Abstract
Inhalation of asbestos fibers causes pulmonary inflammation and eventual pulmonary fibrosis (asbestosis). Although the underlying molecular events are poorly understood, protease/antiprotease and oxidant/antioxidant imbalances are believed to contribute to the disease. Implicated in other forms of pulmonary fibrosis, the matrix metalloproteinases (MMPs) have not been examined in asbestosis. We therefore hypothesized that MMPs play a pathogenic role in asbestosis development. Wild-type C57BL/6 mice were intratracheally instilled with 0.1 mg crocidolite asbestos, causing an inflammatory response at 1 d and a developing fibrotic response at 7, 14, and 28 d. Gelatin zymography demonstrated an increase in MMP-9 (gelatinase B) during the inflammatory phase, while MMP-2 (gelatinase A) was profoundly increased in the fibrotic phase. Immunohistochemistry revealed MMP-9 in and around bronchiolar and airspace neutrophils that were often associated with visible asbestos fibers. MMP-2 was found in fibrotic regions at 7, 14, and 28 d. No increases in RNA levels of MMP-2, MMP-9, or MMP-8 were found, but levels of MMP-7, MMP-12, and MMP-13 RNA did increase at 14 d. The MMP inhibitors, TIMP-1 and TIMP-2, were also increased at 7–28 d after asbestos exposure. To confirm the importance of MMP activity in disease progression, mice exposed to asbestos were given daily injections of the MMP inhibitor, GM6001. MMP inhibition reduced inflammation and fibrosis in asbestos-treated mice. Collectively, these data suggest that MMPs contribute to the pathogenesis of asbestosis through effects on inflammation and fibrosis development.
Keywords: asbestos, extracellular superoxide dismutase, matrix metalloproteinase, pulmonary fibrosis
Pulmonary fibrosis due to asbestos fiber inhalation (asbestosis) remains a significant occupational health problem in the United States and worldwide. Although use of asbestos in the United States has been drastically reduced (1), a long latency between exposure and disease development predicts a continued appearance of new asbestosis cases (2). Unfortunately, the molecular mechanisms underlying this disease are not well understood.
A number of studies have implicated growth factors (3), oxidant stress (4, 5; reviewed in Refs. 6 and 7), and inflammation (reviewed in Ref. 8) in the development of asbestosis. However, the role of proteolytic imbalances, particularly among the matrix metalloproteinases (MMPs), is yet to be determined in asbestos-induced pulmonary fibrosis.
The MMPs are a family of endopeptidases that can degrade several components of the extracellular matrix and also control the activity of several diverse proteins that function in immunity, repair, and disease progression. At least 24 distinct members have been identified in mammals thus far, and all share characteristic features such as a prodomain and active site zinc-binding domain. Latency is maintained through coordination of the active site zinc with the thiol of a conserved cysteine in the prodomain. Disruption of this cysteine–zinc bond can occur by a number of mechanisms, including proteolytic cleavage and oxidative modification of the cysteine (reviewed in Ref. 9), leading to activation and autocatalytic cleavage of the prodomain (10). MMP activity can be silenced by various mechanisms, including endocytosis and by endogenous inhibitors such as tissue inhibitors of metalloproteinases or TIMPs (11).
Several studies have implicated MMPs in human and experimental interstitial lung disease. In human idiopathic pulmonary fibrosis (IPF), levels of MMP-2 (gelatinase A) and MMP-9 (gelatinase B) are elevated in lungs (12, 13) and BAL fluid (14) of affected patients. MMP-2 and MMP-9 proteolytically cleave denatured collagen (gelatin) and have some ability to cleave native type IV collagen, the key structural component of the basement membrane (15). These MMPs also appear to participate in the regulation of chemokine activity, indicating a role in immunologic activation (16–18). IPF studies also revealed an increase in MMP-8 (collagenase-2) in BAL fluid obtained from these patients (19). Other investigations have demonstrated increases in TIMP-2 (12, 14), which apart from its inhibitory capabilities can actually activate MMP-2 through a cell-surface mechanism (20).
Experimental models of fibrosis have shown similar patterns of MMP expression. Bleomycin-induced fibrosis in rabbits exhibits an early increase in MMP-9 followed by a more chronic expression of MMP-1 and MMP-2, as well as TIMP-1 and TIMP-2 (21). On the other hand, experimental lung silicosis also displayed an increase in MMP-2 and MMP-9 but showed reductions in TIMP-1 and TIMP-2 (22). Other investigators using the bleomycin model in mice demonstrated increased expression of MMP-12 (macrophage metalloelastase) in the lung (23, 24). The metalloproteinase inhibitor, batimastat, inhibits the development of bleomycin-induced pulmonary fibrosis (25), suggesting that MMPs are directly contributing to the development of pulmonary fibrosis.
An interesting role was identified for MMP-7, also known as matrilysin. Unexpectedly, this epithelial-derived MMP is highly upregulated in human IPF lungs when compared with controls by microarray analysis (26). Experiments in MMP-7–null mice revealed a marked decrease in the transepithelial efflux of neutrophils and a mildly attenuated fibrotic response compared with wild-type mice. MMP-7 cleaves the heparan sulfate proteoglycan syndecan-1 from the surface of alveolar epithelial cells, leading to the release of neutrophil chemoattractants (27).
Asbestos modulates MMP activity as well. Asbestos fibers induce MMP-8 (collagenase-2) release from neutrophils in vitro (28). In vivo, expression of lung MMP-2 was increased in rats exposed to both chrysotile asbestos and cigarette smoke, but not to asbestos alone. On the other hand, MMP-1 (collagenase-1) was increased in rats exposed to chrysotile asbestos alone, with no augmentation by cigarette smoke (29). However, this study did not examine fibrosis development, since it used the less fibrogenic chrysotile asbestos fibers (8).
Based on the above evidence, we hypothesized that MMPs contribute to the development of asbestos-induced pulmonary fibrosis. To examine the role of MMPs in asbestosis, we treated C57BL/6 wild-type mice with a single intratracheal instillation of crocidolite asbestos or inert particulate (titanium dioxide) and collected lung tissue and BAL fluid at 1, 7, 14, and 28 d. Using gelatin zymography and immunohistochemistry, we found elevations in MMP-9 and both the latent and active forms of MMP-2 in asbestos-treated mice. We also identified an increase in MMP-7, MMP-12, and MMP-13 mRNAs at 14 d in asbestos-treated mice. A broad spectrum MMP inhibitor (GM6001) inhibited inflammation and development of fibrosis in these mice. These studies suggest that MMP activity contributes to the development of asbestosis and that MMPs may regulate inflammation and interstitial fibrosis development.
