Abstract
Maternal tobacco use is associated with adverse developmental outcomes in offspring, including hyperactivity. Animal studies attempting to model this phenomenon have primarily used continuous s.c. nicotine infusion as the method of nicotine administration, which does not model the intermittent bolus delivery of nicotine associated with smoking in humans. The purpose of the present experiment was to examine the locomotor activity of pre-weanling offspring of pregnant rats exposed to an i.v. nicotine dosing protocol that approximates the pattern of nicotine exposure in moderate to heavy smokers. Pregnant rats were administered an i.v. bolus of 0.03 mg/kg nicotine (N=13) or saline (N=10) every 14 min for 16 hr/day, resulting in a total daily dose of 2 mg/kg (base), from gestational day 4 to delivery. Pups from each litter were tested for spontaneous locomotor activity on postnatal days (PND) 19–21 and nicotine-induced locomotor activity on PND 22. Mean birth weight was significantly lower in nicotine-exposed pups compared to controls, but body weights were equivalent between groups by the time of behavioral testing. Mean total distance traveled, vertical counts, and stereotypy counts were lower on PND 19 in nicotine-exposed pups compared to controls, but only the difference in mean stereotypy counts was statistically significant. Within session analysis revealed that both distance traveled and stereotypy were significantly decreased in nicotine-exposed pups in the first five minutes of the session on PND 19. Total time spent in the center of the field was also lower in nicotine exposed pups. Nicotine-induced increases in activity on PND 22 did not differ according to gestational exposure. These findings demonstrate that prenatal nicotine exposure in a model that mimics the pattern of nicotine exposure from cigarette smoking in humans results in offspring that exhibit low birth weight and hypoactivity in a novel environment.
1. Introduction
Smoking during pregnancy is an important public health problem associated with a range of adverse neonatal and developmental outcomes (Stratton et al. 2001). Babies born to women who smoke during pregnancy are, on average, 200–240 g lighter than those born to women who do not smoke (Kleinman et al. 1988; MacArthur and Knox 1988). An increased risk of spontaneous abortion, premature birth, perinatal death, and sudden infant death syndrome have been correlated with smoking during pregnancy (Armstrong et al. 1992; DHHS 2001). Some data also suggest that smoking during pregnancy is associated with adverse behavioral development, including attention deficit hyperactivity disorder (ADHD) (Milberger et al. 1996; Milberger et al. 1998), cognitive deficits (Ernst et al. 2001; Niaura et al. 2001b), conduct disorder (Wakschlag et al. 1997), and an increased risk of tobacco dependence in offspring of women who smoke during pregnancy (Cornelius et al. 2000; Niaura et al. 2001a).
Animal models of prenatal nicotine exposure are critical for studying the role of nicotine in the effects of maternal smoking on adverse behavioral outcomes in offspring. Numerous studies have shown a wide range of behavioral impairments in rodents prenatally exposed to nicotine, including impaired performance in the radial arm maze and Morris water maze (Levin et al. 1993; Sorenson et al. 1991; Yanai et al. 1992), lower response and reinforcement rates under operant schedules of food delivery (Martin and Becker 1971), poorer operant discrimination performance (Martin and Becker 1971), and reduced pre-pulse inhibition of acoustic startle response (Popke et al. 1997). Although several animal studies have found that offspring prenatally exposed to nicotine can also exhibit hyperactivity (Ajarem and Ahmad 1998; Fung and Lau 1989; Martin and Becker 1970; Peters et al. 1979; Vaglenova et al. 2004), other studies have reported conflicting results (see Table 1). Some studies report hyperactivity in only a subset of rats (less than 20%), with no significant overall effect of prenatal nicotine exposure (Richardson and Tizabi 1994; Tizabi et al. 1997). Other studies have reported inconsistent effects across different measures of activity (Tizabi et al. 2000) or postnatal time points (Paulson et al. 1994; Schlumpf et al. 1988). Many studies have failed to show any significant effect of prenatal nicotine on locomotor activity (Gaworski et al. 2004; Martin and Becker 1970; Martin and Martin 1981; Paulson et al. 1993; Shacka et al. 1997), and some studies reported decreased activity (Peters and Tang 1982; Romero and Chen 2004). The reasons for this marked variability across studies are not clear; differences in the daily nicotine dose, route of administration, strain and sex of the subject, postnatal time point of testing, and testing procedures could all play a role.
Table 1.
Effects of prenatal nicotine administration in rodents on birth weight and locomotor activity of offspring.
| Study | Route | Daily Dose mg/kg | GD Start Point | Pup Birth Weight | Spontaneous Locomotor Activity | Drug-Induced Activity |
|---|---|---|---|---|---|---|
| Fung 1988 | s.c. infusion | 1.5 | 1 | No effect | Increase | Increase |
| Romero et al. 2004 | s.c. infusion | 1.8 | 8 | No effect | Decrease (females only on 3rd session) | |
| Shacka et al. 1997 | s.c. infusion | 2.0 | 7 | No effect | No effect | IncreaseM |
| Richardson et al. 1994 | s.c. infusion | 3.0 | 4 | No effect | No overall effect1 | |
| Sobrian et al. 1995 | s.c. infusion | 5.0 | 8 | No effect | No effect | |
| Richardson et al. 1994 | s.c. infusion | 6.0 | 4 | Decrease | No overall effect1 | |
| Tizabi et al. 1997 | s.c. infusion | 6.0 | 4 | NR | No overall effect1 | |
| Vaglenova et al. 2004 | s.c. infusion | 6.0 | 3 | Decrease | Increase vertical, increase horizontalM | |
| Schlumpf et al. 1988 | s.c. infusion | 6.0 | 12 | NR | No effect PND 11, increase PND 15M | |
| Tizabi et al. 2000 | s.c. infusion | 9.0 | 4 | Decrease | Increase vertical movement only | |
| Ajarem et al. 1998 | s.c. injection | 0.5 | 9 | No effect | Increase | |
| Martin et al. 1976 | s.c. injection | 6.0 | 1 | No effect | No effect (in activity wheel, PND 90+) | |
| Martin et al. 1981 | s.c. injection | 6.0 | 1 | NR | No effect (in activity wheel, PND 90+) | |
| Paulson et al. 1993 | Oral2 | 4.0 | 6 | No effect | No effect | |
| Paulson et al. 1994 | Oral2 | 4.0 | 6 | Decrease | No effect PND 15, increase PND 47 | |
| Peters and Tang 1982 | Oral3 | 6.0 | 0 | Decrease | DecreaseM | |
| Peters et al. 1979 | Oral3 | 6.0 | 0 | Decrease | Increase (in home cage, light phase) | |
| Paulson et al. 1994 | Oral2 | 12.0 | 6 | Decrease | No effect | |
| Paulson et al. 1993 | Oral2 | 12.0 | 6 | Decrease | No effect | |
| Paulson et al. 1994 | Oral2 | 18.0 | 6 | Decrease | No effect | |
| Gaworski et al. 2004 | Inhalation4 | NR5 | 4 | Decrease | No effect |
NR = not reported
Males only
Significant proportion of nicotine exposed pups met criteria for hyperactivity
Gavage, aqueous tobacco extract
In drinking water
Mainstream smoke, 2 hr/day
Serum nicotine concentrations ranged 122–245 ng/ml
Although a variety of routes have been studied, continuous subcutaneous infusion via osmotic pump has been the most common method of maternal nicotine administration in studies of prenatal nicotine exposure. Continuous s.c. nicotine infusion is convenient, reliably provides clinically relevant blood nicotine levels, avoids the high peak nicotine levels associated with intermittent s.c. dosing, and is clinically relevant in that it mimics nicotine exposure from a 24 h nicotine patch (Slotkin 1998). However, it does not produce the repeated spikes of peak serum nicotine levels associated with either smoking or intermittent forms of nicotine replacement therapy (gum, lozenge, inhaler, nasal spray) in humans.
