Abstract
Auditory neurons, the target neurons of the cochlear implant, degenerate following a sensorineural hearing loss. The goal of this research is to direct the differentiation of embryonic stem cells (SCs) into bipolar auditory neurons that can be used to replace degenerating neurons in the deafened mammalian cochlea. Successful replacement of auditory neurons is likely to result in improved clinical outcomes for cochlear implant recipients. We examined two post-natal auditory co-culture models with and without neurotrophic support, for their potential to direct the differentiation of mouse embryonic SCs into characteristic, bipolar, auditory neurons. The differentiation of SCs into neuron-like cells was facilitated by co-culture with auditory neurons or hair cell explants, isolated from post-natal day five rats. The most successful combination was the co-culture of hair cell explants with whole embryoid bodies, which resulted in significantly greater numbers of neurofilament positive, neuron-like cells. While further characterisation of these differentiated cells will be essential before transplantation studies commence, these data illustrate the effectiveness of post-natal explant co-culture, at providing valuable molecular cues for directed differentiation of SCs towards an auditory neuron lineage.
Keywords: embryonic stem cell, differentiation, co-culture, hair cell explant, auditory neuron
INTRODUCTION
The sensory hair cells of the cochlea transduce the mechanical vibrations of sound to the brain via auditory neurons. In addition, these hair cells and their surrounding supporting cells provide an ongoing source of neurotrophins to auditory neurons [1-6]. The loss of inner hair cells in mammals causes a permanent sensorineural hearing loss and initiates a wave of secondary degeneration of auditory neurons, the target neurons of the cochlear implant [7, 8]. While the cochlear implant is capable of directly stimulating residual auditory neurons following a sensorineural hearing loss, the auditory nerve continues to degenerate, resulting in reduced numbers of surviving neurons in humans with chronic deafness [9, 10] and in long-term deafened animals [8, 11]. Given that the efficacy of the cochlear implant relies, at least in part, on a critical number of surviving auditory neurons, current investigations are directed towards the protection and/or replacement of these cells. Greater numbers of auditory neurons are likely to result in improved clinical outcomes for cochlear implant recipients [9, 12].
Numerous experimental studies have reported the rescue of auditory neurons from sensorineural hearing loss-induced degeneration by the exogenous infusion of neurotrophins [13-22]. While significant survival is observed during the delivery of neurotrophins into the cochlea, this survival-promoting effect is lost immediately following the cessation of treatment [23]. In addition, the long-term application of exogenous neurotrophins to the cochlea using a pump-based delivery system is problematic, due to the increased risk of infection associated with reloading or replacing these devices, which are currently used for delivery in experimental animals [24]. Given these difficulties, cell based therapies are presently being investigated as a long-term solution to auditory neuron degeneration in the deafened mammalian cochlea [24]. Stem cells (SCs) are promising candidates for cochlear cell based therapy as they have the potential to provide large numbers of replacement neurons to the degenerating auditory nerve.
In order to direct the in vitro differentiation of SCs into an auditory neuron lineage, we need to replicate the early developmental stages that result in the formation of auditory neurons in vivo. In mammals, auditory neurons arise from the otocyst which is formed from an invagination of the otic placode region of ectoderm at approximately embryonic day eight [25]. In a specialised region of the otocyst, there is upregulation of the transcription factor neurogenin-1, which occurs concurrently with upregulation of the neurotrophins brain derived neurotrophic factor (BDNF) and neurotrophin-3 (NT3). These neurotrophins have been shown to be essential for normal auditory development, including developing auditory neurons [25], demonstrated by the localization of their high affinity receptors TrkB and TrkC respectively, on these cells [1-3, 6, 26]. The upregulation of neurogenin-1 results in the formation of auditory neuron precursors, which is followed by the sequential expression of the proneural genes NeuroD, Brn3a and TrkC as these neural precursors delaminate from the otocyst [25]. Yet to be elucidated is the mechanism(s) causing the initial upregulation of neurogenin-1 and the precise sequence of growth factor-receptor interactions that control gene expression, and ultimately differentiation, into auditory neuron precursors.
Interactions and signalling between developing tissues play an essential role in regulating differentiation in vivo. Given the complexity of cellular signalling pathways in vivo, this environment is often difficult to mimic completely in vitro. Co-culture models provide a method to study differentiation under controlled conditions, with the advantage of being able to replicate some tissue-derived signalling. Co-culture models have previously been used to successfully direct the differentiation of stem or precursor cells into neurons [27-29], haematopoietic cells [30, 31], photoreceptor cells [32], and hepatocytes [33], and to promote cell survival and expansion in vitro [34-38]. However, there are presently no established in vitro or co-culture models for the differentiation of SCs into auditory neurons.
The aim of this research is to direct the differentiation of SCs into auditory neurons that can be used to replace those that degenerate following a sensorineural hearing loss. The current study addresses the first vital steps in this process; the differentiation of embryonic SCs into bipolar neurons. We investigated two co-culture models for their ability to direct the differentiation of mouse embryonic SCs in vitro. Stem cells were induced to form neural precursors via timed exposure to retinoic acid [39], before being co-cultured with dissociated rat auditory neurons or hair cell explants isolated from post-natal day five rat pups. The effect of co-culture with and without BDNF and/or NT3 on the differentiation of these SCs into bipolar neuron-like cells was evaluated using a combination of immunohistochemical and quantification techniques [40].
MATERIALS AND METHODS
Maintenance of embryonic SCs
We used the mouse embryonic SC line R1 B5-EGFP (Tg(GFPU)5 Nagy/J) in this study [41] http://www.mshri.on.ca/nagy/r1.htm. These cells were genetically modified to express green fluorescent protein (GFP). Undifferentiated SCs were grown in standard embryonic SC media comprising Dulbecco's modified Eagle's medium (DMEM; Invitrogen), supplemented with 10% fetal bovine serum (FBS; Invitrogen), 1% penicillin/streptomycin (Invitrogen), 1% nucleosides (Sigma), 1% nonessential amino acids (Invitrogen), 1% L-glutamine (Sigma), 1 mL/L 1000X ß-mercaptoethanol (BME; GIBCO/BRL) and 1 mL/L (1000 units) leukaemia inhibitory factor (LIF; Chemicon). Stem cells were grown at 37°C, 5% CO2 and passaged every 2-3 days using 0.025% trypsin (Invitrogen; diluted in phosphate buffered saline (PBS) containing 0.2% ethylenediaminetetraacetic acid (EDTA), AppliChem). Green fluorescent protein positive SCs were purified prior to differentiation into embryoid bodies using fluorescence activated cell sorting.
Pre-differentiation of embryonic SCs for co-culture
Eight days prior to co-culture, undifferentiated SCs were induced to form embryoid bodies via timed exposure to retinoic acid [39]. Briefly, undifferentiated SCs were transferred into non-adherent bacterial petri dishes containing embryonic SC media without LIF and grown for 4 days in vitro. This resulted in the formation of free-floating embryoid bodies. After 4 days in vitro, embryoid bodies were transferred into fresh embryonic SC media without LIF but containing 0.5 μM retinoic acid, and grown for a further 4 days in vitro. Prior to co-culture embryoid bodies were collected in a 50 mL plastic tube and allowed to settle at the base. The media was carefully removed and the cells were rinsed twice for 5 minutes in 10 mL unsupplemented DMEM. Half of the resulting pellet was used for co-culture as whole embryoid bodies, while the remainder was transferred into 1 mL of 0.025% trypsin for 5 minutes for preparation of dissociated embryoid bodies. Enzymatic digestion was inactivated by addition of 9 mL embryonic SC media to the trypsin, and the pellet collected and resuspended in 1 mL auditory neuron media (comprising 50 mL DMEM, 1.1 mL N1 supplement, and 4.5 g/L glucose). The embryoid bodies were dissociated mechanically using 18G - 23G needles in succession. A viable cell count was performed using trypan blue (Sigma) and the suspension adjusted to ∼50,000 cells/mL in auditory neuron media.
Isolation and preparation of tissues for co-culture
Cochlear tissue for co-culture was isolated from post-natal day five Sprague-Dawley rat pups. Rat organ of Corti explants are immature at this time with the onset of auditory function reported to occur between post-natal day 12 and 14, and mature at approximately post-natal day 22 [42]. Sixteen rat pups (32 cochleae) were required for each co-culture experiment. Following anaesthesia on ice, rat pups were decapitated and the heads rinsed in 70% ethanol. Under sterile conditions, the skull was opened longitudinally, the temporal bone identified, and the bulla removed and placed into a chilled solution of hair cell explant media (comprising 50 mL DMEM/F-12 (Invitrogen), 1.1 mL N1 supplement, and 6 g/L glucose). While still submerged in hair cell explant media, the cochlea was gently dissected from the bulla and the organ of Corti removed from the modiolus. The modiolus and organ of Corti were placed into separate petri dishes and maintained in chilled hair cell explant media until all dissections were completed. All procedures were compliant with the Royal Victorian Eye and Ear Hospital Animal Research and Ethics Committee Guidelines (project approval numbers 02/090A and 03/099A).
