Abstract
Background
The KISS1 protein suppresses metastasis of several tumor models without blocking orthotopic tumor growth, but the mechanism remains elusive. For its role in human sexual maturation, KISS1 protein is secreted and processed to kisspeptins, which bind to the G protein–coupled receptor GPR54. We tested the hypothesis that KISS1 secretion is required for metastasis suppression via GPR54.
Methods
KISS1 containing an internal FLAG epitope with (KFM) or without (KFMδSS) a signal sequence was transfected into C8161.9 human melanoma cells, which do not express endogenous KISS1. Whole-cell lysates and conditioned medium from C8161.9KFM and C8161.9KFMΔSS cells were collected and analyzed for kiss-peptins by immunoprecipitation and enzyme-linked immunosorbent assay. GPR54 levels were measured using real-time reverse transcription–polymerase chain reaction. The ability of conditioned medium from C8161.9KFM and C8161.9KFMΔSS cells to stimulate calcium mobilization in GPR54-expressing Chinese hamster ovary cells (CHO-G) and in C8161.9 cells was evaluated. Metastasis was monitored in athymic mice (groups of 10 per experiment) that were injected with C8161.9KFM or C8161.9KFMΔSS cells labeled with enhanced green fluorescent protein. Survival of mice injected with C8161.9 or C8161.9KFM cells was analyzed by Kaplan–Meier methods.
Results
Full-length KFM and KFM SS were detected in whole-cell lysates of C8161.9KFM and C8161.9KFMΔSS cells, respectively, but kisspeptins were detected only in conditioned medium of C8161.9KFM cells. In vivo, C8161.9KFM, but not C8161.9KFMΔSS, cells were suppressed for metastasis to lung, eye, kidney, and bone, with corresponding differences in mouse survival (median > 120 versus 42 days). C8161.9KFM cells seeded mouse lungs but did not form macroscopic metastases. Conditioned medium from C8161.9KFM, but not C8161.9KFMΔSS, cells stimulated calcium mobilization in CHO-G cells. GPR54 expression was low in C8161.9 cells, which were not stimulated by conditioned medium from C8161.9KFM cells.
Conclusions
KISS1 secretion was required for multiple organ metastasis suppression and for maintenance of disseminated cells in a dormant state. The absence of GPR54 expression in C8161.9 cells (whose metastatic spread was suppressed by KFM) suggests that metastasis suppression is not mediated through this receptor. The results imply the existence of another KISS1 receptor and/or paracrine signaling. The findings raise the possibility that soluble KISS1, kisspeptins, or mimetics could be used to maintain tumor dormancy, rendering treatment of already disseminated tumor cells (i.e., micrometastases) a legitimate target.
The KISS1 metastasis suppressor protein strongly inhibits pulmonary and intraperitoneal metastasis of human melanoma, breast cancer, and ovarian cancer xenograft models while still allowing growth of tumors at orthotopic sites (1-5). For many human cancers, KISS1 mRNA expression is inversely related to tumor grade and metastatic potential and directly related to prognosis (6-13). Despite consistent evidence that KISS1 regulates metastasis, its mechanism of action has only been inferred from previous studies.
The product of the KISS1 gene is secreted and processed to produce polypeptides, termed kisspeptins, through mechanisms that are thought to be similar to the processing of neuropep-tides (14-16). Kisspeptins are ligands for a G protein–coupled receptor, GPR54, through which they are thought to exert several important biologic effects, including regulation of sexual maturity, puberty, and perhaps pregnancy and islet cell function (14-22). One of the GPR54-binding kisspeptins is kisspeptin-54 (KP-54), a 54–amino acid polypeptide fragment of KISS1 that is also known as metastin. Administration of the C-terminal 10 amino acids of metastin (KP-10) to mice that had been injected with GPR54-overexpressing B16 melanoma cells decreased the metastatic potential of these cells (22). These observations led to the hypothesis that KISS1 secretion, processing, and autocrine signaling through GPR54 are necessary for its antimetastatic effects.
CONTEXT AND CAVEATS
Prior Knowledge
The KISS1 gene encodes a secreted protein that, in the context of sexual maturation, is processed to peptides known as kisspeptins. Kisspeptins exert their effects on sexual development by binding to the G protein–coupled receptor GPR54. KISS1 was initially identified as a metastasis suppressor, but whether KISS1 secretion and processing are GPR54 binding are necessary for suppression of metastasis is not known.
Study Design
A human melanoma cell line that does not express KISS1 was transfected with an easily detectable tagged form of KISS1 that was either unaltered or altered so that it could not be secreted. Transfected cells were analyzed in vitro and in mice.
Contributions
Only cells transfected with the secretable tagged form of KISS1 secreted kisspeptins to the medium. Such cells showed less meta-static spread in vivo than the cells transfected with the nonsecretable form of KISS1. GPR54 was expressed at a very low level in the melanoma cell line used in the study.
Implications
KISS1 metastasis suppressor activity requires secretion and processing of the protein, but kisspeptin binding to GPR54 may not be necessary. Kisspeptins may have the potential to modify metastasis in vivo.
Limitations
A single cell line was used. Whether metastasis of the transfected line in mice accurately reflects its behavior in humans is not known. The ability of exogenously supplied kisspeptins to suppress metastasis in vivo was not tested.
To determine whether KISS1 secretion and processing are required to prevent metastasis in metastasis-suppressed tumor cells, we expressed an epitope-tagged version of KISS1 and in metastatic C8161.9 human melanoma cells, which do not express endogenous KISS1 (2). The role of autocrine signaling of KISS1 through GPR54 in C8161.9 cells was determined by assessing levels of GPR54 and the response of GPR54-expressing cells to exogenous KISS1. The importance of KISS1 (and/or kisspeptin) secretion as a critical step in metastasis suppression to multiple organs was evaluated using enhanced green fluorescent protein (GFP)–tagged melanoma cells.
Methods
Generation and Maintenance of Cell Lines and Explants
C8161.9 and MelJuSo are amelanotic human melanoma cell lines that metastasize following intradermal, subcutaneous, intracardiac, or intravenous injection into athymic nude mice (23, 24). MDAMB-435 is a human metastatic breast ductal carcinoma cell line that metastasizes following orthotopic or intracardiac injection in athymic nude mice (25, 26). The SKOV3 ovarian carcinoma cell line generates intraperitoneal metastases following orthotopic injection into the ovaries of athymic nude mice (27, 28). None of these meta-static cells express endogenous KISS1 (1, 2, 4).
C8161.9, MelJuSo, and MDA-MB-435 cells were grown in Dulbecco's modified Eagle medium (DMEM)/F12 growth medium (Invitrogen, Carlsbad, CA) supplemented with 5% fetal bovine serum (FBS, Atlanta Biologicals, Norcross, GA), glutamine, and nonessential amino acids (cDMEM/F12). Neomycin-resistant C8161.9 cells, transfected with pcDNA3.1 vector or pcDNA3.1 containing KFM or KFMΔSS (see below), were maintained in cDMEM/F12 containing 500 μg/mL G418 (Invitrogen). All cells were detached from culture dishes using 2 mM EDTA in Ca2+- and Mg2+-free Dulbecco's phosphate-buffered saline (DPBS). SKOV3 cells were cultured in DMEM (Invitrogen). All cells were confirmed to be negative for Mycoplasma spp infection using a polymerase chain reaction (PCR)–based test (TaKaRa, Shiga, Japan).
Chinese hamster ovary (CHO) cells that coexpress the human chemokine receptor CXCR4 and human GPR54 (CHO-G cells) were previously described (27). These cells were maintained using the same conditions as C8161.9 cells. CHO cells that coexpress CXCR4 and hemagglutinin-tagged human m1 muscarinic acetylcholine receptor (M1) were prepared by stable transfection using pcDNA3.1 vector (Invitrogen). Full-length cDNA of CXCR4 and M1 were obtained from the University of Missouri–Rolla cDNA Resource Center (http://www.cdna.org/).
C8161.9 cells were transduced with a human immunodeficiency virus type 1–based lentiviral vector system that constitutively expresses enhanced GFP (29, 30). Infectious virus stocks were prepared by transfecting 293T human renal epithelial cells with lenti-viruses at multiplicities of infection of approximately 10. The top 10% of fluorescing cells were sorted and collected using a BD FACSAria cell sorter (BD Biosciences Immunocytometry Systems, San Jose, CA).
Cultures of lung explants from mice injected with GFP-tagged cells were established by rinsing whole, freshly dissected lungs in ice-cold DPBS followed by extensive mincing with razor blades. Minced lung tissue was cultured in cDMEM/F12 containing gentamicin (Invitrogen) at 100 μg/mL and puromycin (Fisher Scientific, Hampton, NH) at 1 μg/mL. Tissue debris and unattached cells were aspirated after 1 week. All cells were still GFP positive following selection.
In vitro cell growth was measured as follows. C8161.9 and C8161.9KFM cells were plated in triplicate in 24-well plates at a final seeding density of 2500 cells/500 μL. Cells were allowed to attach overnight, and the medium was replaced (500 μL) the next day. At various times, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide was added to each well at a final concentration of 0.5 mg/mL, and the cells were incubated at 37 °C for 3 hours. Following incubation, medium was removed, 1 mL dimethyl sulf-oxide was added to each well to dissolve formazan crystals, and absorbance at 570 nm was recorded.