Some of the behavioral and physiological effects of chronic nicotine treatment are known to depend upon the dosing regimen used. For example, repeated intermittent s.c. or i.v. nicotine administration in nonpregnant adult rats produces a progressive increase in locomotor activity (locomotor sensitization, (Samaha et al. 2005)), whereas continuous nicotine infusion does not (Faraday et al. 2001; Faraday et al. 2003). In addition, daily doses of nicotine that produce decreases in body weight in nonpregnant adult rats when administered via continuous infusion can produce increases in body weight when administered via intermittent s.c. injections (Morgan and Ellison 1987). In light of the pharmacokinetic differences between continuous nicotine infusion and smoking and the dependence of nicotine's effects on the dosing regimen, it is important to explore alternative dosing regimens in the analysis of the behavioral effects of prenatal nicotine exposure in offspring.
To date, the intravenous route has not been used in studies of the behavioral effects of prenatal nicotine exposure. A nicotine dosing regimen consisting of an i.v. bolus of 0.03 mg/kg every 14 min delivered 16 h/day has been characterized in an attempt to more closely mimic nicotine exposure from maternal smoking (Keyler et al. 2005). This type of regimen produces peak and trough arterial and venous serum nicotine levels comparable to those of smokers, and provides a diurnal pattern similar to typical smoking (Hukkanen et al. 2005; LeSage et al. 2003). The purpose of the present experiment was to conduct an initial assessment of the effects of this novel maternal i.v. nicotine dosing protocol on the locomotor activity of pre-weanling pups.
2. Materials and methods
2.1 Animals
Timed-pregnant female Holtzman Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 200 to 225 g were employed. Rats arrived at gestational day (GD) 3 and were housed individually in a temperature- and humidity-controlled colony room with unlimited access to food and water under a 12-h light/dark cycle (lights off at 10:00 pm). Animal husbandry and experimental protocols were approved by the Institutional Animal Care and Use Committee of the Minneapolis Medical Research Foundation and were in accordance with the 1996 NIH Guide for the Care and Use of Laboratory Animals.
2.2 Surgery
On GD 4, rats were anesthetized using fentanyl/droperidol and a cannula was placed in the right jugular vein approximately 12 hr prior to the beginning of nicotine dosing. The catheter was externalized between the scapulae and attached to a harness assembly that allowed connection to a fluid swivel via a tether for nicotine dosing throughout pregnancy.
2.3 Apparatus
Throughout the nicotine administration period, dams were housed in the colony room in modified “shoebox” cages that were equipped with a counter-balanced swivel arm, allowing unrestricted movement. Infusion pumps were located next to each cage on the same shelf. Infusion pumps were controlled by a computer located within the colony room using Med-PC IV interfacing and software (Med Associates, Inc., St. Albans, VT).
Rat pups were tested in open-field activity chambers (Med Associates, Inc., St. Albans, VT), measuring 43 cm long and 43 cm wide. Each chamber contained three 16-beam photocell arrays placed 2.5 and 8 cm above the chamber floor for measuring horizontal and vertical activity, respectively. Chambers were placed inside a sound-attenuating cubicle equipped with an exhaust fan that provided masking noise and ceiling lights to provide ambient illumination. A computer with open field activity software (Version 4, Med Associates, Inc., St. Albans, VT) was used for operating the apparatus and recording data.
2.4 Drugs
Nicotine bitartrate (Sigma Chemical Co., St. Louis, MO) was dissolved in sterile hepranized saline (30 units/ml). The pH of the solution was adjusted to 7.4 with dilute NaOH. Nicotine doses are expressed as the base.
2.5 Maternal Nicotine Administration
Maternal nicotine treatment began on GD 4 (N=13) via an infusion pump delivering nicotine 0.03 mg/kg/dose over 1 second in a volume of 50 μl for 16 hours/day at 14 minute intervals, for a total dose of 2 mg/kg/day. The 0.03 mg/kg dose was chosen because a) it is well tolerated, b) it is readily self-administered by rats, and c) the serum concentrations associated with repeated bolus doses of this size have been well characterized (LeSage et al. 2003). Control females (N=10) received i.v. saline infusions according to the same regimen. Dosing began at 10 p.m. each day, corresponding to the start of the dark phase of the light cycle, and continued until 2 p.m. of the next day. On the day of delivery, nicotine was replaced with saline, litter variables were measured, and females were allowed to nurse their own pups. Cross-fostering was not employed because previous studies indicate that it is not an important determinant of the effects of gestational nicotine exposure on locomotor activity in offspring (Peters et al. 1979; Schlumpf et al. 1988). Litters were not culled because gestational nicotine exposure at doses similar to that used in the present study has been shown to have no effect on litter size (Romero and Chen 2004; Shacka et al. 1997). However, four litters with fewer than eight pups (three nicotine exposed, two saline exposed) were excluded from further study because a litter size of eight has been the minimum allowed in prior studies.
2.6 Spontaneous Locomotor Activity
On PND 19, 20, and 21, four pups (two male and two female) from each litter (N=10 saline, N=13 nicotine) were assessed for spontaneous locomotor activity. On each day, pups were transported from the colony room to the activity testing room and allowed to acclimate to the room for 30 min. Pups were placed in the center of the activity chamber, which automatically started each 30-min session. At the end of each session, pups were removed from the chamber and immediately returned to their mother in the colony room. Testing occurred between 2–4 hr into the light phase of the light cycle. Chambers were cleaned prior to each session.
2.7 Nicotine-induced locomotor activity
On PD 22, nicotine-induced locomotor activity was assessed in the same pups tested for spontaneous activity. Procedures were identical to those used for spontaneous activity, except that an injection of saline or nicotine (1.0 mg/kg, i.p.) was administered immediately before placement into the activity chamber. The dose was chosen based on prior studies (Shacka et al., 1997; Sobrian et al., 1995). Two pups from each litter (one of each sex) were injected with saline, while the other two pups received nicotine. Injections were given at a volume of 10 ml/kg. Activity was only examined for 15 min because no group differences in spontaneous activity on PND 19–21 were apparent beyond this time point.