Dissociated AN culture
Cultures of dissociated auditory neurons were prepared as previously described by Gillespie and colleagues [43]. Briefly, the modioli were digested in a solution of sterile Ca2+, Mg2+-free Hank's balanced salt solution (Gibco), containing 0.025% trypsin (Calbiochem) and 0.001% DNase (Boehringer Mannheim), and incubated at 37°C. After 30 minutes, enzymatic digestion was terminated by addition of 1 mL FBS and the modioli centrifuged for 10 minutes at 2000 rpm at room temperature. The supernatant was discarded and the tissue was mechanically dissociated in 3 mL Hepes buffered Eagle's medium (HEM; Gibco) containing 0.001% DNAse, by gentle trituration using 18G - 23G needles in succession. The tissue was centrifuged at 2000 rpm for 10 minutes, the supernatant removed and the remaining cell pellet gently resuspended in 3 mL auditory neuron media and pre-plated for 30 minutes at 37°C, 10% CO2. The resulting neuron-enriched cell suspension was collected and then plated into chamber slides (4-well, Nunc Lab Tec II) at 250 μL per well. All wells had been pre-coated with poly-ornithine (500 μg/mL, Sigma) overnight, and laminin (0.01 mg/mL, Invitrogen) for at least 1 hour, and contained 50 ng/mL each of BDNF (Peprotech) and NT3 (Peprotech) (final concentration). Previous experimentation in our laboratory demonstrated that the combined effect of BDNF and NT3 (at a concentration of 50 ng/mL each) was observed to significantly enhance auditory neuron survival in vitro compared to either neurotrophin alone [44]. Auditory neuron cultures were incubated at 37°C, 10% CO2 for at least 1 hour prior to co-culture with pre-differentiated SCs.
Hair cell explant culture
All organ of Corti explants were rinsed briefly in HEM using a plugged, siliconised Pasteur pipette, and then transferred immediately into fresh hair cell explant media. The stria vascularis was carefully removed with fine forceps and the remaining hair cell explant oriented on a 0.4 μm organotypic membrane (Millipore) with Reissner's membrane folded away from the hair cells. Two hair cell explants were oriented on each membrane. The membranes were placed into individual 35 mm tissue culture wells containing 0.5 mL hair cell explant media ± 50 ng/mL BDNF (final concentration) and incubated at 37°C, 10% CO2 for 3 hours prior to co-culture with pre-differentiated SCs. Previous experimentation in our laboratory showed that BDNF promoted the survival of hair cells in whole explant cultures (unpublished observations).
Experimental groups
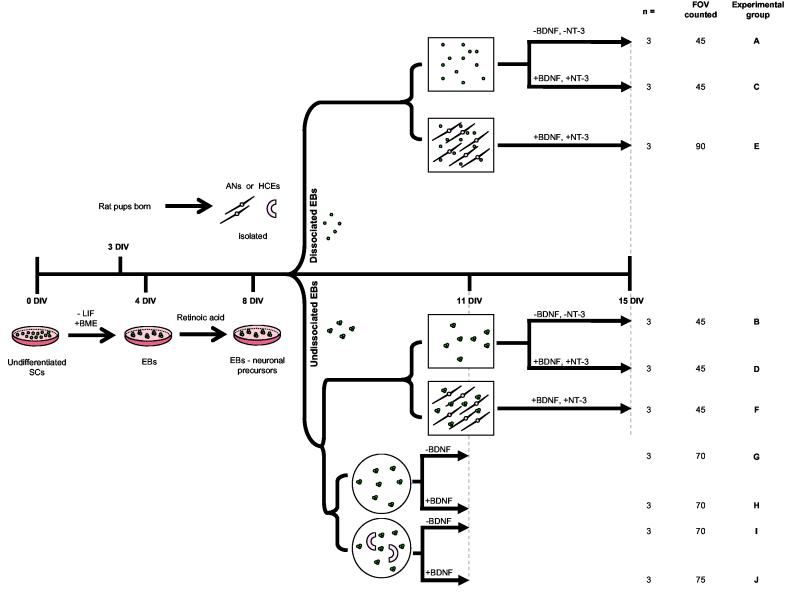
All treatment conditions are summarised in Figure 1 (A-J) and were incubated at 37°C, 10% CO2 for the time periods described. Each experiment was repeated in triplicate using cochlear tissues isolated from two animal litters (born on the same day).
Figure 1.
Auditory co-culture models and control treatments
Stem cells (SCs) were co-cultured with rat post-natal day five auditory neurons or hair cell explants as described. Prior to co-culture, undifferentiated SCs were induced to form embryoid bodies via timed exposure to retinoic acid. Following eight days differentiation, SCs were placed into co-culture either dissociated or whole (illustrated schematically). The square wells represent treatments grown in auditory neuron media. Auditory neuron co-cultures and control treatments were maintained for 7 days in vitro. Circular wells represent hair cell explant co-cultures and relative controls, which were maintained for 3 days in vitro. Co-cultures and control treatments are distinguished pictorially. Following growth in vitro, all wells were fixed and processed for quantitative analysis. The number of times each experiment was repeated (n), the total fields of view (FOV) counted, and the experimental groups (A-J) are detailed on the far right had side of the diagram. DIV = days in vitro; SC = stem cell; LIF = leukemia inhibitory factor; BME = ß-mercaptoethanol; EBs = embryoid bodies; AN = auditory neuron; HCE = hair cell explant; BDNF = brain derived neurotrophic factor; NT3 = neurotrophin-3. Timeline is not to scale.
Co-cultures of SCs with auditory neurons
Pre-differentiated SCs were added to auditory neuron cultures, as either dissociated embryoid bodies (250 μL cells suspended in auditory neuron media) or as whole embryoid bodies (10 μL embryoid bodies + 240 μL auditory neuron media), and grown for 7 days in vitro. Partial media changes were performed after 2, 4 and 6 days in vitro. Controls were set-up identically in 4-well chamber slides, but without dissociated rat auditory neurons. Pre-differentiated SCs were added either as dissociated embryoid bodies (250 μL cells suspended in auditory neuron media) or whole embryoid bodies (10 μL embryoid bodies + 240 μL auditory neuron media) and grown for 7 days in vitro, with partial media changes performed after 2, 4 and 6 days in vitro.
Co-cultures of SCs with hair cell explants
Pre-differentiated SCs were added to hair cell explant cultures as whole embryoid bodies (10 μL embryoid bodies + 490 μL hair cell explant media). The embryoid body/media suspension was carefully plated onto the organotypic membrane so as not to disturb the hair cell explant, and then co-cultured for 3 days in vitro without media changes. Three days was the longest period of time that hair cell explants could be maintained in static cell culture, before the underlying cells of the explant proliferated and disrupted the hair cell growth. Controls were set up identically on organotypic membranes, but without hair cell explants. Pre-differentiated SCs were placed on top of the membrane as whole embryoid bodies (10 μL embryoid bodies in 490 μL hair cell explant media) and grown for 3 days in vitro without media changes. Further controls consisted of growing hair cell explants alone (without whole embryoid bodies).
Quantitative analysis
Auditory neuron co-cultures and control treatments
After 7 days in vitro, all auditory neuron co-culture and control treatment wells were fixed in 250 μL 4% paraformaldehyde (PFA) for 30 minutes and then rinsed three times for 5 minutes in PBS. Following the final rinse, the chambers were removed and the slide mounted in diamidino-2-phenylindole (DAPI) fluorescent mounting medium (Vector). Stem cells were identified in each treatment well by the co-expression of endogenous GFP with the nuclear marker DAPI, using direct fluorescent microscopy. In each well, we counted the number of co-labelled GFP/DAPI positive cells displaying neuron-like or glial-like morphology, within ten randomly generated fields of view. Neuron-like cells were identified based on characteristic auditory neuron bi-polar morphology from previous in vitro experimentation [43] with a soma diameter of between 15-25 μm. Glial-like cells were identified based upon characteristic morphology [45] and typically displayed numerous, short projections, a much larger cell body and a nuclear diameter of 20-35 μm. All quantification was performed blindly and then analysed using multi-factorial ANOVA (SigmaStat 3 software), to detect statistically significant differences in the numbers of neuron-like and glial-like cells produced between the various treatment groups. Any statistically significant effects were then confirmed using a Kruskal-Wallis one-way ANOVA on ranks with Dunn's correction for multiple pairwise comparisons.
