Abstract
Fifteen years ago, Pascual-Leone and colleagues used transcranial magnetic stimulation (TMS) to investigate speech production in pre-surgical epilepsy patients and in doing so, introduced a novel tool into language research. TMS can be used to non-invasively stimulate a specific cortical region and transiently disrupt information processing. These “virtual lesion” studies offer not only the ability to explore causal relations between brain regions and language functions absent in functional neuroimaging, but also spatial and temporal precision not typically available in patient studies. For instance, TMS has been used to demonstrate functionally distinct subregions of the left inferior frontal gyrus; to clarify the relationship between pre-morbid language organisation and susceptibility to unilateral lesions; and to investigate the contribution of both left and right hemisphere language areas in recovery from aphasia. When TMS is used as a measure of functional connectivity, it demonstrates a close link between action words and motor programs; it suggests a potential evolutionary link between hand gestures and language; and it suggests a role in speech perception for the motor system underlying speech production. In combination with functional neuroimaging, it can elucidate the circuits responsible for this involvement. Finally, TMS may even be useful for enhancing recovery in aphasic patients. In other words, TMS has already become an important tool for studying language at both the cognitive and neural levels, and it is clear that further developments in TMS methodology are likely to result in even greater opportunities for language research.
Language can be stimulating. Throughout history poets, preachers, and politicians have utilised language to stimulate their audience. The ability to stimulate language, in contrast, is a modern development largely due to the pioneering work of the neurosurgeons Wilder Penfield and George Ojemann who used direct cortical stimulation in awake neurosurgical patients to probe language (Ojemann, 1979; Penfield and Jasper, 1954). For instance, Ojemann showed that the application of a direct current to a focal brain region could not only evoke spontaneous vocalisations but could also selectively disrupt specific linguistic processes (Ojemann, 1983). Although this work continues to provide novel insights (e.g. Engel et al., 2005; Matsumoto et al., 2004), its utility is limited by its highly invasive nature and questions regarding the generality of the results given the prolonged neurological dysfunction associated with intractable epilepsy.
In 1985 Barker and colleagues (1985) introduced a non-invasive alternative to brain stimulation called transcranial magnetic stimulation (TMS) in which a rapidly changing current within a conducting coil is used to induce a strong, but relatively focal, magnetic field. When the coil is placed on the scalp, the magnetic field induces a physiological response (i.e. depolarisation and/or spiking) in the underlying neural tissue (Jahanshahi and Rothwell, 2000; Pascual-Leone et al., 2000). This introduces transient noise into the neural computation being performed which, in most brain regions, can lead to longer reaction times (RTs) or even errors. Consequently, a TMS-induced change in behaviour can be used to investigate causal relations between specific brain regions and individual cognitive functions (Pascual-Leone et al., 1999; Walsh and Rushworth, 1999).
Like patient studies, TMS can be used to draw causal inferences, as the cortical disruption induced by stimulation can act like a “virtual lesion” lasting from tens of milliseconds up to approximately one hour, depending on the specific type of stimulation (Pascual-Leone et al., 2000). Moreover, TMS avoids some of the well known difficulties of patient studies, which limit their interpretation, including potential differences in pre-morbid ability, compensatory plasticity following the lesion, the large and varied extents of naturally occurring lesions, and damage to sub-adjacent fibres-of-passage. By comparing stimulated to unstimulated trials, participants in TMS experiments act as their own controls, avoiding the potential confound of pre-morbid differences. In addition, there is insufficient time for functional re-organisation to occur during single TMS events. Consequently, the results should not be substantially confounded by any recovery processes (Walsh and Cowey, 1998). Finally, the induced disruption is generally more focal than naturally occurring lesions and does not affect deep white matter pathways.
There are, of course, disadvantages to TMS as well. These include the need to choose appropriate stimulation parameters (single vs. repetitive stimulation, intensity, timing, location, type of coil, coil orientation, etc.), restricted access limited to only surface structures, and the fact that TMS produces both sound and somatosensory stimulation which can also influence behaviour (Walsh and Rushworth, 1999). Occasionally, TMS can cause discomfort or pain, primarily when stimulation affects either muscles on the head or peripheral cranial nerves, although it may be possible to adjust the stimulation parameters to reduce this problem. In addition, the basic physiological mechanisms underlying TMS effects are not yet fully understood (Di Lazzaro et al., 1998; Houlden et al., 1999; Rothwell, 1997), complicating the interpretation of results.
Even so, TMS offers a spatial and temporal resolution rarely available in patient studies and complements the information available from functional neuroimaging techniques such as event-related potentials (ERPs), magnetoencephalograhy (MEG), positron emission tomography (PET) and functional magnetic resonance imaging (fMRI). In addition, TMS can be used with neurological patients to investigate the mechanisms of recovery (Winhuisen et al., 2005) and, in some cases, may even be useful for enhancing recovery in aphasic patients (Naeser et al., 2005a).
TMS can also be used to measure functional connections between linguistic processes and the motor cortex, by measuring motor excitability during various language tasks. In this mode, stimulation of the motor cortex elicits a contraction of specific muscles observable often as a twitch of the muscle and measurable as a motor-evoked potential (MEP) that serves as an index of motor excitability. When a language task enhances MEPs in a region of motor cortex, this provides evidence of a functional link between the task and the motor cortex. In contrast to the use of TMS to disrupt neural computation, this mode of use provides a quantifiable measure of behavioural facilitation (i.e. a positive effect) that may be more sensitive. The lack of a clear understanding of the basis of this apparent “priming,” however, limits the interpretation.
Here, we review these functions of TMS and their role in developing a more complete understanding of the neurological basis of language. Our aim is not to synthesize a single model of language (see, for instance, Levelt, 1999; Price, 2000) but to emphasize the novel contributions of TMS and to highlight its relationship with other tools of cognitive neuroscience. Consequently, we have not attempted to review all TMS studies on language (see Devlin and Watkins, 2006) nor to provide a detailed account of TMS methodology (see Pascual-Leone et al., 1999; Paus, 2005; Walsh and Pascual-Leone, 2003), but instead focus on a few, highly informative areas where TMS has significantly extended our knowledge of either the cognitive or neural basis of language.
Speech production
The first language study to use TMS was by Pascual-Leone and colleagues (1991) who induced speech arrest in pre-surgical epilepsy patients in order to determine whether TMS could be used as a non-invasive alternative to intracarotid amobarbital testing (IAT, Wada and Rasmussen, 1960). Trains (10s) of repetitive TMS (rTMS) were delivered at rates of either 8, 16 or 25Hz over 15 different scalp positions surrounding peri-Sylvian cortex in each hemisphere defined by the International 10–20 electrode system. Patients were asked to count aloud from “one” and 4–6 sec after stimulation of the left inferior frontal cortex, a reproducible speech arrest was observed in each of the six patients. One noted, “I could move my mouth and I knew what I wanted to say, but I could not get the numbers to my mouth” (p. 699, Pascual-Leone et al., 1991). In contrast, no speech arrest was seen during any right hemisphere stimulation. IAT with these same patients revealed left hemisphere language dominance in all six, suggesting that the TMS-induced speech arrest offered a non-invasive alternative for determining language dominance.
Subsequent studies, however, have called into question the usefulness of rTMS in pre-surgical planning. Jennum et al. (1994) were only able to induce complete speech arrest in 14/21 patients, although when slowed speech was included there was still a 95% concordance between the rTMS and IAT findings. In contrast, Michelucci et al. (1994) produced speech arrest in only 7/14 subjects which, they argued, called into question the reliability of rTMS for determining language dominance. Epstein et al. (1996) hypothesized that some of this inter-study variability may have been due to the specific stimulation parameters chosen, and systematically investigated the effects of both intensity and rate of stimulation on speech arrest in normals. As expected, higher intensities led to stronger speech arrest effects, but surprisingly it was the lower rates of stimulation (4–8Hz) that were more reliable at inducing speech arrest than those used in previous studies (16–32Hz, Jennum et al., 1994; Michelucci et al., 1994; Pascual-Leone et al., 1991). Higher frequencies led to prominent facial and laryngeal muscle contractions and significantly increased the discomfort or pain associated with stimulation, making speech arrest more difficult to determine. Stimulation at 4Hz, on the other hand, not only consistently disrupted highly over-learned speech such as counting, it also interfered with reading aloud and spontaneous speech (Epstein et al., 1999). Consequently, this paradigm was used to test the reliability of rTMS relative to IAT in 16 pre-surgical epilepsy patients (Epstein et al., 2000). rTMS indicated left hemisphere language dominance for twelve patients and right dominance for the remaining four while IAT indicated that all sixteen patients were left dominant. Despite the significant correlation between IAT and rTMS (r=0.57, p<0.05), rTMS over-estimated right hemisphere involvement in a considerable proportion of patients. The IAT findings were a better predictor of post-operative language difficulties than the rTMS. As the purpose of determining language dominance is to minimize the impact of the surgical resection on language abilities, rTMS appears to be less reliable than IAT.