2.8 Data Analysis
Comparison of litter variables (size, weight, mean pup weight, deaths) between pups prenatally exposed to saline or nicotine was done by t-tests. Three primary measures of spontaneous activity were recorded, distance traveled, vertical counts (rearing), and stereotypy counts (i.e., non-ambulatory horizontal activity). Each activity measure was examined by a three-factor ANOVA with prenatal exposure, day, and time as factors. If significant main effect of prenatal exposure or prenatal exposure × time interaction was observed, a separate two-factor ANOVA with prenatal exposure and time as factors, followed by Bonferroni post tests, was conducted for each day to examine differences between groups in within-session patterns of activity. In light of the significant hypoactivity seen on PND 19 in pups prenatally exposed to nicotine, time spent in the center of the chamber was compared between groups to begin to examine whether this indicated an anxiety-like effect of prenatal nicotine exposure. The hypothesis was that nicotine exposed pups would exhibit lower time in the center compared to controls. Because this measure was not normally distributed in the prenatal nicotine group, a one-tailed Mann-Whitney U test was conducted for this comparison. Measures of nicotine-induced activity were examined by three-factor ANOVA with prenatal exposure, challenge dose, and time as factors. If significant main effects or interactions were observed, two-factor ANOVA with prenatal exposure and time as factors, followed by Bonferroni post tests, was conducted to examine group differences in within-session activity. Statistical significance was set at p<0.05.
3. Results
3.1 Litter variables
Table 2 shows litter variables for each prenatal exposure group. Litter size was equivalent between groups. Litter weight and mean pup weight were lower in those prenatally exposed to nicotine, but only the difference in mean pup weight was statistically significant. By the time of behavioral testing on PND 19, no significant difference in pup weights was observed (48.1±2.5 g versus 45.3±3.0 g in saline and nicotine exposed groups, respectively). Although a higher death rate was seen in nicotine-exposed litters, this difference only approached significance (t=1.9, p=0.08).
Table 2.
Litter characteristics at birth (mean±SEM).
| Prenatal Exposure | Litter Size | Litter Weight | Mean Pup Weight | Deaths/Litter (PND 0–7)a |
|---|---|---|---|---|
| Saline | 13.1±0.8 | 102.3±4.9 | 8.0±0.3 | 0.6±0.3 |
| Nicotine | 13.6±0.9 | 89.4±5.3 | 6.6±0.2** | 2.4±0.9 |
Includes litters excluded from behavioral testing.
Significantly different from saline, p<0.01
3.1 Spontaneous locomotor activity
Figure 1 shows the mean total distance traveled, stereotypy counts, and vertical counts for each prenatal exposure group during testing on PND 19–21. All three measures were lower in pups exposed to nicotine compared to controls, but only a significant main effect of treatment on mean stereotypy counts was indicated by three-factor ANOVA (F=5.4, p<0.05). There was no significant prenatal exposure × day × time or prenatal exposure × day interaction for any of these measures, but a significant prenatal exposure × time interaction was observed for both distance traveled (F=2.7, p<0.05) and stereotypy (F=3.1, p<0.05).
Fig. 1.
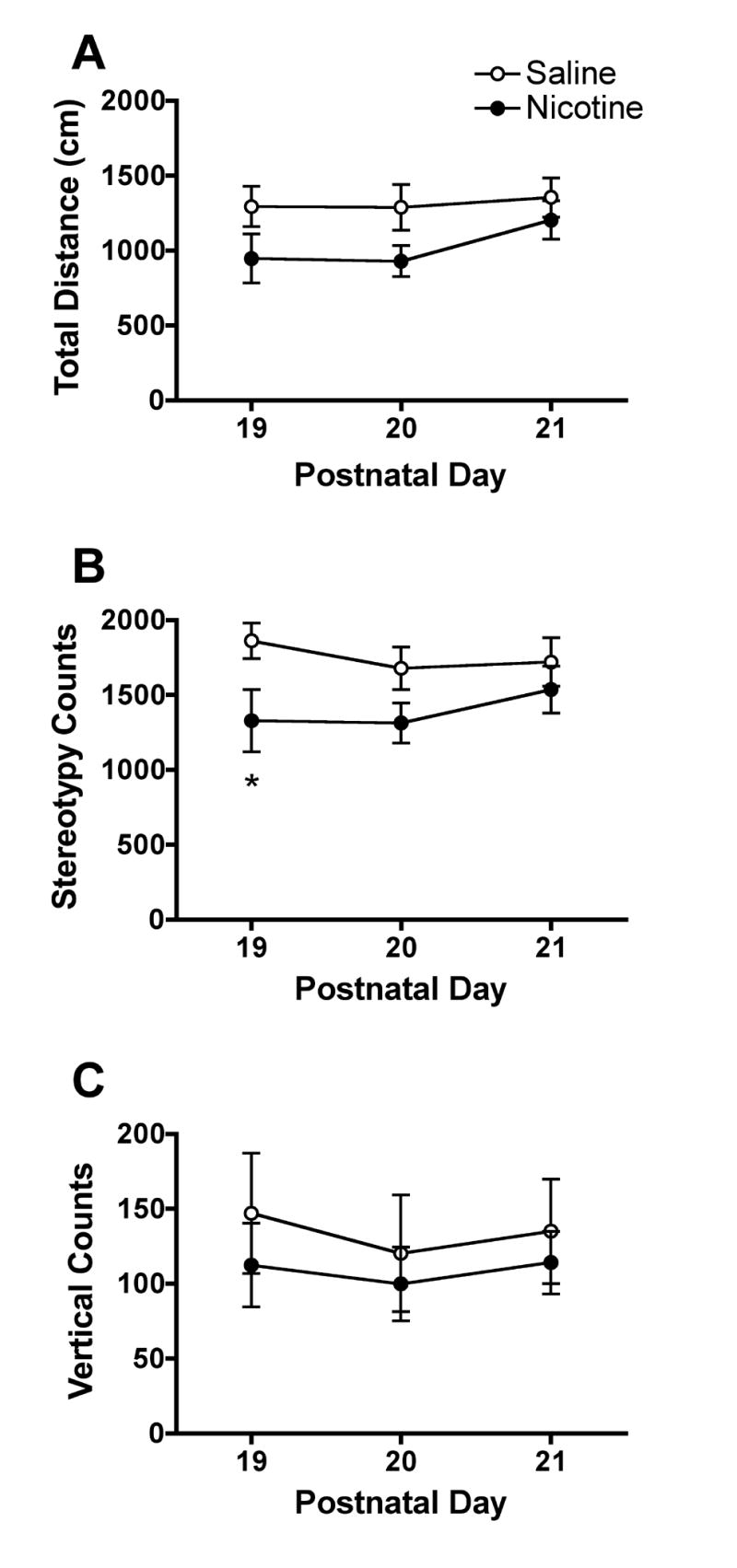
Measures of spontaneous locomotor activity in pups prenatally exposed to saline (open circles) or nicotine (closed circles) on PND 19–21. Each point is the mean±SEM of four pups (two male and two female) from each litter (N=10 saline, N=13 nicotine). A significant main effect of prenatal nicotine exposure was observed for stereotypy counts (F=4.99, p<0.05), with no significant prenatal exposure × day interaction. *Significantly different from saline, p<0.05.