Hair cell explant co-cultures and control treatments
After 3 days in vitro, all organotypic membranes were fixed for 30 minutes in 1 mL 4% PFA and rinsed three times for 5 minutes in PBS. After the final rinse, membranes were immunolabelled for neurofilament protein (68 kDa; Chemicon) using standard techniques. Briefly, all tissues were blocked and permeabilised using 2% goat serum in 0.1% Triton-X (diluted in PBS) for 1 hour. They were then incubated in rabbit anti-neurofilament (1:400) for 1 hour at room temperature on rotation. Following thorough rinsing in PBS, the anti-rabbit secondary antibody Alexafluor 594 (Molecular Probes) was applied for a further hour, at a dilution of 1:200 on rotation at room temperature. Primary and secondary antibodies were diluted in the described blocking solution. Following final rinses in PBS, membranes were placed onto glass slides and mounted with the hair cell explants facing up using Anti-fade (Molecular Probes). IGOR Pro (Wavemetrics®; www.wavemetrics.com) was used to generate a set of random points at which to count the number of neurons present in each treatment group. Each point was equivalent to the field of view under the 20X objective (1.25 mm in diameter). Neuron-like cells were identified within randomly generated fields by neurofilament expression in conjunction with the characteristic bi-polar morphology previously described. All quantification was performed blindly and data analysed using two-way ANOVA (SigmaStat 3 software) to detect statistically significant differences between groups.
Microscopy
All photomicrographs were taken using a Zeiss Axioplan Microscope using both transmitted light and a fluorescent lamp with appropriate filters (Zeiss filter set 00; 488000-0000, Zeiss filter set 02; 488002-0000 and Zeiss filter set 13; 488013-0000), connected to a Zeiss AxioCam 12V monochrome digital camera with AxioVision 3 software.
RESULTS
Stem cells produced neuron-like and glial-like cell morphologies in auditory neuron co-cultures and control treatments
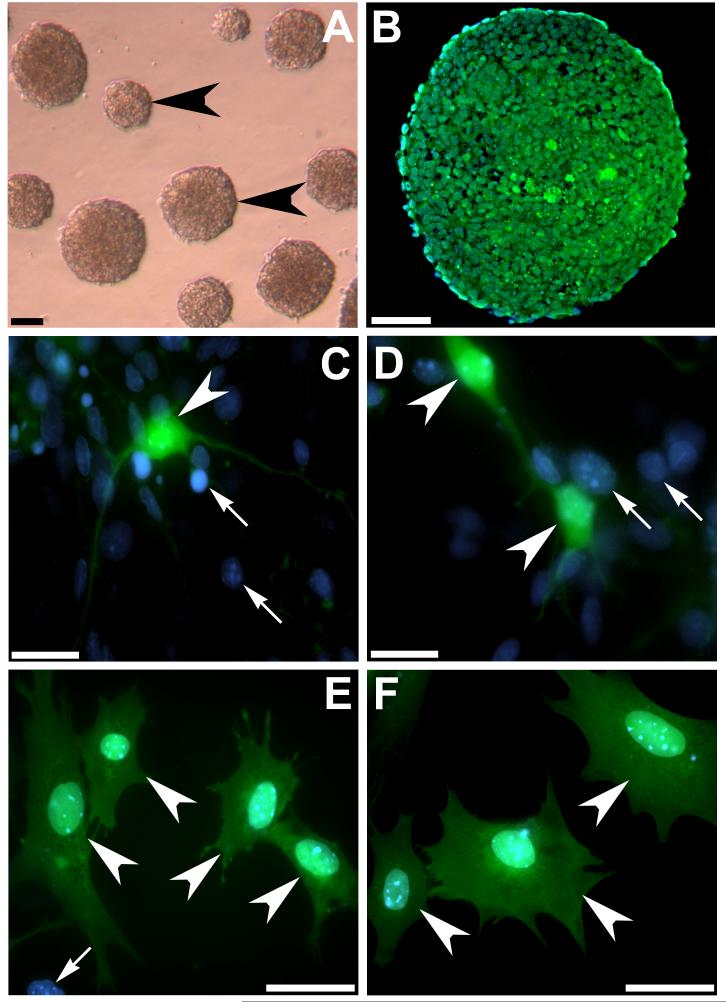
Undifferentiated SCs were induced to form free-floating embryoid bodies for eight days prior to co-culture by removal of LIF from the media and timed exposure to retinoic acid (Figure 2A,B). Following retinoic acid treatment, whole or dissociated embryoid bodies were cultured either with or without dissociated rat auditory neurons for 7 days in vitro. In addition, the effect of neurotrophin exposure on each culture condition was examined. The number of neuron-like cells (Figure 2C,D) and glial-like cells (Figure 2E,F) was counted in each treatment condition (Figure 3 conditions A-F). Stem cells were distinguished from other cell types in the co-culture using direct fluorescent microscopy for the co-expression of GFP (green) and DAPI (blue) (arrowheads, Figure 2C-F). All other cell types were GFP negative and expressed only DAPI (arrows, Figure 2C-E).
Figure 2.
Stem cell morphology and GFP expression in vitro
Mouse embryonic SCs were induced to form free-floating embryoid bodies by removal of LIF from the culture media (arrowheads, A). Photomicrograph of a transverse section through an embryoid body after 8 days in vitro, illustrates endogenous GFP expression (green) in differentiating cells (B). The nuclei are counterstained with DAPI (blue). The co-culture of dissociated and whole embryoid bodies with auditory neurons resulted in neuron-like cells and glial-like cells. Neuron-like cells displayed typical bipolar morphology with a well defined soma (arrowheads C,D) whereas glial-like cells displayed a soma extending beyond the diameter of the nucleus and numerous short processes extending in several directions (arrowheads E,F). Differentiating SCs were identified via colocalisation of endogenous GFP (green) and DAPI (blue). All other cells in co-culture were distinguished by single DAPI label (arrows C,D,E). Scale bars = 100 μm (A); 50 μm (B,E,F); 20 μm (C,D).
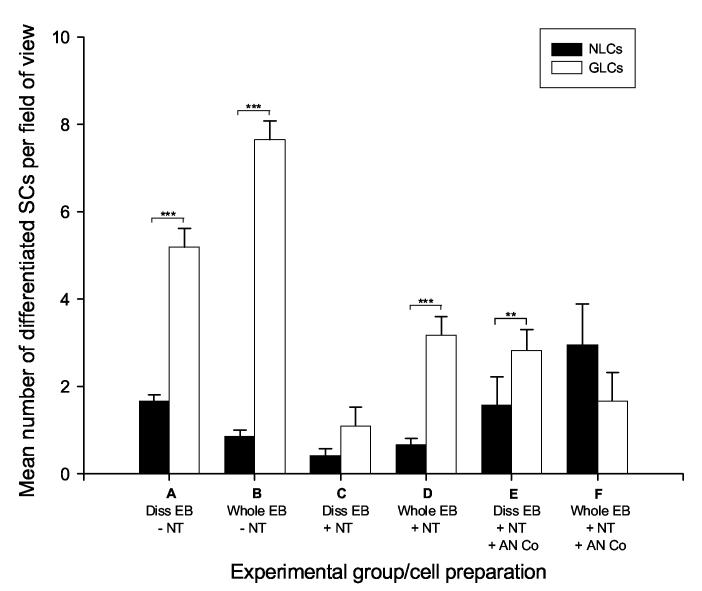
Figure 3.
Generation of neuron-like cells and glial-like cells produced from in vitro differentiation models
The mean number of neuron-like cells and glial-like cells in several differentiation models is represented. In auditory neuron media alone, whole and dissociated embryoid bodies differentiated into significantly more glial-like cells (A,B). Following the addition of BDNF and NT3, the number glial-like cells was reduced, however there were still significantly more glial-like cells formed when BDNF and NT3 were present in embryoid body cell preparations. The co-culture of whole and dissociated embryoid bodies with auditory neurons resulted in improved ratios of neuron-like cells to glial-like cells, which was most pronounced in whole embryoid body preparations. Experimental groups A-F (detailed in Figure 1); error bars indicate SEM; SCs = stem cells; Diss = dissociated; EB = embryoid bodies; NT = neurotrophins BDNF and NT3; AN Co = auditory neuron co-culture; NLCs = neuron-like cells; GLCs = glial-like cells; ** = p≤ 0.01; *** = p≤ 0.001
Effect of media, neurotrophins and auditory neuron co-culture on the differentiation of SCs into neuron-like or glial-like cells
We tested the effect of media alone on the differentiation of either dissociated or whole embryoid bodies into neuron-like or glial-like cells. In the absence of both neurotrophins and auditory neuron co-culture, we observed significantly fewer neuron-like cells in comparison to glial-like cells in both dissociated and whole embryoid body preparations (p≤0.001; Figure 3 conditions A,B). The ratio of neuron-like cells to glial-like cells in these treatments was approximately 1:3 (dissociated embryoid body preparations) and 1:9 (whole embryoid body preparations), strongly favouring the differentiation of glial-like cells in media alone (Figure 3).
