Abstract
Cultured skin substitutes (CSS) consisting of autologous fibroblasts and keratinocytes combined with biopolymers are an adjunctive treatment for large excised burns. CSS containing two cell types are limited by anatomical deficiencies, including lack of a vascular plexus, leading to slower vascularization after grafting than split-thickness autograft. To address this limitation, CSS were prepared containing human keratinocytes, fibroblasts, and dermal microvascular endothelial cells (HDMEC) isolated from a single skin sample. After 16 days in culture, control CSS and CSS containing HDMEC (CSS+EC) were grafted to full-thickness wounds in athymic mice. In CSS+EC in vitro, HDMEC persisted in the dermal substitutes and formed multicellular aggregates. One wk after grafting, HDMEC in CSS+EC organized into multicellular structures, some containing lumens. By 4 wk after grafting, HDMEC were found in linear and circular organizations resembling vascular analogs associated with basement membrane deposition. In some cases, colocalization of HDMEC with mouse perivascular cells was observed. The results demonstrate HDMEC transplantation in a clinically relevant cultured skin model, persistence of HDMEC after grafting, and HDMEC organization into vascular analogs in vitro and in vivo. All cells were derived from the same donor tissue, indicating the feasibility of preparing CSS containing autologous HDMEC for grafting to patients.—Supp, D. M., Wilson-Landy, K., Boyce, S. T. Human dermal microvascular endothelial cells form vascular analogs in cultured skin substitutes after grafting to athymic mice.
Keywords: cultured skin substitute, endothelial cell, angiogenesis, tissue engineering
Cultured skin substitutes (CSS) comprised of autologous dermal fibroblasts and epidermal keratinocytes have been used as an adjunctive therapy in the treatment of large burn wounds with limited donor sites for autograft (1-6). Recent advances in the preparation of CSS have led to improved clinical performance, making cultured skin a desirable alternative therapy for the most severely burned patients (7, 8). Containing just two cell types, CSS are limited by anatomic and physiological deficiencies compared with split-thickness skin autograft. A major limitation of CSS is the lack of a vascular plexus (9), which necessitates vascularization to occur de novo rather than through inosculation of the graft with the wound. This increases the period of nutrient deprivation and susceptibility to microbial contamination for grafted CSS, contributing to higher rates of graft failure compared with autograft. This limitation has been addressed clinically by the extensive use of nutrient and antimicrobial dressing fluids for several days after grafting of CSS (4, 7, 10), but this process is labor-intensive and can subject the grafts to mechanical stresses that influence engraftment. In preclinical studies, genetic modification of keratinocytes in CSS has been used to overexpress vascular endothelial growth factor, an angiogenic cytokine, leading to enhanced vascularization after grafting to mice and improved rates of engraftment (11, 12). These procedural and technical advances address the anatomic deficiency in CSS indirectly, compensating for the lack of blood vessels after grafting by supplying nutrients exogenously or enhancing the ingrowth of vessels from the underlying wound bed. A direct approach to addressing this problem would be to initiate angiogenesis in the skin substitutes in vitro before grafting. This would hypothetically permit vascularization of the CSS to occur through inosculation of existing vessels in the grafts with vessels from the wound bed and neovascularization, as occurs for grafted split-thickness skin (13).
Initiation of angiogenesis in vitro in a cultured skin model requires the addition of cells derived from vascular tissue. Perhaps the most critical cell type for such a model is the endothelial cell, and several methods for the isolation and culture of vascular endothelial cells have been described (14-16). Endothelial cells have been demonstrated to organize into vascular structures under certain culture conditions through the use of biomaterial supports and/or coculture with other cell types. For example, tissue-engineered human blood vessels have been constructed in vitro using mixtures of vascular smooth muscle cells, dermal fibro-blasts, and human umbilical vein endothelial cells (HUVEC) in a collagen matrix (17) or by using cells alone grown along a tubular support for lumen formation (18). A preclinical study involving transplantation of synthetic blood vessels constructed by culture of HUVEC in 3-dimensional collagen/fibronectin gels has been reported (19). These engineered tissues organized into multilayered structures 1–2 months after implantation in mice, illustrating the feasibility of grafting synthetic vessels, but retroviral transduction of the HUVEC resulting in Bcl-2 overexpression was required to promote survival of the transplanted cells (19). In a similar study, human dermal microvascular endothelial cells (HDMEC) in a porous poly-l-lactic acid sponge were implanted subcutaneously in mice and formation of functional microvessels was observed (20). The approaches used in these studies can be applied to the problem of vascular deficiency of cultured skin grafts. For example, preparation of a skin equivalent containing dermal fibroblasts, epidermal keratinocytes, and HUVEC has been reported, but transplantation to wounds was not performed (21).
A potential limitation of previous studies that may impede their clinical application in cultured skin grafting is the reliance on nondermal or nonautologous endothelial cells. HUVEC, used by other investigators to prepare engineered skin equivalents (21), are isolated from human umbilical vein. This is a discarded and therefore widely available tissue source, facilitating the use of HUVEC in preclinical studies. However, these cells are essentially of fetal origin and thus their behavior may differ from that of dermal endothelial cells (16). Preparation of endothelialized skin substitutes for grafting to a patient with a competent immune system ideally should be performed using multiple cell types derived from a single autologous skin sample. The present study was performed as a first step in the introduction of endothelial cells to a clinically relevant cultured skin model.