Figure 2 shows the mean distance, stereotypy, and vertical counts across each 5-min segment of the session on PND 19. For distance scores, two-factor ANOVA indicated no significant main effect of prenatal nicotine exposure, but a significant prenatal exposure × time interaction (F=4.9, p<0.001) was observed. Post hoc tests showed that pups prenatally exposed to nicotine exhibited significantly lower distance scores compared to controls in the first 5 min of the session (t=4.3, p<0.001). For stereotypy, a significant main effect of prenatal nicotine exposure (F=4.7, p<0.05), and a significant prenatal exposure × time interaction (F=4.2, p<0.01) was observed. Post hoc tests showed that pups prenatally exposed to nicotine exhibited significantly less stereotypy compared to controls in the first 5 min of the session (t=4.0, p<0.001). There was no significant effect of prenatal nicotine exposure on vertical activity. No significant main effects or interactions were observed for PND 20 and 21.
Fig. 2.
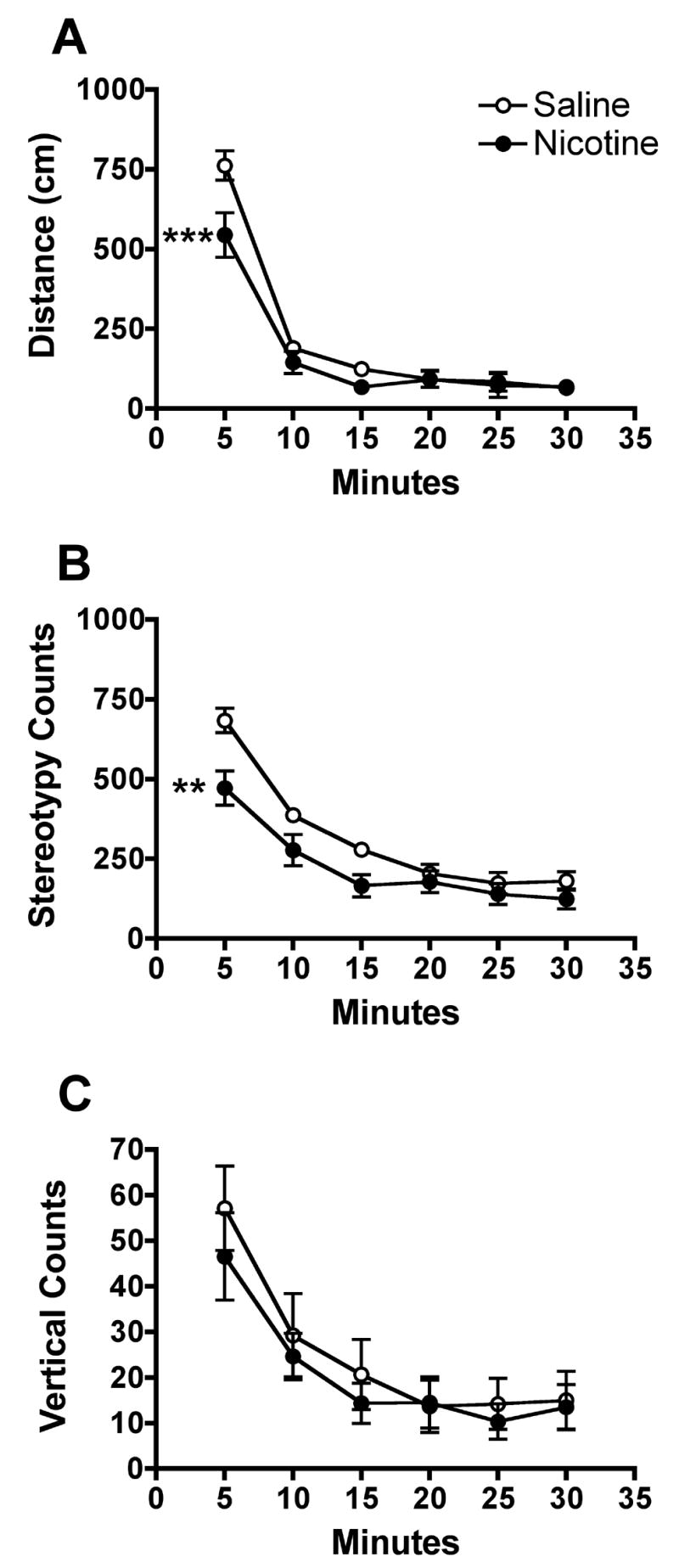
Measures of spontaneous locomotor activity during consecutive 5-min segments of the 30-min activity test session on PND 19. See Fig. 1 for further details. Distance traveled and stereotypy were significantly lower in the first 5 min in pups prenatally exposed to nicotine, **p<0.01, ***p<0.001.
The median total time spent in the center of the chamber on PND 19 was 161.3 sec (308.2, 75th percentile) and 71.5 sec (186.6, 75th percentile) in control pups and those prenatally exposed to nicotine, respectively. Pups prenatally exposed to nicotine exhibited significantly less time in the center compared to controls (U=36.0, p<0.05).
3.2 Nicotine-induced locomotor activity
Figure 3 shows the mean total distance traveled, stereotypy, and vertical counts following a challenge dose of saline or nicotine in each prenatal treatment group on PND 22. A significant main effect of nicotine challenge on all three measures was observed, with significantly greater distance traveled (F=14.4, p<0.01), greater stereotypy (F=21.3, p<0.001) and reduced vertical activity (F=5.6, p<0.05) in pups receiving nicotine compared to controls. There was no significant main effect of prenatal treatment, nor a significant prenatal treatment × challenge interaction.
Fig. 3.