The comparison, however, is not entirely a fair one. IAT affects the functioning over a large region of one hemisphere for several minutes, whereas TMS disruption is far more focal and transient, particularly with rTMS at rates higher than 1Hz. As mentioned above, not all sites are accessible to stimulation as they may be located at different depths from the scalp or oriented differently in the two hemispheres. It may be important, therefore, to independently assess effects in each hemisphere using a variety of stimulation parameters before accepting potentially false negative results with regard to their involvement or relative involvement of each hemisphere in a task. Nonetheless, there is still reason to be optimistic regarding the possibility of using TMS as a reliable alternative to IAT. Studies by Stewart et al. (2001) and Aziz-Zadeh et al. (2005) showed that speech arrest can be induced from two different sites within the inferior frontal cortex. At the more posterior site, stimulation of either the left or right hemisphere induced speech arrest, although the effect was typically stronger on the left. In addition, stimulation at these sites evoked a clear facial muscle response as measured with electromyography (EMG). In contrast, only left hemisphere stimulation of the more anterior site led to speech arrest and it did not evoke an EMG response. The authors hypothesized that the posterior site may correspond to the ventral limb of the precentral gyrus where both motor and premotor regions enervate the mouth and jaw. Consequently, stimulation of either hemisphere can induce speech arrest by interfering with the motor output of speech. The more anterior site may correspond to prefrontal cortex (i.e. Broca’s area in the left hemisphere) where stimulation would be expected to interfere with the formation of an articulatory plan rather than the implementation of the motor sequence. These findings may help to explain the relatively high concordance between rTMS and IAT in some studies (Jennum et al., 1994; Pascual-Leone et al., 1991) but not others (Epstein et al., 2000; Michelucci et al., 1994). Further work is clearly warranted to determine whether anterior inferior frontal stimulation corresponds more closely with IAT and can accurately predict post-operative language deficits following surgical intervention in intractable epilepsy.
As in the “virtual lesion” studies of language, the earliest work examining motor excitability during speech production was aimed at assessing lateralisation of function (Tokimura et al., 1996). MEPs in a hand muscle were measured in response to single pulses of TMS over the hand area of the contralateral motor cortex while subjects read aloud, read silently, spoke spontaneously or made non-speech vocal sounds. The MEP size was facilitated equally for left and right hemisphere stimulations during spontaneous speech, whereas this effect was lateralised to the dominant hemisphere during reading aloud. In contrast, the silent reading condition and the non-speech sound production did not result in changes in excitability. In other words, the authors demonstrated a functional connection between speech output and the hand area of the left motor cortex.
Recent studies confirm and refine this finding. The basic laterality effect has been replicated in several studies (but see Floel et al., 2003; Lo et al., 2003; Meister et al., 2003; Seyal et al., 1999) demonstrating that the functional link between speech production and the hand motor area is left lateralized. In addition, Meister et al. (2003) found evidence that the increased excitability of the hand area during reading aloud was restricted to the hand area as MEPs from the leg area were unchanged by the task (but see Lo et al., 2003).
It seems, then, that speech production increases motor excitability not only in the face area of the left hemisphere, but also in the hand area (see also Saarinen et al., 2006; Salmelin and Sams, 2002). This evidence for a functional link between the hand area and language may reflect the irrepressible use of hand gestures when speaking or indicate an evolutionary link in the development of speech and language through hand gestures (Corballis, 2003; Gentilucci and Corballis, 2006; Rizzolatti and Arbib, 1998).
Speech perception and the motor system
It may seem obvious that speech production affects motor system excitability, but it is potentially surprising that perception should as well. The initial studies to investigate production-perception links focused on visual perception of hand actions, with an aim to providing evidence for an action observation-execution matching mechanism in the human brain (e.g. Gangitano et al., 2004; Strafella and Paus, 2000) akin to the “mirror-neuron” system in the macaque brain (di Pellegrino et al., 1992; Gallese et al., 1996). As in the case of mirror neurons recorded from macaque ventral premotor cortex during auditory perception of actions (Kohler et al., 2002), Aziz-Zadeh et al. (2004) used TMS to show increased excitability of the motor system underlying hand actions while subjects listened to sounds associated with actions performed by the hands. Interestingly, the facilitation of MEP size seen for these bimanual sounds (tearing paper, typing) was lateralised to the left hemisphere. In other words, in both humans and monkeys, hearing a sound known to be related to a particular hand action activates that corresponding motor system.
In the speech domain, the suggestion of motor involvement in perception was not new – Liberman and colleagues (1967) had proposed that phonemes were perceived by mapping them onto the articulatory gestures used in speech production (Liberman and Mattingly, 1985). The TMS studies described below are consistent with a role in speech perception for the motor system underlying speech production, where presumably articulatory gestures are represented; they are, in part, responsible for renewed interest in the motor theory of speech perception.
To date, three studies used TMS to examine the effects of speech perception on the motor system underlying speech production. The first of these found increased MEP size in the lip muscles (orbicularis oris) during visual observation of speech that required lip movements (e.g. /ba/ vs. /ta/, Sundara et al., 2001). Auditory perception of the same syllables, however, did not facilitate motor excitability. In contrast, Watkins and colleagues (2003) found that both visual and auditory perception of speech separately facilitated MEP responses (Figure 1a and b). EMG was recorded from the lips (orbicularis oris) while subjects listened to continuous prose passages while viewing noise or viewed lip movements during continuous speech while listening to white noise. MEP size was significantly facilitated during both auditory and visual speech-perception, but only for stimulation over the left hemisphere and not for the right (Figure 1b). Finally, Fadiga and colleagues (2002) found that auditory presentation of specific phonemes facilitated motor excitability measured from specific muscles used in the production of those phonemes. They measured MEPs from the tongue while subjects passively listened to stimuli with or without consonant sounds that required tongue movements in their production such as the labiodental “rr” in the Italian word “terra” or the lingua-palatal frictative “ff” in the Italian word “zaffo”. MEP size was facilitated when subjects listened to words and nonwords containing the “rr” phoneme (i.e. requiring tongue movements in their production) but not the “ff” phoneme (i.e. which did not require tongue movement in production). These data suggest that the representation of the specific articulatory gesture used to produce a phoneme is “primed” or “excited” by auditory perception of this phoneme. Such conclusions are reminiscent of predictions made by Liberman’s motor theory of speech perception. Whether this increased excitability reflects a corollary of the perception process or in some way aids perception remains to be tested.
Figure 1.
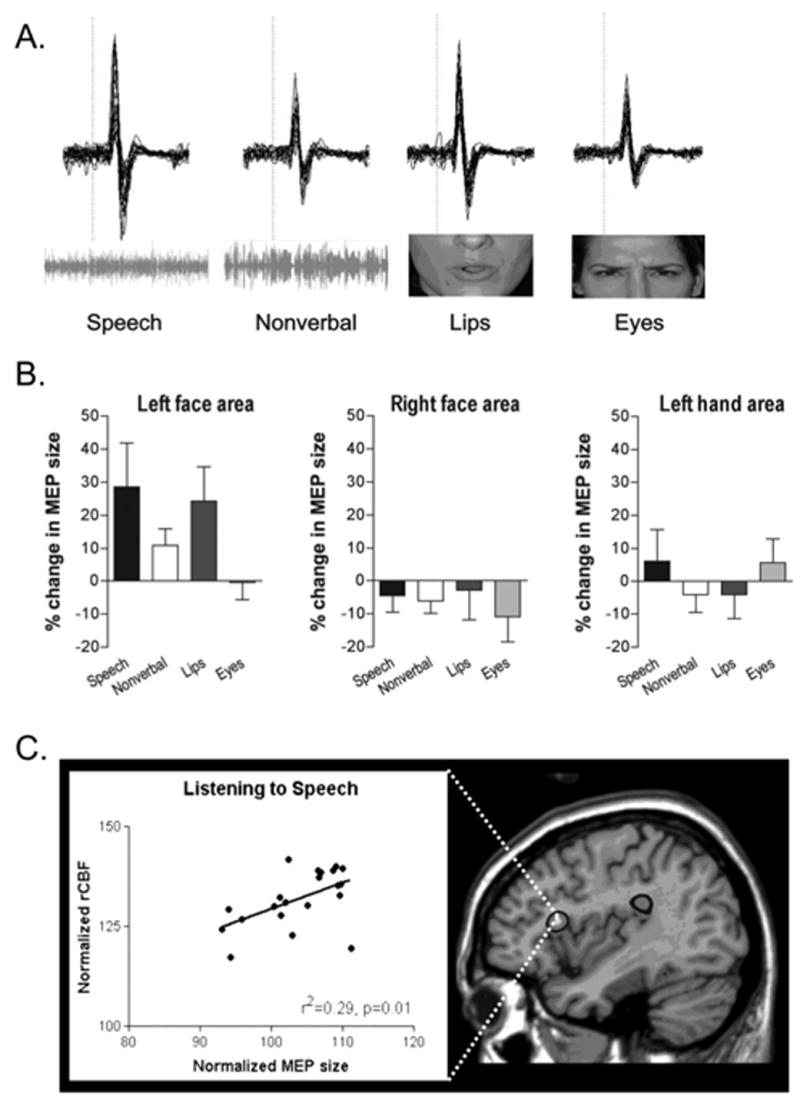
Motor excitability during speech perception. A) Data from stimulation of the left primary motor face area in a single subject when listening to speech, listening to non-verbal sounds, viewing speech, and viewing eye movements. EMG recordings from individual trials are superimposed and the dotted line indicates the time of stimulation. The horizontal bar represents 10msec and the vertical bar 0.5mV. B) Average MEP sizes for the same four stimulus conditions with stimulation to the left face area, right face area, and hand area of motor cortex. The x-axis through the 0% level represents the mean MEP size in the control condition and error bars represent standard error of the mean. Panel A) & B) are modified and reprinted from Neuropsychologia, 41, Watkins, K. W., Strafella, A. P. and Paus, T. “Seeing and hearing speech excites the motor system involved in speech production”, pp. 990–992, Copyright (2003), with permission from Elsevier. C) The relation between regional cerebral blood flow in Broca’s area and the size of the MEP evoked by single pulse TMS over the mouth region of primary motor cortex (left panel). On the right, an activation map showing the anatomical location of the significant positive relationship illustrated in the graph. Reprinted from Journal of Cognitive Neuroscience, 16(6), Watkins, K. W. and Paus, T. “Modulation of motor excitability during speech perception: The role of Broca’s area”, pp. 990–992, Copyright (2004), with permission from MIT Press.
