Abstract
Granulocyte-colony stimulating factor (G-CSF) is an endogenous peptide hormone of the hematopoietic system that has entered Phase I/II clinical trials for treatment of ischemic stroke. Severe intraoperative hypotension can lead to global cerebral ischemia and apoptotic neuron loss within the hippocampus. We tested G-CSF in a rat model of global cerebral ischemia. Global cerebral ischemia was induced in male Sprague-Dawley rats (280–330g) with the 2-vessel occlusion model (hemorrhagic hypotension to a mean arterial pressure of 30–35 mmHg and bilateral common carotid artery occlusion for 8 minutes). Three groups of animals were used: global ischemia without treatment (GI, n=49), global ischemia with G-CSF treatment (GI+G-CSF, n=42), and sham surgery (Sham, n = 26). Rats in the treatment group received G-CSF (50 μg / kg, subcutaneously) 12 hours before surgery, on the day of surgery, and on post-operative Day 1, and were euthanized on Day 2, 3, and 14. Mild hyperglycemia was observed in all groups. T-maze testing for spontaneous alternation demonstrated initial improvement in the G-CSF treatment group but no long-term benefit. Measurement of daily body weight demonstrated an initial trend toward improvement in the G-CSF group. Quantitative Nissl histology of the hippocampus demonstrated equivalent outcomes on Day 3 and 14, which was supported by quantitative TUNEL stain. Immunohistochemistry and Western blot demonstrated an initial increase in phosphorylated-AKT in the GI+G-CSF group on Day 2. We conclude that G-CSF treatment is associated with transient early improvement in neurobehavioral outcomes after global ischemia complicated by mild hyperglycemia, but no long-term protection.
Keywords: G-CSF, global ischemia, hippocampus, CA-1
1. Introduction
Granulocyte-colony stimulating factor (G-CSF) is an endogenous peptide hormone of the hematopoietic system that is currently in Phase I/II clinical trials for ischemic stroke (Shyu et al., 2006, Schabitz et al., 2006). Multiple animal studies have shown that G-CSF has the capacity to provide significant neuroprotection in cerebral ischemia in vivo, in large measure due to strong anti-apoptotic signaling induced by the hormone in the brain (Solaroglu et al., 2006). A number of studies have shown that G-CSF is potentially neuroprotective in hemorrhagic infarction, traumatic brain injury, and spinal cord injury (Solaroglu et al., 2006, Ren et al., 2005), although there is not universal agreement that G-CSF works in trauma (Sakowitz et al., 2006) or neonatal excitotoxicity models (Keller et al., 2006). In vivo G-CSF treatment of global cerebral ischemia has not been published to date.
Global cerebral ischemia is an important clinical problem with few effective treatments (Popp et al., 2006). Intraoperative global cerebral ischemia has been shown to lead to selective hippocampal damage (Block 1999), and hippocampal damage leads to cognitive and memory dysfunction after surgery (Fujikoka et al., 2000). The mechanism of hippocampal neuron loss in global ischemia is thought to be delayed apoptotic cell death (Sugawara et al., 2002, Sugawara et al., 2000). Frequently patients at high risk for global cerebral ischemia can be identified prior to surgery (for example, cardiopulmonary bypass patients), and ideally perioperative therapies to prevent the sequelae of global cerebral ischemia can be developed.
G-CSF is known to induce a variety of pro-survival, anti-apoptotic intracellular cascades in brain ischemia in vivo. These cascades include JAK2 – STAT3, PI3 Kinase – AKT, and ERK 1/2 (Nishiki et al., 2004, Schabitz et al., 2003, Schneider et al., 2005, Solaroglu et al., 2003). Recent work suggests that these intracellular cascades are upregulated selectively in different brain regions, and in different cell types within the brain. In focal ischemia, one of the main mechanisms of G-CSF-induced neuroprotection is inhibition of apoptotic cell death, although other mechanisms such as neurotrophic cell multiplication or anti-inflammatory processes may also be important (Solaroglu et al., 2006). Related growth factors such as Erythropoietin have been shown to be neuroprotective if given before and / or after global cerebral ischemia, in part by anti-apoptotic mechanisms (Zhang et al., 2006, Calapi et al., 2000).
In the present study we tested G-CSF in the 2-vessel occlusion (2VO) model of global cerebral ischemia in rats (Smith et al., 1984). G-CSF was administered in a combined pre- and post-ischemic treatment protocol (described below) in an effort to maximize potential protective effects from the drug, and animals were euthanized for further study on postoperative Day 2, 3 and 14. We hypothesize that G-CSF will improve outcomes in this model by upregulation of anti-apoptotic signaling cascades.