MATERIALS AND METHODS
Cell culture and preparation of cultured skin substitutes
Cultures of epidermal keratinocytes, dermal fibroblasts, and microvascular endothelial cells were isolated from a single human skin sample obtained from a 16-year-old female donor undergoing reduction mammoplasty. Primary cultures of human keratinocytes and fibroblasts were isolated and grown as described (22, 23) with minor modifications to facilitate isolation of HDMEC. The skin was cut into 2 cm × 0.25 cm strips, and the dermis and epidermis were enzymatically separated by incubation with Dispase (Roche Molecular Biochemicals, Indianapolis, IN) for 2.75 h. The epidermal pieces were digested in trypsin-ethylenediaminetetraacetic acid solution (23; Sigma Chemical Co., St. Louis, MO) and the keratinocytes were inoculated into flasks containing lethally irradiated NIH 3T3 cells in keratinocyte growth medium (22, 23). The dermal strips were placed in endothelial cell growth medium (Medium 131+Microvascular Growth Supplement; Cascade Biologics, Portland, OR) supplemented with 10% fetal bovine serum (FBS; Life Technologies, Rockville, MD) and scraped with sterile angled scissors to release microvascular endothelial cells. The cell suspension was centrifuged and the HDMEC were inoculated in endothelial cell growth medium into flasks coated with Attachment Factor (Cascade Biologics). The scraped dermal tissue strips were minced and incubated for 1 h with Collagenase (Worthington Biochemical Corp., Lakewood, NJ) for fibroblast isolation. The digested dermal tissue was centrifuged and the fibroblasts were inoculated into flasks containing fibroblast growth medium (23). After one (keratinocytes and fibroblasts) or two (HDMEC) passages, all cells were cryopreserved in the appropriate growth medium supplemented with 20% FBS plus 10% dimethyl sulfoxide (Sigma). Cells were recovered from cryo-preservation and expanded without additional passage for inoculation of CSS.
CSS were prepared as described (23-26) with modifications for the addition of HDMEC. Dermal substitutes consisted of acellular collagen-glycosaminoglycan (GAG) substrates (∼80 cm2 starting surface area each; n=10 total) inoculated with fibroblasts or a mixture of fibroblasts and HDMEC. For control CSS (n=4), collagen-GAG substrates were inoculated with 5 × 105 fibroblasts/cm2 in fibroblast growth medium. For CSS containing HDMEC (CSS+EC; n=6), 5 × 105 fibroblasts/cm2 were mixed with 5 × 105 HDMEC/cm2 in a 1:1 mixture of fibroblast growth medium and endothelial cell growth medium supplemented with 10 ng/ml recombinant human vascular endothelial cell growth factor (rhVEGF; R&D Systems, Minneapolis, MN). One day later (culture day 0), all dermal substitutes were inoculated with 1 × 106 keratinocytes/cm2 in either control CSS medium (25) or CSS medium supplemented with 10 ng/ml rhVEGF (for CSS+EC). Culture media were changed daily; on culture day 3, all CSS were lifted to the air-liquid interface and rhVEGF was removed from the CSS+EC medium. CSS were cultured for 16 days at 37°C, 5% CO2, with daily changes of nutrient medium. Biopsies for light microscopy and immunohistochemistry were collected at culture day 15. At the end of the culture period, grafts were cut to 2 cm × 2 cm squares for transplantation to mice.
Surface electrical capacitance (SEC)
Measurements of SEC were used to assess in vitro development of epidermal barrier. SEC provides a representation of skin surface hydration that is inversely proportional to electrical impedance. This was measured using the NOVA Dermal Phase Meter (DPM 9003; NOVA Technology, Portsmouth, NH) as described previously (27, 28). Six sets of SEC readings were taken on each CSS at in vitro culture days 8, 10, and 15. Data were collected for 10 s/set from random sites on each CSS. The 10 s values were converted from arbitrary units read by the Dermal Phase Meter to picofarads (pF) as described elsewhere (27). Mean values ± se are presented. Statistical analysis was performed using one-between, one-within repeated measures analysis of variance (RM ANOVA). The between factor was the group (control vs. CSS+EC); the within, or repeated, factor was time (culture day). Significant differences (P<0.001) between control and CSS+EC groups were found at each time point by univariate ANOVAs. Tukey's test was used to show significant differences (P<0.05) between pairs of means.
Grafting to athymic mice
Animal studies were performed with the approval of the University of Cincinnati Institutional Animal Care and Use Committee following National Institutes of Health guidelines. Control CSS and CSS+EC were grafted to 2 cm × 2 cm full-thickness wounds on the flanks of athymic mice (n=15 per group) as described (23, 24) with minor modifications. Each wound was prepared leaving the panniculus carnosus intact. Grafts with overlying nonadherent dressing (N-terface; Winfield Laboratories, Richardson, TX) were sutured to wounds. Grafts were dressed with gauze pads coated with antibiotic ointment, the dressings were covered with OpSite (Smith & Nephew Medical, Hull, UK), and the grafted areas were bandaged with Coban (3M Medical Division, St. Paul, MN). Mice were killed at 1, 2, and 4 wk after surgery (n=5 per group per time point). For mice killed at 4 wk, dressings and sutures were removed after 2 wk. At death, graft biopsies were collected for histological analysis and immunohistochemistry.
Engraftment was confirmed by staining human keratinocytes in frozen CSS sections (see below) by direct immunofluorescence using a fluorescein-labeled anti-human HLAABC antibody (Accurate Chemical & Scientific Corp., Westbury, NY).
Light microscopy and immunohistochemistry
Biopsies of CSS for light microscopy were fixed in 2% glutaraldehyde/2% paraformaldehyde for a minimum of 1 h for in vitro samples or 24 h for in vivo samples. Biopsies were processed, embedded in glycol-methacrylate (plastic) resin, sectioned, and stained with toluidine blue using standard techniques.
Biopsies of CSS for immunohistochemical staining were frozen in M-1 Embedding Matrix (Lipshaw, Pittsburgh, PA). A two-step procedure for imbedding, as described in detail elsewhere (12), was used to ensure that each biopsy was sectioned at a 90° angle to the surface of the epidermis. Cryostat sections (10–12 μm thick) were dehydrated in methanol and fixed in acetone at −20°C. After air-drying, sections were rehydrated in phosphate-buffered saline (PBS) pH 7.6. For light microscopy of frozen sections, hematoxylin and eosin staining was performed using standard procedures.