TMS studies of this type reveal a functional connection between speech comprehension and specific components of the motor system but they do not provide any anatomical information to suggest how this link is mediated. By combining TMS and PET, Watkins and Paus (2004b) investigated the brain regions that mediate the change in motor excitability during speech perception. They obtained measures of motor excitability by TMS over the face area of left motor cortex eliciting MEPs from orbicularis oris muscle during auditory speech perception. These measures were then correlated with regional cerebral blood flow measures across the whole brain obtained simultaneously. Increased motor excitability during speech perception correlated with blood flow increases in the posterior part of the left inferior frontal gyrus (Broca’s area; see Figure 1c), the human homologue of the region containing mirror neurons in the macaque (Kohler et al., 2002). In other words, Broca’s area plays a central role in linking speech perception with speech production, consistent with theories that emphasize the integration of sensory and motor representations in understanding speech (Hickok and Poeppel, 2000; Scott and Wise, 2004).
The results from these studies support the notion that passive speech perception induces activation of brain areas involved in speech production. The increased motor excitability of the speech production system could reflect covert imitative mechanisms or internal speech, which might, in turn, improve comprehension of the percept. From this limited set of initial studies in speech perception, two conclusions arise: increased motor excitability during auditory perception of actions is specific to the effector used to produce the sound (Fadiga et al., 2002), and understanding actions through sound is lateralised to the left hemisphere (Aziz-Zadeh et al., 2004; Watkins et al., 2003).
Syntax, verbs and action
TMS is not limited to studying production and perception mechanisms but can also provide fundamental insights to central aspects of language such as grammar. For instance, TMS has demonstrated a link between grammatical processing and left prefrontal cortex, which is in accord with a number of patient studies (e.g. Shapiro and Caramazza, 2003; Zurif et al., 1972). Sakai et al. (2002) investigated whether, and when, Broca’s area was involved in syntactic processing using a sentence validation task. Participants viewed sentences and had to identify each as correct, grammatically incorrect, or semantically incorrect. All sentences used a simple noun phrase–verb phrase (NP–VP) construction, with the VP appearing 200msec after the NP. TMS to Broca’s area was delivered 0, 150, or 350msec after the VP onset. Relative to sham stimulation, TMS selectively facilitated RTs for syntactic, but not semantic, decisions and the effect was specific to the 150msec time window, which the authors interpreted as strong evidence that Broca’s area is causally involved in syntactic processing.
As mentioned earlier, this type of facilitation effect is somewhat difficult to interpret for two reasons. First, it is clear that stimulation can lead to reductions in RTs due to inter-sensory facilitation, which need to be ruled out carefully (Walsh and Pascual-Leone, 2003). Second, stimulation induces small currents in neural tissue, which are expected to introduce noise and therefore interfere with, rather than facilitate, processing. It is theoretically possible that subthreshold stimulation could “prime” the tissue and thereby enhance processing, but there is no clear physiological evidence to support this hypothesis. Consequently, the fact that Broca’s area stimulation influenced behaviour in the syntactic but not semantic condition suggests that Broca’s area was involved in syntactic processing.
What was particularly intriguing about this study, however, was the timing of the TMS effect – 150msec after the onset of the VP. Electrophysiological studies often report a waveform called the early left anterior negativity (ELAN), which is sensitive to syntactic processes and occurs in roughly that same time window (150–200msec) (Friederici, 2002). The ELAN effect, however, is generally associated with morpho-syntactic violations such as disagreement between a noun and verb form (e.g. “he lie” rather than “he lies”) whereas in Sakai et al.’s 2002 study, the syntactic violation was a disturbance of verb argument structure (e.g. “someone lies snow”). Here, “lies” does not take a direct object because one cannot “lie snow” and this type of syntactic violation is more typically associated with an N400 – P600 complex, occurring much later (Friederici and Kotz, 2003). In other words, there appears to be a timing mismatch between this TMS study and previous ERP findings, with TMS revealing effects that occur considerably before they are seen in ERP. This mismatch may be due to TMS “priming” the region before it was required for the syntactic judgments or it may reflect the fact that synchronized neuronal activity necessary to produce an ERP or MEG signal is delayed relative to the physiological source (Walsh and Cowey, 2000; Walsh and Pascual-Leone, 2003). Clearly, systematic comparisons between the neurophsyiological measures (electrical and magnetic) of the time course of information processing will be necessary to determine the temporal signatures underlying language processes.
The other aspect of grammar that has been investigated with TMS is that of grammatical class. Both neuropsychological (Caramazza and Hillis, 1991) and electrophysiological studies (Federmeier et al., 2000) suggest that there may be different neural substrates for processing nouns and verbs. Cappa and colleagues (2002) postulated that verb-specificity may be due to the close relation between verbs and actions and used TMS to investigate the role of left dorso-lateral prefrontal cortex (DLPFC) in action naming. A set of Italian speaking participants were shown pictures of common objects and asked to either name the object (e.g. “telefono” [a telephone]) or the associated action (“telefonare” [to telephone]). rTMS of left DLPFC decreased naming latencies for verbs relative to right DLPFC and sham stimulation. In contrast, the latencies for object naming were unaffected. Based on this condition-specific facilitation effect, the authors suggested that verbs may be preferentially impaired by left frontal lesions because damage to DLPFC affects action observation and representation which are more tightly linked with verbs than nouns.
The work of Shapiro et al. (2001), however, calls this interpretation into question. In their study, participants were asked to inflect nouns and verbs (e.g. “song” → “songs” or “sing” → “sings”) either before or after 10mins of 1Hz stimulation over left DLPFC. RTs were significantly slowed for verbs, but not nouns. In order to determine whether this effect was due to the action-related meaning of the verbs, a second experiment used pseudowords (e.g. “flonk”) treated as either nouns or verbs. Because pseudowords do not have any associated meaning, the authors reasoned that TMS would only affect RTs if the region was important for processing the grammatical class of verbs rather than words with action-related meanings. Once again, DLPFC stimulation selectively slowed RTs only in the verb condition – a finding interpreted as evidence for a neuroanatomical basis for grammatical categories per se rather than a byproduct of the differences in meaning between nouns and verbs.
Despite the fact that DLPFC stimulation led to facilitation in one study (Cappa et al., 2002) but inhibition in the other (Shapiro et al., 2001), the data strongly suggest that left DLPFC is preferentially important for processing verbs relative to nouns, although its precise role remains unclear. It is theoretically possible that a particular portion of left DLPFC is dedicated to processing the grammatical class of verbs but this seems unlikely given the wide variety of non-linguistic tasks that also engage the region (Duncan and Owen, 2000; Petrides, 2000). In contrast, the relation between verbs and actions is appealing, if for no other reason than verbs imply acts via their thematic roles, even when the action is either unspecified (“flonks”) or not particularly active (“sleeps”). That is, “he flonks” suggests someone (an agent) who is flonking (an action) whereas “the flonks” suggests multiple somethings (no action). Additional evidence for this relation between verbs and actions comes from studies highlighting functional connections between language and the motor system.
fMRI has been used to demonstrate somatotopic brain activation when subjects passively read words that refer to actions executed by different effectors such as the feet (“kick”), hands (“pick”) and mouth (“lick”) (Hauk et al., 2004). The findings suggest a functional link between the meaning of the words and specific motor centres that would be used to execute corresponding actions. Buccino et al. (2005) used TMS to measure this even more directly. In their experiment, MEPs were measured from the hand and foot muscles while subjects listened to sentences related to hand-actions (e.g. “he sewed the skirt”), foot-actions (e.g. “he jumped the rope”) or more abstract actions (e.g. “he forgot the date”). MEPs recorded from hand muscles were significantly modulated by sentences referring to hand actions but not foot or abstract actions. Similarly, sentences with foot, but not hand or abstract, actions modulated MEP responses in the foot muscle. In other words, the size of the MEP in each effector muscle was only affected when listening to sentences containing actions related to that effector. A similar study by Pulvermüller et al. (2005) showed that single pulse TMS over the arm or leg motor cortex in the left hemisphere led to faster reaction times on lexical decisions for actions related to arms (e.g. “folding”) and legs (e.g. “stepping”), respectively. This selective speed-up may have been due to sub-threshold stimulation (i.e. below that necessary to evoke an MEP) “priming” subjects, perhaps by partially activating the representation of actions related to the specific effector being stimulated.