2. Results
2.1 Physiologic parameters, hematological analysis, and mortality
There was no significant difference between the three groups in pH, pCO2, pO2, hemoglobin, hematocrit, or platelets. Mild intraoperative hyperglycemia was noted in each group during surgery, Sham glucose = 232 ± 12 mg / dL, GI glucose = 228 ± 4 mg / dL, GI+G-CSF glucose = 207 ± 14 mg / dL, statistically equivalent on ANOVA. Average mean arterial blood pressure during global ischemia was 32.9 ± 0.4 mmHg for the GI group, and 33.0 ± 0.2 mmHg for the GI+G-CSF group, statistically equivalent on T-test. Pretreatment with G-CSF was associated with a statistically significant increase in the absolute neutrophil count (ANC) on the day of surgery (Figure 1C). White cell differential showed that neutrophils were selectively produced in large number (and other sub-types such as lymphocytes were not over-produced). Measurement of daily body weight demonstrated an initial trend toward improvement of body weight in the G-CSF treatment group compared to the untreated group (Figure 1B), although the two groups had equivalent body weights by Day 14. Three animals (one from each group) died during surgery from anesthetic failure and were excluded from further analysis. The mortality at 24 hours was 0% (0/25) in the sham group, 9% (4/47) in the GI group, and 5% (2/42) in the GI+G-CSF treatment group, statistically equivalent on chi-square analysis.
Figure 1.
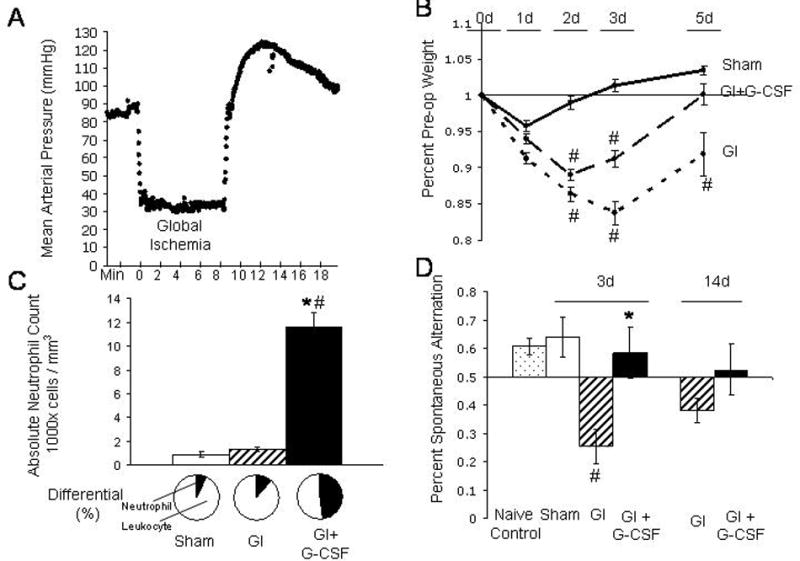
A. Mean arterial pressure (MAP), recorded in two-second intervals, demonstrates the 8-minute period of global ischemia in one of the rats in this study. Average MAP during global ischemia for the GI group (32.9 ± 0.4 mmHg) was equivalent to average MAP for the GI+G-CSF group (33.0 ± 0.2 mmHg) B. Daily body weight, expressed as percent of preoperative body weight. C. Absolute neutrophil count (ANC) and differential on the day of surgery. The GI+G-CSF group demonstrated significantly higher ANC counts on the day of surgery, and the differential confirmed that neutrophils were selectively elevated. D. Spontaneous alternation on T-maze testing. Naive control animals tend to alternate 61 ± 3% of the time when presented with two choices in the T maze. On Day 3, Sham animals (64 ± 7%) demonstrated similar performance to the Naive controls. GI animals (25 ± 6%) fared worse than GI+GCSF animals (58 ± 9%) on Day 3, but performed equivalently by Day 14 (GI = 38 ± 4%, GI+G-CSF = 52 ± 9%). * indicates significance compared to the GI group, # indicates significance compared to sham, p<0.05.
2.2 Histology, TUNEL, Cleaved Caspase 3, and G-CSF-Receptor immunohistochemistry
The G-CSF receptor was observed in the rat hippocampus following global ischemia (Figure 2B). Double staining for G-CSF-R and NeuN confirmed the presence of the receptor on hippocampal neurons. Nissl histology demonstrated delayed cell death characteristic of this model (Figure 2). This cell death began to occur between Day 2 and 3. The sham group had no cell death in CA-1. We observed TUNEL-positive cells first on Day 3 (Figure 3), consistent with Nissl histology (Figure 2). Cleaved Caspase 3 activity (Figure 3, right inset) began on Day 2 (prior to TUNEL activity).
Figure 2.
