Abstract
In songbirds, song learning and production are regulated by the song control system. How the rest of the brain interacts with song nuclei to ensure that song is produced in an appropriate context is not yet clear. In male European starlings (Sturnus vulgaris), breeding context song is sexually motivated whereas non-breeding context song is more broadly socially motivated. Brain regions involved in regulating social behavior might differentially regulate starling song depending upon the context in which it is produced. Here, we compared the number of ZENK-labeled cells in song and social behavior nuclei in starlings singing in either a breeding or a non-breeding context. Numbers of ZENK-labeled cells in HVC related positively to song produced in both contexts. Interestingly, numbers of ZENK-labeled cells in one subdivision of the lateral septum (LS) related negatively to breeding context song but positively to non-breeding context song. In a subdivision of the medial bed nucleus of the stria terminalis (BSTm) ZENK labeling only related positively to non-breeding context song whereas in the ventromedial nucleus of the hypothalamus (VMH) ZENK labeling showed a tighter positive relationship with breeding context song. Together, these findings indicate that social behavior brain regions outside of the song control system regulate singing behavior differently depending upon whether song is sexually or more broadly socially motivated. Breeding context-dependent regulation of song by LS, BSTm, and VMH suggests that these nuclei may be central to adjusting song production so that it occurs in response to appropriate social and environmental stimuli.
Keywords: songbird, season, social context, ZENK, high vocal center, lateral septum, bed nucleus of the stria terminalis, ventromedial nucleus of hypothalamus
INTRODUCTION
Vocal communication plays an important role in mediating successful social interactions between individuals in a variety of contexts. The neural circuitry involved in vocal signal production and perception has been identified in some model systems, including songbirds [see 32]. However, what motivates an animal to communicate can depend upon many factors including presence or absence of conspecifics, dominance status, and season. Often, vocal behavior must be adjusted so that communication occurs in response to appropriate social and environmental stimuli. To date, how the rest of the brain interacts with vocal signal production circuits to ensure that vocal communication occurs in the appropriate context is not yet clear.
Much is already known about how the songbird brain regulates singing behavior. Song learning and production are controlled by a series of cytoarchitecturally distinct, interconnected nuclei including Area X, HVC (used as a proper name), robust nucleus of the arcopallium (RA), and lateral magnocellular nucleus of the anterior nidopallium (lMAN), [e.g. 11,46,52,66]. Collectively, these four regions (and several others) are known as the song control system, and a growing body of literature indicates that activity within song control nuclei is context-dependent. The relationship between singing behavior and activity in HVC, RA, Area X and lMAN can depend upon whether song is sexually or socially motivated [34,42,58] and whether song is directed towards or away from a conspecific [35,37]. Taken together it appears that song nuclei differentially regulate singing behavior depending upon what is motivating a male to sing. However, no research currently implicates the song control system in regulating motivational aspects of singing behavior. Lesions to song nuclei result in deficits in song production, but lesioned birds continue to display motor patterns associated with singing behavior and will assume singing postures, suggesting an intact motivation to communicate [10,51,52,63]. Thus, context-dependent activation of the song control system may be a reflection of context-dependent inputs to song nuclei from areas of the brain regulating the motivation to sing.
In support of this hypothesis are data showing that brain regions outside of the song control system, well known for regulating sexual motivation and social behavior, appear to regulate singing behavior in songbirds. Specifically, neuronal activity within the medial preoptic nucleus (POM), lateral septum (LS), medial bed nucleus of the stria terminalis (BSTm), anterior portions of the hypothalamus (AH), ventromedial nucleus of the hypothalamus (VMH), ventral tegmental area (VTA), and midbrain central gray (GCt) correlates with singing behavior [33,34,44], and lesions to POM and the septum have been shown to modulate song production [1,25,55]. However, the role of these nuclei in the regulation of vocal behavior in songbirds appears to be context-dependent and is influenced by whether song is sexually motivated [33,34,58], sung in response to territorial intrusion [23,44], or observed in gregarious or territorial species [25]). Together, these studies suggest that these seven brain regions are part of a neural circuit that influences the song control system to ensure that singing behavior occurs in an appropriate context in response to appropriate social and/or environmental stimuli.
European starlings (Sturnus vulgaris) provide an excellent model for the study of context-dependent regulation of vocal behavior. Male starlings sing throughout the year, but the social and environmental factors that motivate song production differ depending upon whether it occurs within or outside of a breeding context [see 16 for review]. Outside of a breeding context, in fall and winter, circulating levels of gonadal steroids (i.e. testosterone (T)) are low [6,56], and male and female starlings are found feeding and roosting in large, mixed-sex flocks [20]. At this time, song appears to play no direct role in mate attraction [57], but rather is thought to be important for flock cohesion and establishing/maintaining social hierarchies within the flock [31,64]. Thus, starling song produced in a non-breeding context appears to be broadly socially motivated.
In contrast, breeding context song can be highly sexually motivated. In late winter and early spring, circulating levels of T become elevated [6,56]. Flocks disperse and males compete over limited numbers of appropriate nest sites [30]. Song at this time can play a critical role in mate attraction and nest site defense [e.g. 18,29]. Interestingly, nest site status profoundly influences male singing behavior within a breeding context. Males with nest sites increase their rate of song production in response to the introduction of a female conspecific whereas female introduction has no significant effect on the song rate observed in males without nest sites [57]. Once males with nest sites pair with females, song is restricted to periods immediately prior to copulation [13,19]. Taken together, it appears that nest site ownership significantly influences the degree to which breeding context song is sexually motivated.
In past work on starlings we found that the numbers of cells labeled for the protein products of the immediate early gene (IEG) c-fos (an indirect marker of neural activity) in HVC, RA, POM, BSTm, AH, VMH and VTA related positively only to breeding context song, whereas numbers of Fos-labeled cells in LS related positively only to non-breeding context song [33,34]. Not all brain regions express Fos and the use of multiple indirect markers of neural activity allows for a more complete picture of brain regions regulating behavior than any one IEG alone [see 5,48,58]. Therefore, in the present study we used immunocytochemistry for the protein products of the IEG ZENK (avian homologue of zif-268, egr-1, ngfi-a, and krox-34 [see 49]) to provide a more complete picture of how the brain regulates context-dependent vocal communication in male songbirds.
METHODS
Animals
Starlings were caught on a single farm north of Madison, Wisconsin in fall 2002 and early winter 2003 using fly-in traps. These are the same birds used in a previous study examining song and numbers of Fos-labeled cells [33,34]. The natural photoperiod at the time of capture was <11L. After capture, forty-two adult male and four adult female starlings were housed indoors in single-sex cages (91cm × 47cm × 47 cm) in the University of Wisconsin Department of Zoology animal facilities. Photoperiod and hormone manipulations were used to put birds into either breeding or non-breeding conditions (see below).
Males
Eighteen male starlings were randomly assigned to the non-breeding group. After capture, these birds were housed indoors on a photoperiod matching the natural light/dark cycle. On December 18, 2002, when the outdoor photoperiod was ~10L, non-breeding males were placed into two aviaries (3.7m × 2m × 2.8m) in social groups of 8–10 birds. Behavioral testing of non-breeding males began in January after a one-month habituation period.