We also examined the effect of the neurotrophins BDNF and NT3 on directing the differentiation of whole and dissociated embryoid bodies. In comparison to media alone treatments, these experiments showed a decrease in the number of glial-like cells produced. This was accompanied by a reduction in the total number of differentiated cells counted (Figure 3 conditions C,D). Although whole embryoid body preparations showed significantly fewer neuron-like cells than glial-like cells (p≤0.001), there was no significant difference detected in the mean number of each cell type in dissociated embryoid body preparations. Furthermore, the approximate ratio of neuron-like cells to glial-like cells in dissociated embryoid body preparations was similar with (1:3) and without (1:3) neurotrophin treatment (Figure 3 conditions A,C).
The ability of auditory neurons to promote differentiation toward a neuronal lineage, was assessed by co-culturing both whole and dissociated embryoid bodies with post-natal rat auditory neurons. This strategy improved the proportion of neuron-like cells produced from embryoid bodies (Figure 3 conditions E,F) in comparison to control treatments (Figure 3 conditions A-D), although this increase was not observed to be significantly greater.
Co-cultures of embryoid bodies and hair cell explants promoted differentiation of SCs into bipolar, neurofilament positive, cells
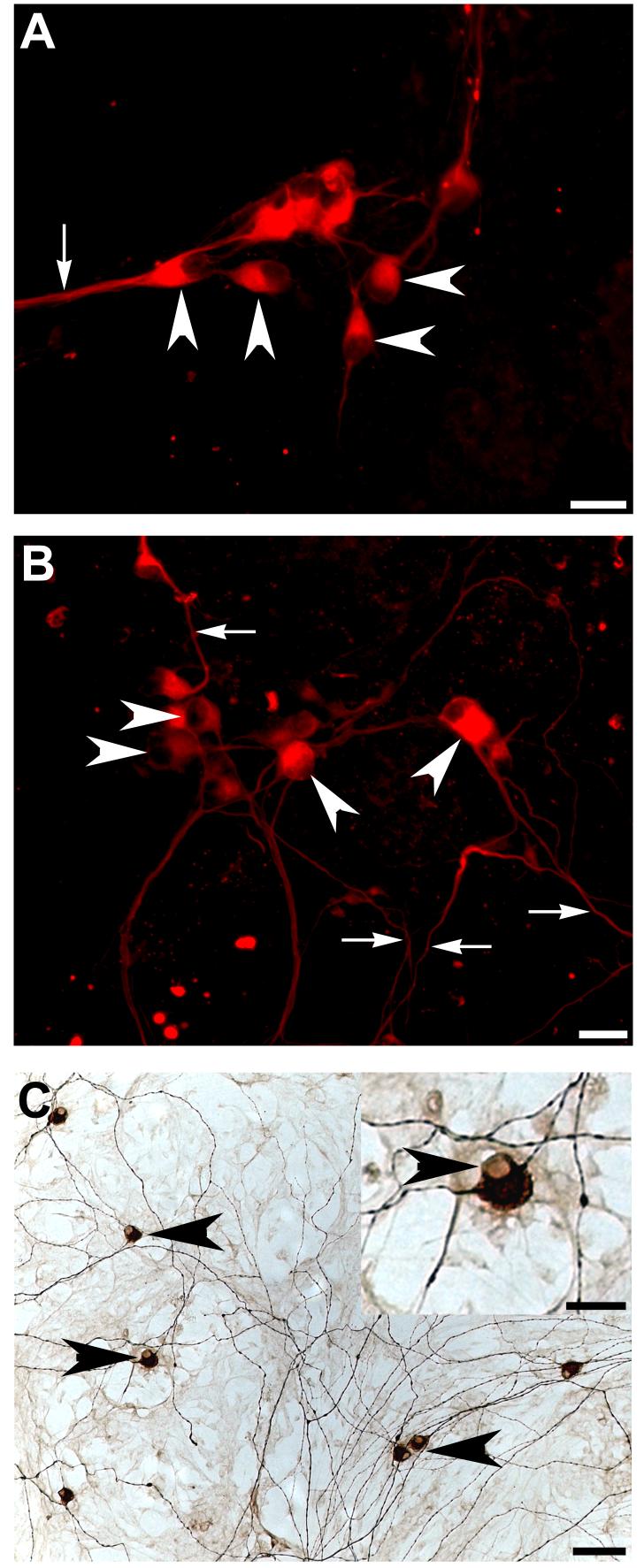
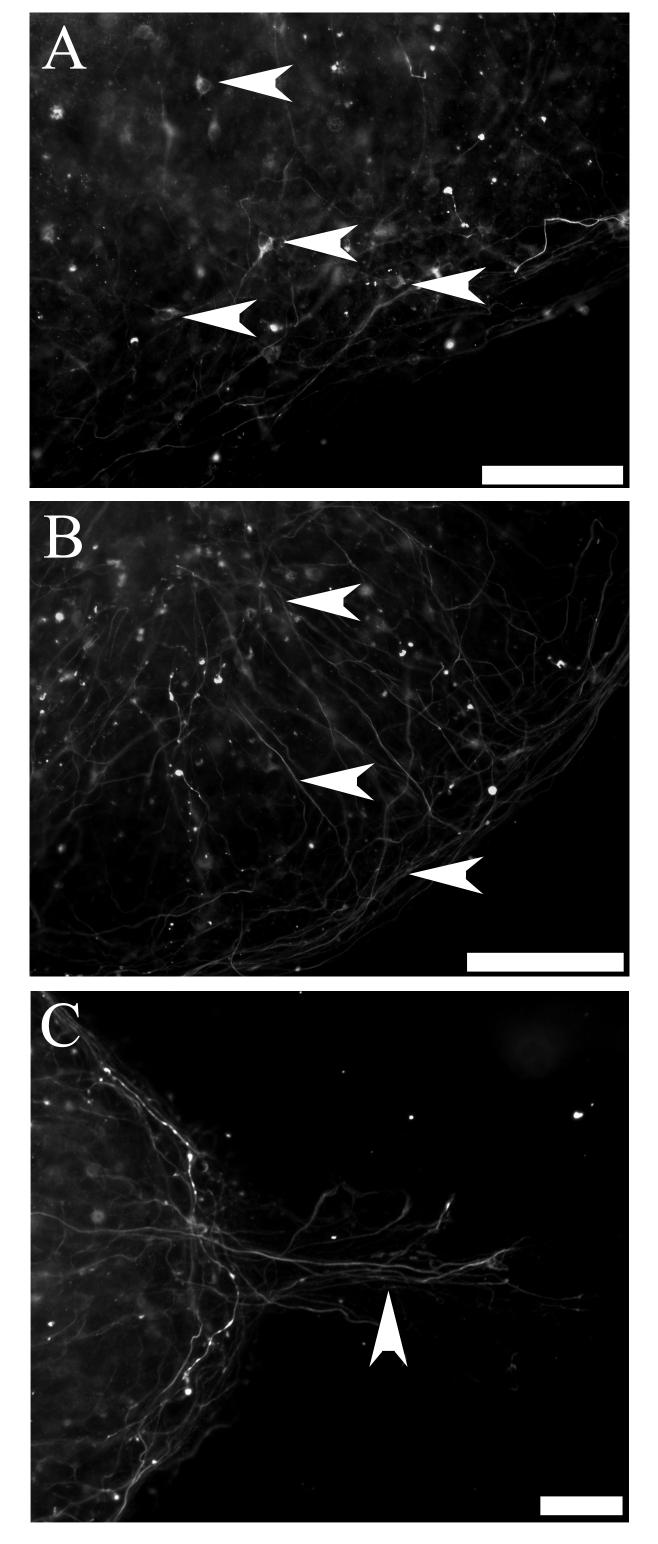
We also investigated post-natal rat hair cell explants for their ability to direct differentiation of whole embryoid bodies into bipolar neurons. Pre-differentiated embryoid bodies were co-cultured with hair cell explants with and without 50 ng/mL BDNF. Fluorescent photomicrographs depict numerous neurofilament positive, bi-polar neuron-like cells produced from co-cultures with hair cell explants (arrowheads Figure 4A,B), which were predominantly observed in the peripheral zone of the embryoid body (arrowheads Figure 5A). While in some cases the processes extending from these cells were observed growing in a directional manner (arrows Figure 4A, arrowhead 5C), in other cases they were observed to be growing in multiple directions (arrows Figure 4B). Many processes were observed growing within embryoid bodies (arrowheads Figure 5B) and a proportion of these processes extended toward adjacent embryoid bodies (arrowhead Figure 5C). Few of these processes were observed growing toward the hair cell explant and no neuron-like cells were observed on organotypic membranes when hair cell explants were cultured alone (data not shown). It is interesting to note that differentiating neuron-like cells were comparable in size, morphology and neurofilament expression to rat auditory neurons grown in culture (Figure 4C).
Figure 4.
