Abstract
The formation of a lumen in three-dimensional mammary epithelial acinar structures in vitro involves selective apoptosis of centrally localized cells that lack matrix attachment. Similarly, apoptosis is induced by forced detachment of mammary epithelial cells from matrix, a process referred to as anoikis. Through microarray analysis, we found that mRNA levels of the proapoptotic BH3-only protein Bmf are up-regulated during both anoikis and acinar morphogenesis. Importantly, down-regulation of Bmf expression by small interfering RNAs is sufficient to prevent anoikis and acinar cell death and promote anchorage-independent growth to a similar extent as down-regulation of another BH3-only protein, Bim, which was previously shown to be required for these processes. Knockdown of the BH3-only proteins Bad or Bid does not suppress anoikis or luminal apoptosis or promote anchorage-independent growth, but protects from other defined apoptotic stimuli, indicating specificity of BH3-only function. Bmf mRNA is significantly up-regulated upon loss of matrix attachment or disruption of the actin cytoskeleton, but not in response to several other stresses. Interestingly, constitutive activation of the Mek/Erk or phosphatidylinositol 3-kinase/Akt pathways suppresses the transcriptional up-regulation of Bmf during anoikis. Thus, Bmf is a central mediator of anoikis in mammary cells and a target of oncogenes that contribute to the progression of glandular epithelial tumors. Finally, Bmf is expressed during involution of the mouse mammary gland, suggesting that Bmf may also critically contribute to developmental processes in vivo.
Keywords: apoptosis, breast, cancer, extracellular matrix
Within a multicellular organism, the balance of cell proliferation and programmed cell death, in particular apoptosis, contributes to the control of cell numbers at defined locations and also plays an important role in maintaining tissue homeostasis (1). Alteration of cell death regulation can result in inappropriate survival and, ultimately, disease. Survival of epithelial cells depends on adhesion to extracellular matrix (ECM) and is controlled through coordinated integrin and growth factor signaling (2). Upon deprivation of ECM attachment, normal epithelial cells undergo a specific form of cell death termed “anoikis” (3). Apoptosis has also been reported to be required for clearing of terminal end buds and ducts during pubertal development and involution of the mammary gland (4, 5) and, more generally, is implicated in the cavitation process of 3D structures (6, 7). Filling of luminal space of glandular structures is an often observed, yet still poorly understood, hallmark of early tumorigenesis. Apoptosis of matrix-deprived cells that proliferate in the lumen of glandular structures may thus serve as an initial barrier of epithelial tumor formation and progression (8). It is therefore important to identify the molecular targets and mechanisms implicated in anoikis and to understand how oncogenes target the molecules critical for apoptosis.
The Bcl-2 family of proteins comprises antiapoptotic (such as Bcl-2 or Bcl-xL) and proapoptotic members that function coordinately to control caspase-dependent death within a cell. The proapoptotic members of the family fall into two categories: multidomain Bax-like molecules (such as Bax or Bak) and BH3-only proteins (such as Bad, Bid, Bik, Bim, Bmf, Hrk, Noxa, and Puma), which contain a single BH3 (Bcl-2 homology 3) domain and function as sensors or sentinels in response to cell damage signals. Members of this family are regulated at several different levels, such as (transcriptional) control of expression or posttranslational modification(s). BH3-only proteins antagonize the function of antiapoptotic proteins, and some members may additionally promote activity of Bax-like multidomain proteins (9, 10). In light of the complex regulation of BH3-only proteins (11), further knowledge on functional BH3-only specificity in response to defined apoptotic stimuli is required.
The BH3-only factor Bmf was originally identified as an actin cytoskeleton-associated member of the BH3-only family and proposed to function in anoikis based on its relocalization to mitochondria upon matrix detachment (12). However, it remains to be determined whether Bmf indeed plays a functional role in anoikis. More recently, Bmf has been functionally implicated in the apoptotic response to histone deacetylase (HDAC) inhibitors and TGF-β treatment (13, 14).