Although none of these studies specifically investigated verbs, their results nonetheless provide additional evidence for the close relation between verbs and actions by demonstrating a functional link between the meaning of individual verbs and regionally-specific enhanced excitability of the motor cortex. As mentioned previously, stimulation of left dorso-lateral prefrontal cortex (DLPFC) preferentially affected verbs relative to nouns (Cappa et al., 2002; Shapiro et al., 2001). This may indicate that the functional link with hand or leg regions of motor cortex is mediated via DLPFC in much the same way the ventrolateral prefrontal cortex mediates the link between perceiving speech and the mouth region of motor cortex (Watkins and Paus, 2004a).
Functional anatomy of Broca’s area
Another area where TMS has provided novel insights concerns the functional organisation of Broca’s area. Although traditionally associated with both speech production and syntactic processing, Broca’s area is part of a larger region that plays an important role in processing meaning, sounds, and syntax as well as many non-linguistic functions (Hagoort et al., 2004; Levy and Anderson, 2002; Zurif et al., 1972). Functional imaging studies have suggested that within the left inferior frontal gyrus (LIFG), the site of Broca’s area, there is a rostro-caudal division of labor for semantic and phonological processing that was not apparent from previous neuropsychological studies (Buckner et al., 1995; Fiez, 1997). This claim has received considerable support from recent TMS studies that not only confirm this division-of-labor, but also clarify the specific regional contributions to semantic and phonological processing. Devlin et al. (2003) investigated whether stimulation of rostral LIFG interfered with simple semantic decision such as deciding whether a visually presented word referred to man-made (e.g. “kennel”) or natural object (e.g. “dog”). Relative to no stimulation, TMS significantly increased RTs in the semantic task, but not when participants focused on visual properties of the presented words. Similarly, Kohler et al. (2004) used fMRI-guided rTMS to stimulate rostral LIFG and showed that semantic decisions were significantly slowed, consistent with the claim that rostral LIFG is necessary for semantic processing. The other half of this division-of-labor was investigated by Nixon and colleagues (2004) who examined whether stimulation of caudal LIFG interfered with a phonological working memory task. Participants saw a word on a computer screen (e.g. “knees”) and then held it in memory during a 1–2 sec delay before deciding whether it sounded the same as a subsequently presented non-word (e.g. “neaze”). rTMS during the delay period selectively increased the error rate during the phonological task, but not of a comparable visual working memory task. Aziz-Zadeh et al. (2005) also included a phonological task in their study of speech arrest by investigating “covert speech arrest,” which was measured by participants silently reading a visually presented word and counting its syllables. Once again, rTMS over caudal LIFG increased RTs relative to unstimulated trials, consistent with a role in phonological processing. Taken together these studies significantly extend the previous neuroimaging results by demonstrating that rostral LIFG is necessary for semantic processing while caudal LIFG is necessary for phonological processing.
A number of functional imaging studies, however, have shown that phonological and semantic tasks commonly engage both rostral and caudal LIFG (Barde and Thompson-Schill, 2002; Devlin et al., 2003; Gold and Buckner, 2002) raising the possibility that both regions are necessary for both types of processing. In other words, LIFG may act as a single functional region that is required for semantic and phonological processing with regional shifts in the peak activation, or there may be sub-regions within LIFG specialized for semantic and phonological processing but co-activated due to incidental processing (Price et al., 1996; Raichle et al., 1994). The TMS results do not distinguish between these possibilities as each of the single dissociations would be predicted by both accounts. Consequently, Gough and colleagues (2005) designed a TMS experiment to test for a double dissociation between semantic and phonological processing in LIFG. Participants saw two letter strings presented simultaneously on a computer screen and had to decide whether they meant the same (e.g. “idea-notion”), sounded the same (e.g., “nose-knows”), or looked the same (e.g. “fwtsp-fwtsp”). Relative to no stimulation, TMS of rostral LIFG selectively increased response latencies when participants focused on the meaning of simultaneously presented words (i.e. semantics) but not when they focused on the sound pattern of the words (i.e. phonology). In contrast, the opposite dissociation was observed with stimulation of caudal LIFG, where stimulation selectively interfered with the phonological, but not semantic, task (Figure 2). Neither site of stimulation affected the RTs in the visual control task. In other words, the authors demonstrated a functional double dissociation for semantic and phonological processing within LIFG in sites separated by less than 3cm. Although this double dissociation was first suggested by functional imaging, it required the spatial precision of TMS to independently disrupt the regions and clarify their distinct contributions to word processing.
Figure 2.

Effects of stimulation on rostral and caudal LIFG. A) The bar plots show the mean normalized TMS effects as percent change in reaction times from the non-TMS baseline during synonym judgments (left), homophone judgments (middle), and visual matching (right). Error bars indicate the standard error of the mean and significant differences are indicated with an * (p<0.05). B) The bottom panel shows the location of stimulation sites for four participants on their mean structural image with rostral locations marked with crosses and caudal locations marked with circles. Next to it is a 3D rendering with the stimulation sites shown as ovals representing the spatial 85% confidence interval. Stimulation sites were on average 2.5cm apart on the cortical surface. From Gough et al. (2005), copyright 2005 by the Society for Neuroscience.
TMS and aphasia
Finally, TMS has been particularly beneficial in evaluating the neural mechanisms of compensation following aphasic brain injury. Questions of central importance to both cognitive and clinical neuroscience include “What are the mechanisms that support recovery following damage?” and “Can these be enhanced to improve outcomes?” In both cases, TMS is providing new insights.
It is often assumed that following left hemisphere damage, homologue areas in the right hemisphere are recruited to (at least partially) take over lost functions. For instance, Coltheart (1980) suggested that following extensive left hemisphere lesions, the right hemisphere is capable of supporting partial reading ability primarily limited to high frequency, concrete nouns (e.g. “apple” but not “cognition”). Functional imaging studies confirm that such patients activate their right hemisphere when reading, but the activation is also present in neurologically normal control subjects (Price et al., 1998). To explore whether right hemisphere involvement in reading was qualitatively different between “right hemisphere readers” and controls, Coslett and Monsul (1994) delivered a single TMS pulse to the right temporo-parietal junction at 145msec after the onset of a visual word. In the patient but not controls, this significantly reduced the number of correctly read words from 17/24 without TMS to 5/24 with TMS. Such a dramatic effect on accuracy using single pulse stimulation is rare, if not unique, and suggests that reading processes in this patient were particularly fragile. Moreover, the pattern of errors induced by TMS was an exaggeration of normal difficulty effects (i.e. more errors for low than high frequency items) – exactly opposite to what one would expect for “right hemisphere reading.” If the right hemisphere selectively supports highly frequent, concrete items, then stimulation of this hemisphere should preferentially impair those items. In contrast, right hemisphere stimulation affected low frequency items to a greater extent. So while the findings do not support the right-hemisphere reading hypothesis, they do provide clear evidence that in this patient, reading relied on the right temporo-parietal junction to a greater extent than in normals.
Other studies have investigated the role of the right inferior frontal gyrus in patients recovering from either left hemisphere strokes (Winhuisen et al., 2005) or brain tumours (Thiel et al., 2005) using a combination of positron emission tomography (PET) and TMS. In both cases, PET was used to identify activation in the left and right inferior frontal gyri for each subject as they performed either a verb-picture matching or a word generation task. The activations were then used to individually target TMS to the site of activated tissue in each hemisphere. In one study, all eleven stroke patients showed LIFG activation and stimulating this region increased latencies or errors in 10/11 patients (Winhuisen et al., 2005). 5/11 patients showed stronger activation in RIFG than LIFG and of these, four had longer response latencies with RIFG stimulation. In the other study, all 14 of the tumour patients showed LIFG activation with 7/14 also showing RIFG activation (Thiel et al., 2005). Once again, a majority of patients (11/14) showed latency increases with LIFG stimulation while the five patients with the most rightward activation asymmetry also showed a latency increase with RIFG stimulation. Taken together these findings demonstrate that in most patients, LIFG remains essential for word generation tasks even after left hemisphere damage. Moreover, in a subset of patients RIFG is also essential, but only in those showing the strongest rightward asymmetries.