Neurofilament positive bipolar cells were identified in hair cell explant co-cultures
Whole embryoid bodies were observed to differentiate into bipolar, neurofilament positive neuron-like cells when co-cultured with hair cell explants (arrowheads A,B). While some of these cells extended processes in the same direction (arrows A), others were observed growing in multiple directions (arrows B). Neurofilament positive cells displayed the characteristic bipolar morphology of early post-natal rat auditory neurons grown in vitro and immunolabelled with neurofilament protein (arrowheads, C). In particular, note the characteristic labelling around the nucleus (arrowhead, inset C). Scale bar = 20μm (A,B, inset C); 50 μm (C).
Figure 5.
Growth of neurofilament positive neuron-like cells on organotypic membranes
Fluorescent photomicrographs illustrating neurofilament labelling of embryoid bodies grown on organotypic membranes. (A) Growth of bipolar, neurofilament positive neuron-like cells (arrowheads) toward the periphery of the embryoid body. (B) Growth of neurofilament positive processes throughout the embryoid body (arrowheads). (C) Lower magnification image illustrating the rudimentary directional growth of neurofilament positive processes (arrowhead) away from the centre of the embryoid body, toward an adjacent embryoid body. Scale bars = 200 μm (A, B, C).
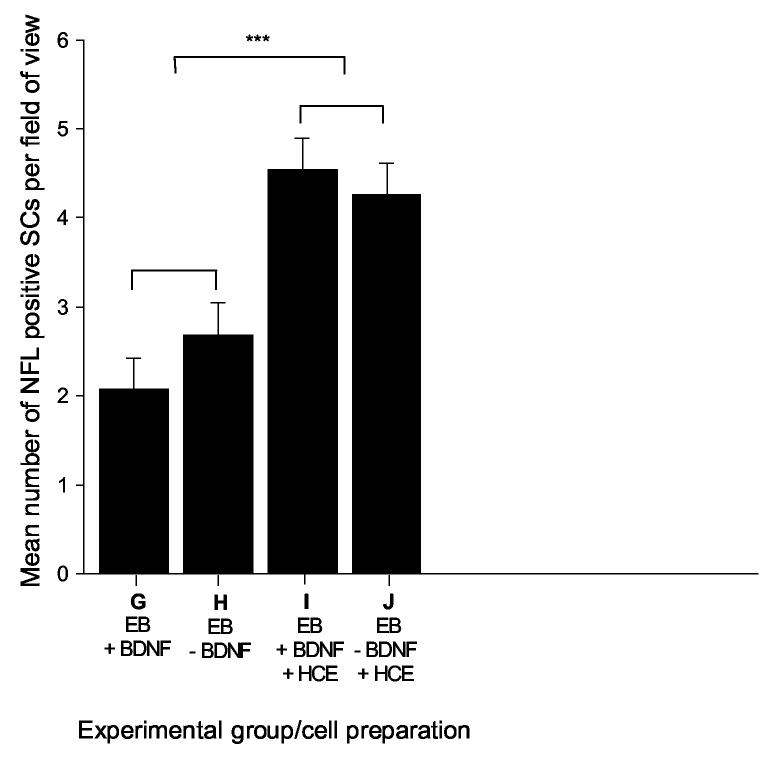
We used a two-way ANOVA to detect significant differences in the number of neurofilament positive neuron-like cells generated from the described treatment conditions. Statistical analyses showed that significantly more neurofilament positive, bi-polar cells were formed when whole embryoid bodies were grown in co-culture with hair cell explants, in comparison to control treatments grown without hair cell explants (p≤0.001; Figure 6). We did not detect any significant effect from the addition of BDNF to the various treatments either in the presence or absence of hair cell explants (Figure 6G,H versus 6I,J).
Figure 6.
Co-cultures of embryoid bodies and hair cell explants produced significantly more neurofilament positive bipolar neuron-like cells compared to controls
When whole embryoid bodies were co-cultured with early post-natal rat hair cell explants, significantly greater numbers of neurofilament positive, bipolar neuron-like cells were observed in comparison to control treatments grown in the absence of hair cell explants. There was no significant difference observed in the number of neuron-like cells formed in the presence or absence of 50 ng/mL BDNF, either with or without co-culture with hair cell explants. Experimental groups G-J (detailed in Figure 1); error bars indicate SEM; NFL = neurofilament 68 kDa; SCs = stem cells; EB = embryoid bodies; BDNF = brain derived neurotrophic factor; HCE = hair cell explant; *** = p≤0.001.
DISCUSSION
The aim of this research is to differentiate SCs into auditory neurons that can be used to replace those that degenerate following a sensorineural hearing loss. We investigated two co-culture models for their ability to provide the correct combination of tissue-derived signals required for the directed differentiation of embryonic SCs toward an auditory neuron lineage. Despite minor methodological differences between the two co-culture models, both were effective at promoting the differentiation of neuron-like cells from whole embryoid body preparations, in comparison to respective controls treatments. Collectively, these results demonstrate the potential use of co-cultures, in particular hair cell explants, at directing the differentiation of SCs into bipolar neuron-like cells. In the future, a purified population of these cells may have the potential to provide replacement neurons to the deafened mammalian cochlea.
Co-cultures of hair cell explants with embryoid bodies promoted differentiation of neuron-like cells
The co-culture of hair cell explants with whole embryoid bodies resulted in significantly greater numbers of neuron-like cells in comparison to control treatments. In these experiments, differentiated neuron-like cells expressed a neuron specific protein in a pattern comparable to dissociated neurons cultured from both post-natal day five rats (Figure 4C) and mice [46]. The neural differentiation in hair cell explant/whole embryoid body co-cultures may be related to several factors, including the specific role of hair cells and supporting cells in development, and the precise changes in neurotrophin expression patterns in developing organ of Corti tissue explants.
Auditory hair cells provide essential trophic support to auditory neurons during development [1-4, 6] in the form of the neurotrophins BDNF and NT3. The expression gradient of these neurotrophins in the cochlea is not static, and is hypothesized to be reversed between embryonic development and adulthood [47]. Specifically, BDNF is expressed in the apical region of the cochlea during development and NT3 is expressed at the base [2, 48], with the relative levels of BDNF and NT3 reported to be evenly distributed at birth [48]. In addition, studies by Stankovic and colleagues [5] have illustrated that the supporting cells of the organ of Corti also provide critical trophic support to auditory neurons via erbB-neuregulin signalling. In this way, it is plausible that the hair cell explants provide both the combination and concentration of trophic support required for the differentiation and survival of auditory neurons precursors in vitro.
The soma of the neurofilament positive, neuron-like cells were observed primarily around the periphery of the embryoid body and growing out onto the organotypic membrane. Neuron-like cells were often observed growing in close proximity to one another and were rarely observed growing alone. In addition, neurofilament positive processes were observed growing both within the embryoid bodies and projecting away from the centre onto the organotypic membrane. A proportion of these processes were observed growing toward one another in the dish and in a rudimentary directional manner, however, we did not observe any significant growth of new processes toward hair cell explants, as has been reported when auditory neurons are co-cultured with hair cell explants [49]. If stem cell therapy is to be used for auditory neuron replacement in the future, it is imperative that newly transplanted neurons form functional and tonotopic connections with second order neurons in the cochlear nucleus [50]. Such a therapy would require the directional growth of new neurons. While further in vivo investigations will be required in order to properly test the capacity of differentiated cells to form tonotopic connections in the deafened auditory system, these observations suggest that differentiated neuron-like cells are capable of rudimentary directional growth in vitro.
Brain derived neurotrophic factor has been reported to direct the differentiation of central nervous system SCs into neurons [51]. It is therefore interesting to note that we did not observe any significant effect of exogenous BDNF in the hair cell explant co-culture model. This may be due to several factors including (but not limited to) a lack of BDNF receptors on differentiating SCs in this model, competitive binding or receptor saturation by other factors in the culture medium, or lack of homology between mouse SC neurotrophin receptors and the human isoform of the protein. Further experimentation will be necessary in order to test these hypotheses.
The differentiation of SCs into neuron-like cells or glial-like cells under various in vitro conditions
The differentiation of whole and dissociated embryoid bodies in auditory neuron media, with and without neurotrophins and auditory neuron co-culture, yielded several interesting findings. These included the spontaneous differentiation of embryoid bodies into glial-like cells, the reduction in overall numbers of differentiated cells following the addition of neurotrophins or auditory neuron co-culture, and the reversed ratio of neuron-like cells to glial-like cells in auditory neuron co-culture treatments.