Immortalized, nontransformed MCF-10A human mammary epithelial cells undergo anoikis when detached from matrix and also form acinus-like structures through a series of morphogenetic events when cultured on reconstituted basement membrane (15). Previous studies from our laboratory have demonstrated a functional role for the BH3-only protein Bim in MCF-10A anoikis and lumen formation (16, 17). In addition, we have recently found that Bim is required for apoptotic processes involved in lumen formation during pubertal expansion of the mammary gland (5), validating the use of the MCF-10A system to model in vivo processes associated with matrix detachment (18).
Here we address questions relating to the specificity and functional activities of BH3-only proteins by using small interfering (si) RNAs to knock down selected BH3-only factors. We find that functional loss of Bmf, but not of Bad or Bid, is sufficient to confer protection from cell death both in MCF-10A anoikis and 3D morphogenesis. Moreover, we show that expression of Bmf is controlled downstream of matrix attachment and oncogenic pathways. Our findings also point to a function for Bmf as an epithelial tumor suppressor and a potential mediator in mammary gland involution. Taken together, our report contributes to the knowledge of how cell death, induced by the BH3-only protein Bmf, may be regulated in development and targeted in oncogenesis.
Results
Up-Regulation of Bmf During Anoikis and Acinar Morphogenesis.
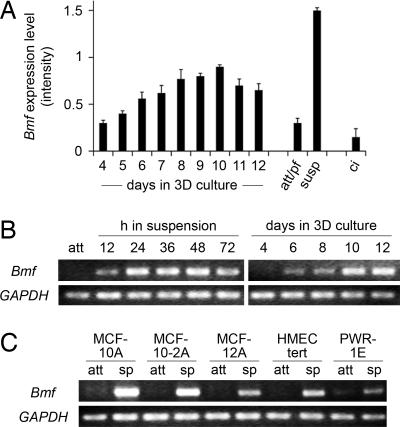
To identify common regulators of apoptotic events associated with both anoikis and lumen formation during mammary morphogenesis in vitro, we analyzed mRNA expression by using cDNA microarrays. RNA was isolated from MCF-10A cells in 3D culture on reconstituted basement membrane and from proliferating or confluent monolayers, as well as cells detached from matrix. Detailed description and analysis of microarray data will be reported elsewhere (T.S., N.L.S., E.S.L., O.P.V. and J.S.B., data not shown). To specifically identify genes that may regulate the apoptotic program during anoikis and morphogenesis, but are not primarily affected by the cell cycle, we selected genes that are up-regulated in suspension and morphogenesis, but are unaffected or down-regulated in contact-inhibited cells [supporting information (SI) Fig. 5 A and B ]. Candidates in the cell death gene ontology category included two proapoptotic BH3-only family members, namely Bmf (Bcl-2-modifying factor) and Bim (Bcl2L11), as well as the Forkhead transcription factor FOXO3A and the small GTPase RhoB (Fig. 1A and SI Fig. 5C Top). Given the potential of one of the identified candidates, Bmf, in anoikis regulation (12) and its homology to the previously reported mediator, Bim (16, 17), we focused on characterizing its functional role(s) and transcriptional regulation in subsequent studies.
Fig. 1.
Up-regulation of Bmf RNA levels in anoikis and morphogenesis. (A) Microarray expression profile for Bmf (U133B array, probeset ID 226530_at). Shown is the relative signal intensity for RNA samples from a 3D culture time course, from attached subconfluent (proliferating) cell monolayers (att/pf), from cells cultured in suspension for 24 h (susp), and from confluent (contact-inhibited) cell monolayers (ci). Error bars equal ± SD of three replicate microarray samples. (B) Bmf is up-regulated in suspension and morphogenesis. RT-PCR products for Bmf and GAPDH were derived from MCF-10A RNA samples from attached (att) cells, cells placed in suspension (Left), or cells grown in 3D culture (Right). (C) Bmf is up-regulated in diverse epithelial cell lines upon matrix detachment. RT-PCR products for Bmf and GAPDH were derived from RNA samples from indicated cell lines cultured as attached monolayers (att) or in suspension (sp) for 24 h (HMECtert) or 36 h (all other lines), respectively.