Why do only some patients show evidence of compensatory right hemisphere involvement? One possibility is that this reflects differences in pre-morbid language organisation. To investigate this, Knecht and colleagues (2002) first identified a set of neurologically normal participants who varied in their degree of language lateralization. Functional transcranial Doppler sonography (fTCD) was used to measure hemispheric perfusion increases during a word generation task across a large sample of the population (Knecht et al., 2000) and then 20 subjects were selected who covered the full range of hemispheric dominance from strongly left to strongly right lateralized. Each performed a picture-word verification task before and after 10mins of 1Hz stimulation over either left or right Wernicke’s area. Participants with left, but not right, language dominance were significantly slowed by left hemisphere stimulation while the opposite pattern was observed for right hemisphere stimulation. In addition, the amount of interference correlated with the degree of language lateralisation – in other words, strongly lateralised subjects were more severely affected by unilateral TMS than those with more bilateral language organisation. Pre-morbid differences, therefore, render the right hemisphere more or less receptive for language before any re-organisation takes place and may play an important role in determining the likelihood of right hemisphere compensation following left-sided damage. In addition, these findings provide strong evidence for the hypothesis that crossed aphasia – that is, aphasia resulting from purely right hemisphere lesions – is a result of atypical pre-morbid organisation.
Finally, among the most intriguing patient studies are those in which TMS is used to actually enhance recovery. In such studies, longer term effects are induced by stimulation sessions repeated daily for over a week and sometimes for several weeks. Hoffman et al. (1999), for example, hypothesized that auditory hallucinations are due to over-activation in auditory cortex and used a 4-day schedule of 1Hz stimulation over left auditory association cortex to suppress activity in the region. After treatment, all three patients reported reduced auditory hallucinations and in two of the patients, the effect lasted for 2+ weeks. A similar approach was adopted in a series of studies by Naeser, Martin and colleagues (Martin et al., 2004; Naeser et al., 2005a; Naeser et al., 2005b) who investigated whether rTMS could be used to improve recovery in chronic non-fluent aphasics. In such patients, strong right hemisphere activation is often observed, even in the absence of behavioural improvements (Naeser et al., 2004). In order to reduce this potentially maladaptive right hemisphere activation, 10 mins of 1Hz rTMS was delivered to each of four different right hemisphere peri-Sylvian sites including rostral RIFG, caudal RIFG, posterior superior temporal gyrus, and the mouth area of primary motor cortex in six non-fluent patients. Only following rostral RIFG stimulation were the patients able to correctly name more pictures than before TMS (Martin et al., 2004). Consequently, this region was targeted for 20mins each day with a ten-day regime of 1Hz rTMS in four of the patients to determine whether a lasting facilitation could be achieved (Naeser et al., 2005b). Immediately following the final rTMS session, picture naming performance was significantly enhanced in each patient but the critical finding was that these effects were still present two months later without any additional TMS sessions nor intervening speech therapy (Figure 3a, Naeser et al., 2005b). One patient was seen again eight months after TMS treatment and her performance remained stable at a level significantly better than before treatment (Figure 3b, Naeser et al., 2005a). Admittedly these are preliminary findings based on only a small set of patients (n=4) and no control groups. The results are nonetheless remarkable in that they demonstrate a very long lasting effect of TMS – far beyond transient first-order effects of stimulation. Presumably TMS modulated activity throughout the language system via cortico-cortico spreading (Ilmoniemi et al., 1997; Paus et al., 1997), and this activity was sufficient to promote plastic changes (i.e. re-organisation) that improved performance. Although speculative, the underlying mechanism is similar to Ramachandran et al’s. 1995 explanation for why visual perception of limb movements can help patients recover from phantom-limb syndrome. In that case, the induced sensori-motor plasticity was driven by an endogenous top-down signal due to the illusion of moving the phantom arm, whereas in the aphasic patients, TMS was an exogenous source of input driving the plasticity. Regardless of the mechanism, the results are encouraging. If these findings can be independently replicated and shown to rely on a controlled regimen of TMS “treatments” then rTMS might become an important part of rehabilitative therapy to improve recovery, at least in some aphasic patients.
Figure 3.
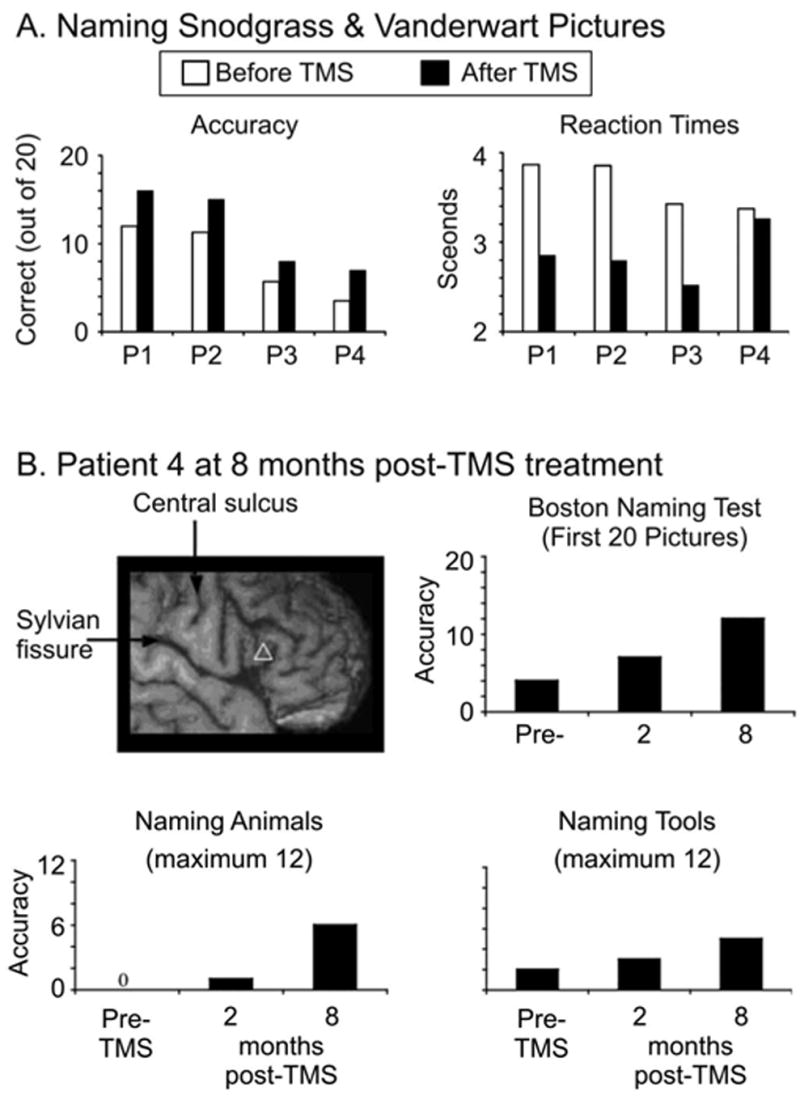
Effects of rTMS treatment on aphasic patients. A) Picture naming accuracies and response times for four patients (P1-4) shown before (white bars) and after (black bars) 10 sessions of rTMS to RIFG. Reprinted from Brain and Language, 93, Naeser et al., “Improved picture naming in chronic aphasia after TMS to part of right Broca's area: an open-protocol study,” p. 101, Copyright (2005), with permission from Elsevier. B) The white triangle indicates the site of RIFG stimulation in a single patient who was followed up two and eight months post-TMS treatment. The bar plots show her picture naming accuracy improving after treatment. From Naeser et al. (2005a), permission pending.
In summary, TMS studies of patients challenge the notion that homologous regions assume lost language functions following left hemisphere lesions – at the very least, the story is considerably more complicated. For one thing, TMS confirms the importance of residual left hemisphere function (Thiel et al., 2005; Winhuisen et al., 2005), as suggested by previous functional imaging studies (Karbe et al., 1998; Musso et al., 1999; Warburton et al., 1999). In fact, left hemisphere stimulation interfered with performance more consistently than right hemisphere stimulation, which only affected the subset of patients with the strongest rightward asymmetries. The reason for these asymmetries remains unclear, but one factor likely to play a role is pre-morbid language organisation (Knecht et al., 2002). Another complicating finding is that in some cases, right hemisphere stimulation interfered with performance (Coslett and Monsul, 1994; Thiel et al., 2005; Winhuisen et al., 2005) while in others, it improved performance (Martin et al., 2004; Naeser et al., 2005a; Naeser et al., 2005b). There are, of course, significant differences between the studies including the types of patients and the type of TMS; nonetheless, the findings demonstrate that one cannot draw a simple conclusion regarding right hemisphere involvement in recovery. Understanding these differences poses a major challenge for cognitive neuroscience and may require adopting more sophisticated models of recovery that move beyond the simple notions of “homologous transfer of function” and “necessary and sufficient” brain regions (Friston and Price, 2003; Price and Friston, 2002).
