In the process of meiosis, a diploid cell produces four haploid gametes, each of which carries but one copy of each chromosome. Before the two meiotic divisions, each set of homologs is composed of two fully replicated chromosomes, and thus four chromatids. At the first meiotic division, one homolog (composed of two sister chromatids) is segregated into each of the two daughter cells. The second meiotic division then separates the two chromatids comprising each chromosome. Unfortunately, the very purpose of meiosis, distributing the four chromatids into four gametes, creates a problem for greedy geneticists who would like to be able to recover all four of the original chromatids! In this issue of PNAS, Francis et al. provide an elegant and efficient solution to this problem in Arabidopsis thaliana (1).
The need to recover all four chromatids can be seen by considering the case where a pair of homologous chromosomes undergoes a single recombination (or cross-over) event. Because each such event involves only two of the four chromatids, only half of the four products of meiosis will carry a recombined chromosome. The other two products of meiosis will carry chromatids that are unrecombined and could have arisen from a meiosis in which no cross-over event had occurred at all! This problem only gets worse if two or more cross-overs occur per chromosome arm. Thus, recovering just one of the four products of meiosis often tells us very little about the number of exchanges that actually took place before the meiotic division.
There are algebraic approaches to solving this problem (2), but the best solution is to recover all four products of meiosis using a technique known as tetrad analysis (3). Tetrad analysis not only greatly facilitates genetic mapping but provides clear information on the number and distribution of exchanges. This issue is of special importance when one is concerned about phenomena such as cross-over interference, the process that regulates the spacing of exchanges along the length of meiotic chromosomes. Most critically, tetrad analysis allows a meiotic biologist to study a phenomenon known as gene conversion, where a DNA sequence from one homolog is replaced by corresponding sequence from the other homolog (converting a copy of allele “a” into allele “A”). The demonstration of gene conversion by Mitchell (4), and its association with sites of genetic recombination (5, 6), was critical to the development of modern molecular models of recombination (7–12). Unfortunately, until quite recently, tetrad analysis was possible only in a small number of unicellular systems, and thus geneticists have long hoped for a simple system for tetrad analysis in a multicellular eukaryote.
Francis et al. (1) report the development of exactly such a system of tetrad analysis in A. thaliana. The work expands upon the discovery of a mutant called quartet (qrt1) that causes the four products produced by each pollen meiosis to remain attached to each other, making tetrad analysis possible (13–15). Studies using this mutant have mapped the centromeres genetically in this organism and provided a thorough understanding of recombination in Arabidopsis (16, 17). However, as useful as having all four products of meiosis wrapped in a little floral box might be, one is still left with the problem of how to genotype the four spores (14). Ideally, one wants to be able to determine the genotype of each spore by just looking at it. Francis et al. achieve this goal by creating a large number of precisely mapped transgene constructs in the qrt1 mutant background (1). Each of these constructs encodes one of three fluorescent proteins (red, yellow, or cyan) under control of a pollen-specific promoter. Each insertion was mapped precisely onto the Arabidopsis genome sequence, and the authors used only those insertions that map in intergenic regions, noting that “it is important that disruption of genes by the marker does not confound the results.”
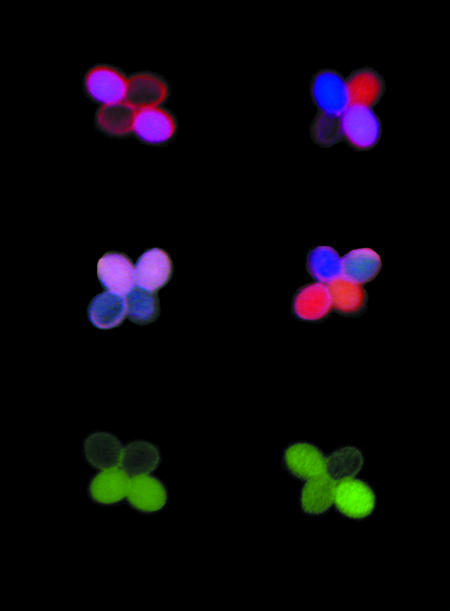
Fig. 1 provides a dramatic demonstration of the power of this technique. Look first at Fig. 1 Top, which shows the segregation of two linked insertions in a plant heterozygous for constructs encoding the red and cyan fluorescent proteins. In this case, the two transgene insertions are located on the same homolog, and so segregation without recombination between these markers yields a tetrad with two pink pollen grains and two colorless pollen grains. As described in the figure legend, the Top Right image displays the results of a single cross-over between these two insertions. Thus, this simple color-based assay allows one to quickly measure the frequency of recombination between two linked sites in either wild-type or meiotic mutant-bearing plants by a simple color-based assay of the pollen. As shown in Fig. 1 Middle, this method also allows the use of three linked insertions to measure the frequency of crossing over in two adjacent intervals, and the frequency of double cross-over events (the latter metric being crucial to any study of cross-over interference).