We observed spontaneous differentiation of embryoid bodies into glial-like cells following seven days growth in vitro in auditory neuron media alone. Similar findings have been reported in neural stem cell neurospheres (embryoid bodies), where gene expression analyses illustrate the prominence of glial-associated genes [52, 53]. Although the present study did not investigate the mechanism(s) behind enhanced glial-like cell differentiation in this model, these results are logical when considered in the context of normal nervous system development. The treatment of undifferentiated SCs with retinoic acid is reported to upregulate several ectodermal genes while suppressing mesodermal genes [54, 55], thereby mimicking early nervous system development. Given the evidence that both neurons and glia are derived from a common neural precursor cell [56, 57] and that the number of glial cells vastly exceeds the number of neurons in vivo [58], it is plausible that the in vitro model described mimics the in vivo scenario. An integral and early mechanism in the differentiation of neurons and glia in numerous SC populations is the activation of the receptor protein Notch [59-63]. Notch activation in undifferentiated SCs results in the upregulation of glial cell differentiation and the concomitant suppression of neuronal differentiation [62, 63]. Once activated, this signalling is irreversible [61]. In addition, glial cells are capable of promoting their own differentiation by secretion of soluble factors into the local environment, thus once gliogenesis is activated, it is self-perpetuating [64]. For example, bone morphogenetic proteins secreted by glial cells are reported to promote SC differentiation into glial cells in vitro [64]. Moreover, these proteins have been reported to strongly induce the differentiation of central nervous system SCs into glial cells [65-70]. The high production of glial-like cells under the described conditions may be beneficial to cell transplantation studies in the central nervous system that require the generation of glia. For example, glial cell transplants have been reported to myelinate [71] and promote functional recovery following spinal cord injury in animal models [72-74].
The ratio of neuron-like cells to glial-like cells in these treatments is also noteworthy. While there is a marked reduction in the total number of cells counted following the addition of neurotrophins, the approximate ratio of neuron-like cells to glial-like cells in dissociated embryoid body cell preparations is similar with and without neurotrophin treatment (Figure 3, condition A versus C). Interestingly, co-culture with rat auditory neurons improved the ratio of neuron-like cells to glial-like cells in treatments containing auditory neurons with whole and dissociated embryoid bodies, suggesting an important role of tissue-derived signalling in directing SC differentiation. Although these findings were not statistically different, they reflect the capacity of co-culture to alter the ratio of differentiated cells in culture. The auditory neuron co-culture requires further methodological refinement before it can be used effectively to direct auditory neuron differentiation.
Methodological considerations for future co-culture experimentation
Given the absence of reports on directed differentiation of SCs into an auditory neuron lineage to date, we hypothesized that co-cultures would provide an excellent starting point. While our data suggest that co-cultures hold promise for the directed differentiation of SCs toward an auditory neuron lineage, there are several methodological difficulties with co-culture which will need to be addressed for future experimentation and before these cells can be used for transplantation. Like many SC differentiation strategies, the described co-cultures produced a heterogeneous population of cells. While not problematic in these early stages of our experimentation, this difficulty will need to be addressed if a pure population of auditory neurons is to be differentiated for transplantation studies. A related problem is the retrieval of cells from the co-culture once they have been directed to differentiate into the desired lineage. While GFP transfection has enabled the rapid identification of SCs in co-culture models, expression of this protein is routinely downregulated or lost upon differentiation [75], rendering cell purification problematic. These difficulties could be overcome by the design of co-culture systems that enable the separation of cell populations within the same dish. Such models would confer the benefit of cell-cell mediated signalling (which is difficult to reproduce with commercially available supplements) without the problem of retrieving differentiated cells. Moreover, the use of conditioned media to direct the differentiation of SCs would also offer these advantages, with the added advantage of driving differentiation in a manner which accurately reflects in vivo development. In the long-term, the findings of these co-cultures will need to be applied to human (rather than animal) stem cell lines. This reiterates the importance of designing co-culture models than enable the rapid and efficient separation of cell types in co-culture. It also highlights the requirement to identify the factors that cause directed differentiation in vitro, so that these factors may, in the future, be applied to pure populations of human stem cells for transplantation.
SUMMARY
There is often a prolonged delay between the loss of hearing and the implementation of treatment, resulting in progressive degeneration of the auditory nerve. The aim of this research is to generate a population of replacement cells for auditory nerve degeneration, by directed differentiation of SCs into auditory neurons. While SC research is directed at cell replacement in many biological systems, to our knowledge this is the first report describing the differentiation of SCs toward an auditory neuron lineage using in vitro models. Particularly promising was the co-culture of whole embryoid bodies with hair cell explants, which resulted in significantly greater numbers of bipolar cells displaying a morphology and neural protein expression analogous to mammalian auditory neurons grown in vitro. While further investigations are necessary to determine whether the neuron-like cells generated from these treatments are functional and possess the characteristic electrophysiological properties of auditory neurons in vivo, hair cell explant/embryoid body co-cultures hold promise for the identification of factors promoting auditory neuron differentiation in vitro. The maintenance of a healthy population of auditory neurons is important for cochlear implant function and future hair cell regeneration studies, both of which rely on critical numbers of surviving auditory neurons for their efficacy.
ACKNOWLEDGEMENTS
The authors extend their sincere thanks to the following individuals for their contributions to this manuscript: to Ms J. Hardman (The Bionic Ear Institute) for her technical assistance in establishing both co-culture models; to Mr M. Hildebrand (Murdoch Childrens Research Institute) for his assistance in growing the R1 SC line; and to Dr A. Nagy (Mt Sinai Medical Hospital, Toronto) for provision of the mouse embryonic SC line R1 B5-EGFP (Tg(GFPU)5 Nagy/J) used in this study. This work was supported by the University of Melbourne, the National Institutes of Health (N01-DC-3-1005), the Royal Victorian Eye and Ear Hospital and the Bionic Ear Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ernfors P, Merlio JP, Persson H. Cells Expressing mRNA for Neurotrophins and their Receptors During Embryonic Rat Development. Eur J Neurosci. 1992;4(11):1140–1158. doi: 10.1111/j.1460-9568.1992.tb00141.x. [DOI] [PubMed] [Google Scholar]
- 2.Pirvola U, Ylikoski J, Palgi J, Lehtonen E, Arumae U, Saarma M. Brain-derived neurotrophic factor and neurotrophin 3 mRNAs in the peripheral target fields of developing inner ear ganglia. Proc Natl Acad Sci. 1992;89:9915–9919. doi: 10.1073/pnas.89.20.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ylikoski J, Pirvola U, Moshnyakov M, Palgi J, Arumae U, Saarma M. Expression patterns of neurotrophin and their receptor mRNAs in the rat inner ear. Hear Res. 1993;65:69–78. doi: 10.1016/0378-5955(93)90202-c. [DOI] [PubMed] [Google Scholar]