Summary and future directions
After 15 years of language research using TMS, the field is still in its infancy but even so several important themes have begun to emerge. When used in its virtual lesion mode, TMS offers the spatio-temporal accuracy to complement the information from imaging and patient studies, making TMS an essential tool for studying language at both the cognitive and neural levels. Such studies have helped to clarify the role of the different regions of LIFG in semantic and phonological processing (Gough et al., 2005), to illustrate the critical relationship between pre-morbid language organisation and susceptibility to unilateral lesions (Knecht et al., 2002), and to demonstrate that left hemisphere activation in aphasic patients is more consistently critical for performance than right hemisphere activation (Thiel et al., 2005; Winhuisen et al., 2005). When TMS is used as a measure of functional connectivity, it demonstrates a close link between action words and motor programs (Pulvermuller et al., 2005); it suggests a potential evolutionary link between hand gestures and language (Meister et al., 2003); and demonstrates that speech perception potentiates the specific parts of the motor system engaged to produce equivalent movements (Fadiga et al., 2002; Watkins and Paus, 2004a; Watkins et al., 2003). TMS even offers the potential for enhancing recovery processes and aiding rehabilitation (Martin et al., 2004; Naeser et al., 2005b).
Recent developments in TMS methodology offer even greater opportunities, particularly for investigating cortical connectivity, and suggest that the application of these tools could help to trace the neural circuitry underlying human language processing. The combination of TMS and other imaging modalities such as PET, fMRI, and EEG/MEG offers the potential to identify both functional and anatomical connectivity (e.g. Fox et al., 1997; Ilmoniemi et al., 1997; Paus et al., 1997; Ruff et al., 2006), to investigate the neural mechanisms underlying TMS effects (Bohning et al., 2000), and to probe the time course of modulatory TMS effects (Komssi and Kahkonen, 2006). Similarly, multifocal stimulation can be used to explore both functional connectivity (e.g. Munchau et al., 2002) and the specific mechanisms of recovery (Price and Friston, 2002). Over the next 15 years, the field seems poised to expand enormously in virtually all areas of language research, building on the early successes and developing novel methods capable of answering an even wider range of questions.
Acknowledgments
Our thanks to Margaret Naesser, Sonja Kotz and two anonymous reviewers for their helpful suggestions. Support for this work was provided by the Wellcome Trust (JTD) and the Medical Research Council (KEW).
References
- Aziz-Zadeh L, Cattaneo L, Rochat M, Rizzolatti G. Covert speech arrest induced by rTMS over both motor and nonmotor left hemisphere frontal sites. J Cogn Neurosci. 2005;17:928–38. doi: 10.1162/0898929054021157. [DOI] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Iacoboni M, Zaidel E, Wilson S, Mazziotta J. Left hemisphere motor facilitation in response to manual action sounds. Eur J Neurosci. 2004;19:2609–12. doi: 10.1111/j.0953-816X.2004.03348.x. [DOI] [PubMed] [Google Scholar]
- Barde LH, Thompson-Schill SL. Models of functional organization of the lateral prefrontal cortex in verbal working memory: evidence in favor of the process model. J Cogn Neurosci. 2002;14:1054–63. doi: 10.1162/089892902320474508. [DOI] [PubMed] [Google Scholar]
- Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1:1106–7. doi: 10.1016/s0140-6736(85)92413-4. [DOI] [PubMed] [Google Scholar]
- Bohning DE, Shastri A, Wassermann EM, Ziemann U, Lorberbaum JP, Nahas Z, et al. BOLD-f MRI response to single-pulse transcranial magnetic stimulation (TMS) J Magn Reson Imaging. 2000;11:569–74. doi: 10.1002/1522-2586(200006)11:6<569::aid-jmri1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Buccino G, Riggio L, Melli G, Binkofski F, Gallese V, Rizzolatti G. Listening to action-related sentences modulates the activity of the motor system: a combined TMS and behavioral study. Brain Res Cogn Brain Res. 2005;24:355–63. doi: 10.1016/j.cogbrainres.2005.02.020. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Raichle ME, Petersen SE. Dissociation of human prefrontal cortical areas across different speech production tasks and gender groups. Journal of Neurophysiology. 1995;74:2163–2173. doi: 10.1152/jn.1995.74.5.2163. [DOI] [PubMed] [Google Scholar]
- Cappa SF, Sandrini M, Rossini PM, Sosta K, Miniussi C. The role of the left frontal lobe in action naming: rTMS evidence. Neurology. 2002;59:720–3. doi: 10.1212/wnl.59.5.720. [DOI] [PubMed] [Google Scholar]
- Caramazza A, Hillis A. Lexical organization of nouns and verbs in the brain. Nature. 1991;349:788–790. doi: 10.1038/349788a0. [DOI] [PubMed] [Google Scholar]
- Coltheart M. Deep dyslexia: A review of the syndrome. In: Coltheart M, Patterson K, Marshall J, editors. Deep Dyslexia. London: Routledge and Kegan Paul; 1980. [Google Scholar]
- Corballis MC. From mouth to hand: gesture, speech, and the evolution of right-handedness. Behav Brain Sci. 2003;26:199–208. doi: 10.1017/s0140525x03000062. discussion 208–60. [DOI] [PubMed] [Google Scholar]
- Coslett HB, Monsul N. Reading with the right hemisphere: evidence from transcranial magnetic stimulation. Brain Lang. 1994;46:198–211. doi: 10.1006/brln.1994.1012. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Matthews PM, Rushworth MF. Semantic processing in the left inferior prefrontal cortex: a combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J Cogn Neurosci. 2003;15:71–84. doi: 10.1162/089892903321107837. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Watkins KE. Investigating language organisation with TMS. In: Wasserman E, Epstein C, Ziemann U, Lisanby S, Paus T, Walsh V, editors. Oxford Handbook of Transcranial Stimulation. Oxford: Oxford University Press; 2006. [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, et al. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res. 1998;119:265–8. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Exp Brain Res. 1992;91:176–80. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000;23:475–83. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Engel AK, Moll CK, Fried I, Ojemann GA. Invasive recordings from the human brain: clinical insights and beyond. Nat Rev Neurosci. 2005;6:35–47. doi: 10.1038/nrn1585. [DOI] [PubMed] [Google Scholar]
- Epstein CM, Lah JK, Meador K, Weissman JD, Gaitan LE, Dihenia B. Optimum stimulus parameters for lateralized suppression of speech with magnetic brain stimulation. Neurology. 1996;47:1590–1593. doi: 10.1212/wnl.47.6.1590. [DOI] [PubMed] [Google Scholar]
- Epstein CM, Meador KJ, Loring DW, Wright RJ, Weissman JD, Sheppard S, et al. Localization and characterization of speech arrest during transcranial magnetic stimulation. Clin Neurophysiol. 1999;110:1073–9. doi: 10.1016/s1388-2457(99)00047-4. [DOI] [PubMed] [Google Scholar]
- Epstein CM, Woodard JL, Stringer AY, Bakay RA, Henry TR, Pennell PB, et al. Repetitive transcranial magnetic stimulation does not replicate the Wada test. Neurology. 2000;55:1025–7. doi: 10.1212/wnl.55.7.1025. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Craighero L, Buccino G, Rizzolatti G. Speech listening specifically modulates the excitability of tongue muscles: a TMS study. Eur J Neurosci. 2002;15:399–402. doi: 10.1046/j.0953-816x.2001.01874.x. [DOI] [PubMed] [Google Scholar]
- Federmeier KD, Segal JB, Lombrozo T, Kutas M. Brain responses to nouns, verbs and class-ambiguous words in context. Brain. 2000;123(Pt 12):2552–66. doi: 10.1093/brain/123.12.2552. [DOI] [PubMed] [Google Scholar]
- Fiez JA. Phonology, semantics, and the role of the left inferior prefrontal cortex. Human Brain Mapping. 1997;5:79–83. [PubMed] [Google Scholar]
- Floel A, Ellger T, Breitenstein C, Knecht S. Language perception activates the hand motor cortex: implications for motor theories of speech perception. Eur J Neurosci. 2003;18:704–8. doi: 10.1046/j.1460-9568.2003.02774.x. [DOI] [PubMed] [Google Scholar]
- Fox P, Ingham R, George MS, Mayberg H, Ingham J, Roby J, et al. Imaging human intra-cerebral connectivity by PET during TMS. Neuroreport. 1997;8:2787–91. doi: 10.1097/00001756-199708180-00027. [DOI] [PubMed] [Google Scholar]