To validate the Bmf expression pattern observed in the microarrays, RT-PCR analysis was performed on RNA samples derived from attached or suspended cells, as well as acinar structures, confirming significant transcriptional up-regulation of Bmf in anoikis and morphogenesis (Fig. 1B).
To further investigate whether transcriptional up-regulation of Bmf upon matrix detachment is a general feature of epithelial cells, RNA samples from several other human epithelial cell lines (mammary: MCF-10−2A, MCF-12A, HMECtert; prostate: PWR-1E) were analyzed for Bmf expression. In all examined lines, Bmf expression was up-regulated upon matrix detachment (Fig. 1C), indicating that transcriptional control through adhesion is likely to function as a general regulatory mechanism for Bmf expression in epithelial cells.
Suppression of Anoikis and Luminal Cell Death by Knockdown of Bmf, but Not of Bad or Bid.
To examine the functional role of Bmf in anoikis and luminal cell death, and more broadly the potential contribution of other BH3-only factors (in particular, Bad, Bid, and, as a reference control, Bim), stable cell lines expressing retro- and lentiviral small hairpin (sh) RNAs were generated in the MCF-10A background.
Knockdown of endogenous BH3-only proteins by shRNA vectors (or synthetic siRNA SMARTpools) was demonstrated by immunoblotting analysis (SI Fig. 6 A–C). We were unable to monitor Bmf protein knockdown because all commercially available antibodies failed to reliably detect Bmf protein in MCF-10A cells. However, the efficacy of the Bmf shRNA vector and siRNA duplexes in down-regulating Bmf protein was confirmed in cells expressing a 3x-FLAG-tagged variant of human Bmf (SI Fig. 6 D). In addition, we found that knockdowns were highly specific for each BH3-only protein, e.g., knockdown of Bmf did not reduce the levels of Bim (data not shown).
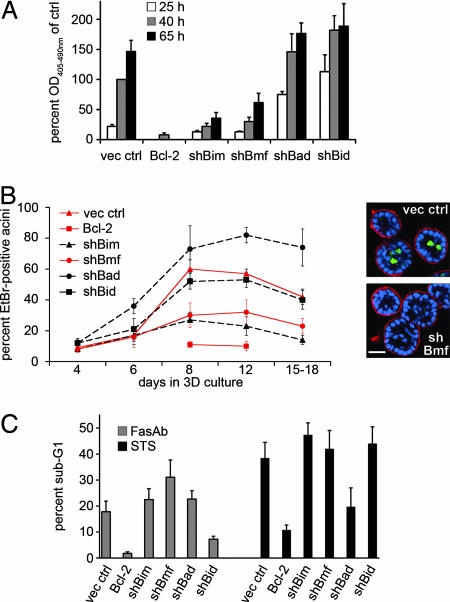
To investigate the role of the above BH3-only proteins in apoptosis upon detachment from matrix, we examined the extent of DNA fragmentation in cells expressing shRNAs targeting Bim, Bmf, Bad, and Bid, as well as cells expressing Bcl-2 as an antiapoptotic control. Knockdown of either Bmf or Bim [as shown previously for Bim by using synthetic siRNA (17)] inhibited cell death in suspension (Fig. 2A). Inhibition of apoptosis in suspended cells by the knockdown of Bmf was also confirmed by immunofluorescent detection of cleaved caspase-3 (control: 18 ± 5%, shBmf: 6 ± 1% at 40 h in suspension; data not shown). Knockdown of either Bad or Bid did not prevent DNA fragmentation in suspended cells (Fig. 2A).
Fig. 2.
