Humans require a minimum daily intake of essential micronutrients, vitamins, and minerals to maintain optimal health. Micronutrient malnutrition, the dietary insufficiency of one or more micronutrients, has far-reaching negative health consequences at all stages of life and was a pervasive health issue for all countries at the turn of the 20th century. Micronutrient malnutrition has been significantly alleviated for those populations in developed countries as a result of programs established in the mid-20th century that fortified processed foods with the necessary micronutrients. Similar fortification efforts have had only modest success in developing countries because an industrial level of agriculture, food-processing, and distribution that is limited or lacking in many of the targeted countries is required. Micronutrient malnutrition thus has remained a widespread and persistent global health problem in developing countries where it continues to exact an enormous toll on individuals, populations, and society (1).
In the past decade, a complementary approach to fortification of processed foods, termed biofortification, has been undertaken to deliver the necessary daily micronutrients directly in the staple crops being grown and consumed by at-risk populations in developing countries (2). All plants have the biochemical activities necessary to synthesize or accumulate a near full complement of essential dietary micronutrients (the exceptions being vitamins D and B12). Unfortunately, the plant-based foods most abundantly consumed by at-risk populations (e.g., rice, wheat, cassava, and maize) contain levels of several individual micronutrients that are insufficient to meet minimum daily requirements, even when the crop is the most abundant component of the diet. Biofortification seeks to increase the levels of specific, limiting micronutrients directly in crops by combined breeding and genetic-engineering approaches. Significant progress has been made in biofortification of vitamin E and provitamin A in plants (3–5), and in this issue of PNAS, the work of Diaz de la Garza et al. (6) extends the approach to include folate, one of the most important of the B vitamins in the human diet.
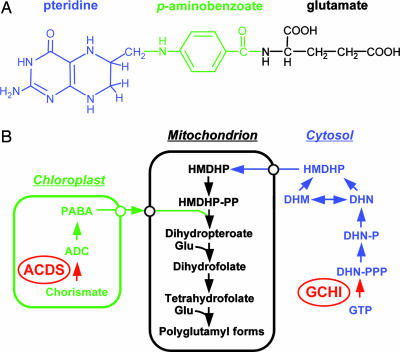
The recommended dietary allowance for folate ranges from 400 μg per day for adults to 600 μg per day for pregnant women (7). Plant-based foods are the primary source of folate in the human diet. Folate is a complex molecule that is assembled from three different molecular components: pteridine, para-amino benzoic acid (PABA), and glutamate. The pteridine moiety is synthesized in the plant cytosol, PABA is synthesized in the chloroplast, and folates are synthesized from these two precursors in the plant mitochondria (Fig. 1). In addition to biosynthetic enzymes, membrane-bound transporters for folates and various pathway intermediates must be present (8). Previous studies (9, 10) have shown that overexpression of the first committed enzyme of the cytosolic pteridine pathway, GTP cyclohydrolase I (GCHI), resulted in a 100- to 1,000-fold increase in pteridine levels but only a 2- to 3-fold increase in folates. In tomato fruit engineered for GCHI overexpression, endogenous PABA levels were depleted, suggesting that PABA synthesis might limit folate levels in the transgenic lines. Consistent with this hypothesis, folate levels could be further increased in tomato fruit overexpressing CGHI by exogenous application of PABA (9).
Fig. 1.
Structure and biosynthesis of folates in plants. (A) The chemical structure of monoglutamyl tetrahydrofolate is shown. The pteridine-, PABA-, and glutamate-derived moieties are color-coded. (B) The plant folate biosynthetic pathway is shown. The pteridine pathway leading to hydroxymethyldihydropterin (HMDHP) is shown in blue, the pathway leading to p-aminobenzoate is shown in green, and steps localized in the mitochondria are in black. Open circles indicate possible transporters. Red arrows indicate the two enzymes (GCHI and ADCS) engineered by Diaz de la Garza et al. (6). DHN, dihydroneopterin; -P, monophosphate; -PP, pyrophosphate; -PPP, triphosphate; DHM, dihydromonapterin.
Diaz de la Garza et al. (6) report engineering of PABA levels in tomato fruit by overexpression of the nuclear-encoded, chloroplast-targeted enzyme aminodeoxychorismate (ADC) synthase (ADCS). The best ADCS transgenic lines accumulated PABA in tomato fruit at levels nearly 20-fold higher than controls, although folate levels were still unchanged relative to controls. When the ADCS-overexpressing lines were crossed to previously characterized GCHI-overexpressing lines (9), the total folate level of fruit was increased up to 25-fold relative to controls. Indeed, the folate levels reported for ADCS/GCHI double transgenics were several times higher than those for green leafy vegetables, which are considered some of the best sources of folate in the diet. The level of folate accumulated in ADCS/GCHI transgenic fruit tissue (840 μg to 100 g) would be more than sufficient to provide the entire recommended dietary allowance of folate for a pregnant woman (600 μg per day).
Although folate levels were substantially increased in the ADCS/GCHI double transgenics, PABA and pteridines were still present at elevated levels, implying that other pathway activities or membrane-bound transporters still constrain folate synthesis in the transgenic fruit. In considering the future implementation of folate biofortification in the food supply, the accumulation of folate pathway intermediates, PABA and pteridines, in ref. 6 must be considered. In this regard, it is important to stress that PABA is already classified as a “generally recognized as safe” food additive and has been shown to have low toxicity at intake levels >10 times that present in a 100-g serving of ADCS/GCHI double transgenics. For pteridines, such concerns are less clear, although it is important to note that humans do both synthesize and degrade pteridines and that the pteridine levels in transgenic fruit represent <10% of the total pteridines excreted daily in human urine (11).
It should also be stressed that the primary goal of the experiments reported by Diaz de la Garza et al. (6) was to maximize the production of folates in plant tissues rather than to minimize accumulation of pathway intermediates. They have accomplished this goal by driving the expression of both ADCS and GCHI from strong fruit-specific promoters and selecting lines with the highest expression level for each transgene for subsequent crosses (6, 9). The high levels of expression selected for each gene construct undoubtedly contribute to the elevated levels of pathway intermediates in ADCS/GCHI double transgenics. However, as with all transgenic experiments, a range of expression levels was observed for both ADCS and GCHI overexpression lines (6, 9), and crossing lines with more modest levels of overexpression might lead to a reduction in accumulation of intermediates, while still allowing significant enhancement of the level of folates in plant tissues. Alternatively, subsequent engineering efforts to apply the folate enhancement technology so clearly demonstrated by Diaz de la Garza et al. to biofortify the folate level of staple crops could use promoters with more moderate expression levels to achieve folate increases while limiting accumulation of pathway intermediates. Regardless of these issues for future implementation of folate biofortification, the work of Diaz de la Garza et al. (6) and others (9, 10, 12, 13) has opened the door for enhancing folate levels in a wide range of agricultural crops, including those consumed as staples by populations in developing countries. Although folate biofortification has clear potential beneficial impacts in developed countries, given the greater prevalence and dire consequences of folate deficiency in developing countries (14, 15), the benefits to be derived from folate biofortification in world staple crops have the potential to be proportionately greater.
Footnotes
The authors declare no conflict of interest.
See companion article on page 4218.
References
- 1.Darnton-Hill I, Webb P, Harvey PW, Hunt JM, Dalmiya N, Chopra M, Ball MJ, Bloem MW, de Benoist B. Am J Clin Nutr. 2005;81:1198S–1205S. doi: 10.1093/ajcn/81.5.1198. [DOI] [PubMed] [Google Scholar]
- 2.Bouis HE. Proc Nutr Soc. 2003;62:403–411. doi: 10.1079/pns2003262. [DOI] [PubMed] [Google Scholar]
- 3.DellaPenna D, Pogson B. Annu Rev Plant Biol. 2006;57:711–738. doi: 10.1146/annurev.arplant.56.032604.144301. [DOI] [PubMed] [Google Scholar]
- 4.Shintani D, DellaPenna D. Science. 1998;282:2098–2100. doi: 10.1126/science.282.5396.2098. [DOI] [PubMed] [Google Scholar]
- 5.Ye X, Al-Babili S, Kloti A, Zhang J, Lucca P, Beyer P, Potrykus I. Science. 2000;287:303–305. doi: 10.1126/science.287.5451.303. [DOI] [PubMed] [Google Scholar]
- 6.Diaz de la Garza RI, Gregory JF, III, Hanson AD. Proc Natl Acad Sci USA. 2007;104:4218–4222. doi: 10.1073/pnas.0700409104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey LB, Gregory JF., III J Nutr. 1999;129:779–782. doi: 10.1093/jn/129.4.779. [DOI] [PubMed] [Google Scholar]
- 8.Klaus SM, Kunji ER, Bozzo GG, Noiriel A, Diaz de la Garza R, Basset GJ, Ravanel S, Rebeille F, Gregory JF, III, Hanson AD. J Biol Chem. 2005;280:38457–38463. doi: 10.1074/jbc.M507432200. [DOI] [PubMed] [Google Scholar]
- 9.Diaz de la Garza R, Quinlivan EP, Klaus SM, Basset GJ, Gregory JF, III, Hanson AD. Proc Natl Acad Sci USA. 2004;101:13720–13725. doi: 10.1073/pnas.0404208101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hossain T, Rosenberg I, Selhub J, Kishore G, Beachy R, Schubert K. Proc Natl Acad Sci USA. 2004;101:5158–5163. doi: 10.1073/pnas.0401342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Messahel S, Pheasant AE, Pall H, Kerr AM. Eur J Pediatr Neurol. 2000;4:211–217. doi: 10.1053/ejpn.2000.0308. [DOI] [PubMed] [Google Scholar]
- 12.Basset GJ, Quinlivan EP, Ravanel S, Rebeille F, Nichols BP, Shinozaki K, Seki M, Adams-Phillips LC, Giovannoni JJ, Gregory JF, III, Hanson AD. Proc Natl Acad Sci USA. 2004;101:1496–1501. doi: 10.1073/pnas.0308331100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Basset GJ, Ravanel S, Quinlivan EP, White R, Giovannoni JJ, Rebeille F, Nichols BP, Shinozaki K, Seki M, Gregory JF, III, Hanson AD. Plant J. 2004;40:453–461. doi: 10.1111/j.1365-313X.2004.02231.x. [DOI] [PubMed] [Google Scholar]
- 14.Rush D. Am J Clin Nutr. 2000;72:212S–240S. doi: 10.1093/ajcn/72.1.212S. [DOI] [PubMed] [Google Scholar]
- 15.Sifakis S, Pharmakides G. Ann NY Acad Sci. 2000;900:125–136. doi: 10.1111/j.1749-6632.2000.tb06223.x. [DOI] [PubMed] [Google Scholar]