- Friederici AD. Towards a neural basis of auditory sentence processing. Trends Cogn Sci. 2002;6:78–84. doi: 10.1016/s1364-6613(00)01839-8. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Kotz SA. The brain basis of syntactic processes: functional imaging and lesion studies. Neuroimage. 2003;20 (Suppl 1):S8–17. doi: 10.1016/j.neuroimage.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Price CJ. Degeneracy and redundancy in cognitive anatomy. Trends Cogn Sci. 2003;7:151–152. doi: 10.1016/s1364-6613(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga L, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Gangitano M, Mottaghy FM, Pascual-Leone A. Modulation of premotor mirror neuron activity during observation of unpredictable grasping movements. Eur J Neurosci. 2004;20:2193–202. doi: 10.1111/j.1460-9568.2004.03655.x. [DOI] [PubMed] [Google Scholar]
- Gentilucci M, Corballis MC. From manual gesture to speech: A gradual transition. Neurosci Biobehav Rev. 2006;30:949–960. doi: 10.1016/j.neubiorev.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Gold BT, Buckner RL. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron. 2002;35:803–12. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Gough PM, Nobre AC, Devlin JT. Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. J Neurosci. 2005;25:8010–6. doi: 10.1523/JNEUROSCI.2307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort P, Hald L, Bastiaansen M, Petersson KM. Integration of word meaning and world knowledge in language comprehension. Science. 2004;304:438–41. doi: 10.1126/science.1095455. [DOI] [PubMed] [Google Scholar]
- Hauk O, Johnsrude I, Pulvermuller F. Somatotopic representation of action words in human motor and premotor cortex. Neuron. 2004;41:301–7. doi: 10.1016/s0896-6273(03)00838-9. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Towards a functional neuroanatomy of speech perception. Trends Cogn Sci. 2000;4:131–138. doi: 10.1016/s1364-6613(00)01463-7. [DOI] [PubMed] [Google Scholar]
- Hoffman RE, Boutros NN, Berman RM, Roessler E, Belger A, Krystal JH, et al. Transcranial magnetic stimulation of left temporoparietal cortex in three patients reporting hallucinated "voices". Biol Psychiatry. 1999;46:130–2. doi: 10.1016/s0006-3223(98)00358-8. [DOI] [PubMed] [Google Scholar]
- Houlden DA, Schwartz ML, Tator CH, Ashby P, MacKay WA. Spinal cord-evoked potentials and muscle responses evoked by transcranial magnetic stimulation in 10 awake human subjects. J Neurosci. 1999;19:1855–62. doi: 10.1523/JNEUROSCI.19-05-01855.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilmoniemi RJ, Virtanen J, Ruohonen J, Karhu J, Aronen HJ, Naatanen R, et al. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport. 1997;8:3537–40. doi: 10.1097/00001756-199711100-00024. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Rothwell J. Transcranial magnetic stimulation studies of cognition: An emerging field. Experimental Brain Research. 2000;131:1–9. doi: 10.1007/s002219900224. [DOI] [PubMed] [Google Scholar]
- Jennum P, Friberg L, Fuglsang-Frederiksen A, Dam M. Speech localization using repetitive transcranial magnetic stimulation. Neurology. 1994;44:269–273. doi: 10.1212/wnl.44.2.269. [DOI] [PubMed] [Google Scholar]
- Karbe H, Thiel A, Weber-Luxenburger G, Herholz K, Kessler J, Heiss WD. Brain plasticity in poststroke aphasia: what is the contribution of the right hemisphere? Brain Lang. 1998;64:215–30. doi: 10.1006/brln.1998.1961. [DOI] [PubMed] [Google Scholar]
- Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, et al. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123(Pt 12):2512–8. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Knecht S, Floel A, Drager B, Breitenstein C, Sommer J, Henningsen H, et al. Degree of language lateralization determines susceptibility to unilateral brain lesions. Nat Neurosci. 2002;5:695–9. doi: 10.1038/nn868. [DOI] [PubMed] [Google Scholar]
- Kohler E, Keysers C, Umilta MA, Fogassi L, Gallese V, Rizzolatti G. Hearing sounds, understanding actions: action representation in mirror neurons. Science. 2002;297:846–8. doi: 10.1126/science.1070311. [DOI] [PubMed] [Google Scholar]
- Kohler S, Paus T, Buckner RL, Milner B. Effects of left inferior prefrontal stimulation on episodic memory formation: a two-stage fMRI-rTMS study. J Cogn Neurosci. 2004;16:178–88. doi: 10.1162/089892904322984490. [DOI] [PubMed] [Google Scholar]
- Komssi S, Kahkonen S. The novelty value of the combined use of electroencephalography and transcranial magnetic stimulation for neuroscience research. Brain Res Brain Res Rev. 2006;52:183–92. doi: 10.1016/j.brainresrev.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Levelt WJ. Models of word production. Trends Cogn Sci. 1999;3:223–232. doi: 10.1016/s1364-6613(99)01319-4. [DOI] [PubMed] [Google Scholar]
- Levy BJ, Anderson MC. Inhibitory processes and the control of memory retrieval. Trends Cogn Sci. 2002;6:299–305. doi: 10.1016/s1364-6613(02)01923-x. [DOI] [PubMed] [Google Scholar]
- Liberman AM, Cooper FS, Shankweiler D, Studdert-Kennedy M. Perception of the speech code. Psychol Rev. 1967;74:431–461. doi: 10.1037/h0020279. [DOI] [PubMed] [Google Scholar]
- Liberman AM, Mattingly IG. The motor theory of speech perception -- revised. Cognition. 1985;21:1–36. doi: 10.1016/0010-0277(85)90021-6. [DOI] [PubMed] [Google Scholar]
- Lo YL, Fook-Chong S, Lau DP, Tan EK. Cortical excitability changes associated with musical tasks: a transcranial magnetic stimulation study in humans. Neurosci Lett. 2003;352:85–8. doi: 10.1016/j.neulet.2003.08.031. [DOI] [PubMed] [Google Scholar]
- Martin PI, Naeser MA, Theoret H, Tormos JM, Nicholas M, Kurland J, et al. Transcranial magnetic stimulation as a complementary treatment for aphasia. Semin Speech Lang. 2004;25:181–91. doi: 10.1055/s-2004-825654. [DOI] [PubMed] [Google Scholar]
- Matsumoto R, Nair DR, LaPresto E, Najm I, Bingaman W, Shibasaki H, et al. Functional connectivity in the human language system: a cortico-cortical evoked potential study. Brain. 2004;127:2316–30. doi: 10.1093/brain/awh246. [DOI] [PubMed] [Google Scholar]
- Meister IG, Boroojerdi B, Foltys H, Sparing R, Huber W, Topper R. Motor cortex hand area and speech: implications for the development of language. Neuropsychologia. 2003;41:401–6. doi: 10.1016/s0028-3932(02)00179-3. [DOI] [PubMed] [Google Scholar]
- Michelucci R, Valzania F, Passarelli D, Santangelo M, Rizzi R, Buzzi AM, et al. Rapid-rate transcranial magnetic stimulation and hemispheric language dominance: usefulness and safety in epilepsy. Neurology. 1994;44:1697–700. doi: 10.1212/wnl.44.9.1697. [DOI] [PubMed] [Google Scholar]
- Munchau A, Bloem BR, Irlbacher K, Trimble MR, Rothwell JC. Functional connectivity of human premotor and motor cortex explored with repetitive transcranial magnetic stimulation. J Neurosci. 2002;22:554–61. doi: 10.1523/JNEUROSCI.22-02-00554.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso M, Weiller C, Kiebel S, Muller SP, Bulau P, Rijntjes M. Training-induced brain plasticity in aphasia. Brain. 1999;122 ( Pt 9):1781–90. doi: 10.1093/brain/122.9.1781. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Baker EH, Hodge SM, Sczerzenie SE, Nicholas M, et al. Overt propositional speech in chronic nonfluent aphasia studied with the dynamic susceptibility contrast fMRI method. Neuroimage. 2004;22:29–41. doi: 10.1016/j.neuroimage.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Helm-Estabrooks N, et al. Improved naming after TMS treatments in a chronic, global aphasia patient--case report. Neurocase. 2005a;11:182–93. doi: 10.1080/13554790590944663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, et al. Improved picture naming in chronic aphasia after TMS to part of right Broca's area: an open-protocol study. Brain Lang. 2005b;93:95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Nixon P, Lazarova J, Hodinott-Hill I, Gough P, Passingham R. The inferior frontal gyrus and phonological processing: an investigation using rTMS. J Cogn Neurosci. 2004;16:289–300. doi: 10.1162/089892904322984571. [DOI] [PubMed] [Google Scholar]
- Ojemann GA. Individual variability in cortical localization of language. J Neurosurg. 1979;50:164–9. doi: 10.3171/jns.1979.50.2.0164. [DOI] [PubMed] [Google Scholar]
- Ojemann GA. Brain organisation for language from the perspective of electrical stimulation mapping. Behav Brain Sci. 1983;6:189–206. [Google Scholar]
