embryonic development and viability. Nature 421:942-947. [DOI] [PubMed] [Google Scholar]
- 67.Yamasaki, L., R. Bronson, B. O. Williams, N. J. Dyson, E. Harlow, and T. Jacks. 1998. Loss of E2F-1 reduces tumorigenesis and extends the lifespan of Rb1(+/−) mice. Nat. Genet. 18:360-364. [DOI] [PubMed] [Google Scholar]
- 68.Zhou, Z., A. Flesken-Nikitin, C. G. Levine, E. N. Shmidt, J. P. Eng, E. Y. Nikitina, D. M. Spencer, and A. Y. Nikitin. 2005. Suppression of melanotroph carcinogenesis leads to accelerated progression of pituitary anterior lobe tumors and medullary thyroid carcinomas in Rb+/− mice. Cancer Res. 65:787-796. [PubMed] [Google Scholar]
- 69.Ziebold, U., E. Y. Lee, R. T. Bronson, and J. A. Lees. 2003. E2F3 loss has opposing effects on different pRB-deficient tumors, resulting in suppression of pituitary tumors but metastasis of medullary thyroid carcinomas. Mol. Cell. Biol. 23:6542-6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ziebold, U., T. Reza, A. Caron, and J. A. Lees. 2001. E2F3 contributes both to the inappropriate proliferation and to the apoptosis arising in Rb mutant embryos. Genes Dev. 15:386-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zochbauer-Muller, S., A. F. Gazdar, and J. D. Minna. 2002. Molecular pathogenesis of lung cancer. Annu. Rev. Physiol. 64:681-708. [DOI] [PubMed] [Google Scholar]