Biological effects and specificity of BH3-only knockdowns. (A) Knockdown of Bmf, but not Bad or Bid, prevents anoikis. DNA fragmentation was assayed in MCF-10A cells expressing empty vector control (vec ctrl), Bcl-2 or shRNA vectors for Bim (shBim), Bmf (shBmf), Bad (shBad), or Bid (shBid) that were cultured in suspension for indicated times. Data are represented as the mean absorbance readings at 405–490 nm relative to control cells at 40 h in suspension. (B) Knockdown of Bmf, but not Bad or Bid, prevents luminal cell death. (Left) Cell lines described in A were cultured in Matrigel, and luminal cell death was assessed by EtBr staining on indicated days. (Right) Acinar structures derived from vector control and shBmf cells were stained for cleaved caspase-3 (green) and laminin 5 (red) on day 8 and analyzed by confocal microscopy (nuclei in blue). (C) Knockdown of Bad or Bid protects against defined apoptotic stimuli. Cell lines described in A were subjected to FasAb or STS treatment and analyzed by flow cytometry of propidium iodide-stained samples. Data are represented as the mean percentages of subG1 DNA content cells. All error bars equal ± SD of at least three independent experiments. (Scale bar: 25 μm.)
Additional independent shRNA vectors for Bim and Bad induced effects similar to those described above (data not shown). Also, shRNA effects were reproduced in experiments by using transient transfection of siRNA SMARTpools targeting Bmf and Bid (SI Fig. 7 A). These control experiments support the conclusion that the observed phenotypes were not due to off-target effects.
To examine the functional role of Bmf, Bad, and Bid in luminal cell death of acini-like structures in 3D, knockdown cell lines were cultured on exogenous basement membrane, and cell death was investigated over the time course of culture. Individual knockdown of Bmf (or Bim, as shown in ref. 16) significantly prevented apoptosis as assayed by ethidiumbromide (EtBr) staining (Fig. 2B Left) or activation of caspase-3 (control: 48 ± 8%, shBmf: 20 ± 6% at day 8 in morphogenesis) (Fig. 2B Right). Again, knockdown of Bad or Bid did not suppress luminal cell death (Fig. 2B Left).
In summary, our data demonstrate that individual reduction of Bmf (or Bim) levels specifically inhibits cell death during MCF-10A anoikis and acinar morphogenesis, whereas reduction of other BH3-only proteins, namely Bad and Bid, does not.
Validation of Bad and Bid Knockdowns and Functional Rescue of Bmf Knockdown.
To confirm that the knockdowns of Bad and Bid are sufficient to confer apoptotic protection in MCF-10A cells, we analyzed the response of the BH3-only knockdown lines to a series of diverse cell death stimuli. Treatment with activating anti-Fas antibody (FasAb) or staurosporine (STS) triggered apoptosis in control cells, whereas cells overexpressing Bcl-2 were largely protected against both stimuli. Interestingly, only cells with Bid knockdown were significantly and specifically protected against FasAb-induced apoptosis, whereas only cells with Bad knockdown exhibited partial, but significant, protection against STS-induced cell death (Fig. 2C, P < 0.001 by t test). These results validate the biological functionality of the Bid and Bad knockdowns and suggest that the inability of these shRNAs to protect in anoikis and morphogenesis reflects an absence of a functional role of Bid and Bad in these processes, rather than a failure to protect from apoptotic stimuli.
To further examine specificity of the Bmf shRNA vector, we conducted functional rescue experiments with cells expressing different combinations of Bmf cDNA and shRNA vectors. Cells overexpressing Bmf were sensitized to apoptosis upon matrix detachment (SI Fig. 7 B Upper), indicating that Bmf is an important contributor to anoikis. Whereas human Bmf, even when overexpressed, was still targeted by the Bmf shRNA vector, shRNA-resistant mouse Bmf (three mismatches within 19 nucleotide human targeting sequence; SI Fig. 7 B Lower) was capable of completely reverting the protection provided by the shBmf vector in suspension (SI Fig. 7 B Upper). Interestingly, knockdown of Bim in cells overexpressing human or mouse Bmf was still capable of at least partially suppressing anoikis (SI Fig. 7 B Upper). These results further confirm that our findings with the Bmf shRNA vector are specifically related to Bmf, and not a consequence of nonspecific, i.e., off-target, effects.