Construct Design of KFM, KFMΔSS, and KGM••
Based on the proposed model of KISS1 processing (Fig. 1) and the inability to detect N-terminal or C-terminal epitope-tagged KISS1 in mammalian cells (unpublished data••), a hydrophilic 8–amino acid FLAG epitope was inserted between the R66–R67 dibasic cleavage site and the metastin sequence of full-length KISS1, to create KFM (Fig. 1, A). The putative secretion signal sequence was deleted from the KFM construct to create KFMΔSS. Finally, the FLAG epitope was replaced with GFP to create KGM, which was used in studies of intracellular localization.
Fig. 1.

Detection and processing of KFM, in which an internal FLAG epitope has been inserted into KISS1 upstream of the 54–amino acid metastin sequence. A) Schematic diagrams of KFM and KFMΔSS (secretion signal deleted). Consensus prohormone convertase dibasic recognition sequences (RK, RR, and GKR) are shown. B) Detection of KFM and KFMΔSS in whole-cell C8161.9 cell variant lysates by immunoprecipitation with anti-metastin polyclonal antiserum followed by immunoblotting with anti-FLAG antibody. β-actin was used as a loading control. C) Detection of KISS1 and processed kisspeptins from C8161.9 cell variants in conditioned medium by immunoprecipitation with anti-metastin polyclonal antiserum followed by immunoblotting with anti-FLAG antibody. D) Predicted processing of KFM to kisspeptins and the mature metastin product. The size of kisspeptins detected in (C) approximate the predicted sizes shown. Metastin is not detected because polyacylamide gels are generally not suitable for detection of small polypeptides. E) Sequences of secreted kisspeptins identified in conditioned medium by electrospray ionization mass spectroscopy/mass spectroscopy. All the bands isolated from the sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel contained sequences within the metastin polypeptide. The sequence shown shows that 39 of the 54 metastin amino acids were found and properly oriented. Detection of sequences N-terminal to metastin in several of the larger bands suggests that at least some KISS1 processing occurs extracellularly.
KISS1 and GPR54 mRNA Quantification
RNA was collected from cells grown to confluence on 10-cm tissue culture plates using Trizol reagent (Invitrogen). RNA pellets were resuspended in diethylpyrocarbonate-treated water and stored at −80 °C. Reverse transcription–polymerase chain reaction (RT–PCR) was performed using One-Step RT-PCR Master Mix Reagents (Applied Biosystems [ABS], Foster City, CA) and ABS premade primer probe sets for mitochondrial ribosomal protein S9 (Hs00396989_m1), KISS1 (Hs00158486_m1), and GPR54 (Hs00261399_m1). Real-time RT–PCR was performed with the ABS 7500 real-time PCR system using the thermal profile recommended by the manufacturer. Human placental polyadenylated mRNA (Ambion, Austin, TX) was used as a positive control for GPR54 expression.
Detection of KFM and Secreted Kisspeptins
C8161.9 and variant cells (C8161.9vector, C8161.9KFMΔSS, C8161.9KFM) were grown to confluence in cDMEM/F12 on 10-cm tissue culture plates. The medium was removed and replaced with serum-free medium (5 mL). After 48 hours, medium was collected and centrifuged (18 000 g at 4 °C) to remove cellular debris. The remaining cells were lysed in lysis buffer (25 mM Tris–HCl [pH 7.4], 50 mM β-glycerol phosphate, 0.5 mM EDTA, 5% glycerol, 0.1% Triton X-100, 1 mM sodium orthovanadate, 1 mM benzamidine, protease inhibitor cocktail containing aprotinin, leupeptin, and phenylmethylsulfonyl fluoride [Roche; Indianapolis, IN]) at xxxx. Secreted kisspeptins were immunoprecipitated from conditioned medium (1 mL) with rabbit polyclonal antiserum that recognizes KP-54 (αKP-54; provided by N. Ghaffari-Tabrizzi and used at 1 : 500) and protein A/G beads (Santa Cruz Biotechnology, Santa Cruz, CA).
KISS1 proteins immunoprecipitated from conditioned medium or whole-cell lysates (lysis buffer contains) were separated by 18% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE), transferred to a polyvinylidenefluoride membrane using semidry transfer, and probed with an anti-FLAG monoclonal antibody (M2, Sigma-Aldrich, St Louis, MO; used at 1 : 500). Immunoblots were incubated with a horseradish peroxidase–labeled secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). Bound antibody was detected using chemiluminescence (ECL-Plus; Amersham Biosciences, Piscataway, NJ). BioRad Kaleidoscope Precision Plus protein standards (Hercules, CA) were run on the gels to allow approximate molecular weight determination.
Secreted kisspeptins from C8161.9KFM cells were evaluated by capture enzyme-linked immunosorbent assay (ELISA) using polyclonal αKP-54 (10 μg/mL) as the primary antibody and horseradish peroxidase–labeled FLAG M2 monoclonal antibody (FLAG M2-HRP, Sigma-Aldrich; used at 1 : 500) for detection. The αKP-54 antibody (10 μg/mL) was placed into microtiter plates overnight at 4 °C. Conditioned medium (1 : 50) from C8161.9, C8161.9vector, C8161.9KFMΔSS, and C8161.9KFM cells was added to the wells, and the plates were incubated at room temperature for 3 hours before addition of FLAG M2-HRP (horseradish peroxidase) for 1 hour at room temperature. Colorimetric detection was performed by addition of 3,3′,5,5 ′-tetramethylbenzidine substrate (Sigma-Aldrich) for 15 minutes followed by addition of 50 μL of sulfuric acid (1 M) to stop the reaction.
Kisspeptins were batch purified from 50 mL of conditioned medium collected from C8161.9KFM and C8161.9KFMΔSS cells (ten 10-cm plates each) by incubating the medium overnight at 4 °C with αKP-54 polyclonal antiserum that was linked to an agarose matrix by its amino terminus according to the manufacturer's instructions (Pierce AminoLink Plus). The agarose beads were loaded onto a gravity flow column, and kisspeptins were eluted with 0.15 M glycine (pH 2.5). Eluted fractions containing kiss-peptins, as determined by western blotting with anti–FLAG M2 monoclonal antibody, were combined and dialyzed using a 3.5 molecular weight cutoff Pierce Slide-A-Lyzer Cassette against two changes (1000 mL each) of 10 mM Tris (pH 7.5) followed by one change of 1000 mL water. The samples were evaporated, resus-pended in Laemmli sample buffer, and resolved on 18% SDS–PAGE. Gels were stained with Coomassie blue, and visible bands were excised and submitted to the University of Alabama at Birmingham (UAB) Mass Spectrometry and Proteomics Shared Facility where they were digested with either trypsin or Glu-C proteases and identified by matrix-assisted laser desorption/ionization-time of flight mass spectroscopy (MALDI-TOF) and/or electrospray ionization mass spectroscopy/mass spectroscopy (ESI-MS/MS). Partial overlapping sequences derived from digested kisspeptin bands were aligned (Fig. 1, E). Bands without mass spectroscopy data and/or sequence determination are thought to be KISS1 or kisspeptins but could not be identified unambiguously because they did not produce an adequate signal.
Signaling Responses in Kisspeptin-Treated Cells
CHO and C8161.9 and C8161.9KFM cells were exposed for 5 minutes to combinations of chemically synthesized ligands for various receptors. These ligands included KP-10 (100 nM; Genemed Synthesis Inc, San Francisco, CA); stromal-derived factor 1 (SDF-1), the ligand for CXCR4 (100 nM; Leinco Technologies, St Louis, MO); and epidermal growth factor (EGF; 10 nM; Sigma-Aldrich) for 5 minutes. When KP-10 or conditioned medium from C8161.9KFM cells was used in combination with SDF-1, cells were exposed to KP-10 or conditioned media 3 minutes before the addition of SDF-1. Cell lysates were collected and subjected to im munoblot analysis as described above using antibodies directed against total AKT and phosphorylated AKT (pAKT; Cell Signaling Tech nology, Danvers, MA). Immunoblots were incubated with horseradish peroxidase–labeled secondary antibody (Jackson ImmunoResearch Laboratories), and antibody binding was visualized using chemiluminescence (ECL-Plus; Amersham Biosciences).
Cell stimulation was assessed by measuring transient cytoplasmic release of Ca2+ as previously described (27). Briefly, CHO cells transfected with CXCR4 and GPR54 or with CXCR4 and M1 (control) were loaded with the calcium indicator dye Fura-2-AM (Molecular Probes, Eugene, OR) at 37 °C for 30 minutes, washed in PBS containing 0.1% bovine serum albumin, and resuspended in Hank's balanced salt solution. The cells (300 μL of suspension at 2 × 106 cells/mL) were then placed in a thermostatted microcuvette and exposed sequentially to conditioned medium from C8161.9vector and C8161.9KFM cells (30 μL each) and to SDF-1 (100 nM final concentration) after a 3-minute interval, as previously described (31). The fluorescence of free Fura-2 (excitation 380 nm, emission 510 nm) and Fura-2 bound to Ca2+ (excitation 340 nm, emission 510 nm) was monitored in a spectrofluorometer (F2500, Hitachi, Parsippany, NJ); the ratio of absorbance at 340 nm to that at 380 nm reflects intracellular calcium mobilization.