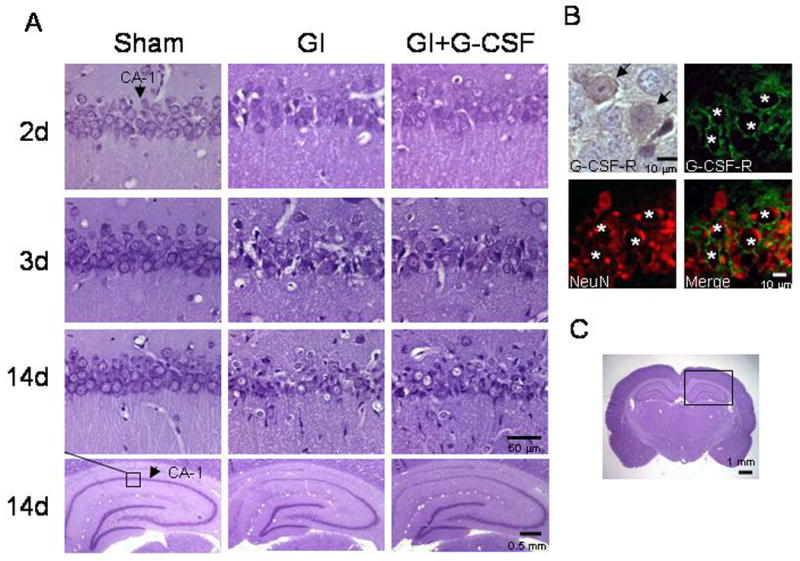
A. Nissl histology from the CA-1 region of each group at Day 2, 3, and 14, demonstrating similar extent of injury between GI and GI+G-CSF groups. Arrows denote CA-1. B. Immunohistochemical stain (brown) of the G-CSF-receptor in CA-1 of the hippocampus after global ischemia. Fluorescent stain demonstrates the G-CSF receptor (green) co-stained with the neuron nuclear marker NeuN (Red) at the border of CA-1 / 2. C. Whole brain photomicrograph showing the hippocampus.
Figure 3.
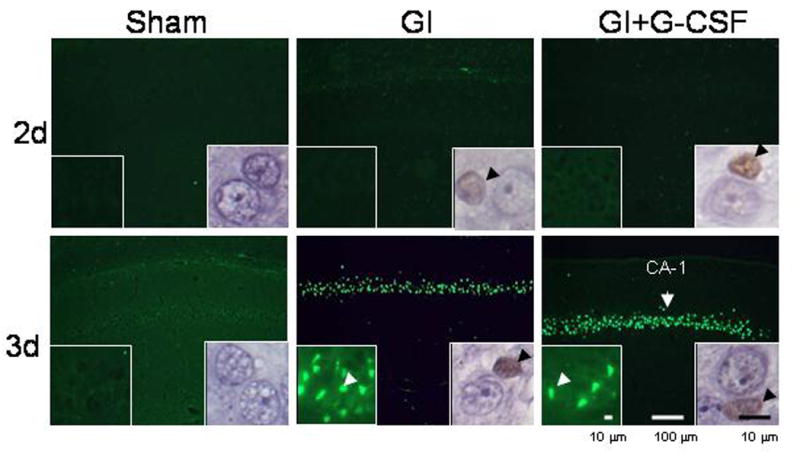
TUNEL photomicrographs demonstrate the onset of apoptosis in the CA-1 region on Day 3 after global ischemia. Left lower insets show TUNEL stain at high magnification, and arrowheads indicate examples of TUNEL-positive cells. Right-lower insets show immunohistochemical stain for cleaved caspase 3, a cellular mediator of apoptosis. Cleaved caspase 3 activity proceeded TUNEL activity.
2.3 Quantified Cell Count
Quantified TUNEL count demonstrated similar degrees of apoptosis in the GI and GI+G-CSF groups on Day 3 (Figure 4A). Quantified Nissl histology on Day 3 and Day 14 demonstrated equivalent histological outcomes in both the GI and GI+G-CSF groups (Figure 4B).
Figure 4.
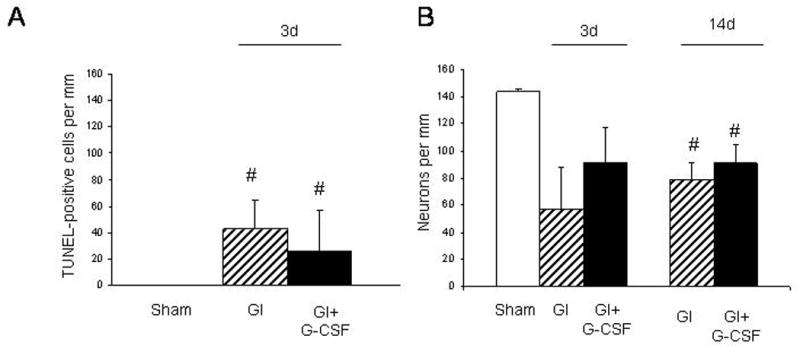
A. Hippocampal CA-1 cell count of TUNEL-positive cells on Day 3 demonstrated similar numbers of apoptotic neurons in the GI and GI+G-CSF groups. B. Hippocampal CA-1 count of viable neurons on Nissl stain demonstrated similar cell survival in both the GI and GI+G-CSF groups on Day 3 and Day 14. # indicates a statistically significant difference compared to the Sham group, p < 0.05.