Transcriptional Regulation of Bmf Expression.
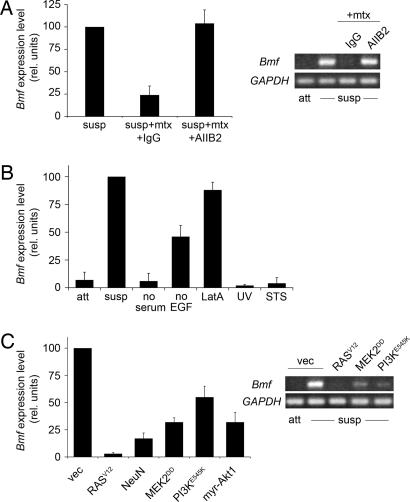
Quantitative real-time RT-PCR analysis confirmed induction of Bmf expression following matrix detachment. Addition of exogenous basement membrane proteins derived from Engelbreth–Holm–Swarm tumor cells (Matrigel) to suspended cells largely blocked Bmf induction, whereas addition of a function-blocking antibody for the β1-integrin subunit (AIIB2) prevented the matrix-mediated suppression of Bmf induction (Fig. 3A). Therefore, cell-matrix adhesion by integrin signaling represents one of the main inputs controlling Bmf mRNA expression.
Fig. 3.
Transcriptional regulation of Bmf expression. (A) Loss of β1-integrin interaction triggers up-regulation of Bmf. (Left) Total RNA samples derived from MCF-10A cells cultured in suspension for 40 h (susp) or in suspension for 40 h with addition of 5% Matrigel basement membrane extract (mtx), and either control isotype IgG or β1-integrin blocking antibody (AIIB2) (75 μg/ml) were subjected to real-time RT-PCR analysis for Bmf expression. (Right) Representative examples of semiquantitative RT-PCR analysis are shown. (B) Bmf expression is controlled downstream of actin cytoskeleton integrity. Total RNA samples derived from MCF-10A cells cultured as attached monolayers (att), in suspension (susp; 24 h), starved for either serum or EGF (24 h), treated with Latrunculin A (LatA) (24 h) or STS (9 h), or irradiated with UV (sample collected at 9 h) were subjected to real-time RT-PCR analysis. (C) Up-regulation of Bmf in suspension is suppressed by oncogenic signaling through Erk and Akt. (Left) Total RNA samples derived from MCF-10A cells expressing empty vector (vec), RASV12, NeuN, MEK2DD, PI3KE545K, or myr-Akt1 and placed in suspension for 40 h were subjected to real-time RT-PCR analysis. (Right) Representative examples of semiquantitative RT-PCR analysis for indicated cell lines are shown. Bmf expression levels were normalized to GAPDH levels and represented relative to control cells in suspension. All error bars equal ± SD of at least three independent experiments.
To further investigate the transcriptional regulation of Bmf, we examined expression of Bmf mRNA under a variety of different culture conditions (Fig. 3B). Serum withdrawal or treatment with the apoptotic stimuli STS or UV irradiation did not result in detectable induction of Bmf levels. Withdrawal of EGF caused an induction of Bmf compared with control cells growing in full media. Finally, inhibition of actin polymerization by treatment of attached cells with Latrunculin A induced significant up-regulation of Bmf levels (Fig. 3B). In summary, disruption of the actin microfilaments or, to a lesser extent, EGF withdrawal elicited induction of Bmf in attached cells to a similar extent as that detected in suspended cells lacking integrin engagement. Thus, expression of Bmf mRNA appears to be regulated downstream of a program linked to actin cytoskeleton integrity.