MATERIALS AND METHODS
Asbestos Exposures and Sample Collection
All animal experiments were reviewed and approved by the University of Pittsburgh Institutional Animal Care and Use Committee. 8–10 wk old male C57BL/6 mice (Taconic, Germantown, NY) were treated with a single 0.1-mg dose of crocidolite asbestos (obtained from the National Institutes of Environmental Health Sciences) or 0.1 mg titanium dioxide (Sigma, St. Louis, MO) suspended in 0.9% saline by intratracheal instillation as previously described (30). Mice were killed at 1, 7, 14, or 28 d, as indicated. Mice being treated with the metalloproteinase inhibitor GM6001 (0.2 mg/mouse, ∼ 10 mg/kg; EMD Biosciences/Calbiochem, La Jolla, CA) were intraperitoneally injected every day for 13 d beginning 1 d before asbestos instillation. Mice treated with GM6001 were killed at 13 d after asbestos instillation. Bronchoalveolar lavage (BAL) fluid was obtained, and total protein, total white blood cell counts, and differential counts were obtained as described previously (30). Lungs were either flash-frozen and stored at −80°C, dried for hydroxyproline analysis, or inflation fixed with 10% buffered formalin and paraffin-embedded for histologic analysis as previously described (5).
Total protein homogenates were isolated from some lungs, which were thawed on ice and homogenized and sonicated in 10 vols of 50 mM potassium phosphate, pH 7.4, 0.3 M potassium bromide, 3 mM diethylenetriaminepentacetic acid, and 0.5 mM phenylmethylsulfonylfluoride. Samples were centrifuged at 20,000 × g for 20 min, and supernatants were collected. The insoluble fraction remaining after supernatant recovery was extracted in 50 mM Tris-HCl, 150 mM NaCl, 10 mM CHAPS buffer containing 100 μM DCI, and 10 μM E-64 protease inhibitors. CHAPS detergent aids in the extraction of MMPs (31). All homogenates were stored at −80°C.
RNA isolates from lungs were prepared using the Qiagen RNeasy Maxi kit as per the manufacturer's instructions (Qiagen, Valencia, CA). Briefly, whole frozen lungs were homogenized in buffer RLT containing 0.143 M β-mercaptoethanol. After centrifugation (3,000 × g for 5 min), supernatants were recovered and mixed with 70% ethanol. The mixtures were added to an RNeasy Maxi column and centrifuged again. After several washes of the column, RNase-free DNase (Qiagen) was added to the filter membranes for 15 min to remove contaminating DNA. Additional washes were followed by recovery of RNA from the filter with two elutions using 0.8 ml water each time.
Gelatin Zymography
Gelatin zymography was performed as described previously (14, 32). Briefly, equivalent protein amounts of lung homogenate or BAL fluid were resolved by nonreducing SDS-PAGE through 10% acrylamide 0.1% gelatin gels (Invitrogen, Carlsbad, CA). After electrophoretic separation of proteins, the gels were washed twice, 20 min each, in 2.5% Triton X-100. Gels were then incubated in 50 mM Tris-HCl, pH 7.8, 5 mM CaCl2, 1 μM ZnCl2, and 1.25% Triton X-100 for 20–24 h and then stained with Coomassie Brilliant Blue R-250 (Fisher Scientific, Pittsburgh, PA). Gelatinolytic activity was observed as clear bands against a blue background. Identities of the bands as MMP-2 and MMP-9 were validated by comparison with purified MMP-2 (EMD Biosciences) and MMP-9 (Chemicon, Temecula, CA). Densitometry was performed using the Gel Logic 100 Imaging System and Kodak 1D software (Kodak, Rochester, NY).
Western Blots
Equivalent protein amounts of BAL fluid and lung homogenates were resolved by reducing SDS-PAGE as described previously (33) and blotted onto PVDF membranes. Antibody against EC-SOD (1:10,000 dilution) and horseradish peroxidase–linked anti-rabbit secondary antibody were used in conjunction with ECL reagents (Amersham Biosciences, Piscataway, NJ) to detect EC-SOD. Antibody against TIMP-1 (1:1,000; Triple Point Biologics, Forest Grove, OR) with anti-rabbit secondary, and antibody against TIMP-2 (1:200; Chemicon) with anti-mouse secondary were also used. Densitometry was performed as described for the zymograms and normalized to β−actin detected with antibody (1:5,000; Sigma) as a loading control.
Immunohistochemistry
Immunohistochemistry was performed on 5-μm sections from paraffin-embedded lung blocks. Slides were deparaffinized and incubated in 6% hydrogen peroxide in methanol to inactivate endogenous peroxidases and then 0.1% pepsin for antigen retrieval. After blocking with protein blocking solution, slides were incubated with either anti–MMP-2 (4 μg/ml) or anti–MMP-9 (2 μg/ml) antibody or with an equivalent amount of normal goat IgG as a negative control for 60 min (all three antibodies from R&D Systems, Minneapolis, MN). Slides were incubated in biotinylated anti-goat antibody (1:500) for 30 min followed by 30 min in ABC-HRP reagent (Vector Laboratories, Burlingame, CA). Slides were developed with the Nova Red (Vector Laboratories) staining kit and counterstained with hematoxylin (Vector Laboratories). All slides for comparison were stained on the same day and developed for the same amount of time.
Histologic Scoring
Standard hematoxylin and eosin staining was performed as previously described (5). Slides were scored by a pathologist (T.D.O.) blinded to sample groups. Individual fields were examined with a light microscope at high-power (×400) magnification. Every other field was scored in the entire lung, beginning peripherally. To be counted, each field had to contain terminal bronchioles/alveolar tissue in > 50% of the field. Scoring in each field was based on the percentage of terminal bronchioles/alveolar tissue with interstitial fibrosis according to the following scale: 0 = no fibrosis, 1 = up to 25%, 2 = 25–50%, 3 = 50–75%, 4 = 75–100%. Scores were calculated for each animal, and the group scores were averaged together for statistical comparisons.