The remaining twenty-four males were assigned to the breeding condition group. After capture, these birds were housed on 6L for two months and then 11L for approximately six months. This photoperiodic regime causes birds to become photosensitive (a condition in which they respond to T with courtship and mating behavior) [14]. Two weeks prior to introduction to an outdoor aviary (identical to those used for the non-breeding groups), birds were implanted with T (see below). After implant surgery, breeding condition males were placed into outdoor aviaries in social groups of 12 birds and given two weeks to habituate to the outdoor aviaries. Behavioral observations for the present study took place at a time when the outdoor photoperiod was ~13L.
Stimulus females
To stimulate sexually motivated singing behavior in breeding condition males, a female was introduced to the outdoor aviary immediately prior to the start of behavioral testing. As described in the Introduction, non-breeding context song plays no role in immediate mate attraction. However, to control for the possibility that the introduction of a novel conspecific might induce ZENK expression in some of the brain regions examined, a stimulus female was introduced to non-breeding condition males as well. To maintain ecological validity of the male singing behavior observed, breeding condition females were released into the aviaries containing breeding condition males and non-breeding condition females were released into aviaries containing non-breeding condition males. Breeding condition stimulus females were housed indoors on an 11L photoperiod. Non-breeding condition females were housed indoors on a 6L photoperiod.
Testosterone implants
Breeding condition males were given subcutaneous T-implants two weeks prior to being introduced into the test aviary based on pilot studies indicating males often do not, or are slow to exhibit a full suite of courtship behavior in captivity. Control implants were not given to non-breeding males because the decision to T-treat breeding males was made after non-breeding birds had been tested. Using isoflurane gas anesthesia, each bird was lightly anesthetized and two fourteen mm lengths of silastic tubing (Dow Corning, i.d., 1.47mm; o.d. 1.96mm), packed for ten mm with crystalline testosterone proprionate (Sigma) and sealed with silastic glue, were placed under the skin just posterior to the last rib on the left side. Males recovered from surgery on a heated surface and were then placed back on an 11L photoperiod until introduction to the outdoor aviary. In a study that is in progress in our lab, breeding condition males implanted with T and housed on an 11L photoperiod for 4 weeks had circulating levels of T which were significantly higher than those in un-manipulated, non-breeding males housed on a 6L photoperiod (breeding condition males (n=7) mean = 705.39 pg/ml, sd = 476.69); non-breeding condition males (n=7) mean = 60.58 pg/ml, sd = 91.68: Z = 3.13, p = 0.002) (Heimovics and Riters, unpublished data).
Behavioral testing
Aviaries used for behavioral testing (see above) were adjacent to one another and birds were in acoustic, but not visual, contact with conspecifics. Six nestboxes and branches for perching were placed in each aviary prior to the habituation period and remained throughout the duration of the study. Food and water were provided ad libitum. Prior to the day of behavioral testing for the present study, males were observed for nine days over a two-week period as part of a separate behavioral analysis. Specifically, over a nine-day period they were presented daily with females in different reproductive conditions and an observer noted their behavior. Behavioral testing procedures were identical for breeding and non-breeding males.
Behavior data collection for the present study began three days after completion of these observations. Behavior data for the present study were collected on a single day for fifty minutes. A complete description of methods used for behavioral testing is published elsewhere [34]. A point sampling method [see 17] was used to measure the proportion of time each male spent singing after the introduction of a female conspecific. During the observation period on each test day, a weak beep was emitted from a timer to indicate each minute. For fifty minutes it was noted at every beep whether or not each individual male was singing. Whether or not a male entered or exited a nestbox, other forms of courtship behavior (wing-waving and nest material-gathering), and measures of overt aggression (i.e. chasing, displacing, pecking, and attacking) were also noted throughout the fifty-minute period.
Tissue processing
Ten minutes after the conclusion of behavioral observations all males in a social group were sacrificed via rapid decapitation and their brains were dissected out of their skulls. Brain tissue from all individuals was collected within twenty minutes. These parameters have been shown in the past to work well to examine the relationship between behavior and ZENK-labeling in songbirds [e.g. 58]. Brains were fixed in a 5% acrolein solution, cryoprotected, and frozen at −80°C until processing. At the time of sacrifice, testis length and width were measured and the presence of T implants in breeding condition males was verified. Brains were cut in the coronal plane using a cryostat. Three series of 30μm sections were collected into phosphate-buffered saline (PBS; 0.1M, 7.5 pH) from just rostral to the tractus septomesencephalicus to just caudal to the song control nucleus RA. One series of sections was counterstained with Thionin, a second was collected for ZENK immunocytochemistry (ICC), and the remaining third was used as part of other studies [see 33,34].
ZENK immunocytochemistry
After the completion of behavioral testing of breeding and non-breeding social groups, thirty-four males were selected to be included in the ZENK immunocytochemistry (ICC) presented here (breeding condition n=16; non-breeding condition n=18). ZENK ICC was run in two batches (batch one n=14; batch two n=20). Each batch included both breeding (males with and without nestboxes) and non-breeding condition birds whose singing behavior fell along a continuum; from very few to many point samples with song.
Details of methods used for ICC have been published elsewhere [34]. Briefly, for ZENK ICC tissue was incubated in a 2% normal goal serum (NGS; Pel-Freeze Biologicals; Rogers, Arkansas) primary antibody solution (1:5000, ZENK Ab = erg-1, (c19) Santa Cruz), 2% NGS secondary antibody solution (1:500 goat anti-rabbit, Jackson ImmunoResearch Laboratories), and visualized using Vector SG (Vector Laboratories). Sections were then mounted on gel-coated slides, dehydrated, and coverslipped.
Quantification
A researcher blind to each individual’s breeding condition, nestbox status, and singing behavior quantified numbers of ZENK-labeled cells. Counts were performed using a digital Spot camera (Diagnostic Instruments Inc.) connecting a microscope to a PC computer. To determine which cells were to be counted as labeled, Meta Vue software (Universal Imaging Corp.) was used to set a cell-count threshold outside of any brain region of interest. The same threshold was used for all brain regions within a batch but, because differences between ICC batches might lead to differences in density of labeling, a unique threshold was set for each batch. For all brain regions, numbers of ZENK-labeled cells were counted bilaterally on three serial sections using Meta Vue Integrated Morphometry Analysis. Automated cell-count values were averaged and the mean value for each region used for statistical analyses. In cases where dust particles and/or overlapping cells were present, the researcher manually adjusted the total number of labeled cells used to calculate the mean. In the few cases of tissue damage or lost sections, counts were made on a fourth serial section. If tissue damage was extensive, that individual was dropped from the analysis of that region.
Numbers of ZENK-labeled cells were counted in Area X, HVC, RA, POM, LS, the medial septum (MS), BSTm, AH, VMH, nucleus taenia of the amygdala (TnA), GCt, and VTA (see Fig. 1A-E for locations and Table 1 for size of the areas in which cells were counted). For POM, counts were made on sections containing the nucleus of the pallial commissure (Fig. 1B). Cell counts within LS were made at the level of the anterior commissure (CoA) within three zones (caudal part, ventrolateral zone (LSc.vl), caudal part, ventral zone (LSc.v), rostral part (LSr)) based on Goodson [27] (Fig. 1C). Research in quail has identified populations of BSTm neurons located dorsal and ventral to CoA [4]. To explore the possibility that these populations are functionally distinct, counts were made separately within each sub-region (Fig. 1C). In songbirds, the precise location of the homologue to mammalian AH has not been identified. Here we examined numbers of ZENK-labeled cells within a region referred to descriptively as anterior hypothalamus (AH) by Goodson [27] (Fig. 1C).
Fig. 1.
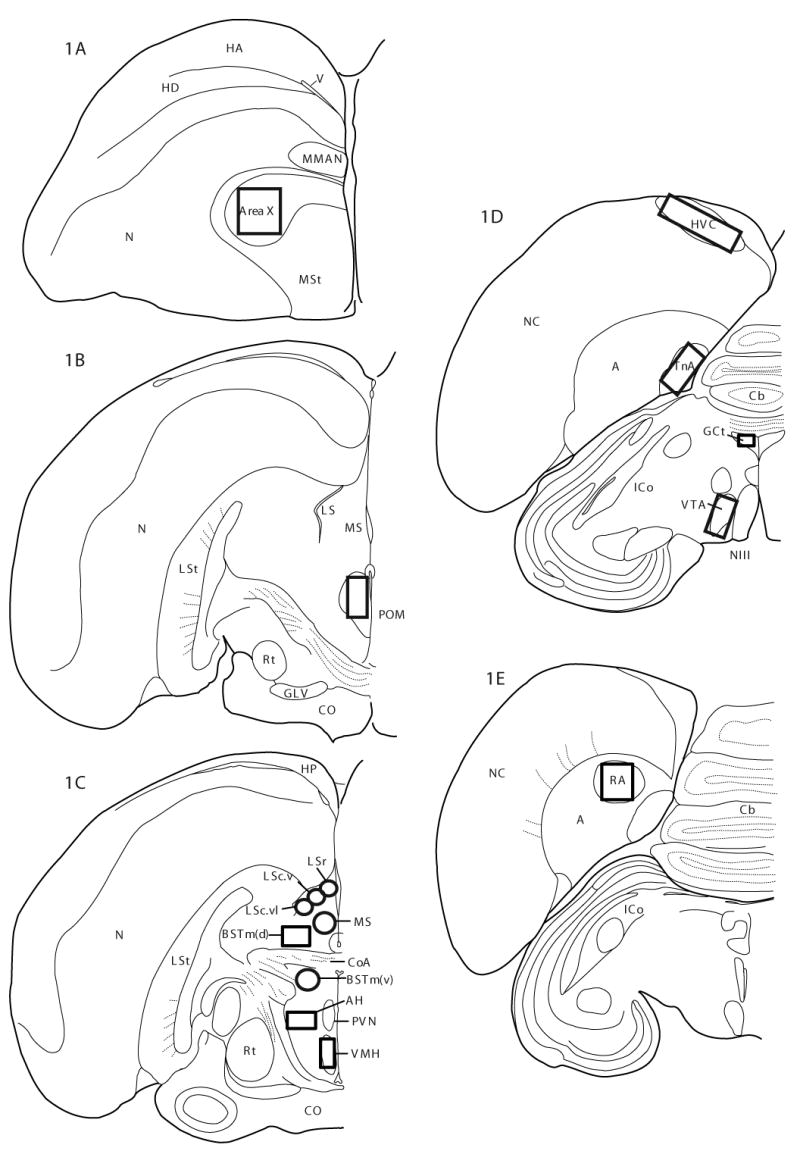
Boxes/circles indicate areas in which ZENK-labeled cells were counted. Abbreviations: A, arcopallium; AH, anterior hypothalamus; BSTm(d), medial bed nucleus of the stria terminals dorsal to CoA; BSTm(v), medial bed nucleus of the stria terminalis ventral to CoA; Cb, cerebellum; CoA, anterior commissure; CO, optic chiasm; GCt, mesencephalic central gray; GLV, nucleus geniculatus lateralis, pars ventralis; HA, apical part of the hyperpallium; HD, densocellular part of the hyperpallium; HP, hippocampus; ICo, nucleus intercollicularis; LS, lateral septum; LSc.v, lateral septum, caudal part, ventral zone; LSc.vl, lateral septum, caudal part, ventrolateral zone; LSr, lateral septum, rostral part; LSt, lateral striatum; MMAN, medial magnocellular nucleus of the anterior nidopallium; MS medial septum; MSt, magnocellular part of the medial striatum; NIII, third cranial nerve; N, nidopallium; NC, caudal nidopallium; POM, intermediate zone of the medial preoptic nucleus; RA, robust nucleus of the acropallium Rt, nucleus rotundus; TnA, nucleus taeniae of the amygdala; V, ventricle; VMH, ventromedial nucleus of the hypothalamus; VTA, ventral tegmental area.
Table 1.
Size of region in which ZENK-labeled cells were counted for each nucleus.
| NUCLEUS | SIZE |
|---|---|
| Area X | area = 0.33 × 0.54mm |
| POM | area = 3.7 × 2.4mm |
| LS | |
| rostral part | diameter = 0.33mm |
| caudal part, | |
| ventral zone | diameter = 0.33mm |
| caudal part, | |
| ventrolateral zone | diameter = 0.33mm |
| MS | diameter = 0.33mm |
| BSTm | |
| dorsal | area = 0.33mm × 0.66mm |
| ventral | diameter = 0.53mm |
| AH | area = 0.27mm × 0.48mm |
| VMH | area = 0.27mm × 0.48mm |
| HVC | area = 0.3 × 0.56mm |
| TnA | area = 0.17 × 0.50mm |
| GCt | area = 0.23 × 0.20mm |
| VTA | area = 0.42 × 0.30mm |
| RA | area = 2.6 × 2.6 mm |
Statistical analysis
Data were analyzed using Statistica 6.0 software (Stat Soft Inc., Tulsa, OK). To normalize the point sampled song data, the number of point samples with singing out of 50 was arcsine transformed for each individual using the following equation: 2*arcsine square root # observed songs/50 [41]. Numbers of ZENK-labeled cells were log transformed when cell count data did not meet the assumptions of parametric statistics. Simple linear regression was used to determine the extent to which brain activity related to male singing behavior. Independent Student’s t-tests were used to compare the numbers of ZENK-labeled cells in the brains of breeding condition males with versus without nestboxes.
RESULTS
Behavior
Forty-two males were used for behavioral testing. Statistical analyses of all forty-two birds’ behavior (i.e. nestbox ownership, song, other courtship behaviors, and aggression) have been published previously [34]. No behaviors other than song and nestbox status appeared to relate to numbers of ZENK-labeled cells in the brain regions presently examined.
Of the males included in the ZENK ICC, six T -implanted males were observed entering and exiting nestboxes and were categorized as “breeding condition with nestbox (BCbox).” The remaining ten T-implanted males not observed entering and exiting nestboxes were categorized as “breeding condition without nestbox (BCno box).” All eighteen non-breeding males were categorized as “non-breeding condition (NBC).”
ZENK Immunolabeling and Behavior
Of the twelve nuclei examined in the present analysis, simple linear regression indicated no significant relationships or trends for relationships between numbers of ZENK-labeled cells in Area X, RA, POM, MS, AH, TnA, GCt, and VTA and singing behavior in either a breeding or a non-breeding context. Additionally, for these twelve nuclei independent Student’s t-tests revealed no significant effects or trends toward effects of nestbox status on the numbers of ZENK-labeled cells in Area X, HVC, RA, MS, AH, VMH, and TnA. Details of these analyses will not be discussed further. Simple linear regression analyses also indicated no significant relationships between numbers of ZENK-labeled cells in any of the brain regions examined and the order in which birds were sacrificed.
Outliers
For one BCno box male, the number of point samples with song/mean number of ZENK-labeled cells value for three brain regions (HVC, LSc.v, and LSc.vl) was found to be ±2 standard deviations from the mean (arcsine all song=0, log HVC ZENK=4.80, log LSc.v ZENK=1.47, log LSc.vl ZENK=2.04). Additionally, for one NBC male the song/cell count value for one brain region (LSc.vl) was also ±2 standard deviations from the mean (arcsine all song=1.33, log LSc.vl ZENK=2.14). These four data points are statistical outliers and were excluded from simple linear regression analyses. Thus, p-values and R2-values for HVC, LSc.v, and LSc.vl presented below are derived from a reduced data set.
ZENK and singing behavior
Simple linear regression indicated significant linear relationships between song and numbers of ZENK-labeled cells in HVC, LSc.v, LSc.vl, BSTm(v), and VMH. Singing behavior by both breeding and non-breeding males related positively to numbers of ZENK-labeled cells in HVC (Fig. 2A. Breeding: R2=0.81, p=0.0003; Non-breeding: R2=0.27, p=0.03). No relationship between numbers of ZENK-labeled cells in LSr and song in either context was observed (Fig. 2B. Breeding: R2=0.03, p=0.51; Non-breeding: R2=0.17, p=0.13). However, numbers of ZENK-labeled cells in LSc.v were negatively related to breeding context song and positively related to non-breeding context song (Fig. 2C and 3A. Breeding: R2=0.37, p=0.02; Non-breeding: R2=0.34, p=0.02). Numbers of ZENK-labeled cells in LSc.vl were also negatively related to breeding context song, but no significant relationship between ZENK in LSc.vl and non-breeding context song was observed (Fig. 2D. Breeding: R2=0.55, p=0.002; Non-breeding: R2=0.25, p=0.07). No relationship between ZENK-labeled cells in BSTm(d) and song in either context was observed (Fig. 2E. Breeding: R2=0.03, p=0.52; Non-breeding: R2=0.03, p=0.56). However, numbers of ZENK-labeled cells in BSTm(v) were positively related to non-breeding, but not breeding, context song (Fig. 2F and 3B. Breeding: R2=0.08, p=0.32; Non-breeding: R2=0.34, p=0.02). The numbers of ZENK-labeled cells in VMH were positively related to breeding, but not non-breeding, context song (Fig. 2G and 3C. Breeding: R2=0.36, p=0.02; Non-breeding: R2=0.22, p=0.07).
Fig. 2.
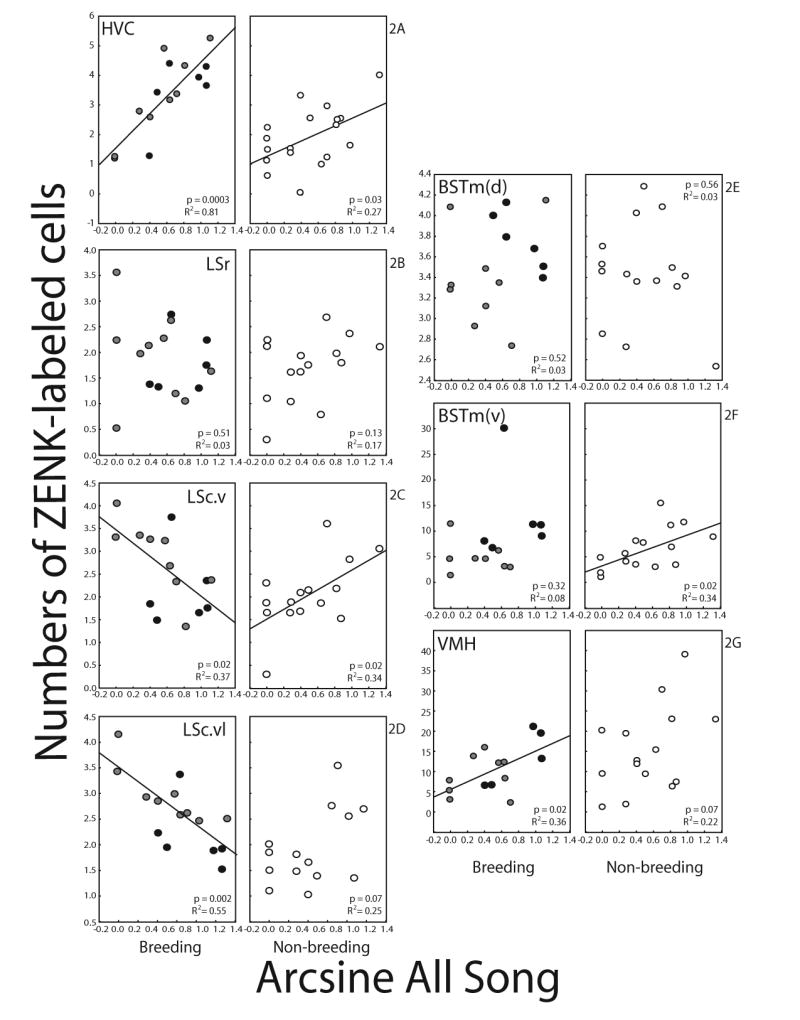
Plots showing the relationships between song production and mean number of ZENK-labeled cells in (A) HVC, (B) LSr, (C) LSc.v, (D) LSc.vl, (E) BSTm(d), (F) BSTm(v), and (G) VMH in breeding and non-breeding condition males. Each point represents one individual. Black dots are breeding condition males with nestboxes; gray dots, breeding condition males without nestboxes; white dots, non-breeding condition males. Presence of regression line indicates significant (p<0.05) linear relationships.
Fig. 3.
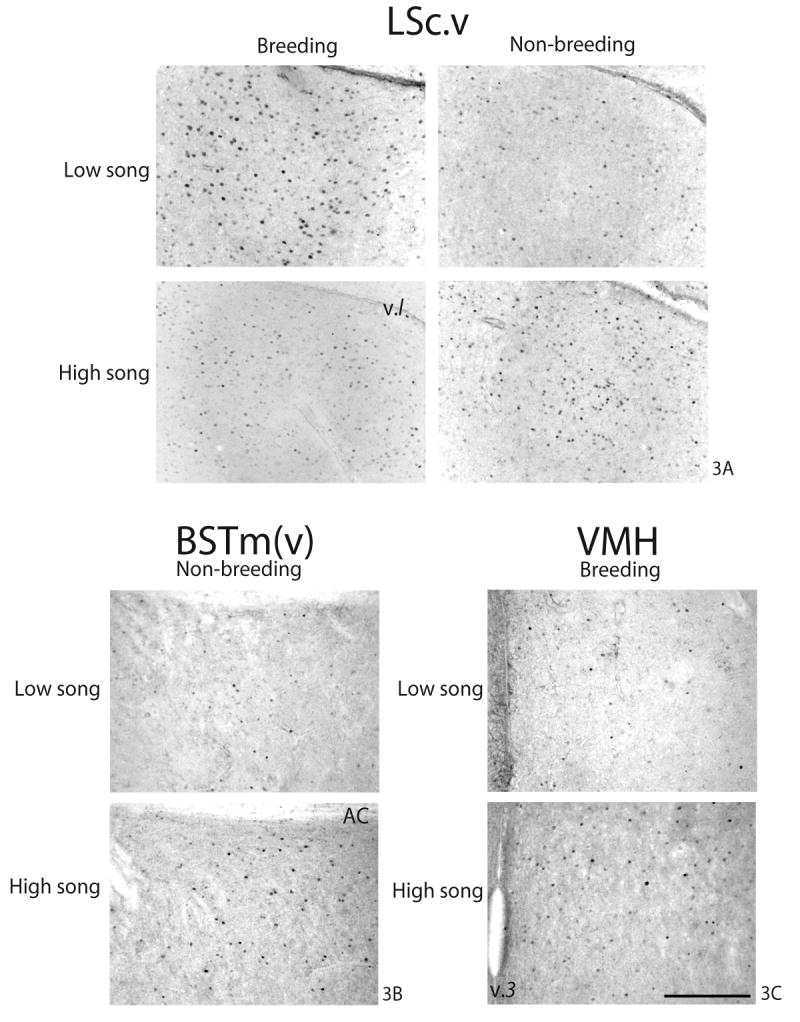
Photomicrographs of significant ZENK immunolabeling findings observed within (A) LSc.v, (B) BSTm(v), and (C) VMH in breeding and non-breeding condition males observed singing at low and high rates. v.l, lateral ventricle; AC, anterior commissure; v.3, third ventricle. Scale bar in bottom of 3C = approximately 0.2mm.
ZENK and nestbox status
Independent Student’s t-tests indicated that in POM (Fig. 4A. t13=4.15, p=0.001), LSc.vl (Fig. 4B. t13=2.28, p=0.04), BSTm(v) (Fig. 4C. t12=2.42, p=0.03), GCt (Fig. 4D. t14=2.97, p=0.01), and VTA (Fig. 4E. t14=3.82, p=0.002) there was a significant difference in the mean numbers of ZENK-labeled cells for BCbox and BCno box males. In POM, BSTm(v), GCT, and VTA, BCbox males had significantly greater numbers of ZENK-labeled cells than BCno box males. In contrast, in LSc.vl BCbox males had significantly fewer numbers of ZENK-labeled than BCno box males.
Fig. 4.
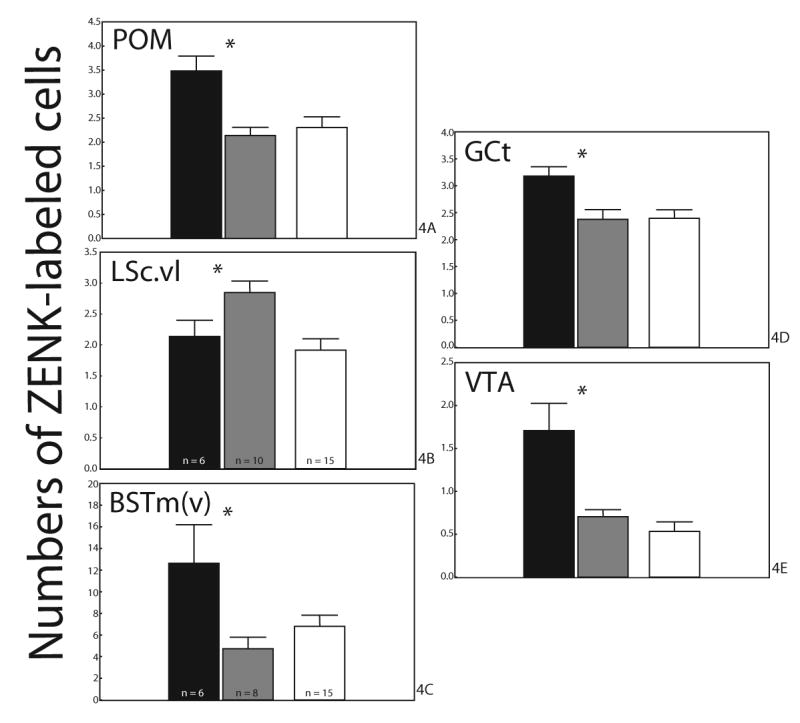
Mean numbers of ZENK-labeled cells counted in (A) POM, (B) LSc.vl, (C) BSTm(v), (D) GCt, and (E) VTA. Black bars are breeding condition males with nestboxes; gray bars, breeding condition males without nestboxes; white bars, non-breeding condition males. Asterisk indicates significant differences between breeding condition males with nestboxes and those without nestboxes (p<0.05; Student’s t-tests). Data from non-breeding condition males were not statistically compared to breeding condition males due to methodological differences.
DISCUSSION
The results presented here reveal novel patterns of neural activity associated with song and add to a growing body of research indicating that the songbird brain differentially regulates song production depending upon the context in which it occurs. Furthermore, these data support the hypothesis that nuclei outside of the song control system modulate singing behavior, and suggest that these regions might be important for adjusting song production so that it occurs in the appropriate context in response to appropriate social and environmental stimuli.
ZENK and the song control system
The finding that the numbers of ZENK-labeled cells in HVC related positively to both breeding and non-breeding context song is consistent with the established role for this region in the regulation of song production [52]. Although the present study did not reveal context-dependent differences in activity within HVC, Area X, or RA associated with song, past work in zebra finches, house sparrows, and starlings has implicated these regions in the context-dependent regulation of singing behavior [34,35,37,58]. Differences between past work and the present study may relate to differences in the markers of neuronal activity used, species differences, or differences in the specific contexts in which song was produced [see 34 for an in-depth discussion]. Overall, the present data do not rule out a role for HVC, Area X, or RA in the context-dependent regulation of song. Rather, these data indicate that in the contexts examined here activity within a subset of neurons within HVC (i.e., neurons expressing the protein products of ZENK) is associated with male starling song across contexts.
ZENK and brain regions outside the song system
In contrast to HVC, the numbers of ZENK-labeled cells in LSc.v were found to be positively related to singing behavior in a non-breeding context but negatively related to singing behavior in a breeding context. A similar pattern for LSc.vl was observed, but the positive relationship between ZENK in this subregion and non-breeding song failed to reach significance. No relationship between ZENK in LSr and song in either context was found. Taken together, it appears that septal modulation of starling song production is strongly influenced by the context in which it occurs. Data from lesion studies and pharmacological manipulations support this idea. Manipulations of septal arginine vasotocin (AVT) and vasoactive intestinal polypeptide (VIP) differentially influence agonistic versus multipurpose song types [23]. Thus, the opposite relationships between ZENK-labeling in LS and sexually versus broadly socially motivated song types might reflect context-dependent modulation of singing behavior by specific neurotransmitter systems such as AVT or VIP. Lesions to LS have been shown to increase song production by territorial species but decrease song production by gregarious species [25]. This suggests that LS activity inhibits territorial song but facilitates gregarious song. This interpretation is supported by the opposite patterns of ZENK-labeling in LSc.v observed in this study. In starlings, breeding context males compete over a limited number of appropriate nest sites and defend nest sites against intruding males. In contrast, non-breeding context males are extremely social, feeding and roosting in large, mixed-sex flocks [16,20,30,40]. We found a high level of caudal LS activity to be associated with low levels of territorial (i.e. breeding context) song but high levels of gregarious (i.e. non-breeding context) song. Thus, the data presented here support the emerging idea that zones within LS are functionally distinct and that septal modulation of singing behavior can be influenced by social factors [23,25–27,45]. Taken together with past work, it appears that LS may play an important, but complex, role in the regulation of singing behavior and that social, physiological, and environmental factors may significantly influence how the LS modulates song production.
The numbers of ZENK-labeled cells in BSTm(v) related positively only to song produced in a non-breeding context. Studies in mammals and birds implicate the BST and LS in dominance, aggression, and social signaling used to maintain dominance status [7,9,15,36,38,45,65]. In European starlings, it has been suggested that song produced outside breeding context is used to establish and/or maintain social hierarchies within the large, overwintering flock [64]. Thus, positive linear relationships between ZENK in BSTm(v) and LSc.v and non-breeding context song might reflect a conserved role for the BST and LS in the regulation of social signaling and dominance relationships across vertebrates.
Interestingly, the pattern we report here for BSTm(v) differs from a previous study that described a positive linear relationship between Fos in BSTm and only sexually motivated song [33]. Thus, taking the ZENK and Fos studies together it appears that the BST regulates both breeding and non-breeding context singing behavior, but that there are fundamental differences in how this nucleus modulates song production depending upon what motivates song behavior. Interestingly, no relationship between ZENK in BSTm(d) and song in either breeding context was found suggesting that subdivisions within BSTm may be functionally distinct.
The numbers of ZENK-labeled cells in VMH related positively to song produced within, but not outside of, a breeding context. These data are comparable to past work in starlings describing a positive linear relationship between Fos in VMH and breeding context song [33]. Interestingly, a similar, but non-significant, pattern was observed between numbers of ZENK-labeled cells in VMH and non-breeding context song. This indicates that VMH may be important to the regulation of male song production independent of the context in which it occurs. Although the role of VMH in non-breeding context song must be examined further, the present data suggest that VMH may be involved in the regulation of sexually motivated singing behavior by male starlings. VMH is classically known to be involved in the regulation of female sexual behaviors [e.g. 12,53], but the present data add to a growing body of evidence suggesting the VMH also plays a role in male reproductive behaviors [21,47,60], including male courtship behavior [8,22].
Our previous studies have shown positive linear relationships between Fos in POM, AH, and VTA and only breeding context song [33,34] but the present data using ZENK do not implicate these nuclei in the regulation of singing behavior in any context. Thus, the protein products of ZENK reported here and our past data using Fos [33,34] reveal distinct patterns of neural activity associated with song, illustrating that the use of two indirect markers of neural activity reveals a better overall picture of brain regions involved in context-dependent regulation of song compared to either alone.
ZENK and c-fos are immediate early genes that are expressed transiently after resting cells are stimulated by extracellular signals such as neurotransmitters. Their protein products are transcription factors that can promote or inhibit the expression of target genes [62]. The downstream targets of ZENK and c-fos are not known at this time but may include those coding for neurotransmitter synthetic enzymes, receptors, or receptor-associated proteins. Thus, different patterns revealed by ZENK versus Fos labeling in POM, BSTm, AH, and VTA may reflect different neurotransmitter systems acting in these regions to modulate sexually motivated versus broadly socially motivated singing behavior.
ZENK labeling and nestbox status
BCbox males had significantly higher numbers of ZENK-labeled cells within POM, BSTm(v), GCt, and VTA and significantly lower numbers of ZENK-labeled cells within LSc.vl than BCno box males. It is doubtful that this pattern is due to differences in endocrine physiology as both BCbox and BCno box males were implanted with T. Instead, these patterns may reflect differences in the sexually motivated and agonistic behavior typically observed in males in these two groups.
BCbox males sang the most and represent the majority of birds displaying other courtship behaviors including wing-waving and nest-material gathering [see 34 for details]. POM, BST, GCT, and VTA are known for their roles in regulating motivated behaviors, including sexually motivated behavior and communication [e.g. 39,43,44,59], and the present data support a role for these nuclei in the regulation of sexually motivated, nestbox-associated behaviors in male songbirds. BCbox males are also typically the dominant individuals within a population [61] and they actively defend nest sites and the area immediately surrounding the nest site entrance from intruders [20,40]. Thus, BCbox males have a higher propensity towards engaging in agonistic encounters with other males. We found BCbox males to have significantly lower numbers of ZENK-labeled cells in LSc.vl than BCno box males. This pattern is consistent with recent work showing agonistic behavior is negatively correlated with IEG-immunoreactivity (IEG-ir) within several zones of the LS (i.e. high levels of aggression were found to be associated with low levels of IEG-ir) [28]. Taken together with our regression data, it appears that the LS may be central to mediating multiple forms of songbird social behavior including vocal communication possibly associated with dominance status.
The mean number of ZENK-labeled cells for NBC males was not compared statistically to either BCbox or BCno box males because of differences in the methods used for behavioral testing (i.e. latency from time of capture to behavioral testing and properties of stimulus females). However qualitatively, the numbers of ZENK-labeled cells in POM, BSTm(v), GCt, and VTA of NBC males were similar to BCno box males. We find this pattern to be particularly interesting given that NBC males had low T and were exposed to a non-breeding condition female and BCno box males had high T are were exposed to a breeding condition female. This qualitative pattern is similar to past work from our lab indicating that POM volume is similar in BCno box and NBC males [57], and should be examined under more controlled conditions in future work. Taken together it appears that nestbox possession (but not endocrine physiology or exposure to a receptive female) may upregulate activity in specific brain regions important to regulating sexually motivated behaviors.
The social behavior network
LS, BST, POM, VMH, VTA and GCt are components of a proposed vertebrate ‘social behavior network’ [50]. These regions are interconnected, steroid hormone sensitive, and are reported to control multiple forms of social behavior including sexual behavior, communication and aggression [see 24 for review]. The data we present here suggest that nodes within the ‘social behavior network’ may be central to the regulation of singing behavior in songbirds.
Tract tracing studies illustrate several indirect routs through which brain regions of the ‘social behavior network’ might modulate activity within the song system. Our laboratory has published data showing the POM connects reciprocally with VTA, GCt, and the locus coeruleus (LoC) [54] and all three of these nuclei have been shown to project directly to the song control system [2,3]. Thus the interconnected nuclei of the ‘social behavior network’ are well positioned to modulate activity of song control nuclei. The present data, taken together with previous reports [33,34], suggest that role of LS, BSTm, POM, AH, VMH, and VTA in the regulation of male starling singing behavior can be breeding context-dependent. Thus, it seems plausible that these regions of the ‘social behavior network’ act in concert with song control nuclei to ensure that singing behavior occurs in the appropriate social context in response to appropriate social and environmental stimuli.
Acknowledgments
The data presented in this paper are based upon work supported by grants from NIMH (R01-MH 65645) to LVR and a graduate research fellowship from NSF to SAH. We gratefully acknowledge Jandra Morrow for assistance in starling capture; Kate Skogen, Jeff Alexander, Chris Elliot, John Irwin, and Martin Lund for animal care taking; Gina Bower, Nate Good, Cindi Kelm, Sarah Schlachet and Hollie Wingate for their help with tissue processing; and Billy Feeny for assistance with illustrations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alger SJ, Riters LV. in press [Google Scholar]
- 2.Appeltants D, Absil P, Balthazart J, Ball GF. Identification of the origin of catecholaminergic inputs to HVc in canaries by retrograde tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Chem Neuroanat. 2000;18:117–133. doi: 10.1016/s0891-0618(99)00054-x. [DOI] [PubMed] [Google Scholar]
- 3.Appeltants D, Ball GF, Balthazart J. The origin of catecholaminergic inputs to the song control nucleus RA in canaries. Neuroreport. 2002;13:649–653. doi: 10.1097/00001756-200204160-00023. [DOI] [PubMed] [Google Scholar]
- 4.Aste N, Balthazart J, Absil P, Grossmann R, Mulhbauer E, Viglietti-Panzica C, Panzica GC. Anatomical and neurochemical definition of the nucleus of the stria terminalis in Japanese quail (Coturnix japonica) J Comp Neurol. 1998;396:141–157. [PubMed] [Google Scholar]
- 5.Ball GF, Tlemcani O, Balthazart J. Induction of the Zenk protein after sexual interactions in male Japanese quail. Neuroreport. 1997;8:2965–2970. doi: 10.1097/00001756-199709080-00032. [DOI] [PubMed] [Google Scholar]
- 6.Ball GF, Wingfield JC. Changes in Plasma-Levels of Luteinizing-Hormone and Sex Steroid-Hormones in Relation to Multiple-Broodedness and Nest-Site Density in Male Starlings. Physiological Zoology. 1987;60:191–199. [Google Scholar]
- 7.Bamshad M, Albers HE. Neural circuitry controlling vasopressin-stimulated scent marking in Syrian hamsters (Mesocricetus auratus) J Comp Neurol. 1996;369:252–263. doi: 10.1002/(SICI)1096-9861(19960527)369:2<252::AID-CNE6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein PL, Zuo M, Cheng MF. Social condition affects the courtship behavior of male ring doves with posterior medial hypothalamic lesions. Behav Neural Biol. 1993;59:120–125. doi: 10.1016/0163-1047(93)90834-5. [DOI] [PubMed] [Google Scholar]
- 9.Bester-Meredith JK, Young LJ, Marler CA. Species differences in paternal behavior and aggression in peromyscus and their associations with vasopressin immunoreactivity and receptors. Horm Behav. 1999;36:25–38. doi: 10.1006/hbeh.1999.1522. [DOI] [PubMed] [Google Scholar]
- 10.Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- 11.Brenowitz EA, Margoliash D, Nordeen KW. An introduction to birdsong and the avian song system. J Neurobiol. 1997;33:495–500. [PubMed] [Google Scholar]
- 12.Coolen LM, Peters HJ, Veening JG. Fos immunoreactivity in the rat brain following consummatory elements of sexual behavior: a sex comparison. Brain Res. 1996;738:67–82. doi: 10.1016/0006-8993(96)00763-9. [DOI] [PubMed] [Google Scholar]
- 13.Cuthill I, Hindmarsh A. Increase in Starling Song Activity with Removal of Mate. Animal Behaviour. 1985;33:326–327. [Google Scholar]
- 14.Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. J Biol Rhythms. 2001;16:365–380. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- 15.Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav Evol. 2000;55:53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- 16.Eens M. Understanding the Complex Song of the European Starling: An Integrated Ethological Approach. Advances in the Study of Behavior. 1997;26:255–435. [Google Scholar]
- 17.Eens M, Pinxten R. Extra-Pair Courtship in the Starling Sturnus-Vulgaris. Ibis. 1990;132:618–619. [Google Scholar]
- 18.Eens M, Pinxten R, Verheyen RF. Male Song as a Cue for Mate Choice in the European Starling. Behaviour. 1991;116:210–238. [Google Scholar]
- 19.Eens M, Pinxten R, Verheyen RF. Variation in Singing Activity During the Breeding Cycle of the European Starling Sturnus-Vulgaris. Belgian Journal of Zoology. 1994;124:167–174. [Google Scholar]
- 20.Feare C. The starling. Oxford University Press, Oxford [Oxfordshire]; New York: 1984. x, 315, 316 of plates pp. [Google Scholar]
- 21.Fernandez-Guasti A, Swaab D, Rodriguez-Manzo G. Sexual behavior reduces hypothalamic androgen receptor immunoreactivity. Psychoneuroendocrinology. 2003;28:501–512. doi: 10.1016/s0306-4530(02)00036-7. [DOI] [PubMed] [Google Scholar]
- 22.Friedman D, Crews D. Role of the anterior hypothalamus-preoptic area in the regulation of courtship behavior in the male Canadian red-sided garter snake (Thamnophis sirtalis parietalis): intracranial implantation experiments. Horm Behav. 1985;19:122–136. doi: 10.1016/0018-506x(85)90013-3. [DOI] [PubMed] [Google Scholar]
- 23.Goodson JL. Territorial aggression and dawn song are modulated by septal vasotocin and vasoactive intestinal polypeptide in male field sparrows (Spizella pusilla) Horm Behav. 1998;34:67–77. doi: 10.1006/hbeh.1998.1467. [DOI] [PubMed] [Google Scholar]
- 24.Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm Behav. 2005;48:11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodson JL, Eibach R, Sakata J, Adkins-Regan E. Effect of septal lesions on male song and aggression in the colonial zebra finch (Taeniopygia guttata) and the territorial field sparrow (Spizella pusilla) Behav Brain Res. 1999;101:167–180. [PubMed] [Google Scholar]
- 26.Goodson JL, Evans AK, Lindberg L. Chemoarchitectonic subdivisions of the songbird septum and a comparative overview of septum chemical anatomy in jawed vertebrates. J Comp Neurol. 2004;473:293–314. doi: 10.1002/cne.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodson JL, Evans AK, Lindberg L, Allen CD. Neuro-evolutionary patterning of sociality. Proc R Soc B. 2005;272:227–235. doi: 10.1098/rspb.2004.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodson JL, Evans AK, Soma KK. Neural responses to aggressive challenge correlate with behavior in nonbreeding sparrows. Neuroreport. 2005;16:1719–1723. doi: 10.1097/01.wnr.0000183898.47160.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gwinner H, Van’t Hof T, Zeman M. Hormonal and behavioral responses of starlings during a confrontation with males or females at nest boxes during the reproductive season. Hormones and Behavior. 2002;42:21–31. doi: 10.1006/hbeh.2002.1795. [DOI] [PubMed] [Google Scholar]
- 30.Gwinner H, Van’t Hof T, Zeman M. Hormonal and behavioral responses of starlings during a confrontation with males or females at nest boxes during the reproductive season. Horm Behav. 2002;42:21–31. doi: 10.1006/hbeh.2002.1795. [DOI] [PubMed] [Google Scholar]
- 31.Hausberger M, Richardyris MA, Henry L, Lepage L, Schmidt I. Song Sharing Reflects the Social-Organization in a Captive Group of European Starlings (Sturnus-Vulgaris) Journal of Comparative Psychology. 1995;109:222–241. [Google Scholar]
- 32.Hauser MD, Konishi M. The design of animal communication. xi. MIT Press; Cambridge, Mass: 1999. p. 701. [Google Scholar]
- 33.Heimovics SA, Riters LV. Breeding context-dependent relationships between song and cFOS labeling within social behavior brain regions in male European starlings (Sturnus vulgaris) in press. doi: 10.1016/j.yhbeh.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heimovics SA, Riters LV. Immediate early gene activity in song control nuclei and brain areas regulating motivation relates positively to singing behavior during, but not outside of, a breeding context. J Neurobiol. 2005;65:207–224. doi: 10.1002/neu.20181. [DOI] [PubMed] [Google Scholar]
- 35.Hessler NA, Doupe AJ. Social context modulates singing-related neural activity in the songbird forebrain. Nat Neurosci. 1999;2:209–211. doi: 10.1038/6306. [DOI] [PubMed] [Google Scholar]
- 36.Irvin RW, Szot P, Dorsa DM, Potegal M, Ferris CF. Vasopressin in the septal area of the golden hamster controls scent marking and grooming. Physiol Behav. 1990;48:693–699. doi: 10.1016/0031-9384(90)90213-n. [DOI] [PubMed] [Google Scholar]
- 37.Jarvis ED, Scharff C, Grossman MR, Ramos JA, Nottebohm F. For whom the bird sings: context-dependent gene expression. Neuron. 1998;21:775–788. doi: 10.1016/s0896-6273(00)80594-2. [DOI] [PubMed] [Google Scholar]
- 38.Jasnow AM, Davis M, Huhman KL. Involvement of central amygdalar and bed nucleus of the stria terminalis corticotropin-releasing factor in behavioral responses to social defeat. Behav Neurosci. 2004;118:1052–1061. doi: 10.1037/0735-7044.118.5.1052. [DOI] [PubMed] [Google Scholar]
- 39.Jurgens U. The role of the periaqueductal grey in vocal behaviour. Behav Brain Res. 1994;62:107–117. doi: 10.1016/0166-4328(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 40.Kessel B. A study of the breeding biology of the European starling (Sturnus vulgaris L.) in North America. American Midland Nautralist. 1957;58:257–331. [Google Scholar]
- 41.Lehner PN. Handbook of ethological methods. xix. Cambridge University Press; Cambridge ; New York: 1996. p. 672. [Google Scholar]
- 42.Liu WC, Nottebohm F. Variable rate of singing and variable song duration are associated with high immediate early gene expression in two anterior forebrain song nuclei. Proc Natl Acad Sci U S A. 2005;102:10724–10729. doi: 10.1073/pnas.0504677102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu YC, Salamone JD, Sachs BD. Lesions in medial preoptic area and bed nucleus of stria terminalis: differential effects on copulatory behavior and noncontact erection in male rats. J Neurosci. 1997;17:5245–5253. doi: 10.1523/JNEUROSCI.17-13-05245.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maney DL, Ball GF. Fos-like immunoreactivity in catecholaminergic brain nuclei after territorial behavior in free-living song sparrows. J Neurobiol. 2003;56:163–170. doi: 10.1002/neu.10227. [DOI] [PubMed] [Google Scholar]
- 45.Maney DL, Erwin KL, Goode CT. Neuroendocrine correlates of behavioral polymorphism in white-throated sparrows. Horm Behav. 2005;48:196–206. doi: 10.1016/j.yhbeh.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Margoliash D. Functional organization of forebrain pathways for song production and perception. J Neurobiol. 1997;33:671–693. doi: 10.1002/(sici)1097-4695(19971105)33:5<671::aid-neu12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 47.McGinnis MY, Williams GW, Lumia AR. Inhibition of male sex behavior by androgen receptor blockade in preoptic area or hypothalamus, but not amygdala or septum. Physiol Behav. 1996;60:783–789. doi: 10.1016/0031-9384(96)00088-1. [DOI] [PubMed] [Google Scholar]
- 48.Meddle SL, King VM, Follett BK, Wingfield JC, Ramenofsky M, Foidart A, Balthazart J. Copulation activates Fos-like immunoreactivity in the male quail forebrain. Behav Brain Res. 1997;85:143–159. doi: 10.1016/s0166-4328(97)87581-x. [DOI] [PubMed] [Google Scholar]
- 49.Mello CV. Mapping vocal communication pathways in birds with inducible gene expression. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2002;188:943–959. doi: 10.1007/s00359-002-0347-1. [DOI] [PubMed] [Google Scholar]
- 50.Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–257. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 51.Nordeen KW, Nordeen EJ. Long-term maintenance of song in adult zebra finches is not affected by lesions of a forebrain region involved in song learning. Behav Neural Biol. 1993;59:79–82. doi: 10.1016/0163-1047(93)91215-9. [DOI] [PubMed] [Google Scholar]
- 52.Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- 53.Rand MS, Crews D. The bisexual brain: sex behavior differences and sex differences in parthenogenetic and sexual lizards. Brain Res. 1994;663:163–167. doi: 10.1016/0006-8993(94)90474-x. [DOI] [PubMed] [Google Scholar]
- 54.Riters LV, Alger SJ. Neuroanatomical evidence for indirect connections between the medial preoptic nucleus and the song control system: possible neural substrates for sexually motivated song. Cell Tissue Res. 2004;316:35–44. doi: 10.1007/s00441-003-0838-6. [DOI] [PubMed] [Google Scholar]
- 55.Riters LV, Ball GF. Lesions to the medial preoptic area affect singing in the male European starling (Sturnus vulgaris) Horm Behav. 1999;36:276–286. doi: 10.1006/hbeh.1999.1549. [DOI] [PubMed] [Google Scholar]
- 56.Riters LV, Eens M, Pinxten R, Ball GF. Seasonal changes in the densities of alpha(2)-noradrenergic receptors are inversely related to changes in testosterone and the volumes of song control nuclei in male European starlings. Journal of Comparative Neurology. 2002;444:63–74. doi: 10.1002/cne.10131. [DOI] [PubMed] [Google Scholar]
- 57.Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal changes in courtship song and the medial preoptic area in male European starlings (Sturnus vulgaris) Horm Behav. 2000;38:250–261. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- 58.Riters LV, Teague DP, Schroeder MB, Cummings SE. Vocal production in different social contexts relates to variation in immediate early gene immunoreactivity within and outside of the song control system. Behavioural Brain Research. 2004;155:307–318. doi: 10.1016/j.bbr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 59.Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- 60.Sakata JT, Gonzalez-Lima F, Gupta A, Crews D. Repeated interactions with females elevate metabolic capacity in the limbic system of male rats. Brain Res. 2002;936:27–37. doi: 10.1016/s0006-8993(02)02491-5. [DOI] [PubMed] [Google Scholar]
- 61.Sartor JJ, Ball GF. Social suppression of song is associated with a reduction in volume of a song-control nucleus in European starlings (Sturnus vulgaris) Behav Neurosci. 2005;119:233–244. doi: 10.1037/0735-7044.119.1.233. [DOI] [PubMed] [Google Scholar]
- 62.Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- 63.Sohrabji F, Nordeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biol. 1990;53:51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- 64.Summers RW, Westlake GE, Feare CJ. Differences in the Ages, Sexes and Physical Condition of Starlings Sturnus-Vulgaris at the Center and Periphery of Roosts. Ibis. 1987;129:96–102. [Google Scholar]
- 65.Wang Z. Species differences in the vasopressin-immunoreactive pathways in the bed nucleus of the stria terminalis and medial amygdaloid nucleus in prairie voles (Microtus ochrogaster) and meadow voles (Microtus pennsylvanicus) Behav Neurosci. 1995;109:305–311. doi: 10.1037//0735-7044.109.2.305. [DOI] [PubMed] [Google Scholar]
- 66.Wild JM. Neural pathways for the control of birdsong production. J Neurobiol. 1997;33:653–670. doi: 10.1002/(sici)1097-4695(19971105)33:5<653::aid-neu11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]