To examine the signaling requirements for Bmf induction, we monitored expression of Bmf mRNA in a variety of transformed MCF-10A lines. Ectopic expression of either an activated, oncogenic allele of Ras (RASV12) or NeuN (HER2/ErbB2) resulted in significant suppression of Bmf induction upon matrix detachment when compared with vector control cells (Fig. 3C), in good correlation with suppression of anoikis (SI Fig. 8). Given the contribution of Mek/Erk and phosphatidylinositol 3-kinase (PI3K)/Akt branches downstream of integrins and growth factor receptors in MCF-10A survival signaling (17, 19), we concentrated on these two signaling branches to probe their contribution to transcriptional regulation of Bmf. Therefore, we investigated effects of either specific activation of the Erk signaling branch by a constitutively active form of Mek2 (MEK2DD) or the Akt signaling branch by an activated, oncogenic mutant of the p110α catalytic subunit of PI3K (PI3KE545K), or by activated, myristoylated Akt1 (myr-Akt1) on Bmf expression upon matrix detachment. Effects on Bmf induction in suspension again paralleled the suppression of DNA fragmentation (SI Fig. 8), with MEK2DD and myr-Akt1 cells significantly reducing the level of Bmf induction during anoikis and PI3KE545K cells suppressing, albeit to a smaller extent, up-regulation of Bmf (Fig. 3C). Taken together, oncogenic transformation by RASV12 or NeuN, or activation of either the Erk or Akt signaling pathways, is sufficient to suppress up-regulation of Bmf in suspension. Our findings thus place the transcriptional regulation of Bmf under the control of two major pathways known to contribute to epithelial tumorigenesis when deregulated.
Function of Bmf as Suppressor of Transformation in Vitro.
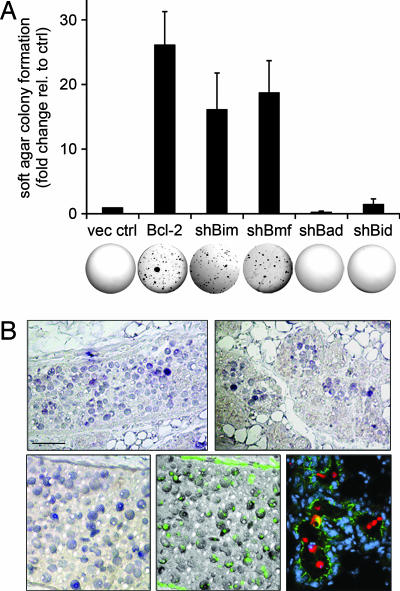
Based on our findings of transcriptional regulation of Bmf by oncogenic pathways and the functional role of Bmf in cell death processes relevant to oncogenesis, we examined whether reduction of BH3-only proteins is sufficient to confer anchorage-independent growth potential to MCF-10A cells. Retroviral vectors encoding either Bim, Bmf, Bad, or Bid shRNAs or Bcl-2 cDNA were transduced into cells expressing a proliferative oncogene, HPV16 E7 (20), and assayed for colony formation in soft agar. Whereas E7-expressing cells infected with vector control formed few colonies (typically <50), E7 cells overexpressing Bcl-2 gave rise to 20- to 30-fold more colonies (Fig. 4A). Interestingly, down-regulation of Bmf or Bim strongly enhanced colony formation of E7 cells (15- to 20-fold) (Fig. 4A). No colony formation was detected for MCF-10A wild-type or Bcl-2 cells or for cells with knockdown of Bmf or Bim alone, respectively (data not shown). Remarkably, E7 cells with reduced expression of Bad or Bid only gave rise to a few colonies in soft agar (Fig. 4A), paralleling our findings for BH3-only specificity in anoikis and lumen formation. Functionality of Bad and Bid knockdowns in the E7 background was validated by protection from STS or FasAb-induced apoptosis, respectively (data not shown).
Fig. 4.