Quantitative RT-PCR
Reagents for RT-PCR for MMP-2, MMP-9, MMP-8, MMP-12, and MMP-13 were purchased from Applied Biosystems (Foster City, CA), except when noted otherwise. Reverse transcriptase reactions for these MMPs were performed on 1 μg RNA using PCR Buffer II, 5 mM MgCl2, 1 mM nucleotide mix (Promega, Madison, WI), 1.0 U RNAsin, 2.5 U MuLV reverse transcriptase enzyme, and 3 μg random primers or oligo dT primers. Reverse transcriptase was performed in a thermocycler programmed for 42°C for 40 min, 99° for 5 min, and 5° for 5 min. Quantitative (real-time) PCR was performed by adding Universal PCR Buffer and Taqman primer/probe assay reagent specific for MMP-2, MMP-9, MMP-8, MMP-12, and MMP-13 according to manufacturer's instructions. GAPDH primer/probes were used as a loading control. The default program was performed on an ABI Prism 7000 (Applied Biosystems) and consisted of 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min.
MMP-7 cDNA was prepared from 4 μg RNA using the SuperScript III First-Stand Synthesis System for RT-PCR (Invitrogen) as per the instructions supplied by the manufacturer. The thermocycler program was 25°C for 10 min, 50°C for 50 min, and 85°C for 5 min, also as recommended by the manufacturer. RNase was added to degrade RNA during a 20-min incubation at 37°C. Samples were stored at −20°C before further use. Quantitative PCR was performed as described above except that Taqman primer/probe assay reagent for MMP-7 was used. A 7900HT FAST Real-Time PCR System machine (Applied Biosystems) was used.
Hydroxyproline Assay
Whole lungs were dried and acid hydrolyzed in sealed, oxygen-purged glass ampoules containing 2 ml of 6 N HCl for 24 h at 110°C and subjected to hydroxyproline quantitation using chloramines T as previously described (5).
Statistics
All comparisons between two groups were performed with the Student's t test. Comparisons between more than two groups were performed with one way ANOVA followed by Tukey's post test. A P value < 0.05 was considered significant.
RESULTS
MMP-2, MMP-7, MMP-9, MMP-12, and MMP-13 Levels Are Increased after Pulmonary Asbestos Injury
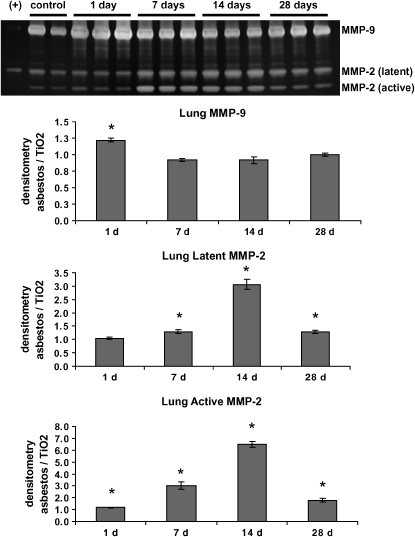
Because of their described role in other fibrotic lung diseases (12, 26), MMP-2 and MMP-9 activity were assessed using gelatin zymography in lung homogenates and BAL fluid from mice intratracheally instilled with crocidolite asbestos fibers. While a representative gel for lung homogenates is shown in Figure 1, densitometry analysis was performed on a larger number of titanium dioxide– and asbestos-treated animals (n = 3–6 for each group) at 1, 7, 14, and 28 d. Titanium dioxide was used as a particulate control that does not cause pulmonary fibrosis (5). Identification of MMP-2 and MMP-9 was made by comparison with the purified enzymes loaded next to the samples being tested.
Figure 1.
MMP-2 and MMP-9 are increased in lung homogenates after asbestos exposure. Wild-type C57BL/6 mice were exposed to asbestos and killed at 1, 7, 14, and 28 d. Gelatin zymography of lung homogenates was performed as described in Materials and Methods. A representative gel is shown, but densitometry was performed on zymograms comparing controls and asbestos samples at each time point (n = 3–6). Densitometry was normalized as the average intensity of the asbestos bands divided by the average of the control (titanium dioxide, TiO2) bands at the same time point. If the asbestos levels of MMPs were equal to control levels, the value would therefore equal 1.0. The identification of bands as MMP-2 and MMP-9 was made through comparison with purified MMP-2 and MMP-9 (+). Latent and active MMP-2 were increased at all time points, while MMP-9 was significantly increased at 24 h only. *P < 0.05 Student's t test, comparing asbestos with control.
In the lung after asbestos exposure, MMP-9 levels were significantly (though modestly) increased at 1 d over control values and returned to baseline levels at the other time points (Figure 1). In contrast, levels of latent MMP-2 increased at 7, 14, and 28 d, while active MMP-2 was increased at all time points examined, with the most striking increases at 7 and 14 d (Figure 1). The ratio of active to latent MMP-2 was also significantly increased at all time points due to this increase in active MMP-2 (densitometry not shown).
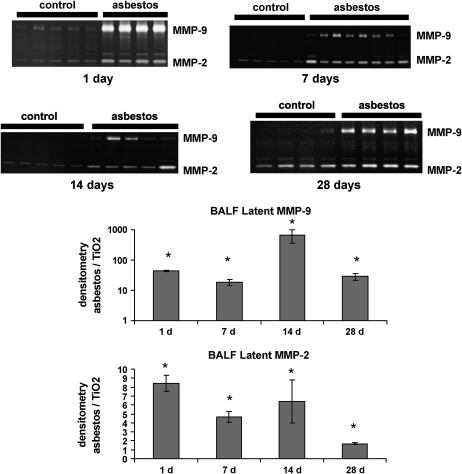
In the BAL fluid, MMP-9 and only the latent form of MMP-2 were detected by zymography (Figure 2). Densitometry shows that MMP-2 and MMP-9 were significantly elevated at all time points after asbestos exposure. As in the lung, the largest increase in MMP-9 was found at 1 d. The largest increase in MMP-2 was noted at 7 and 14 d. Overall, gelatin zymography revealed that MMP-2 and MMP-9 levels were elevated in both lung parenchyma and alveolar lining fluid after asbestos exposure.
Figure 2.
MMP-2 and MMP-9 are increased in BAL fluid after asbestos exposure. Gelatin zymography and densitometry was performed as in Figure 1. Only the latent forms of MMP-2 and MMP-9 were found in BAL fluid. There are significant increases in MMP-2 and MMP-9 at all time points after asbestos exposure. *P < 0.05, Student's t test, comparing asbestos to the control (titanium dioxide, TiO2).
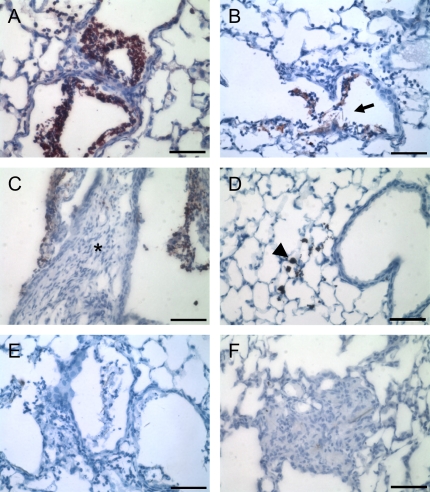
The location of these two gelatinases in the lung was determined by immunohistochemistry. Asbestos-exposed mice revealed neutrophilic influx and some edema and interstitial thickening at 1 d (inflammatory phase), followed by progressive fibrosis at 7, 14, and 28 d (fibrotic phase), similar to results we have published previously (5). We observed the most intense staining for MMP-9 at 1 d (Figure 3A), and signal for this MMP was localized to the bronchiolar airspaces of asbestos-treated mice but not titanium dioxide–treated mice (Figure 3A versus 3D). Staining was associated with neutrophils aggregating around asbestos fibers (Figure 3B). There was no observable increase in MMP-9 staining in alveolar septa of the asbestos-treated mice at any of the time points (Figures 3A–3C), although occasional MMP-9 staining was observed in airspaces at 14 d, again associated with bronchiolar neutrophils (Figure 3C). Similar results were found at 7 and 28 d (data not illustrated). This localization likely accounts for the 24-h increase of MMP-9 in lung homogenates and the persistent abundance of MMP-9 in BAL fluid.
Figure 3.
Immunohistochemistry for MMP-9 in asbestos-exposed mouse lungs. Sections were incubated with anti–MMP-9 antibody (A–D) or with normal IgG as a negative control (E, F). (A, B) One day after asbestos exposure, bronchiolar neutrophils surrounding visible asbestos fibers (fibers at arrow) stained strongly for MMP-9 (red color). (C) Bronchiolar neutrophils retained MMP-9–positive staining at 14 d. However, no interstitial MMP-9 was observed, either in normal alveolar septa or in areas of fibrosis (asterisk). Identical staining patterns were observed at 7 and 28 d. (D) Titanium dioxide–treated mice did not display any MMP-9 staining. Macrophages that have engulfed these particles are clearly visible (arrowhead). Normal goat IgG revealed no staining at 24 h (E), 14 d (F), or 7 d (data not illustrated). Bar equals 50 μm.
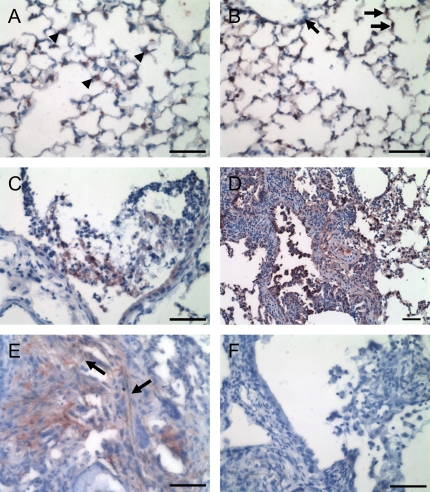
Investigation into the localization of MMP-2 revealed markedly different results for this protease. While titanium dioxide–treated mice exhibited punctuate intracellular staining for MMP-2 localizing to type II epithelial cells and bronchiolar epithelium (Figure 4A), asbestos-treated mice exhibited a more diffuse and often extracellular localization over alveolar surfaces (Figure 4B). MMP-2 also had a more prolonged expression pattern, as it was located in both normal alveoli and particularly in fibrotic areas of lung at 7 and 28 d (Figures 4D–4E). Similar results were found at 14 d (data not shown). In addition, MMP-2 also localized to areas of bronchiolar inflammation, as was seen for MMP-9 (Figure 4C).
Figure 4.
Immunohistochemistry for MMP-2 in asbestos-exposed mouse lungs. (A) Titanium dioxide–treated mice exhibited punctuate localization of MMP-2 in type II epithelial cells (arrowheads). (B) At 1 d, asbestos-treated mice exhibited the same type II cell staining, but in addition had a more diffuse staining over alveolar septa (arrows). (C) Also at 1 d, asbestos-treated mice had MMP-2 around bronchiolar neutrophils. (D) After 7 d, there is a significant increase in interstitial MMP-2 in areas of fibrosis. (E) As late as 28 d, fibrotic regions retain MMP-2 expression, here in fibrosis with visible asbestos fibers (arrows). (F) Normal goat IgG revealed no staining. Bar equals 50 μm.
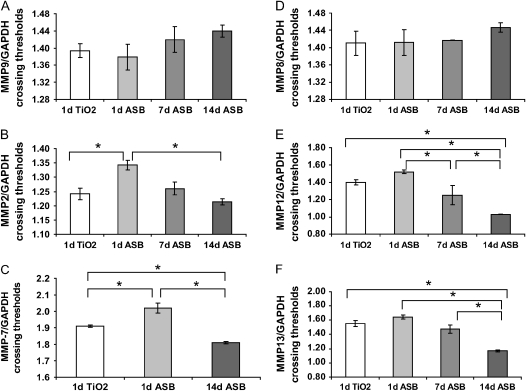
Quantitative RT-PCR was performed for MMP-2, MMP-9, MMP-7, MMP-8, MMP-12, and MMP-13 to determine RNA levels of these enzymes (Figure 5). These data are presented as crossing threshold ratios of target gene divided by GAPDH as a loading control. As such, later crossing thresholds denote decreased levels of RNA compared with GAPDH. MMP-9 levels were not different from controls at any of these time points (Figure 5A). MMP-2 RNA levels were not increased at 1, 7, or 14 d, and actually showed a decrease in message levels at 24 h (Figure 5B). Notably, MMP-7 RNA was significantly decreased at 1 d and then significantly elevated at 14 d after asbestos treatment (Figure 5C). In terms of the two murine collagenases, MMP-8 was not significantly changed at any time after asbestos treatment, whereas MMP-13 RNA was significantly increased at 14 d after asbestos (Figures 5D and 5F). MMP-12 RNA was also increased at 14 d compared with the titanium dioxide controls and with all other time points after asbestos (Figure 5E).
Figure 5.