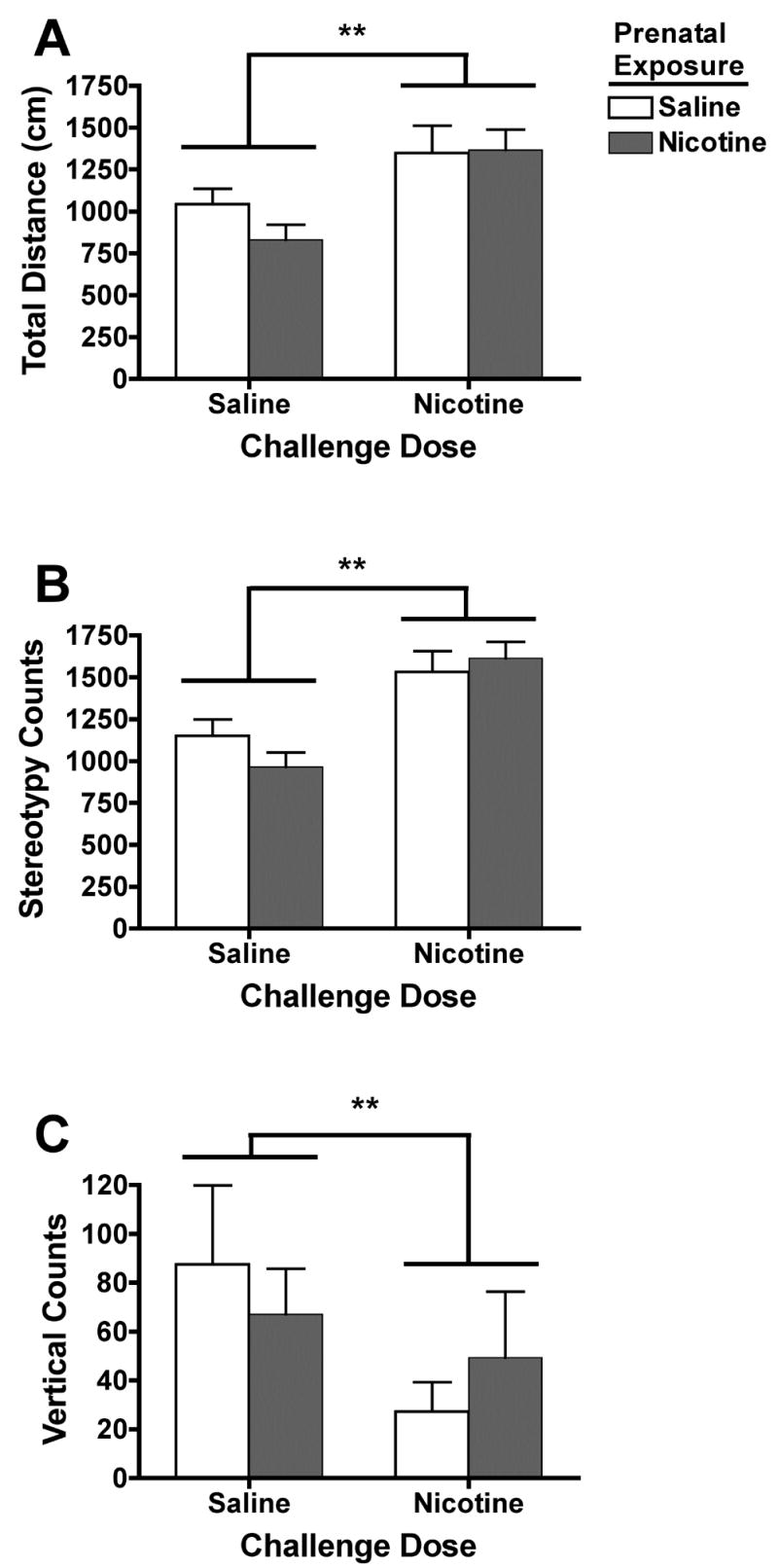
Measures of nicotine-induced locomotor activity in pups prenatally exposed to saline (open bars) or nicotine (shaded bars) following a nicotine challenge dose (1.0 mg/kg) on PND 22. There was a significant main effect of nicotine challenge on all three measures of activity, **p<0.01. There was no main effect of prenatal treatment or significant prenatal treatment by challenge dose interaction.
Figure 4 shows the mean distance traveled, stereotypy, and vertical counts during each consecutive 5-min segment of the session on PND 22. There was a significant nicotine challenge × time interaction on all three measures (distance traveled, F=5.7, p<0.01; stereotypy, F=4.3, p<0.05; vertical activity, F=8.7, p<0.01). There was no significant prenatal treatment × challenge × time interaction for any of the measures. Post hoc tests indicated significantly greater distance traveled in pups receiving nicotine at the 10- (t=5.4, p<0.001, prenatal saline; t=4.3, p<0.01, prenatal nicotine) and 15-min time points (t=4.0, p<0.01, prenatal saline; t=6.6, p<0.001, prenatal nicotine) compared to controls. Greater stereotypy was observed in nicotine challenged pups compared to controls at the 10- (t=5.0, p<0.001) and 15-min time points (t=6.1, p<0.001) in pups prenatally treated with nicotine, but only at the 15-min time point (t=3.7, p<0.01) in pups prenatally treated with saline. Thus, horizontal activity declined less rapidly in pups receiving a nicotine challenge compared to saline controls, regardless of prenatal treatment. Lower vertical activity was observed in nicotine challenged pups compared to controls at the 5-min time point (t=3.7, p<0.01) in pups prenatally treated with saline. However, marked variability was apparent in the vertical activity data.
Fig. 4.
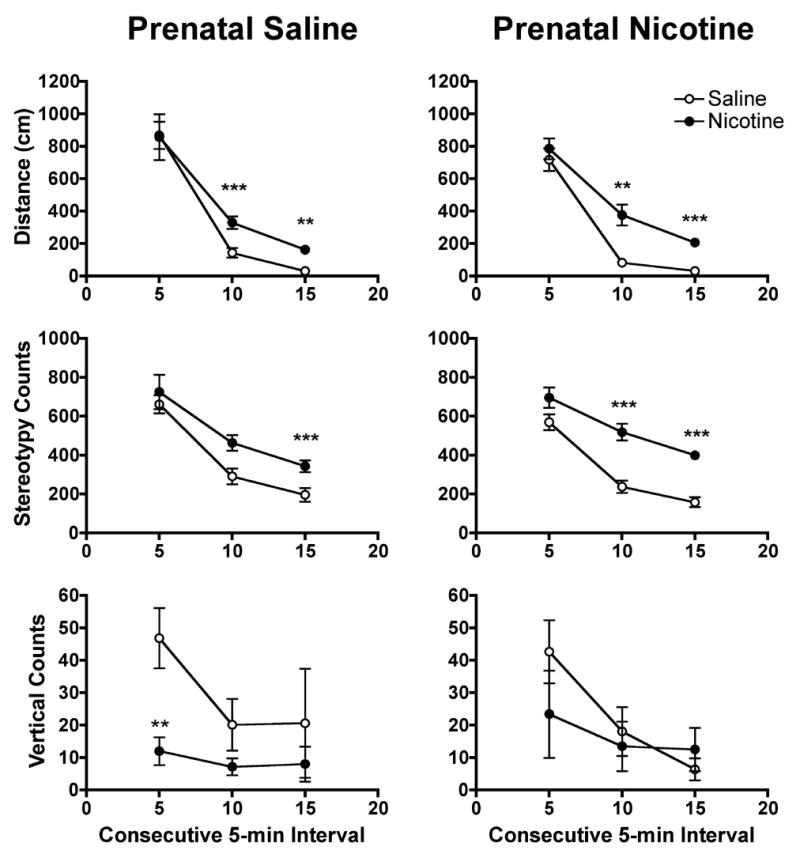
Measures of nicotine-induced locomotor activity during consecutive 5-min segments of the test session on PND 22. The left column of panels shows data from pups prenatally exposed to saline, while the right column show data from nicotine exposed pups. See Fig. 1 for further details. Within each prenatal treatment group, distance traveled and stereotypy were significantly higher at the 10 and 15 min time points in pups challenged with nicotine compared to those challenged with saline, **p<0.01, ***P<0.001.
4. Discussion
The primary findings of the present study are that a) prenatal i.v. nicotine treatment resulted in offspring with lower birth weight, b) nicotine exposed pups were hypoactive compared to controls upon initial exposure to a novel open field on PND 19, c) time spent in the center of the open field on PND 19 was lower in nicotine-exposed pups compared to controls, and d) nicotine-induced increases in locomotor activity on PND 22 did not differ between nicotine exposed pups and controls. The present study is the first to examine the behavioral effects of a prenatal i.v. nicotine dosing regimen that mimics the pattern of nicotine intake from moderate to heavy smoking in humans.