2.4 Neurobehavioral Studies
T-maze testing for spontaneous alternation demonstrated a significantly worse performance in the untreated animals on Day 3 compared to the G-CSF treated animals. The sham animals on Day 3 performed similarly to the Naive control animals. By Day 14 there was no statistically significant difference between the GI and GI+G-CSF groups on the T-maze test. (Figure 1D).
2.5 Western Blotting and Immunohistochemistry
Immunohistochemical analysis for p-STAT3, p-AKT 1/2/3, and p-ERK 1/2 demonstrated a qualitative increase only in p-AKT 1/2/3 in the G-CSF treatment animals (Figure 5A). Western blot analysis of p-AKT demonstrated a trend towards increase in p-AKT 1/2/3 on Day 2, and a statistically significant increase in the G-CSF-treated animals compared to untreated animals on Day 3 (Figure 5B).
Figure 5.
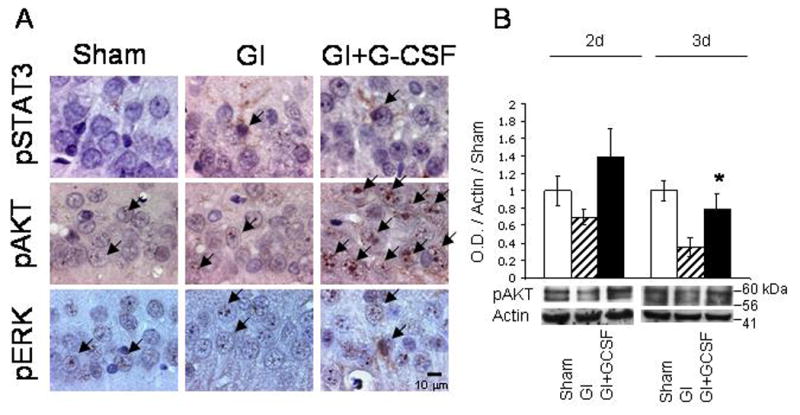
A. Immunohistochemical stain for markers of neuroprotective intracellular cascades, phosphorylated-STAT3, phosphorylated-AKT 1/2/3, and phosphorylated ERK 1/2. Of these three pathways, only phosphorylated-AKT 1/2/3 was qualitatively up-regulated in the GI+G-CSF treatment group. B. Western blot analysis of phosphorylated-AKT 1/2/3 demonstrated a qualitative increase in p-AKT 1/2/3 in the G-CSF group on Day 2, and a statistically significant increase on Day 3. * indicates statistically significant difference with respect to Group GI, p<0.05.
3. Discussion
G-CSF has been shown to be beneficial in animal studies in a variety of brain injury models (Solaroglu et al., 2006), and is presently in Phase I/II trials for ischemic stroke in humans (Shyu et al., 2006, Schabitz et al., 2006). In the present study we found that treatment with G-CSF was associated with a transient improvement in T-maze spontaneous alternation and a trend towards improved body weight, but that it conferred no long-term benefit (Figure 1B,D). Additionally there was no apparent histological benefit to hippocampal neurons (Figure 2A, 4A, 4B). These results are somewhat surprising, given that G-CSF has been shown to be neuroprotective in a variety of brain injury models (Solaroglu et al., 2006).
The initial positive outcome in T-maze performance (Figure 1D) may relate to subtle G-CSF-induced protection in brain regions other than the hippocampus. The G-CSF receptor is widely distributed in other brain regions (Schneider et al., 2005), and protection in the cortex may have led to improved T-maze performance. Under the conditions employed in this study, brain damage in the 2VO global ischemia protocol was mostly limited to the CA-1 region of the hippocampus. Other brain regions, including the cortex, occasionally demonstrated mild damage, although there was no obvious trend or qualitative difference between groups in other brain regions.
Interpretation of results from spontaneous alternation on a T-maze is somewhat controversial. In principle the spontaneous alternation task reflects the performance of working or short-term memory (Hughes 2004). However, previous reports have noted that spontaneous alternation on a T-maze likely reflects multiple behavioral aspects, including complex cortical function related to exploratory behavior (Gherli 2001). Regardless, the untreated and G-CSF treatment groups achieved similar long-term performance (Figure 1D), which suggests that there was no long-term functional benefit to administration of G-CSF.
Hippocampal neurons in CA-1 were not protected from injury in the G-CSF-treatment group (Figure 2, 3, 4), and several factors may explain this observation. The immunohistochemical and Western blot studies of p-STAT3, p-AKT, and p-ERK 1/2 suggest that G-CSF may have had only a small effect on baseline physiology of hippocampal neurons (Figure 5), in spite of the fact that multiple doses of G-CSF were administered before and during apoptosis. Of the neuroprotective intracellular cascades examined, only the PI3K-AKT cascade was slightly elevated in the G-CSF treatment group (Figure 5). Perhaps activation of this pathway alone was not sufficient to afford long-term protection against apoptotic cell death. Notably, we did not detect an increase in either JAK-STAT or ERK 1/2, both of which have been shown to play important roles in G-CSF-related neuroprotection (Solaroglu et al., 2006).