Quantitative RT-PCR for MMP-2, MMP-7, MMP-9, MMP-8, MMP-12, and MMP-13 in asbestos-exposed mouse lungs. (A) Crossing thresholds for MMP-9 were divided by crossing thresholds (ct) for GAPDH and did not reveal any significant differences between groups. Results were averaged for each group (n = 1–3 for each group). Lower crossing thresholds denote greater RNA expression of the target gene. (B) MMP-2/GAPDH ct was significantly greater at 1 d (24 h) asbestos compared with 24 h titanium dioxide (TiO2), denoting less MMP-2 RNA at that time point. (C) Ct of MMP-7 RNA was lower 14 d after asbestos, revealing a significant increase in MMP-7 levels in the 14 d asbestos group compared with both 1 d titanium dioxide and 1 d asbestos. (D) MMP-8/GAPDH ct did not reveal any significant differences. (E) MMP-12/GAPDH ct and (F) MMP-13/GAPDH ct levels showed significantly increased RNA levels of these MMPs at 14 d. *P < 0.05, as determined by one-way ANOVA and Tukey's post test.
TIMP-1 and TIMP-2 Are Increased after Asbestos Exposure
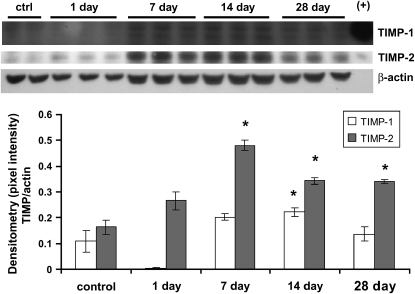
We also examined TIMPs in our asbestos studies. Western blotting of lung homogenates from mice exposed to asbestos revealed virtually no TIMP-1 at 24 h (Figure 6). However, TIMP-1 levels were significantly elevated at 14 d, with a trend toward increased levels at 7 and 28 d. TIMP-2 levels were significantly elevated at 7, 14, and 28 d after asbestos exposure.
Figure 6.
TIMP-1 and TIMP-2 are increased after asbestos exposure. Western blotting was performed on lung homogenates to determine levels of TIMP-1 and TIMP-2. Positive controls are on the right side of the blot. Both TIMPs showed increases that were most pronounced at 7–28 d after asbestos exposure. Densitometry was normalized to β-actin as a loading control. *P < 0.05, one-way ANOVA followed by Tukey's post test, asbestos compared with control (titanium dioxide).
MMP Inhibition Protects against Asbestos-Induced Fibrosis
A broad spectrum MMP inhibitor was used to determine the importance of metalloproteinases in asbestosis. GM6001 inhibits both MMP activity and disease development in murine models of asthma (17) and emphysema (34). GM6001 was intraperitoneally injected (0.2 mg per mouse, ∼ 10 mg/kg) every day beginning 1 d before asbestos instillation. Mice were intratracheally instilled with asbestos and killed 13 d later.
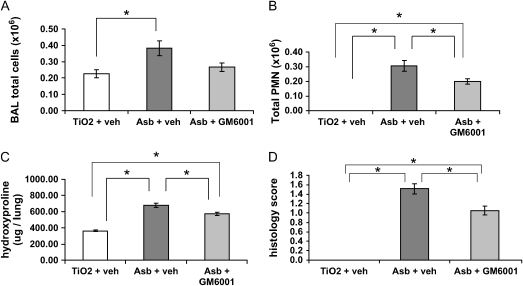
As we have shown previously (5), asbestos treatment led to an increase in BAL fluid white blood cell counts and especially neutrophils compared with titanium controls (Figure 7A). In contrast, asbestos-exposed mice, when treated with GM6001, did not have a significant increase in BAL white blood cells compared with controls (Figure 7A). Furthermore, the number of neutrophils infiltrating the lung in response to asbestos was significantly decreased in the GM6001 group compared with the asbestos/vehicle group (Figure 7B). These findings suggest that MMPs have a role in the inflammatory response to asbestos.
Figure 7.
MMP inhibition reduces inflammation and fibrosis after asbestos exposure. Wild-type mice were injected daily (intraperitoneally) with the broad-spectrum MMP inhibitor GM6001 beginning on Day 0. Mice were exposed to asbestos on Day 1 and killed on Day 13. (A) Total BAL fluid cells were not significantly increased in the asbestos + GM6001 group compared with TiO2 + vehicle controls. (B) BAL fluid neutrophils were significantly decreased in the asbestos + GM6001 group compared with asbestos + vehicle group. (C) Hydroxyproline levels were also significantly decreased after MMP inhibition, indicating decreased fibrosis. (D) The decrease in fibrosis was also observed using blinded histologic scoring. *P < 0.05 one-way ANOVA and Tukey's post test.
MMP inhibition also ameliorated the development of pulmonary fibrosis (Figure 7C). Total hydroxyproline was used as an indicator of collagen deposition in the lungs of mice treated with asbestos. Although both the asbestos/vehicle and asbestos/GM6001 groups exhibited significant increases in fibrosis compared with titanium controls, the asbestos/GM6001 group had a significant reduction (15%) in fibrosis compared with asbestos/vehicle (P < 0.01). Histologic scoring by a pathologist (T.D.O.) blinded to treatment group revealed a similar protection by GM6001 (Figure 7D). Therefore, inhibition of MMPs leads to a reduction in both inflammation and fibrosis in our experimental model of asbestosis.
DISCUSSION
Asbestosis is a debilitating fibrosing condition of the lung due to the inhalation of asbestos. Although exposure to asbestos in the United States has decreased, the long latency between exposure and disease development means that additional cases will continue to be diagnosed (2, 35). As asbestos has many of the histologic hallmarks of IPF, a better understanding of asbestos pathogenesis may aid in our understanding of IPF as well.
MMPs have been implicated in both human IPF (14) and experimental models of pulmonary fibrosis, including bleomycin- (21) and silica-induced models (22). In particular, MMP-2, MMP-7, and MMP-9 have been extensively studied in the context of pulmonary fibrosis (12, 26). However, these MMPs have not been studied in the context of asbestos-induced pulmonary fibrosis. In this study, we sought to determine whether MMPs are increased in response to a single intratracheal instillation of crocidolite asbestos and what role MMPs play in the development of asbestosis.
We describe here increases in MMP-2 and MMP-9 after asbestos instillation. MMP-9 was increased in lung homogenates at Day 1 only, but MMP-2 was increased at 1, 7, 14, and 28 d (Figures 1 and 2). Importantly, the active form of MMP-2 was particularly abundant during the chronic fibrosis phase (7–28 d) of this model. This suggests a large conversion from latent to active form, which could be due to increased proteolytic or oxidative activation of this MMP (9). TIMP-2 is required for the proteolytic activation of latent MMP-2 by MT1-MMP, and its parallel increase in response to asbestos suggests that cell-surface activation was likely responsible for the high levels of active MMP-2 we detected in our model. In the airspaces, as sampled by BAL fluid, an increase in both gelatinases was observed at all time points, with the most profound MMP-9 increases at 1 d (Figures 1 and 2).