- Pascual-Leone A, Bartres-Faz D, Keenan JP. Transcranial magnetic stimulation: studying the brain-behaviour relationship by induction of 'virtual lesions'. Philosophical Transactions of the Royal Society London B. 1999;354:1229–1238. doi: 10.1098/rstb.1999.0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Gates JR, Dhuna A. Induction of speech arrest and counting errors with rapid-rate transcranial magnetic stimulation. Neurology. 1991;41:697–702. doi: 10.1212/wnl.41.5.697. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V, Rothwell J. Transcranial magnetic stimulation in cognitive neuroscience --virtual lesion, chronometry, and functional connectivity. Current Opinions in Neurobiology. 2000;10:232–237. doi: 10.1016/s0959-4388(00)00081-7. [DOI] [PubMed] [Google Scholar]
- Paus T. Inferring causality in brain images: a perturbation approach. Philos Trans R Soc Lond B Biol Sci. 2005;360:1109–14. doi: 10.1098/rstb.2005.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC. Transcranial magnetic stimulation during positron emission tomography: A new method for studying connectivity of the human cerebral cortex. Journal of Neuroscience. 1997;17:3178–3184. doi: 10.1523/JNEUROSCI.17-09-03178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield W, Jasper HH. Epilepsy and the functional anatomy of the human brain. Boston: Little, Brown, and Co; 1954. [Google Scholar]
- Petrides M. The role of the mid-dorsolateral prefrontal cortex in working memory. Exp Brain Res. 2000;133:44–54. doi: 10.1007/s002210000399. [DOI] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: Contributions from fuctional neuroimaging. Journal of Anatomy. 2000;197:335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Degeneracy and cognitive anatomy. Trends Cogn Sci. 2002;6:416–421. doi: 10.1016/s1364-6613(02)01976-9. [DOI] [PubMed] [Google Scholar]
- Price CJ, Howard D, Patterson K, Warburton EA, Friston KJ, Frackowiak RSJ. A functional neuroimaging description of two deep dyslexic patients. Journal of Cognitive Neuroscience. 1998;10:303–315. doi: 10.1162/089892998562753. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJS, Frackowiak RSJ. Demonstrating the implicit processing of visually presented words and pseudowords. Cerebral Cortex. 1996;6:62–70. doi: 10.1093/cercor/6.1.62. [DOI] [PubMed] [Google Scholar]
- Pulvermuller F, Hauk O, Nikulin VV, Ilmoniemi RJ. Functional links between motor and language systems. Eur J Neurosci. 2005;21:793–7. doi: 10.1111/j.1460-9568.2005.03900.x. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Fiez JA, Videen TO, MacLeod AM, Pardo JV, Fox PT, et al. Practice-related changes in human brain functional anatomy during nonmotor learning. Cereb Cortex. 1994;4:8–26. doi: 10.1093/cercor/4.1.8. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Rogers-Ramachandran D, Cobb S. Touching the phantom limb. Nature. 1995;377:489–90. doi: 10.1038/377489a0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Arbib MA. Language within our grasp Trends Neurosci. 1998;21:188–94. doi: 10.1016/s0166-2236(98)01260-0. [DOI] [PubMed] [Google Scholar]
- Rothwell JC. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J Neurosci Methods. 1997;74:113–22. doi: 10.1016/s0165-0270(97)02242-5. [DOI] [PubMed] [Google Scholar]
- Ruff CC, Blankenburg F, Bjoertomt O, Bestmann S, Freeman E, Haynes JD, et al. Concurrent TMS-fMRI and Psychophysics Reveal Frontal Influences on Human Retinotopic Visual Cortex. Curr Biol. 2006;16:1479–1488. doi: 10.1016/j.cub.2006.06.057. [DOI] [PubMed] [Google Scholar]
- Saarinen T, Laaksonen H, Parviainen T, Salmelin R. Motor cortex dynamics in visuomotor production of speech and non-speech mouth movements. Cereb Cortex. 2006;16:212–22. doi: 10.1093/cercor/bhi099. [DOI] [PubMed] [Google Scholar]
- Sakai KL, Noguchi Y, Takeuchi T, Watanabe E. Selective priming of syntactic processing by event-related transcranial magnetic stimulation of Broca's area. Neuron. 2002;35:1177–82. doi: 10.1016/s0896-6273(02)00873-5. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Sams M. Motor cortex involvement during verbal versus non-verbal lip and tongue movements. Hum Brain Mapp. 2002;16:81–91. doi: 10.1002/hbm.10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SK, Wise RJ. The functional neuroanatomy of prelexical processing in speech perception. Cognition. 2004;92:13–45. doi: 10.1016/j.cognition.2002.12.002. [DOI] [PubMed] [Google Scholar]
- Seyal M, Mull B, Bhullar N, Ahmad T, Gage B. Anticipation and execution of a simple reading task enhance corticospinal excitability. Clin Neurophysiol. 1999;110:424–9. doi: 10.1016/s1388-2457(98)00019-4. [DOI] [PubMed] [Google Scholar]
- Shapiro KA, Caramazza A. Grammatical processing of nouns and verbs in left frontal cortex? Neuropsychologia. 2003;41:1189–98. doi: 10.1016/s0028-3932(03)00037-x. [DOI] [PubMed] [Google Scholar]
- Shapiro KA, Pascual-Leone A, Mottaghy FM, Gangitano M, Caramazza A. Grammatical distinctions in the left frontal cortex. J Cogn Neurosci. 2001;13:713–20. doi: 10.1162/08989290152541386. [DOI] [PubMed] [Google Scholar]
- Stewart L, Walsh V, Frith U, Rothwell JC. TMS produces two dissociable types of speech disruption. NeuroImage. 2001;13:472–478. doi: 10.1006/nimg.2000.0701. [DOI] [PubMed] [Google Scholar]
- Strafella AP, Paus T. Modulation of cortical excitability during action observation: a transcranial magnetic stimulation study. Neuroreport. 2000;11:2289–92. doi: 10.1097/00001756-200007140-00044. [DOI] [PubMed] [Google Scholar]
- Sundara M, Namasivayam AK, Chen R. Observation-execution matching system for speech: a magnetic stimulation study. Neuroreport. 2001;12:1341–4. doi: 10.1097/00001756-200105250-00010. [DOI] [PubMed] [Google Scholar]
- Thiel A, Habedank B, Winhuisen L, Herholz K, Kessler J, Haupt WF, et al. Essential language function of the right hemisphere in brain tumor patients. Ann Neurol. 2005;57:128–31. doi: 10.1002/ana.20342. [DOI] [PubMed] [Google Scholar]
- Wada J, Rasmussen T. Intracarotid injection of sodium amytal for the lateralization of cerebral speech dominance: experimental and clinical observations. Journal of Neurosurgery. 1960;17:266–282. doi: 10.3171/jns.2007.106.6.1117. [DOI] [PubMed] [Google Scholar]
- Walsh V, Cowey A. Magnetic stimulation studies of visual cognition. Trends in Cognitive Science. 1998;2:103–110. doi: 10.1016/s1364-6613(98)01134-6. [DOI] [PubMed] [Google Scholar]
- Walsh V, Cowey A. Transcranial magnetic stimulation and cognitive neuroscience. Nature Reviews Neuroscience. 2000;1:73–79. doi: 10.1038/35036239. [DOI] [PubMed] [Google Scholar]
- Walsh V, Pascual-Leone A. Transcranial magnetic stimulation: A neurochronometrics of mind. London: MIT Press; 2003. [Google Scholar]
- Walsh V, Rushworth MFS. The use of transcranial magnetic stimulation in neuropsychological testing. Neuropsychologia. 1999;37:125–135. [PubMed] [Google Scholar]
- Warburton E, Price CJ, Swinburn K, Wise RJ. Mechanisms of recovery from aphasia: evidence from positron emission tomography studies. J Neurol Neurosurg Psychiatry. 1999;66:155–61. doi: 10.1136/jnnp.66.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins K, Paus T. Modulation of motor excitability during speech perception: the role of Broca's area. J Cogn Neurosci. 2004a;16:978–87. doi: 10.1162/0898929041502616. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Paus T. Modulation of motor excitability during speech perception: the role of Broca's area. J Cogn Neurosci. 2004b;16:978–87. doi: 10.1162/0898929041502616. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Strafella AP, Paus T. Seeing and hearing speech excites the motor system involved in speech production. Neuropsychologia. 2003;41:989–94. doi: 10.1016/s0028-3932(02)00316-0. [DOI] [PubMed] [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, et al. Role of the contralateral inferior frontal gyrus in recovery of language function in poststroke aphasia: a combined repetitive transcranial magnetic stimulation and positron emission tomography study. Stroke. 2005;36:1759–63. doi: 10.1161/01.STR.0000174487.81126.ef. [DOI] [PubMed] [Google Scholar]
- Zurif EB, Caramazza A, Myerson R. Grammatical judgements of agrammatic aphasics. Neuropsychologia. 1972;10:405–417. doi: 10.1016/0028-3932(72)90003-6. [DOI] [PubMed] [Google Scholar]