A colorimetric immunoperoxidase procedure was used for visualization of human endothelial cells in CSS prior to grafting instead of immunofluorescent labeling because of high levels of background fluorescence from the collagen-GAG substrate in vitro. CSS sections were incubated for a minimum of 1 h at room temperature with a biotin-conjugated anti-human CD31 antibody (ID Labs, Inc., London, Ontario, Canada) diluted to 5 μg/ml, followed by detection using the Vectastain® Elite ABC Universal kit and the DAB Peroxidase Substrate kit (Vector Labs, Burlingame, CA). Sections were briefly counterstained in a dilute toluidine blue solution.
Direct or indirect immunofluorescence was used for antigen detection in sections of grafted CSS. Human endothelial cells were labeled using a biotin-conjugated anti-human CD31 antibody (5 μg/ml; ID Labs), followed by detection using the Tyramide Signal Amplification Direct (Green) kit (Perkin Elmer Life Sciences, Boston, MA). Mouse endothelial cells were stained either directly using a FITC-conjugated anti-mouse CD31 antibody (12.5 μg/ml; BD PharMingen, San Diego, CA) for green fluorescence or indirectly using a purified rat anti-mouse CD31 antibody (12.5 μg/ml; BD PharMingen), followed by visualization with a Texas Red®-X goat anti-rat IgG antibody (10 μg/ml; Molecular Probes, Eugene, OR) for red fluorescence. Smooth muscle cells were stained using a Cy3-conjugated anti-α smooth muscle actin antibody (5 μg/ml; Sigma). Localization of basement membrane in CSS sections was performed using a rabbit antibody against collagen type IV (6.25 μg/ml; Biodesign International, Saco, ME), followed by staining with Texas Red®-X goat anti-rabbit IgG antibody (10 μg/ml; Molecular Probes). All antibody incubations were performed for a minimum of 1 h at room temperature in a humidified chamber. Samples were washed with PBS and slides were coverslipped using Fluoromount-G mounting media (Southern Biotechnology Associates, Birmingham, AL). In negative controls for all antibody staining, either primary or secondary antibodies were omitted.
Sections were examined using a Microphot-FXA microscope (Nikon, Melville, NY) equipped with epifluorescent illumination and photographed using a Spot-Jr. Digital Camera (Diagnostic Instruments, Sterling Heights, MI).
RESULTS
Human microvascular endothelial cells persist in CSS and form multicellular aggregates in vitro
CSS prepared with cultured fibroblasts and keratinocytes, with or without added HDMEC, were organized into dermal and epidermal tissue compartments, similar to native human skin (Fig. 1). After 15 days of in vitro incubation, the dermal compartments of control CSS and CSS+EC were densely populated with fibroblasts. The epidermal compartments of control CSS were composed of well-stratified epithelial layers, with basal keratinocytes attached to the collagen-GAG substrate and cornified layers at the upper air-exposed surface (Fig. 1A). In contrast, epidermal layers of CSS+EC were more poorly organized than controls (Fig. 1B).
Figure 1.
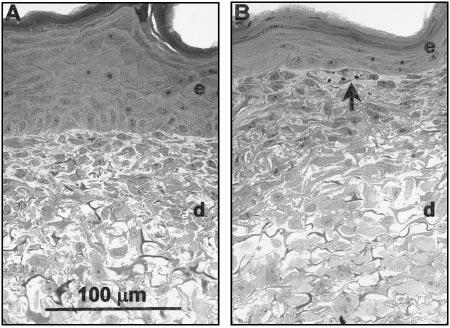
Histologies of CSS at culture day 15. Light microscopic images of plastic-embedded sections stained with toluidine blue are shown. A) Control CSS with well-organized epidermal layer and dermal layer densely packed with fibro-blasts. B) CSS + EC. Arrow indicates ring-like aggregate of cells near the dermal-epidermal junction. e, Epidermal layer; d, dermal layer. Scale bar in panel A (100 μm) is the same for both sections.
Specific staining of human endothelial cells in frozen sections of CSS using antibodies against human CD31 showed that after 15 days of in vitro culture, HDMEC were retained in the dermal compartments of CSS+EC (Fig. 2). Staining for CD31 was localized to the upper regions of the dermal compartments, in proximity to the dermal-epidermal junction. Clusters of CD31-staining cells were observed, suggesting aggregation of HDMEC in the upper dermis and, in some instances, CD31 staining was observed surrounding small holes in the dermal matrix (Fig. 2B). Close examination of the dermal compartments in plastic-embedded histological sections of CSS+EC revealed structures resembling vascular analogs (Fig. 1B). Ring-like aggregates of cells were found near the dermal-epidermal junction in CSS prepared with HDMEC. These structures were not observed in control CSS.
Figure 2.
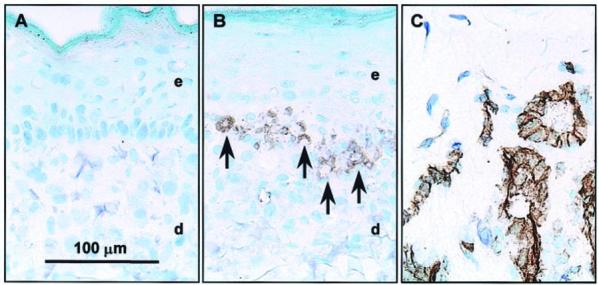
Localization of human endothelial cells in CSS+EC at culture day 15. Shown is immunohistochemical staining of frozen sections for the endothelial cell-specific antigen CD31. Sections were lightly counterstained with toluidine blue. A) Control CSS. B) CSS+EC. C) Normal human dermis shown as a positive control for specificity of staining. The arrows indicate regions of the CSS+EC staining positive for human CD31. e, Epidermal layer; d, dermal layer. Scale bar in panel A (100 μm) is the same for all panels.