Immunohistochemistry supported these results, as the most intense MMP-9 staining was found 1 d after asbestos (Figure 3), while MMP-2 had a more protracted expression pattern (Figure 4). MMP-9 localized to bronchiolar airspaces in conjunction with neutrophils and asbestos fibers, and its levels correlated with the degree of inflammation in the lung. Neutrophils produce and store large quantities of this MMP (15), indicating a possible role for MMP-9 in the inflammatory response to asbestos.
In control mice, MMP-2 was expressed in a few type II epithelial cells. After asbestos injury, staining was more diffuse over the alveolar septa, implying a release from cells into the interstitial space. Furthermore, MMP-2 was found in bronchiolar neutrophil aggregates and most strikingly was observed throughout areas of fibrosis. Similarly to MMP-9, its presence around neutrophils suggests a role in the inflammatory response to asbestos. In addition, MMP-2 is expressed by fibroblasts and has been hypothesized to play a role in the migration of fibroblasts in the lung (reviewed in Ref. 15), which may contribute to fibrosis development.
Interestingly, we did not observe increases in MMP-2 and MMP-9 RNA levels at 1, 7, or 14 d after asbestos exposure compared with controls. This suggests that the increased enzyme levels of these MMPs are not due to increased transcription, but possibly rather due to an increase in translation or release of these MMPs. Because MMP-9 is stored in neutrophils, enhanced release is the most likely process explaining its increased levels.
In contrast, MMP-7 expression was increased at 14 d after asbestos exposure compared with titanium dioxide controls and asbestos-treated mice at 24 h. MMP-7 is increased in human IPF and was found to play a role in bleomycin-induced experimental pulmonary fibrosis (26), but ours is the first analysis of MMP-7 in asbestos-related pulmonary disease. The increased RNA levels could indicate a similar role as in bleomycin lung injury, in which MMP-7 induces influx of neutrophils into the lung and airspaces. We have previously shown that neutrophils are still abundant in asbestos-treated mice 14 d after exposure to the fibers (4). Because it functions to control neutrophil influx, MMP-7 may be partially responsible for this inflammation.
The collagenases, MMP-8 and MMP-13, were also examined in our model system because the collagenase MMP-1 has been implicated in human pulmonary fibrosis (12). Although mice do not produce MMP-1, the other two collagenases may perform the same functions in the mouse. Despite previous reports of increased MMP-8 in BAL fluid from patients with IPF (19), we found that MMP-8 mRNA expression did not change in mice with asbestos treatment. However, this does not rule out a role for this MMP in asbestos injury, as MMP-8 could be transported to the lung by inflammatory cells without de novo synthesis in the lung, as we have demonstrated above for MMP-2 and MMP-9. We did find an increase in MMP-13 RNA levels at 14 d when compared with the controls and with the other time points. This upregulation may be a response to degrade the excess collagen that has accumulated at that time point. However, since significant collagen accumulation was evident at 14 d, it is clear that the upregulation of MMP-13 was not sufficient to prevent fibrosis. It is also notable that, in contrast to our findings, other investigators found a decrease of MMP-8 and MMP-13 in the lungs of mice with induced lung fibrosis (36). However, their model used paraquat and hyperoxia to induce fibrosis, which may differ significantly in its pathogenesis.
We also examined macrophage metalloelastase, or MMP-12. We demonstrated an increase in MMP-12 RNA levels at 14 d compared with the control to the other asbestos time points. These results agree with previous studies in the bleomycin model in which MMP-12 is upregulated in the lung after injury (23, 24). Swaisgood and coworkers hypothesized that MMP-12 plays a role in the degradation of basement membrane, thus contributing to an increase in capillary permeability in the lung (23). Our own previous studies have demonstrated an increase in BAL fluid protein and inflammatory cells in mouse lungs after asbestos treatment, which may be partially due to MMP-12 (5). Furthermore, as macrophages are activated by asbestos fibers, asbestos may also induce these cells to produce more MMP-12 (37).
TIMP levels were also examined using Western blotting. TIMP-1, which preferentially inhibits MMP-9, was not found at 1 d after asbestos exposure, which may allow increased MMP-9 activity at that time point. Interestingly, TIMP-1 deficiency in mice causes increased acute lung injury in response to asbestos, an effect that is associated with exaggerated pulmonary MMP-9 (38). The absence of detectable amounts of TIMP-1 in our studies could similarly potentiate acute lung injury in response to asbestos fibers.
On the other hand, increases in TIMP-1 were found at 7, 14, and 28 d. TIMP-2, which is required for MMP-2 activation but which also preferentially inhibits MMP-2 at higher levels, was also increased at 7 and 14 d over control values. Similar increases in TIMPs at later stages of nonasbestos models of fibrosis have been found by other investigators (12, 14, 21). This increase in TIMPs could be a compensatory response to the increased MMP activity shown in our studies. Alternatively, asbestos-induced increases in TIMPs may inhibit the collagenases responsible for degrading excess collagen, causing an imbalance in proteolytic activities that may lead to fibrosis development. In addition, TIMP-2, while inhibitory of most MMPs, could lead to increased activation of MMP-2 through a cell surface mechanism in which this TIMP brings MMP-2 into close proximity with MT–MMP-1 (20). The increased TIMP-2 levels could therefore be contributing to additional activation of MMP-2. Clearly, TIMPs can have complex functions in biological systems. The specific role of these inhibitors in asbestosis remains to be determined.
MMP inhibition has been used as an experimental therapy in animal studies of a number of pulmonary diseases, including asthma (17), emphysema (34), and bleomycin-induced pulmonary fibrosis (25). We used a broad spectrum MMP inhibitor, GM6001, using daily intraperitoneal injections to attempt to ameliorate disease in our asbestos model. MMP inhibition significantly decreased airspace neutrophil accumulation and the development of fibrosis, thereby protecting against asbestos-induced lung injury.
Other investigators have found similar reductions in fibrosis with MMP inhibition in other models of fibrosis (25). Our findings suggest that one mechanism by which MMP inhibition is protective is by dampening of inflammatory responses to asbestos. One possible explanation is that MMP inhibition could reduce the activity of MMP-7, which is highly upregulated in both human and mouse pulmonary fibrosis and is known to regulate the activity of neutrophil chemoattractants (26, 27). MMP-7 likely plays a similar role in our asbestosis model, as we find both increased MMP-7 and neutrophils after exposure to asbestos.
In addition, inhibition of MMP-2 and MMP-9 may have immunomodulatory effects during asbestos injury. For instance, it is known that collagen fragments such as those resulting from MMP activity are chemoattractants for neutrophils (39). Notably, it has been shown that MMP-2–deficient mice have reduced inflammation in their airspaces compared with wild-type mice in an asthma model of lung disease, indicating a role for this MMP in pulmonary inflammation (17). Furthermore, MMP-9 is capable of modulating immune responses through activation or potentiation of chemokines such as IL-8 (18). Some evidence exists to contradict this role, however, as MMP-9–null mice were not significantly protected from bleomycin-induced pulmonary fibrosis and did not have significantly decreased inflammation (40). Therefore, this MMP might not have a strong role in inflammation in spite of its production in inflammatory cells.
The MMP-9–null mice did have an observed deficit in alveolar bronchiolization after bleomycin injury (40). As bronchiolization may indicate a repair mechanism in the lung, the authors of this study hypothesized that MMP-9 plays a role in the regeneration of normal lung alveolar architecture after injury. MMP-9 may play a similar role in asbestosis. Also, roles for MMP-2 outside of inflammation should not be overlooked, as fibroblast production of MMP-2 in asbestos injury may play a role in fibroblast migration and therefore fibrosis.
In conclusion, we observed large increases in MMP-2, MMP-9, MMP-7, MMP-12, MMP-13, and TIMP-1 and -2 in the course of experimentally induced asbestosis in mice. While MMP-9 appeared to be related temporally to inflammation in the lung, MMP-2 was more strongly associated with time points revealing development of fibrosis. MMP-7 was also upregulated during the fibrotic phase, but its role may be to induce further inflammation by neutrophils, as found by other investigators (27). Indeed, MMP inhibition reduced both inflammation and fibrosis in response to asbestos fibers. MMP-12 and MMP-13 were demonstrated to be upregulated at 14 d. However, this increase apparently could not reverse the fibrotic effects of asbestos, as significant collagen accumulation was noted at that time point. These studies reveal a pathogenic role for MMPs in asbestosis and suggest a potential role in other particulate lung diseases.
Acknowledgments
The authors thank Andy Ghio for providing the crocidolite asbestos used in these studies.
This work was funded by grants R01HL063700–05 (T.D.O.), American Heart Association Established Investigator Award (T.D.O.), HL077555 (W.C.P.), and F30ES013621–01 (R.J.T.)
Originally Published in Press as DOI: 10.1165/rcmb.2005-0471OC on March 30, 2006
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Darcey DJ, Alleman T. Occupational and environmental exposure to asbestos. In: Roggli VL, Oury TD, Sporn TA, editors. Pathology of asbestos-associated diseases, 2nd ed. New York: Springer; 2004. pp. 17–33.
- 2.Kamp DW, Weitzman SA. Asbestosis: clinical spectrum and pathogenic mechanisms. Proc Soc Exp Biol Med 1997;214:12–26. [DOI] [PubMed] [Google Scholar]
- 3.Dai J, Churg A. Relationship of fiber surface iron and active oxygen species to expression of procollagen, PDGF-A, and TGF-beta(1) in tracheal explants exposed to amosite asbestos. Am J Respir Cell Mol Biol 2001;24:427–435. [DOI] [PubMed] [Google Scholar]
- 4.Fattman CL, Tan RJ, Tobolewski JM, Oury TD. Increased sensitivity to asbestos-induced lung injury in mice lacking extracellular superoxide dismutase. Free Radic Biol Med 2006;40:601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan RJ, Fattman CL, Watkins SC, Oury TD. Redistribution of pulmonary EC-SOD after exposure to asbestos. J Appl Physiol 2004;97:2006–2013. [DOI] [PubMed] [Google Scholar]
- 6.Kinnula VL. Oxidant and antioxidant mechanisms of lung disease caused by asbestos fibres. Eur Respir J 1999;14:706–716. [DOI] [PubMed] [Google Scholar]
- 7.Kinnula VL, Fattman CL, Tan RJ, Oury TD. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am J Respir Crit Care Med 2005;172:417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mossman BT, Churg A. Mechanisms in the pathogenesis of asbestosis and silicosis. Am J Respir Crit Care Med 1998;157:1666–1680. [DOI] [PubMed] [Google Scholar]
- 9.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 2004;4:617–629. [DOI] [PubMed] [Google Scholar]
- 10.Van Wart HE, Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci USA 1990;87:5578–5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selman M, Ruiz V, Cabrera S, Segura L, Ramirez R, Barrios R, Pardo A. TIMP-1, -2, -3, and -4 in idiopathic pulmonary fibrosis. A prevailing nondegradative lung microenvironment? Am J Physiol Lung Cell Mol Physiol 2000;279:L562–L574. [DOI] [PubMed] [Google Scholar]
- 12.Fukuda Y, Ishizaki M, Kudoh S, Kitaichi M, Yamanaka N. Localization of matrix metalloproteinases-1, -2, and -9 and tissue inhibitor of metalloproteinase-2 in interstitial lung diseases. Lab Invest 1998;78: 687–698. [PubMed] [Google Scholar]
- 13.Hayashi T, Stetler-Stevenson WG, Fleming MV, Fishback N, Koss MN, Liotta LA, Ferrans VJ, Travis WD. Immunohistochemical study of metalloproteinases and their tissue inhibitors in the lungs of patients with diffuse alveolar damage and idiopathic pulmonary fibrosis. Am J Pathol 1996;149:1241–1256. [PMC free article] [PubMed] [Google Scholar]
- 14.Suga M, Iyonaga K, Okamoto T, Gushima Y, Miyakawa H, Akaike T, Ando M. Characteristic elevation of matrix metalloproteinase activity in idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2000; 162:1949–1956. [DOI] [PubMed] [Google Scholar]
- 15.Corbel M, Belleguic C, Boichot E, Lagente V. Involvement of gelatinases (MMP-2 and MMP-9) in the development of airway inflammation and pulmonary fibrosis. Cell Biol Toxicol 2002;18:51–61. [DOI] [PubMed] [Google Scholar]
- 16.McQuibban GA, Gong JH, Tam EM, McCulloch CA, Clark-Lewis I, Overall CM. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science 2000;289:1202–1206. [DOI] [PubMed] [Google Scholar]
- 17.Corry DB, Rishi K, Kanellis J, Kiss A, Song Lz LZ, Xu J, Feng L, Werb Z, Kheradmand F. Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nat Immunol 2002;3:347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Opdenakker G, Van den Steen PE, Van Damme J. Gelatinase B: a tuner and amplifier of immune functions. Trends Immunol 2001;22:571–579. [DOI] [PubMed] [Google Scholar]
- 19.Henry MT, McMahon K, Mackarel AJ, Prikk K, Sorsa T, Maisi P, Sepper R, Fitzgerald MX, O'Connor CM. Matrix metalloproteinases and tissue inhibitor of metalloproteinase-1 in sarcoidosis and IPF. Eur Respir J 2002;20:1220–1227. [DOI] [PubMed] [Google Scholar]
- 20.Overall CM, Tam E, McQuibban GA, Morrison C, Wallon UM, Bigg HF, King AE, Roberts CR. Domain interactions in the gelatinase A.TIMP-2.MT1-MMP activation complex: the ectodomain of the 44-kDa form of membrane type-1 matrix metalloproteinase does not modulate gelatinase A activation. J Biol Chem 2000;275:39497–39506. [DOI] [PubMed] [Google Scholar]
- 21.Yaguchi T, Fukuda Y, Ishizaki M, Yamanaka N. Immunohistochemical and gelatin zymography studies for matrix metalloproteinases in bleomycin-induced pulmonary fibrosis. Pathol Int 1998;48:954–963. [DOI] [PubMed] [Google Scholar]
- 22.Perez-Ramos J, de Lourdes Segura-Valdez M, Vanda B, Selman M, Pardo A. Matrix metalloproteinases 2, 9, and 13, and tissue inhibitors of metalloproteinases 1 and 2 in experimental lung silicosis. Am J Respir Crit Care Med 1999;160:1274–1282. [DOI] [PubMed] [Google Scholar]
- 23.Swaisgood CM, French EL, Noga C, Simon RH, Ploplis VA. The development of bleomycin-induced pulmonary fibrosis in mice deficient for components of the fibrinolytic system. Am J Pathol 2000;157:177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swiderski RE, Dencoff JE, Floerchinger CS, Shapiro SD, Hunninghake GW. Differential expression of extracellular matrix remodeling genes in a murine model of bleomycin-induced pulmonary fibrosis. Am J Pathol 1998;152:821–828. [PMC free article] [PubMed] [Google Scholar]
- 25.Corbel M, Caulet-Maugendre S, Germain N, Molet S, Lagente V, Boichot E. Inhibition of bleomycin-induced pulmonary fibrosis in mice by the matrix metalloproteinase inhibitor batimastat. J Pathol 2001;193:538–545. [DOI] [PubMed] [Google Scholar]
- 26.Zuo F, Kaminski N, Eugui E, Allard J, Yakhini Z, Ben-Dor A, Lollini L, Morris D, Kim Y, DeLustro B, et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci USA 2002;99:6292–6297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 2002;111:635–646. [DOI] [PubMed] [Google Scholar]
- 28.Hedenborg M, Sorsa T, Lauhio A, Klockars M. Asbestos fibers induce release of collagenase by human polymorphonuclear leukocytes. Immunol Lett 1990;26:25–29. [DOI] [PubMed] [Google Scholar]
- 29.Morimoto Y, Tsuda T, Nakamura H, Hori H, Yamato H, Nagata N, Higashi T, Kido M, Tanaka II. Expression of matrix metalloproteinases, tissue inhibitors of metalloproteinases, and extracellular matrix mRNA following exposure to mineral fibers and cigarette smoke in vivo. Environ Health Perspect 1997;105S:1247–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fattman CL, Tan RJ, Tobolewski JM, Oury TD. Increased sensitivity to asbestos-induced lung injury in mice lacking extracellular superoxide dismutase. Free Radic Biol Med 2006;40:601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang JW, Gottschall PE. Zymographic measurement of gelatinase activity in brain tissue after detergent extraction and affinity-support purification. J Neurosci Methods 1997;76:15–20. [DOI] [PubMed] [Google Scholar]
- 32.Tan RJ, Lee JS, Manni ML, Fattman CL, Tobolewski JM, Zheng M, Kolls JK, Martin TR, Oury TD. Inflammatory cells as a source of airspace extracellular superoxide dismutase after pulmonary injury. Am J Respir Cell Mol Biol 2006;34:226–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oury TD, Schaefer LM, Fattman CL, Choi A, Weck KE, Watkins SC. Depletion of pulmonary EC-SOD after exposure to hyperoxia. Am J Physiol Lung Cell Mol Physiol 2002;283:L777–L784. [DOI] [PubMed] [Google Scholar]
- 34.Choe KH, Taraseviciene-Stewart L, Scerbavicius R, Gera L, Tuder RM, Voelkel NF. Methylprednisolone causes matrix metalloproteinase-dependent emphysema in adult rats. Am J Respir Crit Care Med 2003; 167:1516–1521. [DOI] [PubMed] [Google Scholar]
- 35.Selikoff IJ, Hammond EC, Seidman H. Latency of asbestos disease among insulation workers in the United States and Canada. Cancer 1980;46:2736–2740. [DOI] [PubMed] [Google Scholar]
- 36.Ruiz V, Ordonez RM, Berumen J, Ramirez R, Uhal B, Becerril C, Pardo A, Selman M. Unbalanced collagenases/TIMP-1 expression and epithelial apoptosis in experimental lung fibrosis. Am J Physiol Lung Cell Mol Physiol 2003;285:L1026–L1036. [DOI] [PubMed] [Google Scholar]
- 37.Quinlan TR, BeruBe KA, Hacker MP, Taatjes DJ, Timblin CR, Goldberg J, Kimberley P, O'Shaughnessy P, Hemenway D, Torino J, et al. Mechanisms of asbestos-induced nitric oxide production by rat alveolar macrophages in inhalation and in vitro models. Free Radic Biol Med 1998;24:778–788. [DOI] [PubMed] [Google Scholar]
- 38.Kim KH, Burkhart K, Chen P, Frevert CW, Randolph-Habecker J, Hackman RC, Soloway PD, Madtes DK. Tissue inhibitor of metalloproteinase-1 deficiency amplifies acute lung injury in bleomycin-exposed mice. Am J Respir Cell Mol Biol 2005;33:271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Monboisse JC, Bellon G, Randoux A, Dufer J, Borel JP. Activation of human neutrophils by type I collagen: requirement of two different sequences. Biochem J 1990;270:459–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Betsuyaku T, Fukuda Y, Parks WC, Shipley JM, Senior RM. Gelatinase B is required for alveolar bronchiolization after intratracheal bleomycin. Am J Pathol 2000;157:525–535. [DOI] [PMC free article] [PubMed] [Google Scholar]