- 4.Pirvola U, Arumae U, Moshnyakov M, Palgi J, Saarma M, Ylikoski J. Coordinated expression and function of neurotrophins and their receptors in the rat inner ear during target innervation. Hear Res. 1994;75:131–144. doi: 10.1016/0378-5955(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 5.Stankovic K, Rio C, Xia A, Sugawara M, Adams JC, Liberman MC, Corfas G. Survival of adult spiral ganglion neurons requires erbB receptor signaling in the inner ear. J Neurosci. 2004;24(40):8651–61. doi: 10.1523/JNEUROSCI.0733-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schecterson LC, Bothwell M. Neurotrophin and neurotrophin receptor mRNA expression in developing inner ear. Hear Res. 1994;73:92–100. doi: 10.1016/0378-5955(94)90286-0. [DOI] [PubMed] [Google Scholar]
- 7.Webster M, Webster DB. Spiral ganglion neuron loss following organ of Corti loss: a quantitative study. Brain Res. 1981;212(1):17–30. doi: 10.1016/0006-8993(81)90028-7. [DOI] [PubMed] [Google Scholar]
- 8.Hardie NA, Shepherd RK. Sensorineural hearing loss during development:morphological and physiological response of the cochlear and auditory brainstem. Hear Res. 1999;128:147–165. doi: 10.1016/s0378-5955(98)00209-3. [DOI] [PubMed] [Google Scholar]
- 9.Nadol JB, Jr., Young YS, Glynn RJ. Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1989;98(6):411–6. doi: 10.1177/000348948909800602. [DOI] [PubMed] [Google Scholar]
- 10.Nadol JB., Jr. Patterns of neural degeneration in the human cochlea and auditory nerve: implications for cochlear implantation. Otolaryngol Head Neck Surg. 1997;1173(Pt 1):220–8. doi: 10.1016/s0194-5998(97)70178-5. [DOI] [PubMed] [Google Scholar]
- 11.Leake PA, Hradek GT. Cochlear pathology of long term neomycin induced deafness in cats. Hear Res. 1988;33(1):11–33. doi: 10.1016/0378-5955(88)90018-4. [DOI] [PubMed] [Google Scholar]
- 12.Gantz BJ, Woodworth GG, Knutson JF, Abbas PJ, Tyler RS. Multivariate predictors of audiological success with multichannel cochlear implants. Ann Otol Rhinol Laryngol. 1993;102(12):909–16. doi: 10.1177/000348949310201201. [DOI] [PubMed] [Google Scholar]
- 13.Ernfors P, Duan ML, ElShamy WM, Canlon B. Protection of auditory neurons from aminoglycoside toxicity by neurotrophin-3. Nat Med. 1996;2(4):463–7. doi: 10.1038/nm0496-463. [DOI] [PubMed] [Google Scholar]
- 14.Gillespie LN, Clark GM, Marzella PL. Delayed neurotrophin treatment supports auditory neuron survival in deaf guinea pigs. Neuroreport. 2004;15(7):1121–5. doi: 10.1097/00001756-200405190-00008. [DOI] [PubMed] [Google Scholar]
- 15.Kanzaki S, Stover T, Kawamoto K, Prieskorn DM, Altschuler RA, Miller JM, Raphael Y. Glial cell line-derived neurotrophic factor and chronic electrical stimulation prevent VIII cranial nerve degeneration following denervation. J Comp Neurol. 2002;454(3):350–60. doi: 10.1002/cne.10480. [DOI] [PubMed] [Google Scholar]
- 16.McGuinness SL, Shepherd RK. Exogenous BDNF rescues rat spiral ganglion neurons in vivo. Otol Neurotol. 2005;26(5):1064–72. doi: 10.1097/01.mao.0000185063.20081.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller JM, Chi DH, O'Keefe LJ, Kruszka P, Raphael Y, Altschuler RA. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int J Dev Neurosci. 1997;15(45):631–643. doi: 10.1016/s0736-5748(96)00117-7. [DOI] [PubMed] [Google Scholar]
- 18.Richardson RT, O'Leary S, Wise A, Hardman J, Clark G. A single dose of neurotrophin-3 to the cochlea surrounds spiral ganglion neurons and provides trophic support. Hear Res. 2005;204(12):37–47. doi: 10.1016/j.heares.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Staecker H, Kopke R, Malgrange B, Lefebvre P, Van de Water TR. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. Neuroreport. 1996;7(4):889–94. doi: 10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- 20.Ylikoski J, Pirvola U, Virkkala J, Suvanto P, Liang X-Q, Magal E, Altschuler RA, Miller JM, Saarma M. Guinea pig auditory neurons are protected by glial cell line-derived growth factor from degeneration after noise trauma. Hear Res. 1998;124:17–26. doi: 10.1016/s0378-5955(98)00095-1. [DOI] [PubMed] [Google Scholar]
- 21.Shepherd RK, Coco A, Epp SB, Crook JM. Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss. J Comp Neurol. 2005;486(2):145–58. doi: 10.1002/cne.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wise AK, Richardson R, Hardman J, Clark G, O'Leary S. Resprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. J Comp Neurol. 2005;487(2):147–65. doi: 10.1002/cne.20563. [DOI] [PubMed] [Google Scholar]
- 23.Gillespie LN, Clark GM, Bartlett PF, Marzella PL. BDNF-induced survival of auditory neurons in vivo: cessation of treatment leads to accelerated loss of survival effects. J Neurosci Res. 2003;71:785–790. doi: 10.1002/jnr.10542. [DOI] [PubMed] [Google Scholar]
- 24.Gillespie LN, Shepherd RK. Clinical application of neurotrophic factors: the potential for primary auditory neuron protection. Eur J Neurosci. 2005;22(9):2123–33. doi: 10.1111/j.1460-9568.2005.04430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fritzsch B. Development of inner ear afferent connections: forming primary neurons and connecting them to the developing sensory epithelia. Brain Res Bull. 2003;60(56):423–33. doi: 10.1016/s0361-9230(03)00048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schecterson LC, Bothwell M. Novel roles for neurotrophins are suggested by BDNF and NT-3 mRNA expression in developing neurons. Neuron. 1992;9(3):449–63. doi: 10.1016/0896-6273(92)90183-e. [DOI] [PubMed] [Google Scholar]
- 27.Buytaert-Hoefen KA, Alvarez E, Freed CR. Generation of tyrosine hydroxylase positive neurons from human embryonic stem cells after coculture with cellular substrates and exposure to GDNF. Stem Cells. 2004;22(5):669–74. doi: 10.1634/stemcells.22-5-669. [DOI] [PubMed] [Google Scholar]
- 28.Abouelfetouh A, Kondoh T, Ehara K, Kohmura E. Morphological differentiation of bone marrow stromal cells into neuron-like cells after co-culture with hippocampal slice. Brain Res. 2004;1029(1):114–9. doi: 10.1016/j.brainres.2004.07.092. [DOI] [PubMed] [Google Scholar]
- 29.Wislet-Gendebien S, Hans G, Leprince P, Rigo JM, Moonen G, Rogister B. Plasticity of cultured mesenchymal stem cells: switch from nestin-positive to excitable neuron-like phenotype. Stem Cells. 2005;23(3):392–402. doi: 10.1634/stemcells.2004-0149. [DOI] [PubMed] [Google Scholar]
- 30.Kim SJ, Kim BS, Ryu SW, Yoo JH, Oh JH, Song CH, Kim SH, Choi DS, Seo JH, Choi CW, Shin SW, Kim YH, Kim JS. Hematopoietic differentiation of embryoid bodies derived from the human embryonic stem cell line SNUhES3 in co-culture with human bone marrow stromal cells. Yonsei Med J. 2005;46(5):693–9. doi: 10.3349/ymj.2005.46.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Zhao HP, Lin G, Xie CQ, Nie DS, Wang QR, Lu GX. In vitro hematopoietic differentiation of human embryonic stem cells induced by co-culture with human bone marrow stromal cells and low dose cytokines. Cell Biol Int. 2005;29(8):654–61. doi: 10.1016/j.cellbi.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Sugie Y, Yoshikawa M, Ouji Y, Saito K, Moriya K, Ishizaka S, Matsuura T, Maruoka S, Nawa Y, Hara Y. Photoreceptor cells from mouse ES cells by co-culture with chick embryonic retina. Biochem Biophys Res Commun. 2005;332(1):241–7. doi: 10.1016/j.bbrc.2005.04.125. [DOI] [PubMed] [Google Scholar]
- 33.Fair JH, Cairns BA, Lapaglia M, Wang J, Meyer AA, Kim H, Hatada S, Smithies O, Pevny L. Induction of hepatic differentiation in embryonic stem cells by co-culture with embryonic cardiac mesoderm. Surgery. 2003;134(2):189–96. doi: 10.1067/msy.2003.225. [DOI] [PubMed] [Google Scholar]
- 34.Kadereit S, Deeds LS, Haynesworth SE, Koc ON, Kozik MM, Szekely E, Daum-Woods K, Goetchius GW, Fu P, Welniak LA, Murphy WJ, Laughlin MJ. Expansion of LTC-ICs and maintenance of p21 and BCL-2 expression in cord blood CD34(+)/CD38(−) early progenitors cultured over human MSCs as a feeder layer. Stem Cells. 2002;20(6):573–82. doi: 10.1634/stemcells.20-6-573. [DOI] [PubMed] [Google Scholar]
- 35.Harvey K, Dzierzak E. Cell-cell contact and anatomical compatibility in stromal cell-mediated HSC support during development. Stem Cells. 2004;22(3):253–8. doi: 10.1634/stemcells.22-3-253. [DOI] [PubMed] [Google Scholar]
- 36.Alexanian AR, Kurpad SN. Quiescent neural cells regain multipotent stem cell characteristics influenced by adult neural stem cells in co-culture. Exp Neurol. 2005;191(1):193–7. doi: 10.1016/j.expneurol.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 37.Combelles CM, Fissore RA, Albertini DF, Racowsky C. In vitro maturation of human oocytes and cumulus cells using a co-culture three-dimensional collagen gel system. Hum Reprod. 2005;20(5):1349–58. doi: 10.1093/humrep/deh750. [DOI] [PubMed] [Google Scholar]
- 38.Robinson SN, Ng J, Niu T, Yang H, McMannis JD, Karandish S, Kaur I, Fu P, Del Angel M, Messinger R, Flagge F, de Lima M, Decker W, Xing D, Champlin R, Shpall EJ. Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transplant. 2006;37(4):359–66. doi: 10.1038/sj.bmt.1705258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Developmental Biology. 1995;168(2):342–57. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- 40.Coleman B, Fallon JB, de Silva M, Shepherd RK. Proceedings of the Australian Neuroscience Society. Sydney, Australia: 2006. Hair cell co-culture promotes neural differentiation of mouse embryonic stem cells. [Google Scholar]