Tumor Growth and Metastasis Assays
C8161.9 cells (2 × 105) that were untransfected or had been transfected with pcDNA3.1 (empty vector), KFM, or KFMΔSS were injected into the lateral tail vein or left cardiac ventricle of 3- to 4-week-old female athymic nude mice (10 per group per experiment; Harlan Sprague-Dawley, Indianapolis, IN). Mice were housed at 72 °F and given food and water ad libitum. All mice were killed by cervical dislocation following ketamine : xylazine anesthesia at 4–5 weeks after cell inoculation or if moribund. For survival experiments, mice were killed if moribund or at 120 days after inoculation. All mouse experiments were approved and monitored by the UAB Institutional Animal Care and Use Committee.
For gross examination of metastatic foci, lungs were removed 5 weeks after inoculation and fixed with Bouin's fixative (diluted 1 : 5 in neutral buffered formalin), and the number of metastases per lung was counted using a dissecting microscope (American Optical Company, Buffalo, NY). To evaluate the fate of tumor cells reaching various tissues, fluorescence microscopy was used to analyze GFP-expressing cells. All organs were removed and fixed in ice-cold 4% paraformaldehyde for 48 hours and then stored in 1% paraformaldehyde at 4 °C to maintain fluorescence (32). Organs were photographed, and the size of fluorescent foci was determined using ImagePro Plus 5.1 software (Media Cybernetics, Silver Spring, MD) as previously described (32).
To visualize and quantify metastases derived from the GFP-labeled cells, whole organs were examined by fluorescence microscopy using a Leica MZFLIII dissecting microscope equipped with a ×0.5 objective and GFP fluorescence filters (λexcitation = 480 ± 20 nm; λemission = 510 nm; Leica, Deerfield, IL). Bones were dissected free of soft tissue before imaging. Photomicrographs were collected with a MagnaFire digital camera (Optronics, Goleta, CA) using ImagePro Plus 5.1 software (Media Cybernetics). A map was drawn of the skeletal metastases for each mouse bone that was then transferred to a computerized grid of squares (0.30 mm2). A computer program was used to depict, as a composite image, the number of mice in which tumor encompassed each square in the grid (33).
C8161.9vector and C8161.9KFM cells (1 × 106) were isolated from the lungs of mice injected intravenously with each cell line. Green fluorescent cells (C8161.9 and C8161.9KFM) resistant to puromycin were established in cell culture as previously described (34). Explant cultures were injected orthotopically (i.e., intradermally) in the dorsal flank of 3- to 4-week-old female nu/nu mice (n = 4). Tumors were measured weekly using calipers to assess mean tumor diameter (35). Mice were killed by cervical dislocation following anesthesia with a mixture of ketamine (80–85 mg/kg) and xylazine (14–16 mg/kg) after primary tumors reached an average of 1.2 cm.
To confirm the identity of metastases expressing GFP, lungs were fixed in formalin and embedded in paraffin. Sections (5 μm thick) were cut, dewaxed, and rehydrated, and antigen retrieval was then performed by heating the sections for 10 minutes in 0.01 M citrate buffer before incubating with a rabbit polyclonal antibody against GFP (Molecular Probes; used at a 1 : 250 dilution for 1 hour at room temperature). Slides were then rinsed with 3% H2O2 for 10 minutes and then incubated in 1% goat serum for 1 hour at room temperature. After rinsing with water, an anti-rabbit streptavidin/horseradish peroxidase antibody (Signet Laboratories, Dedham, MA) was added, and antibody binding was visualized using diaminobenzidine (BioGenex, San Ramon, CA). Slides were counterstained with Harris's hematoxylin. Lung sections were analyzed at ×400, and representative pictures were taken.
Immunofluorescence to Determine Intracellular Localization of KISS1
C8161.9vector, C8161.9KFMΔSS, C8161.9KGM, and C8161.9KFM cells were plated on glass coverslips in 24-well plates that had been pre-coated with FBS. After 24 hours, the cells were washed three times in PBS and fixed with 3% paraformaldehyde in PBS for 10 minutes at room temperature. Paraformaldehyde was quenched by treatment with 10 mM ammonium chloride, and cells were permeabilized with 0.1% Triton X-100 in PBS, for 7 minutes at room temperature. The coverslips were washed three times for 2 minutes each with PBS and then blocked with 0.4% fish skin gelatin in PBS and 0.2% Tween 20 for 5 minutes followed by a second block with 2.5% goat serum in PBS and 0.2% Tween 20 for 5 minutes. The cells were incubated with the anti–FLAG M2 monoclonal antibody (1 : 1000), affinity-purified polyclonal antibody to the Golgi protein GM130 (1 : 100; BD Biosciences) (36), or a polyclonal antibody to the Golgi protein giantin (1 : 500; Covance, Berkeley, CA) for 1 hour at room temperature and then with fluorescently labeled secondary antibody (1 : 150; Alexa 488 or Alexa 594; Molecular Probes) for 45 minutes. Coverslips were mounted on slides in 9 : 1 glycerol/PBS with 0.1% p-phenylenediamine (Sigma-Aldrich). Fluorescence patterns were visualized with a Leitz Orthoplan microscope with epifluorescence and Hoffman Modulation Contrast optics from Chroma Technology, Inc (Brattleboro, VT). Optical sections were captured with a charge-coupled device high-resolution camera from Roper Scientific (Tucson, AZ) that was equipped with a camera/computer interface. Images were analyzed on a Macintosh computer using IPLab Spectrum software (Scanalytics Inc, Fairfax, VA).
Statistical Analysis
Statistical analyses were performed using SigmaStat 3.11 (Systat Software, Richmond, CA), and graphs were generated using SigmaPlot 8.0 (Systat Software). Fisher's exact test was used for binomial analysis of the presence or absence of metastasis. Analysis of variance and Holm–Sidak multiple-group posttest comparisons were used to determine the statistical significance of differences in metastatic frequency and size of metastatic foci between transfected and untransfected C8161.9 cells. Kaplan–Meier survivor analysis with log-rank tests was performed to analyze the statistical significance of survival differences between mice injected with parental C8161.9 cells or with C8161.9vector or C8161.9KFM cells followed by a Holm–Sidak multiple-group posttest. All in vivo experiments contained at least 10 mice per group, and most studies were repeated at least twice. All statistical tests were two-sided.
Results
KISS1 Secretion and Processing in C8161.9 Cells
Previously published data (17, 19,22,37-39) indicated that KISS1 (and/or kisspeptins) is likely to be secreted from KISS1-expressing cells. Only KP-54 has been identified by immunoblotting of conditioned medium (40), although KP-54, KP-14, KP-13, and KP-10 have all been detected by MALDI-TOF in concentrated conditioned medium that was purified using reverse-phase HPLC (20, 22, 40). On the basis of the presumptive mechanisms of processing KISS1 to KP-54 and other kisspeptins, we added an internal FLAG epitope to KISS1, generating KFM (Fig. 1, A).
In whole-cell lysates of C8161.9 cells that stably expressed KFM, we detected a single band of approximately 14.5 kDa (Fig. 1, B), slightly smaller than the expected size of 16.7 kDa (Fig. 1, A). A 14.5-kDa band was also observed in conditioned medium (Fig. 1, C), along with several lower molecular weight bands, consistent with predicted processing (Fig. 1, D). To verify that the smaller peptides were derived from KISS1, we subjected the medium to immunoaffinity column purification using an anti–KP-54 antibody followed by ESI-MS/MS. Trypsin digests of kisspeptin bands isolated from conditioned medium contained sequences from KISS1 (Fig. 1, E). Unexpectedly, following Glu-C digestion, several of the larger bands were found to contain sequences proximal to the proposed processing sites for KP-54, suggesting that extracellular processing may occur in C8161.9 cell lines (Fig. 1, E). These results highlight the need to better characterize KISS1 processing and to determine whether all kiss -peptins, or only a subset of them, are responsible for metastasis suppression.
We next examined the intracellular localization of FLAG-tagged KISS1 and kisspeptins by performing immunocytochemical colocalization analysis with two different Golgi markers (36). Although some nonspecific fluorescence was observed in C8161.9vector cells, this signal did not colocalize with giantin or GM130 (Fig. 2). By contrast, in C8161.9KFM cells, specific FLAG immunofluorescence colocalized with both Golgi markers (Fig. 2). Similar localization patterns were observed in C8161.9KGM cells, in which the FLAG in KISS1 was replaced with GFP (Fig. 2).
Fig. 2.