Bmf function as epithelial tumor suppressor in vitro and expression during mammary gland involution. (A) Knockdown of Bmf confers anchorage-independent growth. (Upper) Cells expressing HPV16 E7 and empty vector control (vec ctrl), Bcl-2, or shRNA vectors for Bim, Bmf, Bad, or Bid were plated in soft-agar assays and grown for 5 weeks. Data were obtained as the mean number of colonies per six-well culture of 100,000 cells and expressed relative to the number of colonies obtained from vector control cells. Error bars equal ± SD of at least three independent experiments. (Lower) Representative images of wells are shown. (B) Bmf is expressed in apoptotic cells during involution of the mouse mammary gland. Sections of a wild-type mouse mammary gland at day 3 of involution were probed for Bmf expression (purple) by in situ hybridization (Upper). An overlay image of Bmf expression (purple) and nuclear morphology (DAPI staining, green) is shown (Lower Left). Expression of activated caspase-3 (red) and β-catenin (green) is shown on an independent section (nuclei in blue) (Lower Right). (Scale bars: B Upper and Lower Right, 10 μm; B Lower Left, 5 μm.)
These findings indicate that Bmf and Bim contribute substantially to the suppression of inappropriate epithelial survival, and their absence, in required combination with deregulated proliferation (21), promotes anchorage-independent growth, one of the strongest predictors of in vivo tumor formation.
Expression of Bmf During Mammary Gland Involution.
Based on our results elucidating the functional role and transcriptional regulation of Bmf in mammary epithelial cells in vitro, we also examined the expression of Bmf in the mammary gland in vivo. Tissues for this analysis were derived from the two major phases with pronounced apoptotic activity during mouse mammary gland development: pubertal expansion (weeks 4–8), with apoptotic clearing of cells in the presumptive lumen of terminal end buds; and early stages (days 1–3) of involution following weaning, with clearing of secretory epithelial cells from the lumena of ducts and acini before stromal remodeling.
Bmf expression was not detected by in situ hybridization in terminal end buds at week 5 of pubertal development (data not shown). However, a specific population of cells within the clearing ducts and acini of the involuting mammary gland scored positive for Bmf expression (Fig. 4B Upper). These cells were characterized by fragmented nuclear morphology and activation of caspase-3 (Fig. 4B Lower), and can thus be considered to form part of a population of apoptotic epithelial cells. Controls performed with a correspondingly labeled sense probe for Bmf only resulted in very weak or nonspecific signal accumulation (data not shown). Thus, Bmf expression may also function as an important determinant of cell death during mammary gland involution, consistent with a functional role of Bmf in the context of matrix detachment and lumen formation identified by our in vitro studies.
Discussion
Regulation of epithelial cell death and survival by ECM interactions is tightly controlled to ensure appropriate clearance of detached cells. In this report, we identify a functional role for the proapoptotic BH3-only factor Bmf in mammary epithelial anoikis and in vitro lumen formation during acinar morphogenesis. Moreover, our study has uncovered a hitherto unknown, matrix-mediated transcriptional regulation of Bmf expression that is controlled through the Erk and Akt pathways. Finally, our data also suggest potential roles for Bmf as an epithelial tumor suppressor and a physiological mediator of apoptosis in the mammary gland.
The BH3-only proapoptotic protein family comprises several different members, suggesting that they may either function specifically/individually downstream of defined stimuli or, alternatively, in conjunction (e.g., as a “pool”) to control the apoptotic balance within a cell. Our data indicate that specific BH3-only proteins, namely Bim and Bmf, but not others, such as Bad or Bid, are functionally required for apoptosis during anoikis and morphogenesis. These findings are consistent with both a model requiring a functional “activator” (Bim)/“sensitizer” (Bmf) BH3-only pair to elicit apoptosis (10) and a model in which a threshold of distinct BH3-only proteins (e.g., Bmf and Bim) antagonizes prosurvival molecules (9).
Unexpectedly, reduction in Bad or Bid led to a significant (P < 0.001 by t test) increase in DNA fragmentation at early time points in suspension (Fig. 2A); the molecular basis for this response is currently unknown. However, BH3-only proteins may also perform BH3-independent functions, such as Bid in DNA-damage response (22), or integrate distinct cellular processes with apoptosis, as reported for Bad in glucose metabolism (23). Therefore, down-regulation of individual BH3-only factors may also impinge indirectly on cell death readouts.