Low birth weight in the offspring of smokers, a well documented effect (Kleinman et al. 1988; MacArthur and Knox 1988), is important because it is associated with increased perinatal mortality (DHHS 2001). The deficit in birth weight is dose-related, so that it is greatest in offspring of heavy smokers but also present in the offspring of more moderate smokers. Studies using rodent models of gestational nicotine exposure have also shown a dose-related effect on birth weight. Decreases in birth weight have been reported from animal studies using daily doses of 6 to 9 mg/kg/day nicotine via continuous infusion (Cutler et al. 1996; Richardson and Tizabi 1994; Tizabi et al. 2000; Vaglenova et al. 2004), which produces venous serum nicotine levels higher than in most smokers. Other studies using s.c. injections or a continuous nicotine infusion that delivered a daily dose similar to or higher than that in the present study (1.5 to 5 mg/kg/day) have generally found no effect on birth weight (Fung and Lau 1989; Johns et al. 1993; Levin et al. 1993; Romero and Chen 2004; Shacka et al. 1997; Sobrian et al. 2003). Thus the reduced birth weight observed with a relatively low daily nicotine dose in the present study suggests that prenatal nicotine treatment may be more potent when administered via repeated i.v. boluses in a pattern that mimics maternal smoking. This increased potency may be due to the fact that peak arterial serum concentrations produced by the present i.v. dosing protocol (100 ng/ml; LeSage et al., 2002) are within the range of serum nicotine concentrations produced by a continuous infusion rate of 6.0 mg/kg/day, which has been shown to decrease birth weight (Richarson & Tizabi, 1993). Thus, the present findings suggest that a steady-state serum concentration in this range is not required to produce adverse effects on birth weight.
The hypoactivity observed on the first day of testing differs from the majority of previous studies, representing a variety of routes of administration and doses of nicotine, which showed either no effect or an increase in locomotor activity in rodents prenatally exposed to nicotine (Ajarem and Ahmad 1998; Fung 1988; Vaglenova et al. 2004). However, one study (Peters and Tang 1982) found that prenatal oral administration of nicotine (6.0 mg/kg/day) via drinking water resulted in offspring that exhibited hypoactivity in an open field in the first five minutes of the session, which is quite consistent with the present findings. Because the nicotine solution was the sole fluid source in this study, drinking likely occurred in bouts across the dark phase of the light cycle, resulting in an intermittent diurnal pattern of nicotine delivery comparable to that in the present study. Given the scarcity of studies reporting hypoactivity in offspring prenatally exposed to nicotine, these findings raise the question of whether the diurnal pattern of maternal nicotine administration, in addition to peak serum nicotine concentrations, may play a role in the behavioral effects of prenatal nicotine exposure.
The hypoactivity observed in nicotine-exposed pups on PND 19 could be indicative of higher levels of novelty-induced anxiety in this group compared to controls, since lower activity in a novel open field is associated with other measures of anxiety such as increased defecation and high plasma corticosterone levels (File and Vellucci 1979). Consistent with this possibility, pups prenatally exposed to nicotine in the present study spent significantly less time in the center of the chamber on PND 19, which is considered another indicator of anxiety (Fox et al. 2001). The present findings are consistent with studies showing that prenatal nicotine exposure increases anxiety-like behavior in other animal models. For example, Johns et al. (1982; 1993) has shown that guinea pigs prenatally exposed to nicotine were less likely to enter an unfamiliar stimulus alley compared to controls. In addition, Vaglenova et al. (2004) found that rats prenatally exposed to nicotine exhibited decreased entries and time spent in the open arms of an elevated-plus maze. However, one study in mice found that prenatal nicotine exposure had the opposite effect in the elevated-plus maze (Ajarem and Ahmad 1998). Moreover, Peters and Tang (1982) found that in rats prenatally exposed to nicotine that exhibited hypoactivity in an open field, neither the plasma corticosterone response to stress nor the hypothalamic norepinephrine level was significantly affected. Further studies that directly compare neuropharmacological and behavioral indices of anxiety are needed to confirm whether prenatal i.v. nicotine treatment increases anxiety-like responses in offspring.
In the present study, prenatal nicotine exposure had no effect on measures of nicotine-induced locomotor activity at PND 22. The present findings are consistent with that of Sobrian et al. (1995) who found that the same nicotine challenge dose as that used in the present study (1.0 mg/kg) produced a similar increase in total activity and stereotypy regardless of prenatal treatment. However, Shacka et al. (1997) found that the same nicotine challenge dose only increased activity in 14-day old male rats prenatally exposed to nicotine. These findings are interesting in light of studies showing reduced activity of cholinergic and catecholaminergic systems in rats prenatally exposed to nicotine, an effect one might expect to influence nicotine-induced locomotor activity (Kane et al. 2004; Slikker et al. 2005; Slotkin 1998). The discrepancy between studies may be due to differences in the postnatal time point at which nicotine-induced locomotor behavior was measured, since neuropharmacological sequelae of prenatal nicotine exposure are known to vary over the course of postnatal development.
In order to attribute the present findings to adverse effects of nicotine per se, the effect of nicotine-induced fetal ischemia and hypoxia must be ruled out. The effects of the i.v. dosing regimen in the present study on fetal ischemia and hypoxia are not known. However, the peak arterial concentrations produced with this regimen (100 ng/ml) are equilavent to the steady state nicotine concentrations produced by a continuous infusion rate of 6.0 mg/kg/day nicotine via osmotic minipump (e.g., Richardson & Tizabi, 1993), which falls below the threshold for producing fetal ischemia and hypoxia (Slotkin, 1998). Nonetheless, future studies are needed to determine whether episodic ischemia and hypoxia is produced by the i.v. bolus nicotine regimen used in the present study. It is important to note that, even if ischemia and hypoxia do occur, the present model of maternal smoking would still be valid to the extent that the human fetus experiences these effects during each cigarette.
In summary, prenatal i.v. nicotine administration was associated with low birth weight and hypoactivity in response to an open field in pre-weanling offspring. The present findings provide further support that nicotine may play a role in some of the behavioral effects in offspring of women who smoke during pregnancy. The maternal i.v. nicotine dosing regimen used in the present study mimics the pattern of nicotine exposure from maternal smoking and may therefore provide a useful model for studying the consequences of prenatal nicotine exposure on offspring neurobehavioral development. The reduction in birth weight and hypoactivity observed in the present study with a relatively low daily nicotine dose contrast with the majority of previous studies and suggests that the dosing regimen may influence the physiological and behavioral consequences of prenatal nicotine exposure. However, given the differences in age, strain, and testing conditions between the present a prior studies, future studies that directly compare different maternal dosing regimens are needed to confirm this possibility.