The poorer in vitro organization of the epidermal compartments of CSS+EC vs. controls observed in histological sections corresponded with a reduction in epidermal barrier function as measured by surface electrical capacitance (SEC) (Fig. 3). Surface hydration, reflected by SEC measurements, is inversely proportional to epidermal barrier; hence, a drier cultured skin surface (lower SEC) indicates better barrier development. In control CSS, SEC values dropped significantly during in vitro incubation, suggesting maturation of epidermal barrier, and approached the value for native human skin by the end of the culture period (Fig. 3). SEC values for CSS+EC were statistically greater than controls at each time point measured. The SEC readings for CSS+EC decreased during in vitro culture, indicating drying of the epidermal surface and thus improvement in epidermal barrier with time, but values did not approach those of control CSS or native human skin.
Figure 3.
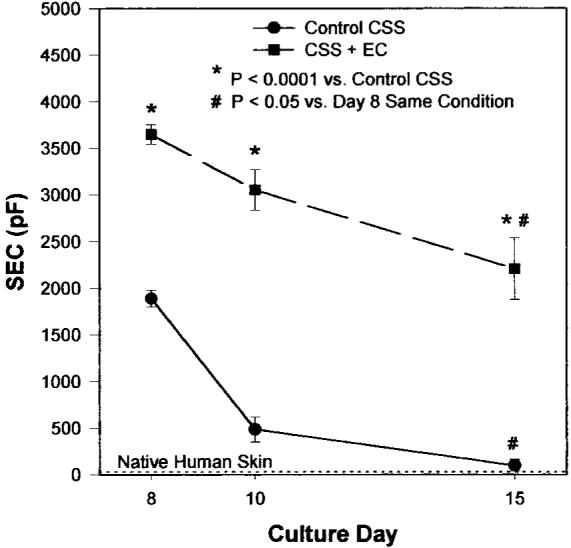
Development of epidermal barrier in vitro is impaired in CSS+EC. Surface electrical capacitance (SEC, pF) of control CSS decreases with time in culture, approaching that of native human skin by 15 days. SEC values for CSS+EC also decrease with time in culture but do not approach values for either control CSS or human skin, indicating a delay or disruption of barrier formation. CSS+EC have significantly wetter surfaces than control CSS at all time points examined in vitro (P<0.001). Plotted are means ± se.
Human microvascular endothelial cells organize into putative vascular analogs after grafting to athymic mice
Control CSS and CSS+EC healed after grafting to full-thickness wounds on the flanks of athymic mice (Fig. 4). No significant differences in engraftment were observed (data not shown).
Figure 4.
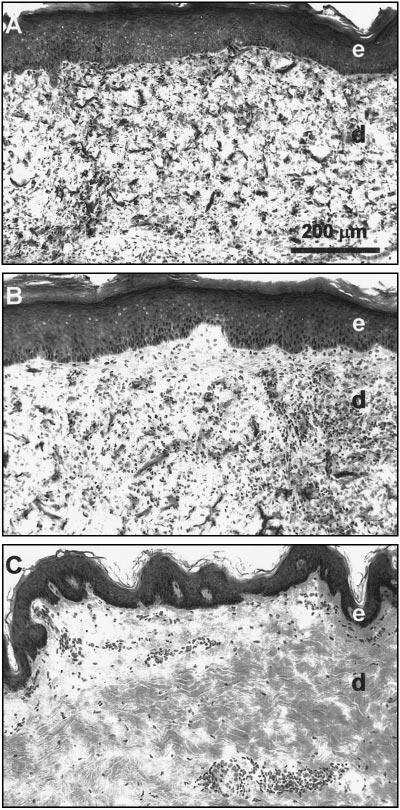
Histologies of CSS 4 wk after grafting to athymic mice. A) Control CSS. B) CSS+EC. C) Normal human skin. Light microscopic images of frozen sections stained with hematoxylin and eosin are shown. e, Epidermal layer; d, dermal layer. Scale bar in panel A (200 μm) is the same for all sections.
Antibodies specific for human or mouse CD31 were used for immunohistochemical localization of human and mouse endothelial cells in sections of CSS excised from mice at multiple times after grafting (Fig. 5). At 1 wk after grafting, clusters of human endothelial cells were observed near the dermal-epidermal junction in CSS+EC and some rings of human endothelial cells were found near the middle of the dermal compartments (Fig. 5A). In contrast, staining for mouse endothelial cells at this time was found mostly in the middle and lower regions of the dermal compartments (Fig. 5B). By 2 wk after grafting, larger rings of human endothelial cells, resembling putative vascular structures, were observed in the dermal compartments of CSS+EC (Fig. 5C). Fewer individual human endothelial cells were found; human CD31 staining appeared to be confined mainly to either ring-like groupings (Fig. 5C) or linear structures (data not shown) within the dermis. Similar multicellular structures were seen 4 wk after grafting (Fig. 5E). In some regions of CSS+EC excised 2 or 4 wk after grafting, cells staining positive for human CD31 were found in close proximity to regions of mouse CD31 staining, indicating colocalization of human and mouse endothelial cells (Fig. 5C-F).
Figure 5.
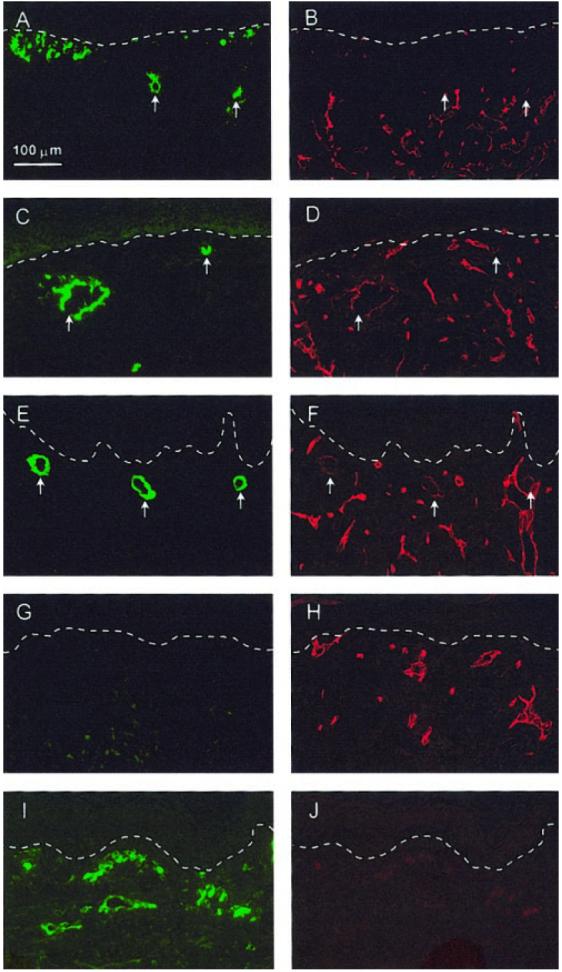
Localization of both human and mouse endothelial cells in CSS after grafting to athymic mice by double-immunofluorescent labeling. A–F) Sections of CSS+EC excised from mice at 1 (A, B), 2 (C, D), or 4 wk (E, F ) after grafting. G, H) Sections of control CSS excised at 4 wk after grafting. I, J) Normal human skin. A, C, E, G, I) Localization of human endothelial cell-specific CD31 (green fluorescence). B, D, F, H, J) Localization of mouse endothelial cell-specific CD31 (red fluorescence) in same sections. Arrows indicate locations of ring-like structures of aggregated human endothelial cells in CSS+EC sections. At 2 and 4 wk after grafting, some colocalization of staining for human and mouse CD31 can be seen (C–F ). Note absence of staining for human CD31 in control CSS (G) and absence of mouse CD31 staining in human skin (J), demonstrating species specificity of antibody staining. Dashed white lines indicate locations of dermalepidermal junctions. Scale bar in panel A (100 μm) is the same for all panels.
Staining for collagen type IV was colocalized with mouse CD31 staining in CSS+EC (data not shown) and control CSS (Fig. 6I, J) at all time points examined, indicating deposition of basement membrane around the dermal microvessels originating from the wound beds of the grafted mice. In CSS+EC, staining for collagen type IV was associated with human CD31 staining in regions where the human endothelial cells were organized into multicellular structures (Fig. 6A-F). At 1 wk after grafting, little or no colocalization of collagen type IV and human CD31 staining was observed (Fig. 6A, B). After 2 wk, colocalization was seen surrounding the larger rings of human endothelial cells (Fig. 6C, D), and this staining was more pronounced at 4 wk after surgery (Fig. 6E, F ).
Figure 6.
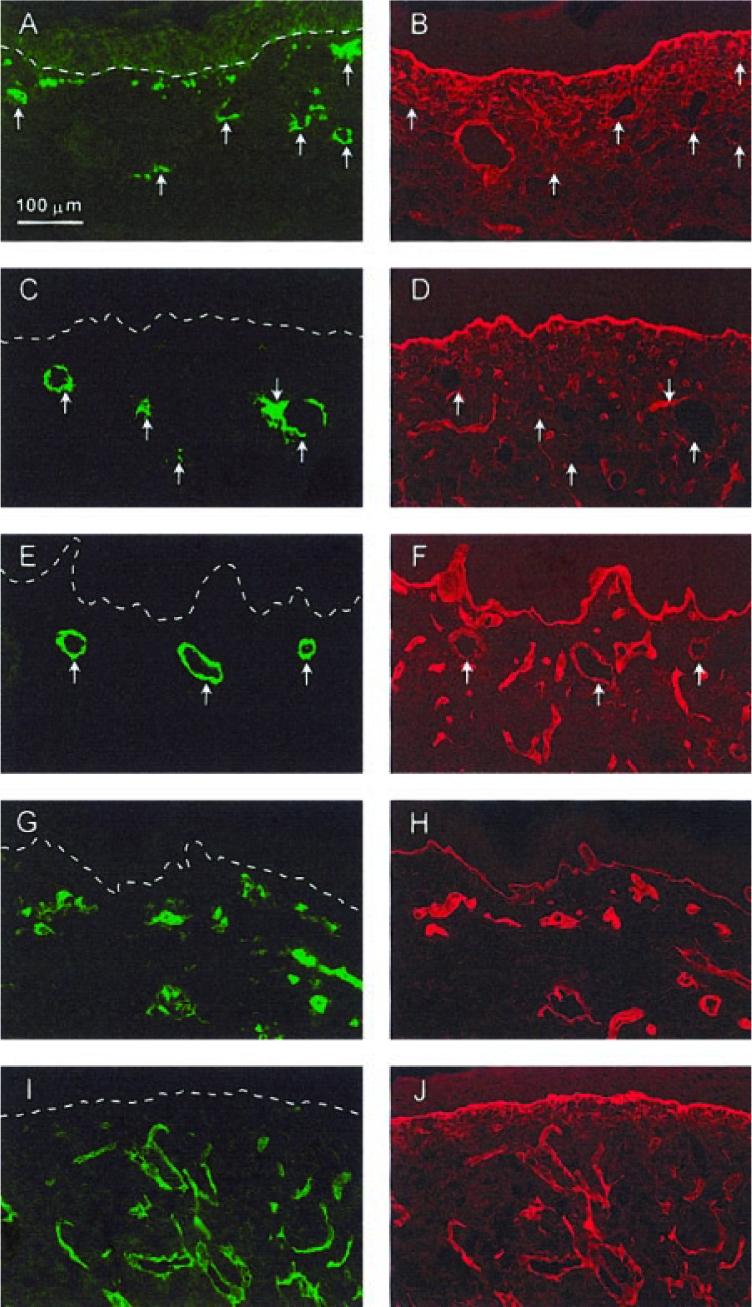
Deposition of basement membrane by endothelial cells in grafted CSS. A–F) Sections of CSS+EC excised from mice at 1 (A, B), 2 (C, D), or 4 wk (E, F), after grafting. G, H) Normal human skin. I, J) Control CSS at 2 wk after grafting. Sections were double-labeled with antibodies for either human (A, C, E, G) or mouse (I) CD31 (green fluorescence) and collagen type IV (B, D, F, H, J ) (red fluorescence). Arrows indicate regions of aggregated human endothelial cells in CSS+EC sections. Note increasing colocalization of collagen type IV staining and human CD31 staining in CSS+EC with increasing time after grafting. Essentially all staining for CD31 in normal human skin or in control CSS at 2 wk after grafting is colocalized with collagen type IV staining. Note also the localization of collagen type IV staining to the dermal-epidermal junction (dashed white lines in A, C, E, G, and I). Scale bar in panel A (100 μm) is the same for all panels.