Several recent studies have reported the common involvement of the cytoskeletally regulated BH3-only factors Bim and Bmf in specific cell death processes. In particular, both Bim and Bmf are transcriptionally induced upon HDAC inhibitor treatment in diverse cancer cell lines (13) and also in response to TGF-β, via SMAD4/p38 MAPK (14). It is interesting to note that in some models individual down-regulation of either Bmf or Bim is sufficient to confer protection from cell death, e.g., of Bmf (but not Bim) for HDAC inhibitors (13) and of Bim (but not Bmf) for TGF-β (24). In other models, only the combined knockdown of Bmf and Bim significantly suppresses apoptosis in a cooperative way (14). In the MCF-10A model system, individual down-regulation of either BH3-only protein, Bmf or Bim, confers similar protection from apoptosis induced by loss of matrix adhesion. Possible reasons for these apparently discrepant requirements among various systems may include differences in cell death kinetics, tissue/cell type, or expression of antiapoptotic members of the Bcl-2 protein family. Similarly, some of these differences may also account for divergent results obtained in other models, such as the proposed involvement of Bad (25) or Bid (26) in anoikis. A recent report has suggested the functional involvement of individual BH3-only proteins, namely Bim and Bad, but not Bid, in neonatal hypoxia-ischemia (27). This finding is reminiscent of our observations in the MCF-10A model, in which the individual deficiency of Bmf or Bim, but not Bad or Bid, inhibits anoikis and lumen formation.
Bmf appears to be coregulated at the transcriptional and posttranslational levels in response to several stimuli, such as anoikis (12) or TGF-β treatment (14). In agreement with previous studies (12), our results also suggest that posttranslational regulation may contribute to Bmf activity because stable expression of Bmf resulted in only a moderate increase in death of attached cells (data not shown), but sensitized cells to anoikis (SI Fig. 7 B Upper).
Evidence from the in vitro studies reported here, in particular the data demonstrating that down-regulation of Bmf suppresses anoikis and luminal cell death and promotes anchorage-independent growth and that Bmf is targeted by oncogenes, strongly supports a function for Bmf as an epithelial tumor suppressor. Our results from analyses of the role of Bmf and Bim suggest that they are more potent inhibitors than other BH3-only proteins, i.e., Bad or Bid, of transformation in mammary epithelial cells under conditions of matrix deprivation. Similarly, a recent report has demonstrated that Bim functions as a tumor suppressor in epithelial solid tumors (28). Bmf is located on chromosome 15q14, a site frequently lost in metastatic breast, lung, and colon carcinoma (29). Our studies raise the possibility that deletion of Bmf would allow survival of tumor cells deprived of matrix interactions outside their natural “niche.”
Our data also suggest that Bmf may be a critical mediator of apoptosis during mammary gland involution. Recent in vivo studies from our laboratory, in agreement with previously published MCF-10A in vitro data (16), have demonstrated a crucial role for Bim in mammary ductal morphogenesis during pubertal development (5). Thus, one may speculate that functions of Bmf and Bim overlap in MCF-10A cells, but show more specific involvement in different developmental stages in vivo; however, their roles in apoptotic clearance of cells may involve deprivation of matrix and growth factor signaling under both conditions. Differences may be explained by the increased complexity of cell populations and stromal environment in vivo.
Interestingly, in addition to revealing transcriptional regulation of Bmf during anoikis and lumen formation, our microarray analysis also identified additional genes that are up-regulated during these processes (SI Fig. 5 B and C). For example, the induction of cytokeratins 1 and 10 suggests the presence of a squamous differentiation program in response to matrix detachment. Indeed, parallel studies from our laboratory have identified such a process during mammary morphogenesis in vivo (5). Definition of the functional role and regulation of other identified candidate genes, similar to our Bmf studies, will further contribute to understanding ECM regulation of cell survival.
Materials and Methods
Cell Culture, