Abstract
Inhibitors of poly(ADP-ribose)polymerase (PARP), a nuclear enzyme involved in regulating cell death and cellular responses to DNA repair, show considerable promise in the treatment of cancer both in monotherapy as well as in combination with chemotherapeutic agents and radiation. We have recently demonstrated that PARP inhibition with 3-aminobenzamide or PJ-34 reduced vascular endothelial growth factor (VEGF)-induced proliferation, migration and tube formation of human umbilical vein endothelial cells (HUVECs) in vitro. Here we show dose-dependent reduction of VEGF- and basic fibroblast growth factor (bFGF)-induced proliferation, migration and tube formation of HUVECs in vitro by two potent PARP inhibitors 5-aminoisoquinolinone-hydrochloride (5-AIQ) and 1,5-isoquinolinediol (IQD). Moreover, PARP inhibitors prevented the sprouting of rat aortic ring explants in an ex vivo assay of angiogenesis. These results establish the novel concept that PARP inhibitors have antiangiogenetic effects, which may have tremendous clinical implications for the treatment of various cancers, tumor metastases and certain retinopathies.
Keywords: Poly(ADP-ribose)polymerase (PARP); angiogenesis; proliferation; migration; tube formation; aortic ring assay; HUVEC; 5-aminoisoquinolinone-hydrochloride (5-AIQ); 1,5-isoquinolinediol (IQD); cancer
Introduction
Poly(ADP-ribose) polymerase (PARP) is a family of nuclear enzymes of eukaryotic cells. PARP plays an important role in regulating DNA repair, gene transcription, cell cycle progression, chromatin function, genomic stability and cell death [1; 2; 3].
In preclinical testing, PARP inhibitors have demonstrated the ability to enhance the efficacy of various chemotherapeutic agents (e.g. methylating agents, DNA topoisomerase inhibitors, cisplatin), as well as radiation, against a broad spectrum of tumors (e.g. glioma, melanoma, lymphoma, colorectal cancer, head and neck tumors; [4; 5; 6; 7; 8; 9; 10; 11; 12; 13; 14; 15; 16; 17; 18; 19; 20]), and were also reported to be protective against untoward effects exerted by certain anticancer drugs [21; 22; 23]. Recently, PARP inhibitors are being considered as promising treatment for cancer both in monotherapy as well as in combination with radiation and chemotherapeutic agents in humans.
Angiogenesis, the process of new blood vessel formation, is crucial for the development and progression of pathophysiological changes associated with a variety of disorders, including various cancers, tumor metastases and retinopathies. We have recently demonstrated that PARP inhibition with 3-aminobenzamide or PJ-34 reduced VEGF-induced proliferation, migration and tube formation of human umbilical vein endothelial cells (HUVEC) in vitro [24]. Here we show that two potent (structurally distinct from 3-AB and PJ-34) PARP inhibitors 5-aminoisoquinolinone-hydrochloride (5-AIQ) or 1,5-isoquinolinediol (5-ISQ) dose-dependently reduce both VEGF- and bFGF-induced proliferation, migration and tube formation of HUVEC in vitro. Additionally, we demonstrate that PARP inhibitors prevent the sprouting of rat aortic ring explants. These results establish the concept that pharmacological inhibition of PARP decreases angiogenesis, and predicts unexpected additional benefits of PARP inhibitors in various cancers and retinopathies.
Materials and Methods
Reagents
Human recombinant VEGF165 was purchased from R&D systems (Minneapolis, MN). Human recombinant basic fibroblast growth factor (bFGF) was from Invitrogen (CA, USA). PARP inhibitors 5-aminoisoquinolinone-hydrochloride (5-AIQ) and 1,5-isoquinolinediol (IQD) were purchased from Calbiochem (San Diego, CA) and Sigma (St. Louis, MO), respectively.
Cell Culture
Human umbilical vein endothelial cells (HUVEC) and endothelial cells growth medium EGM™ were purchased from Cambrex, (Walkersville, MD) and cells were cultured in EGM™ medium. Cells were used within passages 3 to 7. Prior to treatment, cells were conditioned in endothelial basal medium containing 1% FBS (Invitrogen , CA) 6 hrs and then used for the experiments.
Proliferation assay
Endothelial cells (5 X 103) cells were suspended in 100 μl of growth factor free medium containing 1 % FBS and then treated with VEGF 20 ng/ml or FGF 20 ng/ml alone or with PARP inhibitors IQD (4–16 μM) or 5-AIQ (0.3–1.2 μM) and seeded on to 96 well plates and allowed to proliferate for 24–36 hrs. Then BrdU labeling solution was added and incubated further for 12 hrs. After the incubation, the effect of PARP inhibitors on VEGF/FGF induced proliferation of the HUVECs was determined by the extent of BrdU incorporation using ELISA kit following the protocol supplied by the manufacturer (Roche Diagnostics, IN). In brief, after the incubation of cells with BrdU labeling solution, the cells were fixed and incubated with anti-BrdU antibody. After washing, the cells were incubated with secondary antibody conjugated with horse radish peroxidase. Finally, the extent of BrdU incorporation was determined colorimetrically by measuring the absorbance at 450 nm. The treatments were performed in triplicate and the experiment was repeated at least three times, as described [24].
Migration assay
The migration assays were performed in a 24-well modified Boyden chamber as described earlier [24]. In brief, 8 μm cell culture inserts (BD Biosciences) coated with 0.2 % gelatin (Sigma) were placed over the bottom chamber containing 20 ng/ml VEGF/FGF as the chemo-attractant. Growth factor free medium containing 1% FBS served as negative control. 3X104 cells were suspended in 150 μl in growth factor free medium containing 1% FBS. Then the cells were pretreated with PARP inhibitors IQD or 5-AIQ, for 30 min at 37°C in 5% CO2 incubator. Then the cell suspension was added to the upper chamber. After 8 hrs of incubation at 37°C, the non-migrated cells on the upper surface of the filter were removed by gentle swabbing with cotton tipped applicators. The cells that had migrated to the lower side of the chamber were fixed with 100 % methanol for 15 min at room temperature. After complete drying, the cells were stained with 0.5 % Giemsa stain (Sigma). 3–4 fields per insert were counted using 10X objective using Olympus IX81 microscope. The assays were performed in triplicate.
In vitro angiogenesis assay
The effect of PARP inhibits on inhibiting the formation of tube-like structures on growth factor reduced Matrigel (BD Biosciences) were performed as described earlier [24; 25; 26]. In brief, 96 well plates were coated with 50 μl of growth factor reduced Matrigel and allowed to solidify at 37°C in 5% CO2. Cells treated with either VEGF, FGF alone or with IQD and 5-AIQ were carefully overlaid on to the solidified matrix. After 12 hrs of incubation at 37°C in 5% CO2, the medium was carefully aspirated and the cells were fixed with 4% formaldehyde and the images were captured using Olympus IX81 microscope. The tube length was quantified using the NIH Image J software. Results are represented as total tube length (μm) for three photographic fields per experimental condition. Each treatment was performed in duplicate and the set of experiment was independently repeated three times.
Ex vivo rat aortic ring assay
The angioinhibitory properties of PARP inhibitors were further conformed using the cultured aortic ring explants in three-dimensional matrix gel according to the protocol described by Nicosia and Ottinetti (1990) with slight modifications [27]. In brief, thoracic aortas were dissected from 16-week old male Sprague-Dawley rats. Aortas were immediately transferred to the culture dish filled with cold and sterile serum-free Endothelial Cell Basal Medium 2 (EBM-2, Cambrex, USA) containing penicillin 100 IU/mL and streptomycin 100 μg/mL (Gibco, USA). The peri-aortic fibro-adipose tissue was carefully removed with fine micro dissecting forceps and iridectomy scissors, taking special precaution not to damage the aortic wall. The aorta was cut in to 1.0 mm rings and rinsed several times with EBM-2. Washed aortic rings were then embedded between two 125 μL layers of Growth Factor Reduced Matrigel (BD Biosciences, USA) in 48 well tissue culture plates. The matrigel was overlaid with EBM-2 containing penicillin 100 IU/mL, streptomycin 100 μg/mL, 2% FBS and 2 % FBS (Invitrogen, CA). In certain experiments, the rings were incubated with either EBM-2 supplemented with VEGF/FGF (20 ng/ml) alone or with VEGF/FGF + IQD (16 μM) or 5-AIQ (1.2 μM), respectively. The cultures were incubated at 37ºC in a 5% CO2 incubator for 8 days. The medium was replaced every 48 hrs. At the end of 8th day, the sprouting of endothelial cells differentiating into tube-like structures was documented using phase-contrast microscope (Olympus IX80). Each experiment was performed in triplicate. The sprout length was quantified using NIH image software.
Statistical analysis
Values are represented as mean ± SD. The statistical significance of the data was analyzed using one way ANOVA or Student’s t test. P < 0.05 was considered as significant.
Results
PARP inhibitors IQD and 5-AIQ inhibitors VEGF and FGF induced proliferation of HUVECs
As shown in Fig.1 A&B, VEGF and FGF induced the proliferation of HUVECs by ~ 4.5 and 5.5 fold respectively (P < 0.00001 vs. control). On the other hand when HUVECs were pretreated with IQD (Fig. 1A) or 5-AIQ (Fig. 1B), elicited dose dependent inhibition in both VEGF and FGF stimulated cells. Also at the indicated concentrations both IQD and 5-AIQ did not found to have cytotoxic effects on HUVECs (data not shown).
Fig.1. PARP inhibitors decrease VEGF- and FGF-induced proliferation of HUVECs.
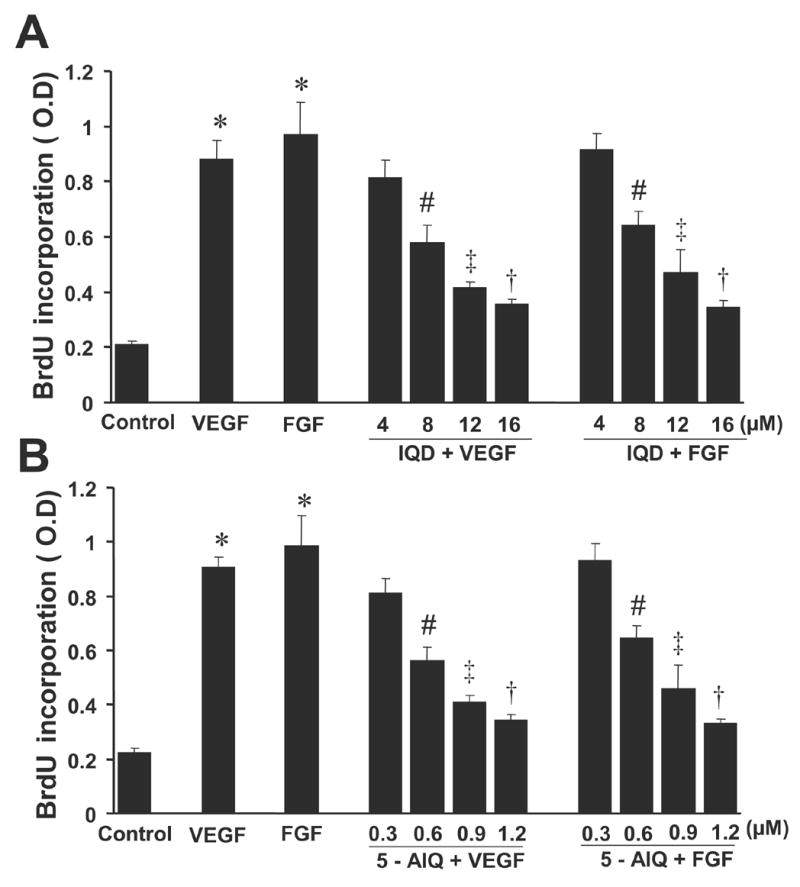
(A) IQD decreases VEGF- and FGF-induced proliferation of HUVECs.
Cells were treated with VEGF or FGF 20 ng/ml alone or with VEGF/FGF + IQD as indicated and the proliferation of HUVECs was determined by measuring the amount of BrdU incorporated using ELISA kit colorimetrically at 450 nm.
(B) 5-AIQ decreases VEGF- and FGF-induced proliferation of HUVECs.
* P < 0.00001 vs. control; # P < 0.05 vs. VEGF or FGF; ‡ P < 0.01 vs. VEGF or FGF; † P< 0.001 vs. VEGF or FGF, n = 3.
PARP inhibitors decrease VEGF- and FGF-induced migration of HUVECs
Both VEGF and FGF when used at 20 ng/ml enhanced the migration of HUVECs by ~ 3.5 fold ( P <0.00001 vs. control) Fig. 2A–C. When HUVECs were pretreated with increasing concentrations of IQD or 5-AIQ (Fig. 2), both these inhibitors were found to decrease the migration of HUVECs in concentration dependent fashion. IQD or 5-AIQ by itself had no effect on the migration of HUVECs and the results were comparable to that of vehicle control (data not shown).
Fig.2. PARP inhibitors attenuate VEGF- and FGF-stimulated migration of HUVECs.
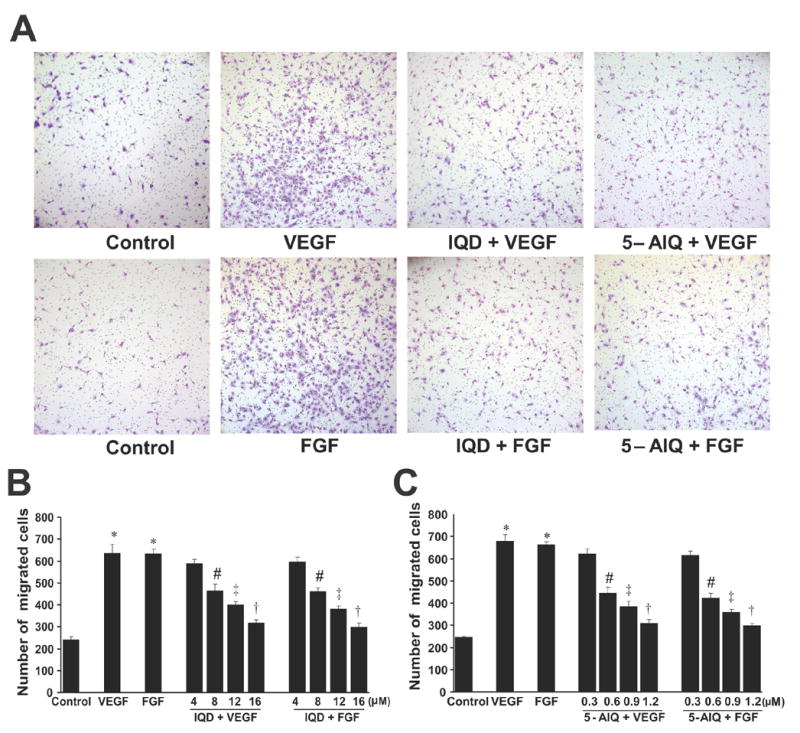
(A) Representative fields of cell culture inserts containing HUVECs migrated in response to the treatments as indicated.
(B) Quantitative data on the effect of IQD as indicated.
(C) Quantitative data on the effect of 5-AIQ as indicated.
* P < 0.00001 vs. control; # P < 0.05 vs.VEGF or FGF; ‡ P < 0.01 vs. VEGF or FGF; † P< 0.001 vs. VEGF or FGF, n = 3.
PARP inhibitors attenuate VEGF- and FGF-induced tube formation of HUVECs
As shown in Fig. 3 A, VEGF and FGF significantly enhanced the tube formation of HUVECs by 2.3 fold over control (P < 0.00001 ) when plated in growth-factor reduced matrigel. Pre-treatment of HUVECs with IQD or 5-AIQ dose-dependently inhibited both VEGF- and FGF-induced tube formations (Figure 3A–C). IQD or 5-AIQ by itself had no effect on the tube formation and the results were comparable to that of vehicle control (data not shown).
Fig.3. PARP inhibitors attenuate VEGF- and FGF-induced tube formation of HUVECs.
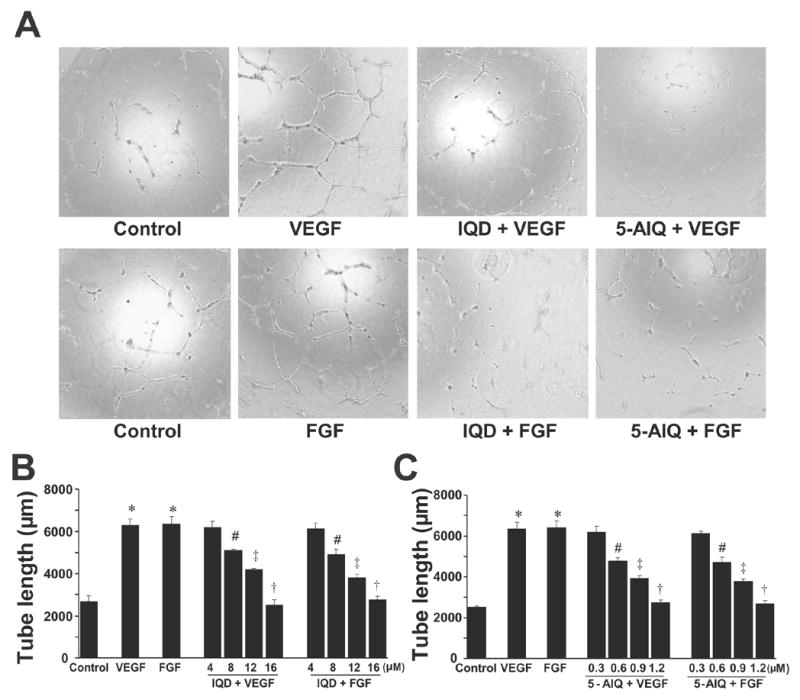
HUVECs were treated with either VEGF/FGF or PARP inhibitors +VEGF/FGF and in vitro angiogenesis assays were performed using growth factor reduced Matrigel as described in the Methods section.
(A) Representative images of the tube formation of HUVECs when treated with treatments as indicated.
(B) Quantitative data on the effect of IQD on the tube formation of HUVECs.
(C) Quantitative data on the effect of 5-AIQ on the tube formation of HUVECs.
* P < 0.00001 vs. control; # P < 0.05 vs. VEGF or FGF; ‡ P < 0.01 vs. VEGF or FGF; † P< 0.001 vs. VEGF or FGF, n = 3.
PARP inhibitors inhibit angiogenesis by preventing the sprouting of rat aortic ring explants
As shown in Fig. 4 VEGF and FGF at 20 ng/ml markedly stimulated the sprouting of rat aortic rings (P < 0.00001 vs. control), which was largely reduced by both IQD (16 μM) or 5-AIQ (1.2 μM).
Fig.4. PARP inhibitors abrogate sprouting of rat aortic rings.
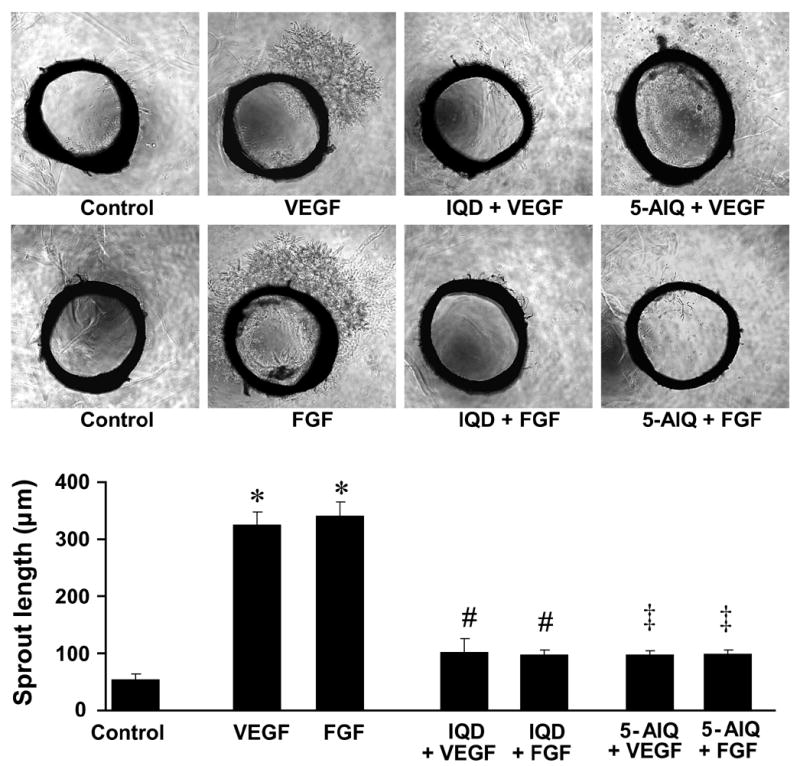
Representative images of rat aortic ring sprouting in response to treatments as indicated in the figure (above) and quantitative data on the sprout lengths (below).
* P < 0.00001 vs. control; # P < 0.05 vs. VEGF or FGF; ‡ P < 0.01 vs. VEGF or FGF; † P< 0.001 vs. VEGF or FGF, n = 3.
Discussion
Inhibitors of PARP show considerable promise in the treatment of cancer both in monotherapy as well as in combination with chemotherapeutic agents and radiation [4; 5; 6; 7; 8; 9; 10; 11; 12; 13; 14; 17; 18; 19]. Targeting PARP may prevent tumor cells from repairing DNA themselves and developing drug resistance, which may make them more sensitive to various chemotherapeutic agents (e.g. methylating agents, DNA topoisomerase inhibitors, cisplatin), as well as radiation therapy. In preclinical testings, PARP inhibitors markedly enhance the effect of the above mentioned chemotherapeutic agents and radiation against a broad spectrum of tumors (e.g. melanoma, glioma, lymphoma, colorectal cancer, head and neck tumors; [4; 5; 6; 7; 8; 9; 10; 11; 12; 13; 14; 17; 18; 19]). Furthermore, PARP inhibitors were also reported to exert cytoprotective effects against cardiotoxicity of doxorubicin [22; 23] and nephrotoxicity of cisplatin [21]. The cytoprotective effects of PARP inhibitors involve inhibition of the pathological overactivation of PARP, and blockade of the cells from entering into necrosis via over-utilization of cellular NAD+ pools eventually leading to cellular energetic failure [1; 2].
Angiogenesis is crucial for the growth and metastasis formation of a variety of cancers, and pathologically increased angiogenesis also contributes to the development of complications (e.g. blindness) associated with various retinopathy (e.g. diabetic retinopathy). We have recently demonstrated that PARP inhibition with 3-aminobenzamide or PJ-34 reduced VEGF-induced proliferation, migration and tube formation of human umbilical vein endothelial cells in vitro [24]. In the present study we confirm these observations using two potent structurally distinct PARP inhibitors 5-aminoisoquinolinone-hydrochloride and 1,5-isoquinolinediol. Additionally, to strengthen the concept, we also demonstrate antiangiogenetic potential of PARP inhibitors against FGF-induced proliferation, migration and tube formation of human umbilical vein endothelial cells, and by using an ex vivo rat aortic ring assay of angiogenesis. Consistently with these results, minocycline (a semisynthetic tetracycline antimicrobial antibiotic), which has recently been described as a potent PARP inhibitor, [28; 29] also inhibits angiogenesis [30]. These results suggest that PARP inhibitors may be beneficial in decreasing angiogenesis-dependent growth of certain types of cancers and cancer metastases.
Additional benefits of antiangiogenetic effects of PARP inhibitors can also be expected in various retinopathies associated with increased angiogenesis (e.g. diabetic retinopathy), where over-activation of PARP was previously demonstrated to contribute to the pathophysiology [31; 32; 33].
Collectively, these results have established the concept that pharmacological inhibition of PARP decreases angiogenesis, and predicts additional benefits of PARP inhibitors in various cancers and retinopathies.
Acknowledgments
This study was supported by the Intramural Research Program of NIH/NIAAA (to P.P.). Dr. Pacher wants to dedicate this work to his beloved mother Iren.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 2.Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–40. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber V, Dantzer F, Ame JC, de Murcia G. Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol. 2006;7:517–28. doi: 10.1038/nrm1963. [DOI] [PubMed] [Google Scholar]
- 4.Delaney CA, Wang LZ, Kyle S, White AW, Calvert AH, Curtin NJ, Durkacz BW, Hostomsky Z, Newell DR. Potentiation of temozolomide and topotecan growth inhibition and cytotoxicity by novel poly(adenosine diphosphoribose) polymerase inhibitors in a panel of human tumor cell lines. Clin Cancer Res. 2000;6:2860–7. [PubMed] [Google Scholar]
- 5.Tentori L, Portarena I, Vernole P, De Fabritiis P, Madaio R, Balduzzi A, Roy R, Bonmassar E, Graziani G. Effects of single or split exposure of leukemic cells to temozolomide, combined with poly(ADP-ribose) polymerase inhibitors on cell growth, chromosomal aberrations and base excision repair components. Cancer Chemother Pharmacol. 2001;47:361–9. doi: 10.1007/s002800000248. [DOI] [PubMed] [Google Scholar]
- 6.Canan Koch SS, Thoresen LH, Tikhe JG, Maegley KA, Almassy RJ, Li J, Yu XH, Zook SE, Kumpf RA, Zhang C, Boritzki TJ, Mansour RN, Zhang KE, Ekker A, Calabrese CR, Curtin NJ, Kyle S, Thomas HD, Wang LZ, Calvert AH, Golding BT, Griffin RJ, Newell DR, Webber SE, Hostomsky Z. Novel tricyclic poly(ADP-ribose) polymerase-1 inhibitors with potent anticancer chemopotentiating activity: design, synthesis, and X-ray cocrystal structure. J Med Chem. 2002;45:4961–74. doi: 10.1021/jm020259n. [DOI] [PubMed] [Google Scholar]
- 7.Tentori L, Portarena I, Bonmassar E, Graziani G. Combined effects of adenovirus-mediated wild-type p53 transduction, temozolomide and poly (ADP-ribose) polymerase inhibitor in mismatch repair deficient and non-proliferating tumor cells. Cell Death Differ. 2001;8:457–69. doi: 10.1038/sj.cdd.4400832. [DOI] [PubMed] [Google Scholar]
- 8.Calabrese CR, Batey MA, Thomas HD, Durkacz BW, Wang LZ, Kyle S, Skalitzky D, Li J, Zhang C, Boritzki T, Maegley K, Calvert AH, Hostomsky Z, Newell DR, Curtin NJ. Identification of potent nontoxic poly(ADP-Ribose) polymerase-1 inhibitors: chemopotentiation and pharmacological studies. Clin Cancer Res. 2003;9:2711–8. [PubMed] [Google Scholar]
- 9.Calabrese CR, Almassy R, Barton S, Batey MA, Calvert AH, Canan-Koch S, Durkacz BW, Hostomsky Z, Kumpf RA, Kyle S, Li J, Maegley K, Newell DR, Notarianni E, Stratford IJ, Skalitzky D, Thomas HD, Wang LZ, Webber SE, Williams KJ, Curtin NJ. Anticancer chemosensitization and radiosensitization by the novel poly(ADP-ribose) polymerase-1 inhibitor AG14361. J Natl Cancer Inst. 2004;96:56–67. doi: 10.1093/jnci/djh005. [DOI] [PubMed] [Google Scholar]
- 10.Curtin NJ, Wang LZ, Yiakouvaki A, Kyle S, Arris CA, Canan-Koch S, Webber SE, Durkacz BW, Calvert HA, Hostomsky Z, Newell DR. Novel poly(ADP-ribose) polymerase-1 inhibitor, AG14361, restores sensitivity to temozolomide in mismatch repair-deficient cells. Clin Cancer Res. 2004;10:881–9. doi: 10.1158/1078-0432.ccr-1144-3. [DOI] [PubMed] [Google Scholar]
- 11.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ, Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–7. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 12.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I, Knights C, Martin NM, Jackson SP, Smith GC, Ashworth A. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 13.Graziani G, Battaini F, Zhang J. PARP-1 inhibition to treat cancer, ischemia, inflammation. Pharmacol Res. 2005;52:1–4. doi: 10.1016/j.phrs.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Graziani G, Szabo C. Clinical perspectives of PARP inhibitors. Pharmacol Res. 2005;52:109–18. doi: 10.1016/j.phrs.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Cheng CL, Johnson SP, Keir ST, Quinn JA, Ali-Osman F, Szabo C, Li H, Salzman AL, Dolan ME, Modrich P, Bigner DD, Friedman HS. Poly(ADP-ribose) polymerase-1 inhibition reverses temozolomide resistance in a DNA mismatch repair-deficient malignant glioma xenograft. Mol Cancer Ther. 2005;4:1364–8. doi: 10.1158/1535-7163.MCT-05-0128. [DOI] [PubMed] [Google Scholar]
- 16.Haince JF, Rouleau M, Hendzel MJ, Masson JY, Poirier GG. Targeting poly(ADP-ribosyl)ation: a promising approach in cancer therapy. Trends Mol Med. 2005;11:456–63. doi: 10.1016/j.molmed.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Tentori L, Leonetti C, Scarsella M, Muzi A, Mazzon E, Vergati M, Forini O, Lapidus R, Xu W, Dorio AS, Zhang J, Cuzzocrea S, Graziani G. Inhibition of poly(ADP-ribose) polymerase prevents irinotecan-induced intestinal damage and enhances irinotecan/temozolomide efficacy against colon carcinoma. Faseb J. 2006;20:1709–11. doi: 10.1096/fj.06-5916fje. [DOI] [PubMed] [Google Scholar]
- 18.De Soto JA, Wang X, Tominaga Y, Wang RH, Cao L, Qiao W, Li C, Xu X, Skoumbourdis AP, Prindiville SA, Thomas CJ, Deng CX. The Inhibition and Treatment of Breast Cancer with Poly (ADP-ribose) Polymerase (PARP-1) Inhibitors. Int J Biol Sci. 2006;2:179–85. doi: 10.7150/ijbs.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Soto JA, Deng CX. PARP-1 inhibitors: are they the long-sought genetically specific drugs for BRCA1/2-associated breast cancers? Int J Med Sci. 2006;3:117–23. doi: 10.7150/ijms.3.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seimiya H. The telomeric PARP, tankyrases, as targets for cancer therapy. Br J Cancer. 2006;94:341–5. doi: 10.1038/sj.bjc.6602951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Racz I, Tory K, Gallyas F, Jr, Berente Z, Osz E, Jaszlits L, Bernath S, Sumegi B, Rabloczky G, Literati-Nagy P. BGP-15 - a novel poly(ADP-ribose) polymerase inhibitor - protects against nephrotoxicity of cisplatin without compromising its antitumor activity. Biochem Pharmacol. 2002;63:1099–111. doi: 10.1016/s0006-2952(01)00935-2. [DOI] [PubMed] [Google Scholar]
- 22.Pacher P, Liaudet L, Bai P, Virag L, Mabley JG, Hasko G, Szabo C. Activation of poly(ADP-ribose) polymerase contributes to development of doxorubicin-induced heart failure. J Pharmacol Exp Ther. 2002;300:862–7. doi: 10.1124/jpet.300.3.862. [DOI] [PubMed] [Google Scholar]
- 23.Pacher P, Liaudet L, Mabley JG, Cziraki A, Hasko G, Szabo C. Beneficial effects of a novel ultrapotent poly(ADP-ribose) polymerase inhibitor in murine models of heart failure. Int J Mol Med. 2006;17:369–75. [PMC free article] [PubMed] [Google Scholar]
- 24.Rajesh M, Mukhopadhyay P, Batkai S, Godlewski G, Hasko G, Liaudet L, Pacher P. Pharmacological inhibition of poly(ADP-ribose) polymerase inhibits angiogenesis. BBRC. 2006 doi: 10.1016/j.bbrc.2006.09.049. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cattaneo MG, Pola S, Francescato P, Chillemi F, Vicentini LM. Human endostatin-derived synthetic peptides possess potent antiangiogenic properties in vitro and in vivo. Exp Cell Res. 2003;283:230–6. doi: 10.1016/s0014-4827(02)00057-5. [DOI] [PubMed] [Google Scholar]
- 26.Rajesh M, Kolmakova A, Chatterjee S. Novel role of lactosylceramide in vascular endothelial growth factor-mediated angiogenesis in human endothelial cells. Circ Res. 2005;97:796–804. doi: 10.1161/01.RES.0000185327.45463.A8. [DOI] [PubMed] [Google Scholar]
- 27.Nicosia RF, Ottinetti A. Growth of microvessels in serum-free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Lab Invest. 1990;63:115–22. [PubMed] [Google Scholar]
- 28.Alano CC, Kauppinen TM, Valls AV, Swanson RA. Minocycline inhibits poly(ADP-ribose) polymerase-1 at nanomolar concentrations. Proc Natl Acad Sci U S A. 2006;103:9685–90. doi: 10.1073/pnas.0600554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szabo C, Pacher P, Swanson A. Novel modulators of poly(ADP-ribose) polymerase. Trends in Pharmacological Sciences. 2006 doi: 10.1016/j.tips.2006.10.003. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamargo RJ, Bok RA, Brem H. Angiogenesis inhibition by minocycline. Cancer Res. 1991;51:672–5. [PubMed] [Google Scholar]
- 31.Zheng L, Szabo C, Kern TS. Poly(ADP-ribose) polymerase is involved in the development of diabetic retinopathy via regulation of nuclear factor-kappaB. Diabetes. 2004;53:2960–7. doi: 10.2337/diabetes.53.11.2960. [DOI] [PubMed] [Google Scholar]
- 32.Obrosova IG, Minchenko AG, Frank RN, Seigel GM, Zsengeller Z, Pacher P, Stevens MJ, Szabo C. Poly(ADP-ribose) polymerase inhibitors counteract diabetes- and hypoxia-induced retinal vascular endothelial growth factor overexpression. Int J Mol Med. 2004;14:55–64. [PubMed] [Google Scholar]
- 33.Pacher P, Szabo C. Role of poly(ADP-ribose) polymerase-1 activation in the pathogenesis of diabetic complications: endothelial dysfunction, as a common underlying theme. Antioxid Redox Signal. 2005;7:1568–80. doi: 10.1089/ars.2005.7.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]