Another possible explanation for the lack of G-CSF-related neuroprotection in the hippocampus relates to the distribution of the G-CSF receptor. We detected the G-CSF receptor in CA-1 and CA-3, although the receptor density was qualitatively less in CA-1, and not all CA-1 neurons were positive after global ischemia (Figure 2B). Previous studies have noted that the distribution of the G-CSF receptor is not uniform throughout all hippocampal sub-regions (Schneider et al., 2005), which is consistent with our observations.
Three doses of G-CSF were given to the treatment group, including a dose 12 hours before global ischemia. The elevated absolute neutrophil count (ANC) in the GI+G-CSF group on the day of surgery (Figure 1C) suggests that the drug reached therapeutic levels in the animals prior to global ischemia. The dose and treatment schedule in our study were designed to cover the period of time between global ischemia (Day 0) and the onset of apoptotic cell death in the hippocampus (Day 2–3), and thereby maximize the potential for therapeutic benefit from the drug. In addition to the three-dose treatment protocol described in this study, we also examined a single-dose, post ischemic G-CSF therapy in preliminary work, but found this to inefficacious for prevention of neuron loss in CA-1 on postoperative Day 3 (data not shown). It is possible that multiple post-ischemic doses of G-CSF on Day 2 and beyond may have resulted in an improved histological outcome in CA-1. It is also possible that neutrophilia associated with G-CSF treatment may have attenuated possible neuroprotective effects, although we did not study this possibility specifically.
Apoptosis played a major role in the death of CA-1 neurons in this study. Cleaved caspase 3, a mediator of apoptosis, was readily apparent on immunohistochemical analysis on Day 2 (Figure 3), and this was followed by TUNEL activity on Day 3 (Figure 3). This pattern of caspase activity preceding TUNEL activity has been noted in previous studies (Zhou et al., 2004).
Post-hoc calculations of statistical power suggest that our study was sufficiently powered to detect a difference between the GI and GI+G-CSF groups at Day 14, if a difference actually existed. The technique used in Figure 4B (Day 14) had a 90% chance of detecting an average difference between the GI and G-CSF groups as small as 39 cells per mm. Other studies of global ischemia that have successfully identified neuroprotective drugs with similar cell counting protocols have demonstrated protective effects that are substantially greater (in proportion) than 39 cells per mm (Zhang et al., 2006).
The global ischemia model used in this study was complicated by transient, statistically equivalent intraoperative hyperglycemia in all experimental groups. This was observed shortly after induction of general anesthesia, and was likely a result of the anesthetic combination used in this study (Ketamine and Xylazine) (Kawai et al., 1997, Saha et al., 2005). Hyperglycemia may have exacerbated blood-brain-barrier breakdown during global ischemia (Dietrich et al., 1993) or possibly increased neuron loss, although we did not study these effects specifically.
In this study we used a previously unpublished modification of the 2VO global ischemia model that requires bilateral femoral arterial catheterization. Previous iterations of the 2VO global ischemia model have included a single arterial line for both inducing hemorrhage and measuring mean arterial pressure (MAP), a venous line for controlled hemorrhage along with an arterial line, and pharmacological reduction of blood pressure (McBean et al., 1998). We used dual (bilateral) femoral arterial lines in this study. This enabled direct, real-time manipulation of MAP, and exceptionally strict control over blood pressure (Figure 1A), with no statistically significant difference in average MAP during global ischemia between the two groups. The parameters for hypotension (30–35 mm Hg) and carotid occlusion (8 minutes) produced a reliably mild-to-moderate injury (Figure 2), with relatively low mortality.
We conclude that G-CSF treatment is associated with transient early improvement in neurobehavioral outcomes after global ischemia complicated by mild hyperglycemia, but that it confers no long-term protection. Hippocampal neurons in CA-1 do not appear to be protected by G-CSF treatment in vivo.
4. Experimental Procedures
4.1 Global ischemia model
Institutional Animal Care and Use Committee (IACUC) approval was obtained for this study. We used the 2-vessel occlusion (2VO) model of global ischemia first described by Smith et al (1984) with modifications. After induction of general anesthesia with ketamine (100 mg/kg IP) and xylazine (20 mg/kg IP) rats (Sprague-Dawley, male, 280–320g, Harlan, Indianapolis, IN) were intubated and ventilated with a small animal ventilator (Kent Scientific, Torrington, CT). Arterial blood gas samples were measured. Temperature was controlled with a heating pad and heat lamp, and maintained at 37 ± 1° C. Bilateral femoral arterial lines were placed under sterile conditions, and the common carotid arteries in the neck were exposed bilaterally. One arterial line was used to monitor continuous mean arterial blood pressure (MAP), and the other line was used to induce transient hypotension. Hypotension was induced by withdrawing blood into a heparinized syringe (Baxter, Deerfield, IL) until the MAP was between 30 and 35 mmHg, with a target pressure of 33 mmHg (Figure 1A). Upon reaching this pressure, the common carotid arteries were then occluded with atraumatic microvascular clamps for 8 minutes. After eight minutes of global cerebral ischemia the carotid artery clamps were removed and the blood was reinfused. All skin sites were closed and the animal was allowed to recover. Sham surgery included placement of arterial lines and exposure of common carotid arteries. Animals were euthanized under general anesthesia on Day 2, 3, and 14 after global ischemia. No animals in this study demonstrated limb dysfunction from ischemia or other cause as a result of the femoral arterial instrumentation.