- 41.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A. 1993;90(18):8424–8. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geal-Dor M, Freeman S, Li G, Sohmer H. Development of hearing in neonatal rats: air and bone conducted ABR thresholds. Hear Res. 1993;69(12):236–42. doi: 10.1016/0378-5955(93)90113-f. [DOI] [PubMed] [Google Scholar]
- 43.Gillespie LN, Clark GM, Bartlett PF, Marzella PL. LIF is more potent than BDNF in promoting neurite outgrowth of mammalian auditory neurons in vitro. Neuroreport. 2001;12(2):275–279. doi: 10.1097/00001756-200102120-00019. [DOI] [PubMed] [Google Scholar]
- 44.Marzella PL, Gillespie LN, Clark GM, Bartlett PF, J KT. The neurotrophins act synergistically with LIF and members of the TGF-B superfamily to promote the survival of spiral ganglion neurons in vitro. Hear Res. 1999;138(12):73–80. doi: 10.1016/s0378-5955(99)00152-5. [DOI] [PubMed] [Google Scholar]
- 45.Morrow T, Song MR, Ghosh A. Sequential specification of neurons and glia by developmentally regulated extracellular factors. Development. 2001;128(18):3585–94. doi: 10.1242/dev.128.18.3585. [DOI] [PubMed] [Google Scholar]
- 46.Whitlon DS, Ketels KV, Coulson MT, Williams T, Grover M, Edpao W, Richter CP. Survival and morphology of auditory neurons in dissociated cultures of newborn mouse spiral ganglion. Neurosci. 2006;138(2):653–62. doi: 10.1016/j.neuroscience.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 47.Davis RL. Gradients of neurotrophins, ion channels, and tuning in the cochlea. Neuroscientist. 2003;9(5):311–6. doi: 10.1177/1073858403251986. [DOI] [PubMed] [Google Scholar]
- 48.Farinas I, Jones KR, Tessarollo L, Vigers AJ, Huang E, Kirstein M, de Caprona DC, Coppola V, Backus C, Reichardt LF, Fritzsch B. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J Neurosci. 2001;21(16):6170–80. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martinez-Monedero R, Corrales CE, Cuajungco MP, Heller S, Edge AS. Reinnervation of hair cells by auditory neurons after selective removal of spiral ganglion neurons. J Neurobiol. 2006;66(4):319–31. doi: 10.1002/neu.20232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coleman B, Hardman J, Coco A, Epp S, de Silva M, Crook J, Shepherd RK. Fate of embryonic stem cells transplanted into the deafened mammalian cochlea. Cell Transplantation. 2006;15(5):369–380. doi: 10.3727/000000006783981819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahmed S, Reynolds BA, Weiss S. BDNF enhances the differentiation but not the survival of CNS stem cell-derived neuronal precursors. J Neurosci. 1995;15(8):5765–78. doi: 10.1523/JNEUROSCI.15-08-05765.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parker MA, Anderson JK, Corliss DA, Abraria VE, Sidman RL, Park KI, Teng YD, Cotanche DA, Snyder EY. Expression profile of an operationally-defined neural stem cell clone. Exp Neurol. 2005;194(2):320–32. doi: 10.1016/j.expneurol.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 53.Jori FP, Galderisi U, Piegari E, Cipollaro M, Cascino A, Peluso G, Cotrufo R, Giordano A, Melone MA. EGF-responsive rat neural stem cells: molecular follow-up of neuron and astrocyte differentiation in vitro. J Cell Physiol. 2003;195(2):220–33. doi: 10.1002/jcp.10249. [DOI] [PubMed] [Google Scholar]
- 54.Bain G, Ray WJ, Yao M, Gottlieb DI. Retinoic acid promotes neural and represses mesodermal gene expression in mouse embryonic stem cells in culture. Biochem Biophys Res Commun. 1996;223(3):691–4. doi: 10.1006/bbrc.1996.0957. [DOI] [PubMed] [Google Scholar]
- 55.Boudjelal M, Taneja R, Matsubara S, Bouillet P, Dolle P, Chambon P. Overexpression of Stra13, a novel retinoic acid-inducible gene of the basic helix-loop-helix family, inhibits mesodermal and promotes neuronal differentiation of P19 cells. Genes Dev. 1997;11(16):2052–65. doi: 10.1101/gad.11.16.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kilpatrick TJ, Bartlett PF. Cloning and growth of multipotential neural precursors: requirements for proliferation and differentiation. Neuron. 1993;10(2):255–65. doi: 10.1016/0896-6273(93)90316-j. [DOI] [PubMed] [Google Scholar]
- 57.Stemple DL, Anderson DJ. Isolation of a stem cell for neurons and glia from the mammalian neural crest. Cell. 1992;71(6):973–85. doi: 10.1016/0092-8674(92)90393-q. [DOI] [PubMed] [Google Scholar]
- 58.Kandel ER, Schwartz JH, Jessell TM. Principles of neural science. Fourth ed. McGraw-Hill; Sydney: 2000. p. 1414. [Google Scholar]
- 59.Chitnis A, Henrique D, Lewis J, Ish-Horowicz D, Kintner C. Primary neurogenesis in Xenopus embryos regulated by a homologue of the Drosophila neurogenic gene Delta. Nature. 1995;375(6534):761–6. doi: 10.1038/375761a0. [DOI] [PubMed] [Google Scholar]
- 60.Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284(5415):770–6. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 61.Morrison SJ, Perez SE, Qiao Z, Verdi JM, Hicks C, Weinmaster G, Anderson DJ. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell. 2000;101(5):499–510. doi: 10.1016/s0092-8674(00)80860-0. [DOI] [PubMed] [Google Scholar]
- 62.Tanigaki K, Nogaki F, Takahashi J, Tashiro K, Kurooka H, Honjo T. Notch1 and Notch3 instructively restrict bFGF-responsive multipotent neural progenitor cells to an astroglial fate. Neuron. 2001;29(1):45–55. doi: 10.1016/s0896-6273(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 63.Schuurmans C, Guillemot F. Molecular mechanisms underlying cell fate specification in the developing telencephalon. Curr Opin Neurobiol. 2002;12(1):26–34. doi: 10.1016/s0959-4388(02)00286-6. [DOI] [PubMed] [Google Scholar]
- 64.Chang MY, Son H, Lee YS, Lee SH. Neurons and astrocytes secrete factors that cause stem cells to differentiate into neurons and astrocytes, respectively. Mol Cell Neurosci. 2003;23(3):414–26. doi: 10.1016/s1044-7431(03)00068-x. [DOI] [PubMed] [Google Scholar]
- 65.Gross RE, Mehler MF, Mabie PC, Zang Z, Santschi L, Kessler JA. Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron. 1996;17(4):595–606. doi: 10.1016/s0896-6273(00)80193-2. [DOI] [PubMed] [Google Scholar]
- 66.Rajan P, McKay RD. Multiple routes to astrocytic differentiation in the CNS. J Neurosci. 1998;18(10):3620–9. doi: 10.1523/JNEUROSCI.18-10-03620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mabie PC, Mehler MF, Kessler JA. Multiple roles of bone morphogenetic protein signaling in the regulation of cortical cell number and phenotype. J Neurosci. 1999;19(16):7077–88. doi: 10.1523/JNEUROSCI.19-16-07077.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yanagisawa M, Takizawa T, Ochiai W, Uemura A, Nakashima K, Taga T. Fate alteration of neuroepithelial cells from neurogenesis to astrocytogenesis by bone morphogenetic proteins. Neurosci Res. 2001;41(4):391–6. doi: 10.1016/s0168-0102(01)00297-8. [DOI] [PubMed] [Google Scholar]
- 69.Adachi T, Takanaga H, Kunimoto M, Asou H. Influence of LIF and BMP-2 on differentiation and development of glial cells in primary cultures of embryonic rat cerebral hemisphere. J Neurosci Res. 2005;79(5):608–15. doi: 10.1002/jnr.20373. [DOI] [PubMed] [Google Scholar]
- 70.Brederlau A, Faigle R, Elmi M, Zarebski A, Sjoberg S, Fujii M, Miyazono K, Funa K. The bone morphogenetic protein type Ib receptor is a major mediator of glial differentiation and cell survival in adult hippocampal progenitor cell culture. Mol Biol Cell. 2004;15(8):3863–75. doi: 10.1091/mbc.E03-08-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu S, Qu Y, Stewart TJ, Howard MJ. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc Natl Acad Sci. 2000;97(11):6126–31. doi: 10.1073/pnas.97.11.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramon-Cueto A, Cordero MI, Santos-Benito FF, Avila J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron. 2000;25(2):425–35. doi: 10.1016/s0896-6273(00)80905-8. [DOI] [PubMed] [Google Scholar]
- 73.Hasegawa K, Chang YW, Li H, Berlin Y, Ikeda O, Kane-Goldsmith N, Grumet M. Embryonic radial glia bridge spinal cord lesions and promote functional recovery following spinal cord injury. Exp Neurol. 2005;193(2):394–410. doi: 10.1016/j.expneurol.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 74.Lee KH, Yoon do H, Park YG, Lee BH. Effects of glial transplantation on functional recovery following acute spinal cord injury. J Neurotrauma. 2005;22(5):575–89. doi: 10.1089/neu.2005.22.575. [DOI] [PubMed] [Google Scholar]
- 75.Elefanty A, Stanley EG. GFP is routinely lost from murine embryonic stem cell lines upon differentiation (personal communication) In: Coleman B, editor. Melbourne: 2005. [Google Scholar]