Intracellular localization of KISS1 to the Golgi complex of transfected C8161.9 melanoma cells. Each column represents a different C8161.9 transfectant. KGM = C8161.9 cells transfected with KISS1 into which enhanced green fluorescent protein (GFP) was inserted N-terminally to metastin. Vector = C8161.9 cells transfected with empty vector. KFM = C8161.9 cells transfected with KISS1 into which the FLAG epitope was inserted N-terminally to metastin. KFMΔSS = C8161.9 cells transfected with KFM lacking the signal sequence. Cells were stained by immunofluorescence with antibodies against FLAG and/or GM130 or giantin, as indicated. For the KGM transfectants, staining for FLAG was omitted; the fluorescence represents GFP. KFM and KGM colocalize with GM130 and giantin, but KFMΔSS shows diffuse cytoplasmic staining. Scale bar = 16 μm.
Biologic Activity of Secreted Kisspeptins
We measured the biologic activity of KFM—the ability to activate GPR54-expressing cells in vitro and to suppress metastasis in vivo—to verify that it retained the biologic activities of KISS1. We previously showed that KP-10 treatment of CHO cells transfected with GPR54 cDNA (CHO-G cells) activated GPR54, resulting in Ca2+ flux as measured by ratiometric fluorescence imaging (31). Signaling in CHO cells transfected with muscarinic type I receptor cDNA (CHO-M) did not respond to KP-10. Here, we found that conditioned medium from C8161.9vector cells did not induce GPR54-dependent Ca2+ mobilization in CHO-G cells (Fig. 3, A), whereas conditioned medium from C8161.9KFM cells did (Fig. 3, B). Treatment of C8161.9 cells with SDF-1 and FBS, a nonspecific stimulant of intracellular calcium mobilization, also stimulated Ca2+ flux (Fig. 3, C). However, treatment of C8161.9 cells with KP-10 (100 nM, Fig. 3, D), with C8161.9vector-conditioned medium (Fig. 3, E), and with C8161.9KFM-conditioned medium (Fig. 3, F) failed to induce Ca2+ flux. C8161.9KFM cells also did not respond to KP-10 or C8161.9vector- or C8161.9KFM-conditioned medium (data not shown).
Fig. 3.

Effect of kisspeptins on Ca2+ signaling. A and B) Chinese hamster ovary cells expressing the G protein–coupled receptor GPR54 (CHO-G cells) (dashed lines) or type 1 muscarinic acetylcholine receptor (solid lines) were exposed to conditioned medium from C8161.9 melanoma cells transfected with empty vector (VM) (A) or with FLAG-tagged KISS1 (KM) (B). Ca2+ signaling was assayed by ratiometric spectrofluorometry. C and D) C8161.9 cells were treated with stromal-derived factor 1 (SDF-1), fetal bovine serum (FBS), and kisspeptin-10 (KP-10) as indicated, and Ca2+ signaling was assessed. E and F) C8161.9 cells were treated with VM, SDF-1, KM, and FBS as indicated, and Ca2+ signaling was assessed. G and H) AKT activation (i.e., phosphorylation on Ser473) was assayed in C8161.9 cells exposed to SDF-1, epidermal growth factor (EGF), KP-10, KM, and PM (conditioned medium from C8161.9 cells) alone or in combination. I) Relative GPR54 mRNA levels in C8161.9 (81), MelJuSo (Mel), MDA-MB-435 (435), and SKOV3 (V3) cells as determined by real-time reverse transcription–polymerase chain reaction and normalized to S9 ribosomal protein. Placenta (Plac) mRNA is used as a positive control.
We also previously showed (31) that CHO-G cells exhibited an impaired AKT-signaling response (i.e., reduced AKT phosphorylation) in response to SDF-1 if previously exposed (within 3–5 minutes) to KP-10. To investigate whether the failure of C8161.9 cells to respond to kisspeptins by stimulating Ca2+ release reflected impaired AKT signaling, cells were treated with SDF-1 or EGF (Fig. 3, G and H). AKT signaling in C8161.9 cells appeared to be functional, based on the finding that Ser473 in AKT became phosphorylated in response to treatment with both growth factors (Fig. 3, G and H). Phospho-AKT (pAKT at Ser473), which represents AKT activation, was absent in C8161.9 cells exposed to secreted kisspeptins (Fig. 3, H) or KP-10 (Fig. 3, G). However, AKT activation by SDF-1 or EGF was not attenuated by pretreatment with KP-10 or secreted kisspeptins (Fig. 3, G H). In addition, KP-10 did not stimulate ERK1/2 signaling, an alternative readout for GPR54 activation, signaling in C8161.9 cells (data not shown). AKT- and ERK-signaling patterns and Ca2+ flux data in C8161.9KFM cells were similar to those in parental C8161.9 cells (data not shown).
The failure of C8161.9 cells to respond to KP-10 or to conditioned medium from C8161.9KFM cells suggested that a KISS1 autocrine loop might not exist. To confirm this, we measured endogenous GPR54 mRNA expression levels in cell lines that were previously shown (1-5) to be metastasis suppressed following KISS1 transfection and reexpression (Fig. 3, I). GPR54 mRNA expression was low to undetectable in C8161.9, MDA-MB-435, and MelJuSo cells but detectable in SKOV3 cells (Fig. 3, I). Expression was highest in the positive control, placenta tissue (Fig. 3, I). C8161.9KFM and C8161.9 cells exposed to KP-10 did not show substantial alterations in GPR54 expression as compared with unstimulated C8161.9 cells (data not shown).
In addition to detecting biologic activities of secreted KISS1 and/or kisspeptins from the conditioned medium of C8161.9KFM cells, we detected secreted and processed KISS1 by immunoblot (Fig. 4, A) and sandwich ELISA (Fig. 4, B) analyses. Introduction of the FLAG epitope did not impair cellular proliferation (data not shown), nor did it impair the ability of KISS1 to suppress metastasis. C8161.9KFM cells formed far fewer metastasesthan C8161.9vector cells (range = 0–25 versus 96–205 metastases, difference > 87%, P<.001) (Fig. 4, C). This reduction in metastasis is consistent with that seen in previously published studies using KISS1 cDNA (5).
Fig. 4.

KISS1 secretion and lung metastasis by C8161.9 human melanoma cells. Cells were not transfected (P) or were transfected with empty vector (V), with FLAG-tagged KISS1 (KFM), or with FLAG-tagged KISS1 lacking the signal sequence (KFMΔSS). Numbers indicate KFM transfectant clones (17, 46, 57, 59, 66) and KFMΔSS transfectant clones (6, 7, 10, 16). Mix indicates uncloned transfectants for each KISS1 construct. A and D) Immunoblot analysis of conditioned medium from P, V, and KFM (A) or P, V, and KFMΔSS (D) cells using immunoprecipitation with anti-metastin polyclonal antiserum followed by immunoblotting with anti–FLAG M2 antibody. B and E) Relative quantification of KISS1 secretion levels by enzyme-linked immunosorbent assay of KFM (B) and KFMΔSS (E) clones. Removal of the putative signal sequence blocks secretion of KISS1 (E). KFM clone 59 is shown on panel E as a positive control for the assay. C and F) Lung colonization in mice injected intravenously with C8161.9 cells transfected with KFM (C) or KFMΔSS (F) was determined by counting surface lung foci. Statistical significance (*** P<.001) was calculated by comparison of experimental values (KFM versus KFMΔSS) with vector control (V) (Holm–Sidak method).
KISS1 Expression, Secretion, and Maintenance of Metastatic Dormancy of Disseminated Cells
Having established that the FLAG epitope did not modify subcellular localization or attenuate metastasis suppression, we next asked at which step(s) in the metastatic process KISS1 blocked lung colonization by C8161.9 cells expressing KFM. To facilitate monitoring of KFM-expressing cells in vivo, C8161.9 and C8161.9KFM cells were transduced with lentivirus containing GFP, mice were injected intravenously with cells with constitutive GFP expression, and the fate of these cells in the lungs was monitored by fluorescence stereomicroscopy (25,33) (Fig. 5, A–H). Equivalent numbers of small fluorescent foci were seen in the lungs of mice injected with C8161.9 and C8161.9KFM cells at 24 hours and 1 week after injection (Fig. 5, A–F). However, 3 weeks after injection, C8161.9-injected mice had macroscopic metastases (mean diameter = 607 μm) that were statistically significantly larger than those of C8161.9KFM-injected mice (mean diameter = 28 μm, difference = 579 μm, 95% confidence interval = 505 to 708 μm, P<.001) (Fig. 5, C, G, and K). Immunohistochemical analysis of GFP expression in lung sections revealed multicellular foci in C8161.9-injected mice (Fig. 5, I) but only single cells or small clusters (1–6 cells per focus) in C8161.9KFM-injected mice (Fig. 5, J). Isolated dormant C8161.9KFM cells (or small foci) that persisted at 5 weeks after injection (Fig. 5, H, K, and L) still expressed KISS1 mRNA (Fig. 5, M). In contrast, C8161.9 formed lethal macroscopic lung metastases (Fig. 5, D, K, and L). Although fewer C8161.9KFM cells remained in the lung at 5 weeks than were found 24 hours after injection, KFM expression did not diminish proportionate viability of cells reaching the lung when compared to parental C8161.9 cells (data not shown).
Fig. 5.

KISS1 secretion and maintenance of dormancy of disseminated melanoma cells in the lung. A–H) Examination of C8161.9, C8161.9KFM, and C8161KFM.59 cell growth in the lung. Mice were injected into the lateral tail vein with cells transduced with a green fluorescent protein (GFP)–containing lentivirus (A–D) or C8161.9 melanoma cells that had been transfected with FLAG-tagged KISS1 (KFM). Images from C8161KFM clone 59 are shown as representative data for the other clones (E–H). Representative lung micrographs were taken at 24 hours (A and E), 1 week (B and F), 3 weeks (C and G), and 5 weeks (D and H). Scale bar = 50 μm. I and J) Immunohistochemical visualization of. GFP-labeled C8161.9 cells in lung using an anti-GFP antibody (brown). Arrows identify macroscopic metastatic foci (I) and microscopic metastatic foci (J) at 5 weeks postinjection. Scale bar = 40 μm. K) Growth of fluorescent C8161.9 cells in the lung. Growth of fluorescent lung foci, such as those depicted in panels A–H , were plotted for a period of 5 weeks for C8161.9 (closed circle) and C8161.9KFM.59 (open circle) cells. C8161.9KFM.59 foci were statistically significantly smaller than C8161.9 metastases after 3 weeks (*** P<.001, Holm–Sidak Method) and remained smaller until the experiment was terminated after 5 weeks. Data are represented as mean lung focus diameter ± SEM from a minimum of 10 mice. Mean focus diameter for C8161.9 was 1020 μm, 95% confidence interval (CI) = 922 to 1117 μm. Mean focus diameter for C8161.9vector was 930 μm, 95% CI = 833 to 1027 μm. L) Size of tumor (cell) foci at 5 weeks for multiple C8161.9KFM (clone 46; mean = 34 μm, 95% CI = 27 to 41 μm; clone 57; mean = 25, 95% CI = 22 to 28 μm; clone 59, mean = 27 μm, 95% CI = 24 to 31 μm) and C8161.9KFMΔSS (clone 6; mean = 1148 μm, 95% CI = 1053 to 1244 μm; clone 7; mean = 1103 μm, 95% CI = 1015 to 1191 μm; clone 16, mean = 1074 μm, 95% CI = 988 to 1134 μm) clones were determined by fluorescence microscopy (as above) following intravenous inoculation of athymic mice. Lung foci (metastases) from C8161.9KFM were statistically significantly smaller than those formed following injection of C8161.9 or C8161KFMΔSS cells (*** P<.001, Holm–Sidak Method). Each group in the experiment contained 10 mice. Determination of KISS1 mRNA levels by real-time reverse transcription–polymerase chain reaction (RT–PCR) in C8161.9KFM and C8161.9KFMΔSS cells isolated from lung tissue 5 weeks after intravenous inoculation of cells. Fluorescent cells were established as cell cultures by combining explants from 2 to 3 lungs each for C8161.9KFM.59 and C8161.9KFMΔSS clones 6, 7, and 16. KISS1 levels were then determined by real-time RT–PCR; KISS1 mRNA levels were normalized to C8161.9vector (V) cells before injection. N) Orthotopic tumor growth of C8161.9 and C8161.9KFM.59 cells isolated from lungs. C8161.9 and C8161.9KFM.59 cells were injected intravenously into athymic mice and cells harvested from the lungs of mice 5 weeks after inoculation. Following establishment of cell cultures, tumor cells (C8161.9 and C8161.9KFM.59) were injected orthotopically into five athymic mice per group, and mean tumor diameters of C8161.9 (closed square) and C8161.9KFM.59 (open square) were determined weekly. O) Kaplan–Meier survival analysis of mice receiving C8161.9 (P), C8161.9vector (V), and KFM-expressing clones (KFM.17, KFM.46, and KFM.59; 10 mice per clone). The experiment was terminated at 120 days after intravenous inoculation or if mice appeared moribund. Differences in survival between mice receiving C8161.9 (mean survival = 38.8 days, 95% CI = 37.8 to 39.9 days), C8161.9vector (mean survival = 42.6 days, 95% CI = 41.6 to 43.4 days), and C8161.9KFM (mean survival clone 17 = 66.7 days, 95% CI = 51.6 to 81.8 days; mean survival clones 46 and 59 ≥ 120 and 118.6 days) cell clones were determined using Kaplan–Meier survival analysis log-rank test, and statistical significance (* P<.001) was calculated by comparing values for KFM clones to C8161.9vector (Holm–Sidak method). P) Frequency of lung metastases in moribund (i.e., cachexic) mice compared to healthy survivors from panel O (10 mice per group). Mean number of lung metastases for C8161.9 (mean = 63 metastases, 95% CI = 50 to 76 metastases), C8161.9vector (mean = 128 metastases, 95% CI = 117 to 140 metastases), and C8161.9KFM clones (clone 17; mean = 13 metastases, 95% CI = 7 to 19 metastases; clone 46; mean = 0 metastases, 95% CI = 0 to 1 metastases; clone 59, mean = 1 metastasis, 95% CI = 0 to 2 metastases) are shown. Statistical significance (*** P<.001) was calculated by comparison of experimental values for KFM versus C8161.9vector control (V) (Holm–Sidak method).
Lung metastases from five mice injected with C8161.9 cells or dormant tumor cells from the lungs of five mice injected with C8161.9KFM cells were isolated from lungs and established in cell culture. The cells were then injected intradermally into athymic mice. Tumors grew progressively from both kinds of cells and at the same rates (Fig. 5, N). C8161.9KFM cells were still viable and tumorigenic. Furthermore, C8161.9KFM cells were able, to the best of our knowledge, to complete all steps of the metastatic cascade before tumor cell arrest at secondary sites (e.g., survival in circulation, attachment, invasion).
We also analyzed the survival of mice injected intravenously with C8161.9, C8161.9vector , or several C8161.9KFM clones. Whereas all 20 mice injected with C8161.9 or C8161.9vector cells died from massive lung metastatic burden within 39–43 days, 30 mice injected with C8161.9KFM cells survived for 67 to more than 120 days, when the experiment was terminated (P<.001) (Fig. 5, O). In mice injected with C8161.9KFM clones, formation of overt, macroscopic metastases was suppressed by more than 90% compared with that in mice injected with C8161.9 or C8161.9vector cells (range = 0–13 versus 63–128 metastases per mouse; P<0.001) (Fig. 5, P). In general, survival time was directly proportional to the level of KFM, and the number of macroscopic metastases was inversely proportional.
Effect of KISS1 on Metastasis to Multiple Organs
Most studies with metastasis suppressors have focused on metastases to a single site, the lung, largely because of the models that have been used. Because melanoma cells metastasize to many other sites, we were able to evaluate whether KISS1 would suppress metastasis to additional tissues. To achieve the widest distribution of tumor cells, C8161.9 cells were introduced into the arterial circulation of mice via intracardiac injection. A substantial fraction of mice injected with C8161.9 or C8161.9vector cells developed large, function-impairing metastases in the bone (28/28 mice), kidney (26/28 mice), and eye (18/28 mice) (Fig. 6, A ). By contrast, fewer than half of the mice injected with C8161.9KFM clones had metastases (P<.001), and the total number of metastases to all organs was reduced by more than 93% compared with mice injected with C8161.9 and C8161.9vector cells (P<.001) (Fig. 6; Table 1).
Fig. 6.
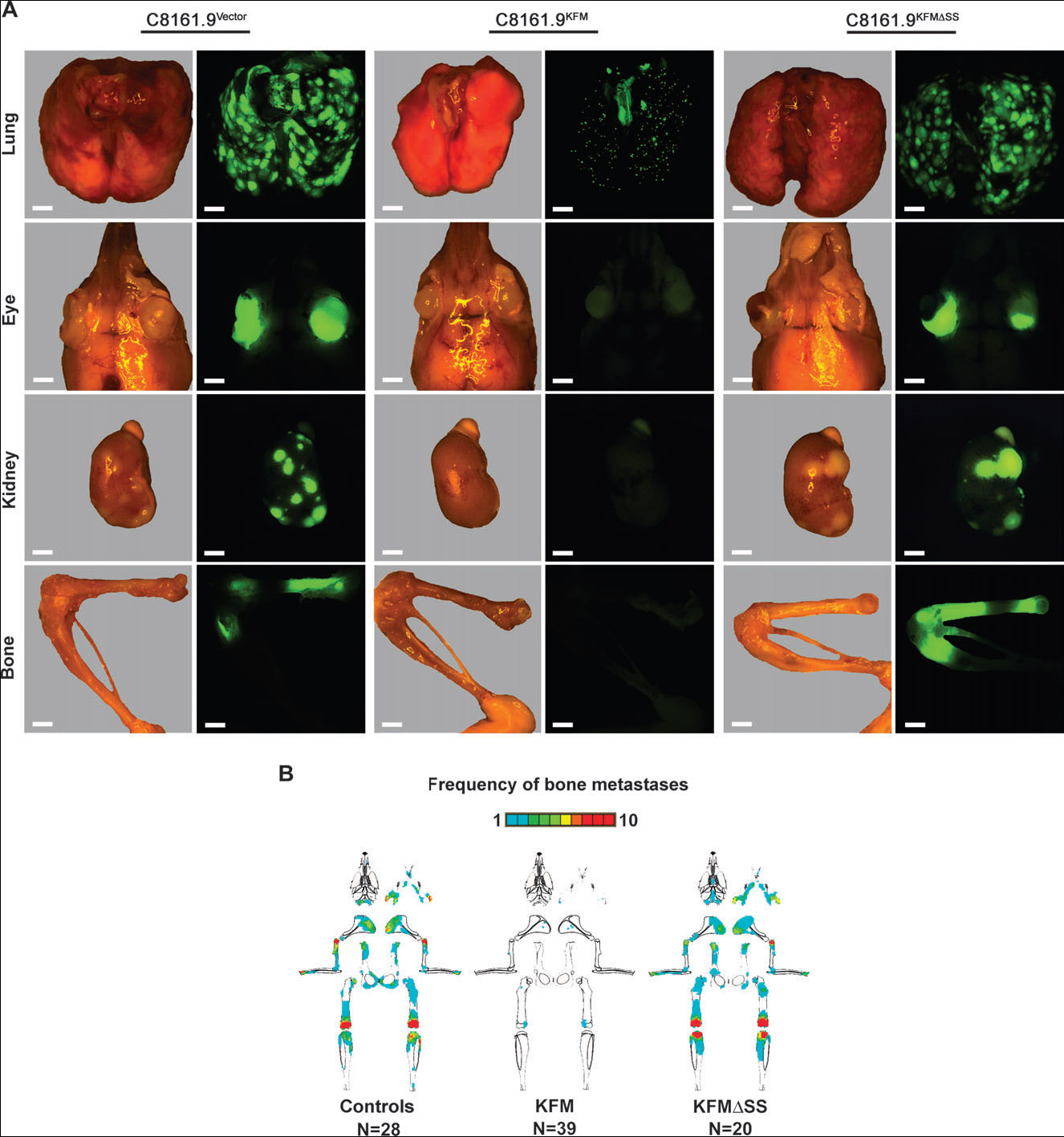
KISS1 suppression of metastasis to multiple organs in mice. Mice were injected in the left cardiac ventricle with C8161.9 cells that had been transfected with empty vector or with FLAG-tagged KISS1 containing (KFM) or lacking (KFMΔSS) the signal sequence and then infected with a green fluorescent protein–containing lentivirus. A) Representative lung, eye, kidney, and bone photomicrographs and fluorescent photomicro-graphs showing metastatic lesions 5 weeks after injection. Most extra-pulmonary sites were devoid of green cells. Scale bar = 2000 μm. B) Graphical mapping of the distribution, relative size, and frequency of fluorescent metastatic foci in bone. Bone maps reflect a data merge of all mice (10 mice per experimental group) from two or three independent experiments. Mice received C8161.9 and C8161.9vector cells (controls); C8161.9KFM clones 46, 57, and 59 (KFM); and C8161.9KFMΔSS clones 6, 7, and 16 (KFMΔSS). The total number of mice examined is shown. A frequency scale is depicted above bone schematic diagrams. If more than 10 mice in a group had metastases at a particular location, the scale reads red .
Table 1.
C8161.9 metastatic incidence and frequency in multiple organs*
| Metastases |
||||||||
|---|---|---|---|---|---|---|---|---|
| Mean (95% confidence interval) |
||||||||
| Lung |
Bone |
Kidney |
Eye |
|||||
| Cells | Incidence†(%) | Size (μm) | Incidence (%) | Frequency‡ | Incidence†(%) | Frequency‡ | Incidence†(%) | Frequency‡ |
| Parental C8161.9 cells | 100 | 1020 (922 to 1117) | 100 | 7.9 (5 to 11) | 92 | 9.7 (5 to 15) | 77 | 1.3 (1 to 2) |
| Empty vector transfectant | 100 | 930 (833 to 1027) | 100 | 11.9 (11 to 18) | 93 | 14.7 (7 to 23) | 53 | 0.7 (0 to 1) |
| KFM46 | 15§ | 34§ (27 to 41) | 44§ | 0.6§ (0 to 1) | 0§ | 0∥ (−) | 0¶ | 0∥ (−) |
| KFM57 | 2§ | 25§ (22 to 28) | 10§ | 0.1§ (−) | 0§ | 0§ (−) | 0¶ | 0∥ (−) |
| KFM59 | 6§ | 27§ (24 to 31) | 20§ | 0.1§ (0 to 1) | 0§ | 0§ (−) | 0¶ | 0∥ (−) |
| KFMΔSS6 | 100 | 1148 (1053 to 1244) | 100 | 12.4 (8 to 17) | 100 | 22.6 (6 to 39) | 100 | 1.5 (1 to 2) |
| KFMΔSS7 | 100 | 1103 (1015 to 1191) | 100 | 6.6 (2 to 11) | 100 | 10.1 (0 to 22) | 86 | 1.0 (1 to 2) |
| KFMΔSS16 | 100 | 1074 (988 to 1134) | 100 | 6.4 (1 to 12) | 100 | 18.4 (1 to 36) | 80 | 1.0 (0 to 2) |
Incidence and frequency were determined 5 weeks after left ventricular injection of mice with C8161.9 cells that had been transfected with empty vector or with FLAG-tagged KISS1 containing (KFM) or lacking (KFMΔSS) the signal sequence and then infected with a green fluorescent protein–containing lentivirus. Three different C8161.9KFM clones (46, 57, 59) and three different C8161.9KFMΔSS clones (6, 7, 16) were used. At least 10 mice were used per experimental group.
Incidence reflects the percentage of mice with observable fluorescent metastases. Lung incidence represents the percentage of mice with metastatic foci _50 μm.
Frequency represents the number of metastatic foci per organ.
Statistically different (P<.001) compared with C8161.9vector for incidence (Fisher's exact test) and frequency (analysis of variance [ANOVA] followed by Holm–Sidak posttest).
Statistically significantly different (P<.01) compared with C8161.9vector for incidence (Fisher's exact test) and frequency (ANOVA followed by Holm–Sidak posttest).
Statistically significantly different (P<.05) compared with C8161.9vector for incidence (Fisher's exact test) and frequency (ANOVA followed by Holm–Sidak posttest).
KISS1 Secretion and Biologic Activity
To test whether secretion is essential for the antimetastatic effects of KISS1, we removed the predicted secretion signal from KFM, creating KFMΔSS (Fig. 1). C8161.9 cells transfected with the KFMΔSS construct produced a single band of approximately 14.9 kDa in whole-cell lysates (Fig. 1, B , right lane). No KISS1 protein could be detected in conditioned medium from these cells by either immunoprecipitation (Figs 1, C and 4, D) or ELISA (Fig. 4, E). Removal of the signal sequence coincided with cytoplasmic, but not Golgi, localization (Fig. 2). Conditioned medium from C8161.9KFMΔSS cells also failed to induce GPR54-dependent Ca2+ mobilization in CHO-G cells (data not shown). We also carried out cell growth and experimental metastasis assays using KFMΔSS and KFM clones with similar mRNA levels of the transfected gene; C8161.9KFMΔSS cells proliferated in vitro to the same extent as C8161.9 and C8161.9KFM cells (data not shown). Of the 73 mice injected intravenously with five independently isolated C8161.9KFMΔSS cell clones (Fig. 4, D and E), four clones (68 mice) developed a similar number of lung metastases as those injected with C8161.9 or C8161.9vector cells (range = 98–200 versus 125–169 metastases) (Fig. 4, F). The fifth C8161.9KFMΔSS clone (15 mice) produced fewer lung metastases than C8161.9vector, but the number was statistically significantly greater than those for all the C8161.9KFM clones (P<.001) (Fig. 4, F). The macroscopic foci formed by C8161.9KFMΔSS cells were similar in size (range = 1102–1148 μm) to those formed by C8161.9 cells (range = 930–1020 μm) (Fig. 5, L). Tumor cells isolated from lungs after 5 weeks after injection still expressed KFMΔSS (Fig. 5, M). C8161.9KFMΔSS were not suppressed for metastasis to other organs (Fig. 6; Table 1).
Discussion
The results presented here confirm that KISS1 secretion is required for suppression of metastases to multiple organs. Our results also demonstrate that KISS1 suppressed lung metastasis by interfering with the last step of the metastatic process, i.e., outgrowth at the secondary site. However, the findings presented also included some surprises about KISS1 function in metastasis suppression, particularly with regard to signaling through GPR54.
The hypothesis that KISS1 activates GPR54 via an autocrine loop to suppress metastasis had been largely inferred but had not been tested directly due to the lack of reliable antibodies to detect and monitor KISS1 and its processed forms in western blots. Using the KFM construct, we were able to detect full-length KISS1 and its presumptive active form, metastin (also known as KP-54) (16) in conditioned medium from C8161.9KFM cells. Moreover, we detected several intermediate processing variants. Even though a predicted model of KISS1 processing has been formulated (Fig. 1, D), neither the intermediate processing steps nor location of processing had been determined. In concordance with this hypothesis, site-directed mutagenesis of the proposed dibasic cleavage sites resulted in the specific loss of smaller kisspeptin products (Nash KT, Stafford LJ, and Welch DR: unpublished data).
Cellular secretion of proteins involves posttranslational modifications in the endoplasmic reticulum and Golgi apparatus. As expected, KFM is associated with the Golgi secretory machinery and is secreted and processed. However, the location of cellular processing has not been determined unequivocally, either here or in previous publications. However, based on findings presented in this paper, it appears that, at least some, proteolytic processing occurs outside the cell. The inability to detect processed KFMΔSS forms either inside the cell or in the extracellular milieu also suggests that some or all KISS1 processing occurs while the protein is localized in the Golgi or outside the cell. Because KFM appears to be smaller than predicted and because conditioned medium contains bands larger than 14.5 kDa (Fig. 1, C), it seems possible that posttranslational Golgi-dependent processing and/or altered protein folding and intramolecular interactions occur. Systematic and comprehensive sequencing analyses of the larger bands will be required to sort through all the potential options.
In vivo evaluation of the fate of C8161.9KFM or C8161.9KFMΔSS cells showed that KISS1 secretion and processing are required to block metastasis to multiple sites. This study is the first, to our knowledge, to show that any metastasis suppressor blocks metastasis to multiple organs. C8161.9KFM cells were able to complete antecedent steps of the metastatic cascade but not to colonize lungs. These cells persisted in mouse lungs for extended times (i.e., at least 120 days). Persistence of dormant disseminated cells has long been recognized in cancer patients in whom dormancy generally confers resistance to proliferation-dependent therapies, such as radiation and chemotherapy (41-43). Once cells break dormancy and proliferate, patient survival drops precipitously. Therefore, the identification of molecular targets that can maintain cells in a dormant state, such as KISS1, or selectively kill dormant cells would have an immediate impact on patient survival. Because many patients have occult metastatic disease by the time of diagnosis, our results raise the possibility that exogenous KISS1 therapy might someday prove useful in patients as a prophylactic adjuvant measure to keep metastatic cells dormant and prolong survival.
Although GPR54 signaling was observed in CHO-G cells in response to kisspeptins, we wanted to determine whether secreted KISS1 could suppress metastasis through autocrine GPR54 signaling. In the absence of reliable, specific antibodies that recognize the receptor, we measured GPR54 mRNA in cell lines (C8161, C8161.9, MelJuSo, MDA-MB-435, and SKOV3) that had previously been shown to be suppressed by KISS1 (1-5). GPR54 was reproducibly detected in SKOV3 ovarian carcinoma cells only. Failure to detect GPR54 in the other three lines, even though KISS1 expression in any of those cell lines suppresses metastasis (2-5), argues against the existence of an autocrine-signaling loop. This conclusion is supported by the lack of intracellular Ca2+ mobilization or failure to attenuate pAKT or ERK1/2 signaling induction in C8161.9 cells following exogenous treatment with KP-10 or with secreted kisspeptins.
However, the failure to detect GPR54 in metastatic tumor cells does not rule out the possibility that autocrine signaling occurs even through extremely low or undetectable levels of GPR54 or through another form of GPR54 that is not detectable using the PCR primers we used. Likewise, lack of autocrine signaling does not rule out the potential for intracrine signaling (44, 45) or paracrine signaling of KISS1 through GPR54 in stromal cells, possibilities that we have discussed elsewhere (16). The possibility of paracrine signaling is supported by recent observations that GPR54 mRNA is detectable in NIH3T3 fibroblasts (data not shown). Further suggestive evidence that differences in the micro-environment are key outcomes from the finding that KFM-expressing cells grow progressively in the skin or in cell culture but remain dormant in the lung (1-4).
We emphasize caution when interpreting and extrapolating our data. Experiments should ideally be done with additional cell lines, preferably incorporating nonmelanomas and immunocompetent metastasis models. Also, to fully understand the mechanisms of dormancy, models that allow longitudinal examination would be preferred. Nonetheless, the fact that active polypeptides of KISS1 are secreted increases the likelihood that their effects could be mimicked. The potential that KISS1 or mimetics could be used in the clinic is especially promising because KISS1 is a natural product that can be administered at high levels to humans without apparent toxicity (46). Thus, the implication is that KISS1 mimics might be administered continuously in a manner analogous to insulin injection for diabetic patients. The finding that KISS1 can inhibit metastatic growth in multiple organs is a particularly important feature for an antimetastatic molecular agent. Finally, the finding that KISS1 can suppress metastasis in the apparent absence of GPR54 in tumor cells opens up the possibility that additional KISS1 metastasis suppressor receptors and signaling pathways exist with the potential to be selectively targeted. It is also likely that targeting of disseminated cells and their interactions with the microenvironment will be important for achieving optimal efficacy of antimetastatic therapies.
Acknowledgments
We thank the following investigators for generously providing reagents: C8161 (F. Meyskens), MelJuSo (J. Johnson), MDA-MB-435 (J. Price), SKOV3 (J. Hickson and D. Yamada), and αKP-54 (N. Ghaffari-Tabrizzi). The authors are grateful for the superb technical assistance provided by Natalia Fralova, Thomasz Szul, Yujiang Jia, and the UAB Comprehensive Cancer Center Mass Spectroscopy (Landon Wilson and Dr Stephen Barnes) and Epitope Recognition Immunoreagent Shared Resource Facilities. We are also grateful for core facilities from the UAB Center for Metabolic Bone Diseases.
Footnotes
Funding for this project was primarily supported by grants from the US Army Medical Research and Materiel Command DAMD17-02-1-0541 (D. R. Welch), the US Public Health Service CA087728 (D. R. Welch), and the National Foundation for Cancer Research. Additional funding was provided by the following grants: CA089019 (UAB Breast SPORE) and P30-CA13148 (UAB Cancer Center support grant). D. R. Hurst is recipient of a National Research Service Award (CA113037), and K. S. Vaidya is recipient of a post-doctoral fellowship from the Susan G. Komen Breast Cancer Research Fund (PDF0601220). All experiments were designed by the authors. All data analysis, writing, and interpretation were done solely by the authors, without restrictions by the sponsoring organizations. This work is submitted in partial fulfillment of the requirements for the UAB Graduate Program in Molecular and Cellular Pathology (K. T. Nash and P. A. Phadke).
References
- 1.Jiang Y, Berk M, Singh LS, Tan HY, Yin LH, Powell CT, et al. KiSS1 suppresses metastasis in human ovarian cancer via inhibition of protein kinase C alpha. Clin Exp Metastasis. 2005;22:369–76. doi: 10.1007/s10585-005-8186-4. [DOI] [PubMed] [Google Scholar]
- 2.Lee J-H, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene. J Natl Cancer Inst. 1996;88:1731–7. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- 3.Lee J-H, Welch DR. Identification of highly expressed genes in metastasis-suppressed chromosome 6/human malignant melanoma hybrid cells using subtractive hybridization and differential display. Int J Cancer. 1997;71:1035–44. doi: 10.1002/(sici)1097-0215(19970611)71:6<1035::aid-ijc20>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 4.Lee J-H, Welch DR. Suppression of metastasis in human breast carcinoma MDA-MB-435 cells after transfection with the metastasis suppressor gene, KiSS-1. Cancer Res. 1997;57:2384–7. [PubMed] [Google Scholar]
- 5.Lee J-H, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, et al. KiSS-1, a novel human malignant melanoma metastasis-suppressor gene [erratum] J Natl Cancer Inst. 1997;89:1549. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- 6.Dhar DK, Naora H, Kubota H, Maruyama R, Yoshimura H, Tonomoto Y, et al. Downregulation of KiSS-1 expression is responsible for tumor invasion and worse prognosis in gastric carcinoma. Int J Cancer. 2004;111:868–72. doi: 10.1002/ijc.20357. [DOI] [PubMed] [Google Scholar]
- 7.Ikeguchi M, Hirooka Y, Kaibara N. Quantitative reverse transcriptase polymerase chain reaction analysis for KiSS-1 and orphan G-protein-coupled receptor (hOT7T175) gene expression in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2003;129:531–5. doi: 10.1007/s00432-003-0469-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeguchi M, Yamaguchi K, Kaibara N. Clinical significance of the loss of KiSS-1 and orphan G-protein-coupled receptor (hOT7T175) gene expression in esophageal squamous cell carcinoma. Clin Cancer Res. 2004;10:1379–83. doi: 10.1158/1078-0432.ccr-1519-02. [DOI] [PubMed] [Google Scholar]
- 9.Masui T, Doi R, Mori T, Toyoda E, Koizumi M, Kami K, et al. Metastin and its variant forms suppress migration of pancreatic cancer cells. Biochem Biophys Res Commun. 2004;315:85–92. doi: 10.1016/j.bbrc.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 10.Ohta S, Lai EW, Pang ALY, Brouwers FM, Chan WY, Eisenhofer G, et al. Downregulation of metastasis suppressor genes in malignant pheochr omocytoma. Int J Cancer. 2005;114:139–43. doi: 10.1002/ijc.20670. [DOI] [PubMed] [Google Scholar]
- 11.Ringel MD, Hardy E, Bernet VJ, Burch HB, Schuppert F, Burman KD, et al. Metastin receptor is overexpressed in papillary thyroid cancer and activates MAP kinase in thyroid cancer cells. J Clin Endocrinol Metab. 2002;87:2399. doi: 10.1210/jcem.87.5.8626. [DOI] [PubMed] [Google Scholar]
- 12.Shirasaki F, Takata M, Hatta N, Takehara K. Loss of expression of the metastasis suppressor gene KiSS1 during melanoma progression and its association with LOH of chromosome 6q16.3-q23. Cancer Res. 2001;61:7422–5. [PubMed] [Google Scholar]
- 13.Stark AM, Tongers K, Maass N, Mehdorn HM, Held-Feindt J. Reduced metastasis-suppressor gene mRNA-expression in breast cancer brain metastases. J Cancer Res Clin Oncol. 2004;131:191–8. doi: 10.1007/s00432-004-0629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beier DR, Dluhy RG. Bench and bedside—the G protein-coupled receptor GPR54 and puberty. N Engl J Med. 2003;349:1589–92. doi: 10.1056/NEJMp038155. [DOI] [PubMed] [Google Scholar]
- 15.Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147:1154–8. doi: 10.1210/en.2005-1282. [DOI] [PubMed] [Google Scholar]
- 16.Nash KT, Welch DR. The KISS1 metastasis suppressor: mechanistic insights and clinical utility. Front Biosci. 2006;11:647–59. doi: 10.2741/1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gottsch ML, Clifton DK, Steiner RA. Kisspepeptin-GPR54 signaling in the neuroendocrine reproductive axis. Mol Cell Endocrinol. 2006;254:91–6. doi: 10.1016/j.mce.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 18.Hauge-Evans AC, Richardson CC, Milne HM, Christie MR, Persaud SJ, Jones PM. A role for kisspeptin in islet function. Diabetologia. 2006;49:2131–5. doi: 10.1007/s00125-006-0343-z. [DOI] [PubMed] [Google Scholar]
- 19.Funes S, Hedrick JA, Vassileva G, Markowitz L, Abbondanzo S, Golovko A, et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312:1357–63. doi: 10.1016/j.bbrc.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 20.Horikoshi Y, Matsumoto H, Takatsu Y, Ohtaki T, Kitada C, Usuki S, et al. Dramatic elevation of plasma metastin concentrations in human pregnancy: metastin as a novel placenta-derived hormone in humans. J Clin Endocrinol Metab. 2003;88:914–9. doi: 10.1210/jc.2002-021235. [DOI] [PubMed] [Google Scholar]
- 21.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Shagoury JK, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–27. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 22.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, et al. Metastasis suppressor gene KiSS1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–7. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- 23.Welch DR, Bisi JE, Miller BE, Conaway D, Seftor EA, Yohem KH, et al. Characterization of a highly invasive and spontaneously metastatic human malignant melanoma cell line. Int J Cancer. 1991;47:227–37. doi: 10.1002/ijc.2910470211. [DOI] [PubMed] [Google Scholar]
- 24.Miele ME, Robertson G, Lee JH, Coleman A, McGary CT, Fisher PB, et al. Metastasis suppressed, but tumorigenicity and local invasiveness unaffected, in the human melanoma cell line MelJuSo after introduction of human chromosomes 1 or 6. Mol Carcinog. 1996;15:284–99. doi: 10.1002/(SICI)1098-2744(199604)15:4<284::AID-MC6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 25.Phadke PA, Mercer RR, Harms JF, Jia Y, Frost AR, Jewell J, et al. Kinetics of metastatic breast cancer cell trafficking in bone. Clin Cancer Res. 2006;12:1431–40. doi: 10.1158/1078-0432.CCR-05-1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips KK, Welch DR, Miele ME, Lee J-H, Wei LL, Weissman BE. Suppression of MDA-MB-435 breast carcinoma cell metastasis following the introduction of human chromosome 11. Cancer Res. 1996;56:1222–6. [PubMed] [Google Scholar]
- 27.Hickson JA, Huo D, Vander Griend DJ, Lin A, Rinker-Schaeffer CW, Yamada SD. The p38 kinases MKK4 and MKK6 suppress metastatic colonization in human ovarian carcinoma. Cancer Res. 2006;66:2264–70. doi: 10.1158/0008-5472.CAN-05-3676. [DOI] [PubMed] [Google Scholar]
- 28.Yamada SD, Hickson JA, Hrobowski Y, Vander Griend DJ, Benson D, Montag A, et al. Mitogen-activated protein kinase kinase 4 (MKK4) acts as a metastasis suppressor gene in human ovarian carcinoma. Cancer Res. 2002;62:6717–23. [PubMed] [Google Scholar]
- 29.Van Tine BA, Kappes JC, Banerjee NS, Knops J, Lai L, Steenbergen RD, et al. Clonal selection for transcriptionally active viral oncogenes during progression to cancer. J Virol. 2004;78:11172–86. doi: 10.1128/JVI.78.20.11172-11186.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen W, Wu X, Levasseur DN, Liu H, Lai L, Kappes JC, et al. Lentiviral vector transduction of hematopoietic stem cells that mediate long-term reconstitution of lethally irradiated mice. Stem Cells. 2000;18:352–9. doi: 10.1634/stemcells.18-5-352. [DOI] [PubMed] [Google Scholar]
- 31.Navenot JM, Wang Z, Chopin M, Fujii N, Peiper SC. Kisspeptin-10-induced signaling of GPR54 negatively regulates chemotactic responses mediated by CXCR4: a potential mechanism for the metastasis suppressor activity of kisspeptins. Cancer Res. 2005;65:10450–6. doi: 10.1158/0008-5472.CAN-05-1757. [DOI] [PubMed] [Google Scholar]
- 32.Harms JF, Budgeon LR, Christensen ND, Welch DR. Maintaining green fluorescent protein tissue fluorescence through bone decalcification and long-term storage. Biotechniques. 2002;33:1197–200. doi: 10.2144/02336bm02. [DOI] [PubMed] [Google Scholar]
- 33.Harms JF, Welch DR. MDA-MB-435 human breast carcinoma metastasis to bone. Clin Exp Metastasis. 2003;20:327–34. doi: 10.1023/a:1024062911144. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg SF, Harms JF, Quon K, Welch DR. Metastasis-suppressed C8161 melanoma cells arrest in lung but fail to proliferate. Clin Exp Metastasis. 1999;17:601–7. doi: 10.1023/a:1006718800891. [DOI] [PubMed] [Google Scholar]
- 35.Welch DR. Technical considerations for studying cancer metastasis in vivo. Clin Exp Metastasis. 1997;15:272–306. doi: 10.1023/a:1018477516367. [DOI] [PubMed] [Google Scholar]
- 36.Nelson DS, Alvarez C, Gao YS, Garcia-Mata R, Fialkowski E, Sztul E. The membrane transport factor TAP/p115 cycles between the Golgi and earlier secretory compartments and contains distinct domains required for its localization and function. J Cell Biol. 1998;143:319–31. doi: 10.1083/jcb.143.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–6. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- 38.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, et al. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci U S A. 2005;102:1761–6. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, et al. AXOR12: a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276:28969–75. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- 40.Bilban M, Ghaffari-Tabrizi N, Hintermann E, Bauer S, Molzer S, Zoratti C, et al. Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. J Cell Sci. 2004;117(Pt 8):1319–28. doi: 10.1242/jcs.00971. [DOI] [PubMed] [Google Scholar]
- 41.Aguirre-Ghiso JA. The problem of cancer dormancy—understanding the basic mechanisms and identifying therapeutic opportunities. Cell Cycle. 2006;5:1740–3. doi: 10.4161/cc.5.16.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naumov GN, Akslen LA, Folkman J. Role of angiogenesis in human tumor dormancy—animal models of the angiogenic switch. Cell Cycle. 2006;5:1779–87. doi: 10.4161/cc.5.16.3018. [DOI] [PubMed] [Google Scholar]
- 43.Ranganathan AC, Adam AP, Aguirre-Ghiso JA. Opposing roles of mitogenic and stress signaling pathways in the induction of cancer dormancy. Cell Cycle. 2006;5:1799–807. doi: 10.4161/cc.5.16.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Re RN, Cook JL. The intracrine hypothesis: an update. Regul Pept. 2006;133:1–9. doi: 10.1016/j.regpep.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 45.Re RN. The intracrine hypothesis and intracellular peptide hormone action. Bioessays. 2003;25:401–9. doi: 10.1002/bies.10248. [DOI] [PubMed] [Google Scholar]
- 46.Seminara SB, DiPietro MJ, Ramaswamy S, Crowley WF, Plant TM. Continuous human metastin 45-54 infusion desensitizes G protein-coupled receptor 54-induced gonadotropin-releasing hormone release monitored indirectly in the juvenile male rhesus monkey (Macaca mulatta): a finding with therapeutic implications. Endocrinology. 2006;147:2122–6. doi: 10.1210/en.2005-1550. [DOI] [PubMed] [Google Scholar]