4.2 Physiological parameters and hematological analysis
Intraoperative blood gas measurements were made with a blood-gas analyzer, and continuous mean arterial blood pressure (MAP) was recorded (Figure 1A). Hematological parameters including hematocrit, hemoglobin, white blood cell count and differential, absolute neutrophil count, blood glucose, other parameters were measured in a blinded manner by the clinical lab at Loma Linda University Medical Center.
4.3 Treatment methods
Granulocyte-colony stimulating factor (G-CSF, Neupogen (R), Amgen, Thousand Oaks, CA) was given to animals in the treatment group 12 hours before surgery, the day of surgery, and on post-operative Day 1. G-CSF (50 μg/kg) was given subcutaneously, following dosing regimens described previously (Lee et al., 2005). The three-dose sequence was chosen to maximize the potential protective effect of the drug, and to mimic a treatment regimen that could theoretically be useful as preoperative therapy in humans. Normal saline injections in similar volumes (approximately 0.05 mL per injection) were given to the Sham and untreated groups on the same dosing schedule as G-CSF.
4.4 Histology and Immunohistochemistry
Rats were euthanized under general anesthesia for histology. Nissl stain and double fluorescent stain of formalin fixed, paraffin embedded tissue followed standard protocols (Shimamura et al., 2006). Antigen retrieval was undertaken by microwave irradiation in 0.1 M sodium citrate, ph=6, for 10 minutes. DAB immunohistochemical stain used the ABC staining kit (Santa Cruz Biotech, Santa Cruz, CA, SC-2018). Antibodies included goat anti-phosphorylated-STAT3 (Santa Cruz Biotech, SC-7993), rabbit anti-phosphorylated-ERK 1/2 (SC-16982), rabbit anti-phosphorylated-AKT 1/2/3 (SC-16646), mouse anti-NeuN (Chemicon International, Temecula CA, MAB377), rabbit anti-G-CSF-receptor (SC-9173), rabbit anti-cleaved caspase 3 (Cell Signaling Technology, Boston, MA #9661), and appropriate secondary antibodies (Jackson Immuno Research, West Grove, PA). Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) was done with a kit (Roche, Indianapolis, IN, #11684795910).
4.5 Quantitative Cell Count
Animals from Day 3 and Day 14 were sacrificed under general anesthesia and processed for Nissl (Day 3: Sham n=7, GI n=6, GI+G-CSF n=5; Day 14: GI n=7, GI+GCSF n=7), and TUNEL (Day 3: Sham n=7, GI n=6, GI+G-CSF n=5). The brains were sectioned into 8 micrometer sections from Bregma -3.3 mm to -4.5 mm to encompass the dorsal hippocampus. Brains were sectioned in a serial manner, and a total of approximately 30 slices per animal were saved, taking care to avoid adjacent slices. From this initial set, random sections were selected with a random number table, and a total of 24 Nissl photomicrographs from the dorsal CA-1 per animal were taken at 100X for the Nissl cell counts. Eight photomicrographs of CA-1 were taken from each animal and counted for the TUNEL study. All sections were analyzed by a blinded investigator.
4.6 Neurobehavioral testing
Prior to sacrifice on Day 3 and Day 14 rats were tested for spontaneous alternation on a T-maze (Gerlai 2001; Hughes 2004) following methods described by Kofler et al (2004) and Ishabi et al (2003) with modifications. The T-maze was constructed from acrylic and silicon glue. The path-width throughout the maze was 10cm. The stem length was 40 cm, and each arm was 46 cm. Animals were placed in the stem of the T-maze, and allowed to freely explore the two arms of the maze. Once an arm was chosen, the animal was placed in the stem of the maze again, and the trial was repeated. A total of ten trials were attempted with each animal over 20–30 minutes. Six different groups of animals were tested: Naive healthy male rats from an unrelated study (n=17), Sham animals on Day 3 (n=12), global ischemia Day 3 (n=11), global ischemia with G-CSF treatment on Day 3 (n=12), global ischemia on Day 14 (n=7), and global ischemia with G-CSF treatment on Day 14 (n=7). Animals tested on Day 14 were not tested on Day 3. Absolute numbers of left and right choices were recorded, as were the sequence of left and right choices. Data are expressed as the rate of spontaneous alternation, with a baseline of alternation of 50%.
4.7 Western blot
Western blotting followed standard protocols (Shimamura et al., 2006). Animals were euthanized under general anesthesia and perfused with PBS (100cc) by trans-cardiac approach. The brains were then removed and hippocampus was isolated bilaterally for analysis. Antibodies included rabbit anti-phosphorylated-AKT 1/2/3 (Santa Cruz Biotech, SC-16646), goat anti-beta actin (SC-1615), and appropriate secondary antibodies. Bands were detected by chemiluminescent kit (Amersham Bioscience, NJ), and recorded on X-ray film (Kodak, Rochester, NY). Bands were quantified by optical density method (Image J), and densities were expressed relative to beta actin and sham.
4.8 Statistical analysis
Data are expressed as mean ± S.E.M. Analysis of variance (ANOVA) supported by Sigma Stat (Systat Software, Richmond, CA) was used for analysis of differences between groups for physiological parameters, hematological parameters, Western blot, daily weight, T-maze results, and cell counts. A chi-squared test was used for a comparison of mortality. Statistical power was calculated for the Day 14 Nissl histology.
Acknowledgments
This study was supported by the Clinician-Scientist Training Program at Loma Linda University and by NIH Grants NS43338, NS45694, NS53407, and HD 43120 to JHZ.
Abbreviations
- (G-CSF)
Granulocyte-colony stimulating factor
- (BBB)
blood-brain barrier
- (2VO)
2 vessel occlusion
- (TUNEL)
Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Block F. Global ischemia and behavioral deficits. Progress in Neurobiology. 1999;58:279–295. doi: 10.1016/s0301-0082(98)00085-9. [DOI] [PubMed] [Google Scholar]
- Calapi G, Marciano MC, Corica F, Allegra A, Parisi A, Frisina N, Caputi AP, Buemi M. Erythropoietin protects against brain ischemic injury by inhibition of nitric oxide formation. European Journal of Pharmacology. 2000;410:349–356. doi: 10.1016/s0014-2999(00)00466-0. [DOI] [PubMed] [Google Scholar]
- Dietrich WD, Alonso O, Busto R. Moderate hyperglycemia worsens acute blood-brain barrier injury after forebrain ischemia in rats. Stroke. 1993;24(1):111–116. doi: 10.1161/01.str.24.1.111. [DOI] [PubMed] [Google Scholar]
- Fujikoka M, Nishio K, Miyamoto S, Hiramatsu KI, Sakaki T, Okuchi K, Taoka T, Fujioka S. Hippocampal damage in the human brain after cardiac arrest. Cerebrovasc Dis. 2000;10(1):2–7. doi: 10.1159/000016018. [DOI] [PubMed] [Google Scholar]
- Gerlai R. Behavioral tests of hippocampal function: simple paradigms, complex problems. Behavioural brain research. 2001;125:269–277. doi: 10.1016/s0166-4328(01)00296-0. [DOI] [PubMed] [Google Scholar]
- Hughes RN. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neuroscience and biobehavioral reviews. 2004;28:487–505. doi: 10.1016/j.neubiorev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Ishibashi S, Kuroiwa T, Endo S, Okeda R, Mizusawa H. Neurological dysfunction versus regional infarction volume after focal ischemia in Mongolian gerbils. Stroke. 2003;34(6):1501–1506. doi: 10.1161/01.STR.0000074034.32371.13. [DOI] [PubMed] [Google Scholar]
- Kawai N, Keep RF, Betz AL. Hyperglycemia and the vascular effects of cerebral ischemia. Stroke. 1997;28(1):149–154. doi: 10.1161/01.str.28.1.149. [DOI] [PubMed] [Google Scholar]
- Keller M, Simbruner G, Urbanek M, Tinhofer I, Griesmaier E, Sarkozy G, Schwendimann L, Gressens P. Systematic application of granulocyte-colony stimulating factor and stem cell factor exacerbates excitotoxic brain injury in newborn mice. Pediatric Res. 2006;59(4):549–553. doi: 10.1203/01.pdr.0000205152.38692.81. [DOI] [PubMed] [Google Scholar]
- Kofler J, Hattori K, Sawada M, DeVries AC, Martin LJ, Hurn PD, Traystman RJ. Histopathological and behavioral characterization of a novel model of cardiac arrest and cardiopulmonary resuscitation in mice. J Neurosci Methods. 2004;136:33–44. doi: 10.1016/j.jneumeth.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Lee ST, Chu K, Jung KH, Ko SY, Kim EH, Sinn DI, Lee YS, Lo EH, Kim M, Roh JK. Granulocyte-colony stimulating factor enhances angiogenesis after focal cerebral ischemia. Brain research. 2005;1058:120–128. doi: 10.1016/j.brainres.2005.07.076. [DOI] [PubMed] [Google Scholar]
- McBean DE, Kelly PA. Rodent models of global cerebral ischemia: a comparison of two-vessel occlusion and four-vessel occlusion. Gen Pharmacol. 1998;30(4):431–434. doi: 10.1016/s0306-3623(97)00284-x. [DOI] [PubMed] [Google Scholar]
- Nishiki S, Hato F, Kamata N, Sakamoto E, Hasegawa T, Kimura-Eto A, Hino M, Kitagawa S. Selective activation of STAT3 in human monocytes stimulated by G-CSF: implication in inhibition of LPS-induced TNF-alpha production. American Jour Cell Physiology. 2004;286(6):1302–1311. doi: 10.1152/ajpcell.00387.2003. [DOI] [PubMed] [Google Scholar]
- Popp E, Bottiger BW. Cerebral resuscitation: state of the art, experimental approaches, and clinical perspectives. Neurol Clin. 2006;24(1):73–87. doi: 10.1016/j.ncl.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Ren JM, Finklestein SP. Growth factor treatment of stroke. Current Drug Targets - CNS & Neurological Disorders. 2005;4:121–125. doi: 10.2174/1568007053544101. [DOI] [PubMed] [Google Scholar]
- Saha JK, Zia J, Grondin JM, Engle SK, Kakubowski JA. Acute hyperglycemia induced by ketamine/xylazine anesthesia in rats: mechanisms and implications for preclinical models. Exp BIol Med. 2005;230:777–784. doi: 10.1177/153537020523001012. [DOI] [PubMed] [Google Scholar]
- Sakowitz OW, Schardt C, Neher M, Stover JF, Unterberg AW, Kiening KL. Granulocyte colony stimulating factor does not affect contusion size, brain edema, or cerebrospinal fluid glutamate concentrations in rats following controlled cortical impact. Acta Neurochir Suppl. 2006;96:139–143. doi: 10.1007/3-211-30714-1_31. [DOI] [PubMed] [Google Scholar]
- Schabitz W, Kollmar M, Schwaninger E, Juettler J, Bardutzky M, Scholzke N. Neuroprotective effect of granulocyte colony stimulating factor after focal cerebral ischemia. Stroke. 2003;34(3):745–751. doi: 10.1161/01.STR.0000057814.70180.17. [DOI] [PubMed] [Google Scholar]
- Schabitz WR, Schneider A. Developing granulocyte-colony stimulating factor for the treatment of stroke: current status of clinical trials. Stroke. 2006;37(4):1654. doi: 10.1161/01.STR.0000227299.62106.0e. [DOI] [PubMed] [Google Scholar]
- Schneider A, Kruger T, Steigleder D, Weber C, Pitzer R, Laag J, Aronowski M, Maurer H, Gassler W, Meir M, Hasselblatt R, Kollmar S, Schwab C, Sommer A, Bach H, Kuhn G, Schabitz A. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. Journal of Clinical Investigation. 2005;115(8):2089–2098. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura N, Matchett G, Yatsushige H, Calvert JW, Okhuma H, Zhang J. Inhibition of integrin alpha(v) beta(3) ameliorates focal cerebral ischemic damage in the rat middle cerebral artery occlusion model. Stroke. 2006;37(7):1902–1909. doi: 10.1161/01.STR.0000226991.27540.f2. [DOI] [PubMed] [Google Scholar]
- Shyu WC, Lin SZ, Lee CC, Liu DD, Li H. Granulocyte colony stimulating factor for acute ischemic stroke: a randomized controlled trial. CMAJ. 2006;174(7):927–933. doi: 10.1503/cmaj.051322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Bendek G, Dahlgren N, Rosen I, Wieloch T, Siesjo BK. Models for studying long-term recovery following forebrain ischemia in the rat: a 2-vessel occlusion model. Acta Neurol Scand. 1984;69:385–401. doi: 10.1111/j.1600-0404.1984.tb07822.x. [DOI] [PubMed] [Google Scholar]
- Solaroglu I, Jadhav V, Zhang JH. A novel neuroprotectant: granulocyte-colony stimulating factor. Stroke. 2006;37:1123–1128. doi: 10.1161/01.STR.0000208205.26253.96. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Kawase M, Lewen A, Noshita N, Gasche Y, Fujimura M, Chan P. Effect of hypotension severity on hippocampal CA1 neurons in a rat global ischemia model. Brain Research. 2000;877:281–287. doi: 10.1016/s0006-8993(00)02684-6. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Lewen A, Noshita N, Gasche Y, Chan P. Effects of global ischemia duration on neuronal, astroglial, oligodendroglial, and microglial reactions in the vulnerable hippocampal CA-1 sub region in rats. Journal of Neurotrauma. 2002;19(1):85–89. doi: 10.1089/089771502753460268. [DOI] [PubMed] [Google Scholar]
- Zhang F, Signore AP, Zhou Z, Wang S, Cao G, Chen J. Erythropoietin protects CA1 neurons against global cerebral ischemia in rats: potential signaling mechanisms. Journal of Neuroscience Research. 2006;83:1241–1251. doi: 10.1002/jnr.20816. [DOI] [PubMed] [Google Scholar]
- Zhou L, del Villar K, Dong Z, Miller CA. Neurogenesis response to hypoxia-induced cell death: map kinase signal transduction mechanisms. Brain Research. 2004;1021:8–19. doi: 10.1016/j.brainres.2004.05.115. [DOI] [PubMed] [Google Scholar]