Acknowledgments
The authors thank Thomas Bramwell for his technical assistance in completing this study. Supported by NIDA grant DA15668.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ajarem JS, Ahmad M. Prenatal nicotine exposure modifies behavior of mice through early development. Pharmacol Biochem Behav. 1998;59:313–318. doi: 10.1016/s0091-3057(97)00408-5. [DOI] [PubMed] [Google Scholar]
- Armstrong BG, McDonald AD, Sloan M. Cigarette, alcohol, and coffee consumption and spontaneous abortion. Am J Public Health. 1992;82:85–87. doi: 10.2105/ajph.82.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius MD, Leech SL, Goldschmidt L, Day NL. Prenatal tobacco exposure: is it a risk factor for early tobacco experimentation? Nicotine Tob Res. 2000;2:45–52. doi: 10.1080/14622200050011295. [DOI] [PubMed] [Google Scholar]
- Cutler AR, Wilkerson AE, Gingras JL, Levin ED. Prenatal cocaine and/or nicotine exposure in rats: preliminary findings on long-term cognitive outcome and genital development at birth. Neurotoxicol Teratol. 1996;18:635–643. doi: 10.1016/s0892-0362(96)00125-0. [DOI] [PubMed] [Google Scholar]
- DHHS U. Women and smoking: A report of the surgeon general. Washington, DC: U.S. Government Printing Office; 2001. [Google Scholar]
- Ernst M, Heishman SJ, Spurgeon L, London ED. Smoking history and nicotine effects on cognitive performance. Neuropsychopharmacology. 2001;25:313–319. doi: 10.1016/S0893-133X(01)00257-3. [DOI] [PubMed] [Google Scholar]
- Faraday MM, Elliott BM, Grunberg NE. Adult vs. adolescent rats differ in biobehavioral responses to chronic nicotine administration. Pharmacol Biochem Behav. 2001;70:475–489. doi: 10.1016/s0091-3057(01)00642-6. [DOI] [PubMed] [Google Scholar]
- Faraday MM, Elliott BM, Phillips JM, Grunberg NE. Adolescent and adult male rats differ in sensitivity to nicotine's activity effects. Pharmacol Biochem Behav. 2003;74:917–931. doi: 10.1016/s0091-3057(03)00024-8. [DOI] [PubMed] [Google Scholar]
- File SE, Vellucci SV. Behavioural and biochemical measures of stress in hooded rats from different sources. Physiol Behav. 1979;22:31–35. doi: 10.1016/0031-9384(79)90399-8. [DOI] [PubMed] [Google Scholar]
- Fox GB, Curzon P, Decker MW. The behavioral assessment of sensorimotor processes in the mouse: Acoustic startle, locomotor activity, rotarod, and beam walking. In: Buccafusco JJ, editor. Methods of Behavior Analysis in Neuroscience. New York: CRC Press; 2001. pp. 27–49. [Google Scholar]
- Fung YK. Postnatal behavioural effects of maternal nicotine exposure in rats. J Pharm Pharmacol. 1988;40:870–872. doi: 10.1111/j.2042-7158.1988.tb06290.x. [DOI] [PubMed] [Google Scholar]
- Fung YK, Lau YS. Effects of prenatal nicotine exposure on rat striatal dopaminergic and nicotinic systems. Pharmacol Biochem Behav. 1989;33:1–6. doi: 10.1016/0091-3057(89)90419-x. [DOI] [PubMed] [Google Scholar]
- Gaworski CL, Carmines EL, Faqi AS, Rajendran N. In utero and lactation exposure of rats to 1R4F reference cigarette mainstream smoke: effect on prenatal and postnatal development. Toxicol Sci. 2004;79:157–169. doi: 10.1093/toxsci/kfh083. [DOI] [PubMed] [Google Scholar]
- Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- Johns JM, Louis TM, Becker RF, Means LW. Behavioral effects of prenatal exposure to nicotine in guinea pigs. Neurobehav Toxicol Teratol. 1982;4:365–369. [PubMed] [Google Scholar]
- Johns JM, Walters PA, Zimmerman LI. The effects of chronic prenatal exposure to nicotine on the behavior of guinea pigs (Cavia porcellus) J Gen Psychol. 1993;120:49–63. doi: 10.1080/00221309.1993.9917861. [DOI] [PubMed] [Google Scholar]
- Kane VB, Fu Y, Matta SG, Sharp BM. Gestational nicotine exposure attenuates nicotine-stimulated dopamine release in the nucleus accumbens shell of adolescent Lewis rats. J Pharmacol Exp Ther. 2004;308:521–528. doi: 10.1124/jpet.103.059899. [DOI] [PubMed] [Google Scholar]
- Keyler DE, Dufek MB, Calvin AD, Bramwell TJ, LeSage MG, Raphael DE, Ross CA, Le CT, Pentel PR. Reduced nicotine distribution from mother to fetal brain in rats vaccinated against nicotine: time course and influence of nicotine dosing regimen. Biochem Pharmacol. 2005;69:1385–1395. doi: 10.1016/j.bcp.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Kleinman JC, Pierre MB, Jr, Madans JH, Land GH, Schramm WF. The effects of maternal smoking on fetal and infant mortality. Am J Epidemiol. 1988;127:274–282. doi: 10.1093/oxfordjournals.aje.a114803. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Keyler DE, Collins G, Pentel PR. Effects of continuous nicotine infusion on nicotine self-administration in rats: relationship between continuously infused and self-administered nicotine doses and serum concentrations. Psychopharmacology (Berl) 2003;170:278–286. doi: 10.1007/s00213-003-1539-2. [DOI] [PubMed] [Google Scholar]
- Levin ED, Briggs SJ, Christopher NC, Rose JE. Prenatal nicotine exposure and cognitive performance in rats. Neurotoxicol Teratol. 1993;15:251–260. doi: 10.1016/0892-0362(93)90006-a. [DOI] [PubMed] [Google Scholar]
- MacArthur C, Knox EG. Smoking in pregnancy: effects of stopping at different stages. Br J Obstet Gynaecol. 1988;95:551–555. doi: 10.1111/j.1471-0528.1988.tb09481.x. [DOI] [PubMed] [Google Scholar]
- Martin JC, Becker RF. The effects of nicotine in utero upon activity in the rat. Psychol Sci. 1970;19:59–60. [Google Scholar]
- Martin JC, Becker RF. The effects of maternal nicotine absorption or hypoxic episodes upon appetitive behavior of rat offspring. Dev Psychobiol. 1971;4:133–147. doi: 10.1002/dev.420040205. [DOI] [PubMed] [Google Scholar]
- Martin JC, Martin DC. Voluntary activity in the aging rat as a function of maternal drug exposure. Neurobehav Toxicol Teratol. 1981;3:261–264. [PubMed] [Google Scholar]
- Martin JC, Martin DC, Radow B, Sigman G. Growth, development and activity in rat offspring following maternal drug exposure. Exp Aging Res. 1976;2:235–251. doi: 10.1080/03610737608257179. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Chen L, Jones J. Is maternal smoking during pregnancy a risk factor for attention deficit hyperactivity disorder in children? Am J Psychiatry. 1996;153:1138–1142. doi: 10.1176/ajp.153.9.1138. [DOI] [PubMed] [Google Scholar]
- Milberger S, Biederman J, Faraone SV, Jones J. Further evidence of an association between maternal smoking during pregnancy and attention deficit hyperactivity disorder: findings from a high-risk sample of siblings. J Clin Child Psychol. 1998;27:352–358. doi: 10.1207/s15374424jccp2703_11. [DOI] [PubMed] [Google Scholar]
- Morgan MM, Ellison G. Different effects of chronic nicotine treatment regimens on body weight and tolerance in the rat. Psychopharmacology (Berl) 1987;91:236–238. doi: 10.1007/BF00217070. [DOI] [PubMed] [Google Scholar]
- Niaura R, Bock B, Lloyd EE, Brown R, Lipsitt LP, Buka S. Maternal transmission of nicotine dependence: psychiatric, neurocognitive and prenatal factors. Am J Addict. 2001a;10:16–29. doi: 10.1080/105504901750160420. [DOI] [PubMed] [Google Scholar]
- Niaura R, Shadel WG, Goldstein MG, Hutchinson KE, Abrams DB. Individual differences in responses to the first cigarette following overnight abstinence in regular smokers. Nicotine Tob Res. 2001b;3:37–44. doi: 10.1080/14622200020032088. [DOI] [PubMed] [Google Scholar]
- Paulson RB, Shanfeld J, Vorhees CV, Cole J, Sweazy A, Paulson JO. Behavioral effects of smokeless tobacco on the neonate and young Sprague Dawley rat. Teratology. 1994;49:293–305. doi: 10.1002/tera.1420490409. [DOI] [PubMed] [Google Scholar]
- Paulson RB, Shanfeld J, Vorhees CV, Sweazy A, Gagni S, Smith AR, Paulson JO. Behavioral effects of prenatally administered smokeless tobacco on rat offspring. Neurotoxicol Teratol. 1993;15:183–192. doi: 10.1016/0892-0362(93)90014-f. [DOI] [PubMed] [Google Scholar]
- Peters DA, Tang S. Sex-dependent biological changes following prenatal nicotine exposure in the rat. Pharmacol Biochem Behav. 1982;17:1077–1082. doi: 10.1016/0091-3057(82)90497-x. [DOI] [PubMed] [Google Scholar]
- Peters DA, Taub H, Tang S. Postnatal effects of maternal nicotine exposure. Neurobehav Toxicol. 1979;1:221–225. [PubMed] [Google Scholar]
- Popke EJ, Tizabi Y, Rahman MA, Nespor SM, Grunberg NE. Prenatal exposure to nicotine: effects on prepulse inhibition and central nicotinic receptors. Pharmacol Biochem Behav. 1997;58:843–849. doi: 10.1016/s0091-3057(97)98985-1. [DOI] [PubMed] [Google Scholar]
- Richardson SA, Tizabi Y. Hyperactivity in the offspring of nicotine-treated rats: role of the mesolimbic and nigrostriatal dopaminergic pathways. Pharmacol Biochem Behav. 1994;47:331–337. doi: 10.1016/0091-3057(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Romero RD, Chen WJ. Gender-related response in open-field activity following developmental nicotine exposure in rats. Pharmacol Biochem Behav. 2004;78:675–681. doi: 10.1016/j.pbb.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Samaha AN, Yau WY, Yang P, Robinson TE. Rapid delivery of nicotine promotes behavioral sensitization and alters its neurobiological impact. Biol Psychiatry. 2005;57:351–360. doi: 10.1016/j.biopsych.2004.11.040. [DOI] [PubMed] [Google Scholar]
- Schlumpf M, Gahwiler M, Ribary U, Lichtensteiger W. A new device for monitoring early motor development: prenatal nicotine-induced changes. Pharmacol Biochem Behav. 1988;30:199–203. doi: 10.1016/0091-3057(88)90444-3. [DOI] [PubMed] [Google Scholar]
- Shacka JJ, Fennell OB, Robinson SE. Prenatal nicotine sex-dependently alters agonist-induced locomotion and stereotypy. Neurotoxicol Teratol. 1997;19:467–476. doi: 10.1016/s0892-0362(97)00063-9. [DOI] [PubMed] [Google Scholar]
- Slikker W, Jr, Xu ZA, Levin ED, Slotkin TA. Mode of action: disruption of brain cell replication, second messenger, and neurotransmitter systems during development leading to cognitive dysfunction--developmental neurotoxicity of nicotine. Crit Rev Toxicol. 2005;35:703–711. doi: 10.1080/10408440591007421. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285:931–945. [PubMed] [Google Scholar]
- Sobrian SK, Ali SF, Slikker W, Jr, Holson RR. Interactive effects of prenatal cocaine and nicotine exposure on maternal toxicity, postnatal development and behavior in the rat. Mol Neurobiol. 1995;11:121–143. doi: 10.1007/BF02740690. [DOI] [PubMed] [Google Scholar]
- Sobrian SK, Marr L, Ressman K. Prenatal cocaine and/or nicotine exposure produces depression and anxiety in aging rats. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:501–518. doi: 10.1016/S0278-5846(03)00042-3. [DOI] [PubMed] [Google Scholar]
- Sorenson CA, Raskin LA, Suh Y. The effects of prenatal nicotine on radial-arm maze performance in rats. Pharmacol Biochem Behav. 1991;40:991–993. doi: 10.1016/0091-3057(91)90117-k. [DOI] [PubMed] [Google Scholar]
- Stratton K, Shetty P, Wallace R, Bondurant S. Clearing the smoke: the science base for tobacco harm reduction--executive summary. Tob Control. 2001;10:189–195. doi: 10.1136/tc.10.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizabi Y, Popke EJ, Rahman MA, Nespor SM, Grunberg NE. Hyperactivity induced by prenatal nicotine exposure is associated with an increase in cortical nicotinic receptors. Pharmacol Biochem Behav. 1997;58:141–146. doi: 10.1016/s0091-3057(96)00461-3. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Russell LT, Nespor SM, Perry DC, Grunberg NE. Prenatal nicotine exposure: effects on locomotor activity and central [125I]alpha-BT binding in rats. Pharmacol Biochem Behav. 2000;66:495–500. doi: 10.1016/s0091-3057(00)00171-4. [DOI] [PubMed] [Google Scholar]
- Vaglenova J, Birru S, Pandiella NM, Breese CR. An assessment of the long-term developmental and behavioral teratogenicity of prenatal nicotine exposure. Behav Brain Res. 2004;150:159–170. doi: 10.1016/j.bbr.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS, Lahey BB, Loeber R, Green SM, Gordon RA, Leventhal BL. Maternal smoking during pregnancy and the risk of conduct disorder in boys. Arch Gen Psychiatry. 1997;54:670–676. doi: 10.1001/archpsyc.1997.01830190098010. [DOI] [PubMed] [Google Scholar]
- Yanai J, Pick CG, Rogel-Fuchs Y, Zahalka EA. Alterations in hippocampal cholinergic receptors and hippocampal behaviors after early exposure to nicotine. Brain Res Bull. 1992;29:363–368. doi: 10.1016/0361-9230(92)90069-a. [DOI] [PubMed] [Google Scholar]