To further characterize the multicellular structures formed by HDMEC in CSS+EC after grafting, sections were double-labeled with antibodies against either human or mouse CD31 and α smooth muscle actin. No colocalization of staining for human CD31 and α smooth muscle actin was observed in CSS+EC 1 wk after grafting (data not shown). By 4 wk, however, some of the larger and more highly organized human endothelial structures in CSS+EC were associated with staining for α smooth muscle actin (Fig. 7A, B), indicating colocalization of mouse smooth muscle cells with grafted HDMEC. Most staining for mouse CD31 was associated with staining for α smooth muscle actin in either CSS+EC (data not shown) or control CSS (Fig. 7C, D).
Figure 7.
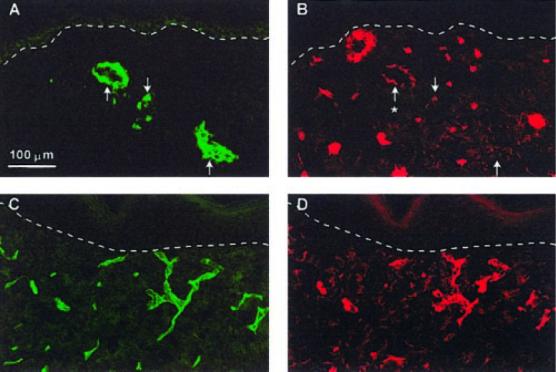
Association of putative vascular analogs in CSS+EC with vascular smooth muscle cells at 4 wk after grafting. Smooth muscle cells were identified by immunostaining with an anti-α smooth muscle actin antibody. A, B) Section of CSS+EC double-labeled with antibodies against human CD31 (A) and α smooth muscle actin (B). C, D) Section of control CSS double-labeled with antibodies against mouse CD31 (C) and α smooth muscle actin (D). Colocalization (asterisk) of human endothelial cell CD31 staining and α smooth muscle actin staining is clearly seen in the larger human endothelial cell vascular analog (A, B). This α smooth muscle actin staining is less intense than that seen in the vessels derived from the mouse vasculature (C, D). Dashed white lines indicate locations of dermal-epidermal junctions. Scale bar in panel A (100 μm) is the same for all panels.
DISCUSSION
Vascularization is critical to engraftment of skin substitutes. Initiation of angiogenesis during the in vitro culture period can prospectively shorten the period of vascularization after grafting. As a step toward development of an in vitro endothelialized cultured skin model, CSS were prepared with human keratinocytes, fibroblasts, and dermal endothelial cells isolated from a single skin biopsy. Endothelial cells were confined to the dermal compartment and were found in aggregates near the dermal-epidermal junction after 15 days of in vitro incubation. Some cells aggregated into ring-like structures suggesting the organization of endothelial cells into primitive vascular analogs. The human endothelial cells persisted after grafting to full-thickness wounds in mice and organized into linear and ring-like structures around lumens in the dermis. At 1 wk after grafting, several clusters of human endothelial cells were found near the dermal-epidermal junction of CSS+EC in addition to small circular aggregates deeper in the dermis. By 2–4 wk after grafting, fewer individual human endothelial cells were observed, with most found in either larger ring-like or linear structures. This suggests aggregation of the human endothelial cells into primitive vascular structures and/or loss of individual cells.
The aggregated human endothelial cells in CSS+EC grafted to mice secreted basement membrane protein. Colocalized staining for human CD31 and collagen type IV increased between 2 and 4 wk after grafting, indicating that basement membrane deposition accompanied organization of human endothelial cells and the development of putative vascular analogs in CSS+EC after grafting. Colocalization of human CD31 and α smooth muscle actin staining was evident by 4 wk after transplantation, indicating association of the human vascular analogs in grafted CSS+EC with mouse smooth muscle cells. Staining with species-specific antibodies suggested some colocalization of human and mouse endothelial cells at 2 and 4 wk after grafting. However, as it was beyond the scope of this qualitative study, it was not definitively determined whether functional connections between the human vascular analogs in grafted CSS+EC and the mouse circulation were present. This determination, to be addressed in future studies, will be critical for analyzing the effect of inclusion of HDMEC on vascularization after grafting.
The results of this study demonstrate the ability to transplant HDMEC in a cultured skin graft and the ability of such a graft to heal a full-thickness wound; however, several issues remain to be addressed. Although endothelial cells were easily identified in sections of CSS+EC in vitro, there were relatively small numbers of HDMEC by the end of the culture period. Whereas the dermis is packed with fibroblasts after 15 days in culture, an equal initial inoculation of HDMEC resulted in a lower final proportion of the dermis populated by endothelial cells. This indicates either a lower proliferation rate of the HDMEC compared with fibroblasts in the dermal substrate or selective loss of these cells during in vitro culture of the skin substitute. Endothelial cell loss by apoptosis in tissue-engineered vascular constructs has been observed by other investigators (19, 20). One group has used genetic modification with a Bcl-2 retrovirus to delay apoptosis of endothelial cells and promote survival after grafting (19, 29). Hypothetically, this endothelial cell loss can also be addressed by modifying the culture conditions or biopolymer matrix used for preparation of CSS.
Another important issue is the poorer organization of the epidermal layers of CSS+EC compared with control CSS. This may have been due to alterations in the dermal matrix resulting from the presence of HDMEC or the secretion of factors by the HDMEC that affect keratinocyte proliferation and/or differentiation. Alternatively, this observation may be a by-product of the increased overall cell inoculation in the dermal compartments of CSS+EC compared with controls. In this study, the total number of fibroblasts inoculated per unit area was held constant between groups, but an equal number of HDMEC was inoculated in the dermal substitutes of CSS+EC grafts. Despite the in vitro observations, differences in epidermal organization were not detected in vivo at any time after transplantation, suggesting that the impairment of barrier development in vitro was overcome after grafting to mice.
To our knowledge, these studies represent the first transplantation of dermal microvascular endothelial cells in a composite cultured skin graft. Because all three cell types (keratinocytes, fibroblasts, and endothelial cells) were obtained from a single skin biopsy, this study demonstrates the feasibility of preparing autologous CSS+EC for grafting to patients. Restoration of a vascular plexus in engineered skin grafts may result in greater efficacy of wound healing as well as reduced morbidity and mortality from extensive skin loss injuries.
Acknowledgments
The authors gratefully acknowledge Andrew Supp for his expert technical assistance. We also thank Jodi Miller, Todd Schuermann, and Gail Macke for reagent preparation and Laura James for assistance with statistical analyses. This work was supported by National Institutes of Health grant #5R01GM050509 to S. Boyce, and Shriners Hospitals for Children Research Grant #8680 to D.M.S.
REFERENCES
- 1.Hansbrough JF, Boyce ST, Cooper ML, Foreman TJ. Burn wound closure with cultured autologous keratinocytes and fibroblasts attached to a collagen-glycosaminoglycan substrate. J. Am. Med. Assoc. 1989;262:2125–30. [PubMed] [Google Scholar]
- 2.Boyce ST, Greenhalgh DG, Kagan RJ, Housinger T, Sorrell M, Childress CP, Rieman M, Warden GD. Skin anatomy and antigen expression after burn wound closure with composite grafts of cultured skin cells and biopolymers. Plast. Reconstr. Surg. 1993;91:632–41. doi: 10.1097/00006534-199304000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Kuroyanagi Y, Kenmochi M, Ishihara S, Takeda A, Shiraishi A, Ootake N, Uchinuma E, Torikai K, Shioya N. A cultured skin substitute composed of fibroblasts and keratinocytes with a collagen matrix: preliminary results of clinical trials. Ann. Plast. Surg. 1993;31:340–51. doi: 10.1097/00000637-199310000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Boyce ST, Goretsky MJ, Greenhalgh DG, Kagan RJ, Rieman MT, Warden GD. Comparative assessment of cultured skin substitutes and native skin autograft for treatment of full-thickness burns. Ann. Surg. 1995;222:743–52. doi: 10.1097/00000658-199512000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zacchi V, Soranzo C, Cortivo R, Radice M, Brun P, Abatangelo G. In vitro engineering of human skin-like tissue. J. Biomed. Mater. Res. 1998;40:187–94. doi: 10.1002/(sici)1097-4636(199805)40:2<187::aid-jbm3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 6.Caruso DM, Schuh WH, Al-Kasspooles MF, Chen MC, Schiller WR. Cultured composite autografts as coverage for an extensive body surface area burn: case report and review of the technology. Burns. 1999;25:771–79. doi: 10.1016/s0305-4179(99)00083-2. [DOI] [PubMed] [Google Scholar]
- 7.Boyce ST, Kagan RJ, Meyer NA, Yakuboff KP, Warden GD. The 1999 Clinical Research Award: cultured skin substitutes combined with Integra Artificial Skin™ to replace native skin autograft and allograft for the closure of excised full-thickness burns. J. Burn Care Rehabil. 1999;20:453–61. doi: 10.1097/00004630-199920060-00006. [DOI] [PubMed] [Google Scholar]
- 8.Boyce ST, Kagan RJ, Yakuboff KP, Meyer NA, Rieman MT, Greenhalgh DG, Warden GD. Cultured skin substitutes reduce donor skin harvesting for closure of excised, full-thickness burns. Ann. Surg. 2002;235:269–279. doi: 10.1097/00000658-200202000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyce ST. Cultured skin substitutes: a review. Tissue Eng. 1996;2:255–266. doi: 10.1089/ten.1996.2.255. [DOI] [PubMed] [Google Scholar]
- 10.Boyce ST, Supp AP, Harriger MD, Greenhalgh DG, Warden GD. Topical nutrients promote engraftment and inhibit wound contraction of cultured skin substitutes in athymic mice. J. Invest. Dermatol. 1995;104:345–349. doi: 10.1111/1523-1747.ep12665374. [DOI] [PubMed] [Google Scholar]
- 11.Supp DM, Supp AP, Bell SM, Boyce ST. Enhanced vascularization of cultured skin substitutes genetically modified to overexpress vascular endothelial growth factor. J. Invest. Dermatol. 2000;114:5–13. doi: 10.1046/j.1523-1747.2000.00824.x. [DOI] [PubMed] [Google Scholar]
- 12.Supp DM, Boyce ST. Overexpression of vascular endothelial growth factor accelerates early vascularization and improves healing of genetically modified cultured skin substitutes. J. Burn Care Rehabil. 2002;23:10–20. doi: 10.1097/00004630-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Young DM, Greulich KM, Weier HG. Species-specific in situ hybridization with fluorochrome-labeled DNA probes to study vascularization of human skin grafts on athymic mice. J. Burn Care Rehabil. 1996;17:305–310. doi: 10.1097/00004630-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Davison PM, Bensch K, Karasek MA. Isolation and growth of endothelial cells from the microvessels of the newborn human foreskin in cell culture. J. Invest. Dermatol. 1980;75:316–321. doi: 10.1111/1523-1747.ep12530941. [DOI] [PubMed] [Google Scholar]
- 15.Karasek MA. Microvascular endothelial cell culture. J. Invest. Dermatol. 1989;93:33S–38S. doi: 10.1111/1523-1747.ep12580906. [DOI] [PubMed] [Google Scholar]
- 16.Hewett PW, Murray JC. Human microvessel endothelial cells: isolation, culture and characterization. In Vitro Cell. Dev. Biol. 1993;29:823–830. doi: 10.1007/BF02631356. [DOI] [PubMed] [Google Scholar]
- 17.L'Heureux N, Germain L, Labbé R, Auger FA. In vitro construction of a human blood vessel from cultured vascular cells: a morphologic study. J. Vasc. Surg. 1993;17:499–509. doi: 10.1067/mva.1993.38251. [DOI] [PubMed] [Google Scholar]
- 18.L'Heureux N, Pâquet S, Labbé R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12:47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 19.Schechner JS, Nath AK, Zheng L, Kluger MS, Hughes CCW, Sierra-Honigmann MR, Lorber MI, Tellides G, Kashgarian M, Bothwell ALM, Pober JS. In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc. Natl. Acad. Sci. USA. 2000;97:9191–9196. doi: 10.1073/pnas.150242297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nör JE, Peters MC, Christensen JB, Sutorik MM, Linn S, Khan MK, Addison CL, Mooney DJ, Polverini PJ. Engineering and characterization of functional human microvessels in immunodeficient mice. Lab. Invest. 2001;81:453–463. doi: 10.1038/labinvest.3780253. [DOI] [PubMed] [Google Scholar]
- 21.Black AF, Berthod F, L'Heureux N, Germain L, Auger FA. In vitro reconstruction of a human capillary-like network in a tissue-engineered skin equivalent. FASEB J. 1998;12:1331–1340. doi: 10.1096/fasebj.12.13.1331. [DOI] [PubMed] [Google Scholar]
- 22.Boyce ST, Ham R. Cultivation, frozen storage and clonal growth of normal human epidermal keratinocytes in serum-free media. J. Tissue Cult. Methods. 1985;9:83–93. [Google Scholar]
- 23.Boyce ST. Methods for the serum-free culture of keratinocytes and transplantation of collagen-GAG-based skin substitutes. In: Morgan JR, Yarmush ML, editors. Methods in Molecular Medicine: Tissue Engineering Methods and Protocols. Humana Press; Totowa, NJ: 1998. pp. 365–389. [DOI] [PubMed] [Google Scholar]
- 24.Boyce ST, Foreman TJ, English KB, Stayner N, Cooper ML, Sakabu S, Hansbrough JF. Skin wound closure in athymic mice with cultured human cells, biopolymers and growth factors. Surgery. 1991;110:866–76. [PubMed] [Google Scholar]
- 25.Swope VB, Supp AP, Greenhalgh DG, Warden GD, Boyce ST. Expression of insulin-like growth factor-1 by cultured skin substitutes does not replace the physiologic requirement for insulin in vitro. J. Invest. Dermatol. 2001;116(5):650–657. doi: 10.1046/j.1523-1747.2001.01325.x. [DOI] [PubMed] [Google Scholar]
- 26.Boyce ST, Christianson DJ, Hansbrough JF. Structure of a collagen-GAG dermal skin substitute optimized for cultured human epidermal keratinocytes. J. Biomed. Mater. Res. 1988;22:939–957. doi: 10.1002/jbm.820221008. [DOI] [PubMed] [Google Scholar]
- 27.Boyce ST, Supp AP, Harriger MD, Pickens WL, Wickett RR, Hoath SB. Surface electrical capacitance as a noninvasive index of epidermal barrier in cultured skin substitutes in athymic mice. J. Invest. Dermatol. 1996;107:82–87. doi: 10.1111/1523-1747.ep12298286. [DOI] [PubMed] [Google Scholar]
- 28.Supp AP, Wickett RR, Swope VB, Harriger MD, Hoath SB, Boyce ST. Incubation of cultured skin substitutes in reduced humidity promotes cornification in vitro and stable engraftment in athymic mice. Wound Rep. Reg. 1999;7:226–237. doi: 10.1046/j.1524-475x.1999.00226.x. [DOI] [PubMed] [Google Scholar]
- 29.Zheng L, Dengler TJ, Kluger MS, Madge LA, Schechner JS, Maher SE, Pober JS, Bothwell ALM. Cytoprotection of human umbilical vein cells against apoptosis and CTL-mediated lysis provided by caspase-resistant Bcl-2 without alterations in growth or activation responses. J. Immunol. 2000;164:4665–4671. doi: 10.4049/jimmunol.164.9.4665. [DOI] [PubMed] [Google Scholar]