Fig. 1.
Visualizing meiotic recombination. The segregation of genes encoding different colors of fluorescent proteins enables the detection of recombination events in pollen tetrads produced by Arabidopsis qrt1 mutants. Plants heterozygous for genes encoding red and cyan fluorescent proteins arrayed on the same chromosome in cis (Top Left) shed tetrads with two pollen grains expressing both red and cyan protein (pink after digitally merging images taken using the appropriate filters). Single cross-overs between the markers (Top Right) can be visualized as tetrads with a cyan, a red, a pink, and a nonfluorescent pollen grain (the nonfluorescent grain shows minor background signal). Using three different fluorescent proteins, cyan, yellow, and red, arrayed in cis (Middle Left) allows the detection of double cross-overs (Middle Right), in this case, a four-chromatid double cross-over results in a tetrad with a cyan, a yellow, and cyan (light blue), a red, and a red and yellow (orange) pollen grain. Finally, heterozygous plants with one fluorescent allele and one nonfluorescent mutant allele of yellow fluorescent protein (Bottom Left) can be used to detect gene conversion events (Bottom Right). The tetrads in this figure were digitally positioned for ease of comparison. [I am indebted to Luke Berchowitz and Gregory P. Copenhaver (University of North Carolina, Chapel Hill, NC) for providing this figure.]
Finally, Fig. 1 Bottom beautifully displays its utility in measuring gene conversion. Heterozygous plants with one fluorescent allele and one nonfluorescent mutant allele of yellow fluorescent protein (Fig. 1 Bottom Left) can be used to detect gene conversion events (Fig. 1 Bottom Right). Until this paper, the frequency of such conversions had been sufficiently low as to escape detection (15). However, using this system, the authors were able to demonstrate six cases of gene conversion among 4,033 tetrads for a frequency of 1/336. As pointed out by the authors, this appears to be the first unambiguous observation of meiotic gene conversion in Arabidopsis using tetrad analysis.
The utility of this advance lies not only in its ability to properly measure exchange (both frequency and distribution) and gene conversion but also in the fact that the assay is rapid, inexpensive, and can be done in essentially isogenic lines. The authors could not be more correct in noting that “experiments that would have been difficult in the past because of laborious crossing schemes can now be done with relative ease.” Such an advance is all of the more critical because it comes at a time when the meiotic recombination studies are flourishing in this system (18–20), cytological analysis of Arabidopsis meiosis has been greatly improved (21), and new meiotic mutants are being rapidly discovered in this system (22–25).
The best solution is to recover all four products of meiosis using a technique known as tetrad analysis.
Specifically, the development of this efficient methodology for tetrad analysis will greatly facilitate the analysis of meiotic recombination in two critical ways. First, it will provide a straightforward means for identifying those gene conversion events that are associated with recombination. The ability to isolate these events and to analyze their structure has proven critical to the analysis of recombination-defective mutants in both yeast (9) and Drosophila (26, 27). As pointed out above, this analysis is critical to determining the actual mechanism of recombination used by a given organism. The two organisms, Saccharomyces cerevisiae and Saccharomyces pombe, for which we do understand how crossing over occurs, employ quite different mechanisms of meiotic recombination (12)! The ability to identify convertants easily in Arabidopsis will provide the important opportunity to determine which, if indeed either, of the two yeast paradigms is conserved in higher organisms. Second, the technique reported here will allow us to address the mechanisms that control the distribution of meiotic exchange in higher eukaryotes, and most importantly address the mechanism of cross-over interference (19). The technique presented by Francis et al. (1) will facilitate the identification of mutants defective in the maintenance of interference and in other mechanisms that control cross-over distribution. These mutants will provide the keys required to understand the mechanisms by which exchange distribution is controlled.
In summary, the fusion of a rapid and efficient method for recombinational analysis (including gene conversion), cytological methods that allow the study of the later stages of meiotic prophase and two meiotic divisions, and an ever-growing arsenal of meiotic mutants combine together to establish Arabidopsis as a premiere system in which to analyze meiotic biology, and in living color to boot!
Footnotes
The author declares no conflict of interest.
See companion article on page 3913.
References
- 1.Francis KE, Lam SY, Harrison BD, Bey AL, Berchowitz LE, Copenhaver GP. Proc Natl Acad Sci USA. 2007;104:3913–3918. doi: 10.1073/pnas.0608936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinstein A. Proc Sixth Intl Congress Genet. 1932;2:206–208. [Google Scholar]
- 3.Hawley RS, Walker MY. Advanced Genetic Analysis. Malden, MA: Blackwell; 2003. [Google Scholar]
- 4.Mitchell MB. Proc Natl Acad Sci USA. 1955;41:215–220. doi: 10.1073/pnas.41.4.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurst DD, Fogel S, Mortimer RK. Proc Natl Acad Sci USA. 1972;69:101–105. doi: 10.1073/pnas.69.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stadler DR, Towe AM. Genetics. 1971;68:401–413. doi: 10.1093/genetics/68.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paques F, Haber JE. Microbiol Mol Biol Rev. 1999;63:349–404. doi: 10.1128/mmbr.63.2.349-404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meselson M, Radding CM. Proc Natl Acad Sci USA. 1975;72:358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szostak JW, Orr-Weaver TL, Rothstein RJ, Stahl FW. Cell. 1983;33:25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- 10.Cromie GA, Hyppa RW, Taylor AF, Zakharyevich K, Hunter N, Smith GR. Cell. 2006;127:1167–1178. doi: 10.1016/j.cell.2006.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neale MJ, Keeney S. Nature. 2006;442:153–158. doi: 10.1038/nature04885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bishop DK. Cell. 2006;127:1095–1097. doi: 10.1016/j.cell.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 13.Preuss D, Rhee SY, Davis RW. Science. 1994;264:1458–1460. doi: 10.1126/science.8197459. [DOI] [PubMed] [Google Scholar]
- 14.Copenhaver GP, Keith KC, Preuss D. Plant Physiol. 2000;124:7–16. doi: 10.1104/pp.124.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Copenhaver GP, Browne WE, Preuss D. Proc Natl Acad Sci USA. 1998;95:247–252. doi: 10.1073/pnas.95.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lam SY, Horn SR, Radford SJ, Housworth EA, Stahl FW, Copenhaver GP. Genetics. 2005;170:807–812. doi: 10.1534/genetics.104.040055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Copenhaver GP, Housworth EA, Stahl FW. Genetics. 2002;160:1631–1639. doi: 10.1093/genetics/160.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones GH, Franklin FC. Cell. 2006;126:246–248. doi: 10.1016/j.cell.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Mezard C, Vignard J, Drouaud J, Mercier R. Trends Genet. 2007;23:91–99. doi: 10.1016/j.tig.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Mercier R, Jolivet S, Vezon D, Huppe E, Chelysheva L, Giovanni M, Nogue F, Doutriaux MP, Horlow C, Grelon M, et al. Curr Biol. 2005;15:692–701. doi: 10.1016/j.cub.2005.02.056. [DOI] [PubMed] [Google Scholar]
- 21.Jones GH, Armstrong SJ, Caryl AP, Franklin FC. Chromosome Res. 2003;11:205–215. doi: 10.1023/a:1022831724990. [DOI] [PubMed] [Google Scholar]
- 22.Franklin FC, Higgins JD, Sanchez-Moran E, Armstrong SJ, Osman KE, Jackson N, Jones GH. Biochem Soc Trans. 2006;34:542–544. doi: 10.1042/BST0340542. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JD, Sanchez-Moran E, Armstrong SJ, Jones GH, Franklin FC. Genes Dev. 2005;19:2488–2500. doi: 10.1101/gad.354705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mercier R, Armstrong SJ, Horlow C, Jackson NP, Makaroff CA, Vezon D, Pelletier G, Jones GH, Franklin FC. Development (Cambridge, UK) 2003;130:3309–3318. doi: 10.1242/dev.00550. [DOI] [PubMed] [Google Scholar]
- 25.Stacey NJ, Kuromori T, Azumi Y, Roberts G, Breuer C, Wada T, Maxwell A, Roberts K, Sugimoto-Shirasu K. Plant J. 2006;48:206–216. doi: 10.1111/j.1365-313X.2006.02867.x. [DOI] [PubMed] [Google Scholar]
- 26.Carpenter AT. Proc Natl Acad Sci USA. 1982;79:5961–5965. doi: 10.1073/pnas.79.19.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blanton HL, Radford SJ, McMahan S, Kearney HM, Ibrahim JG, Sekelsky J. PLoS Genet. 2005;1:e40. doi: 10.1371/journal.pgen.0010040. [DOI] [PMC free article] [PubMed] [Google Scholar]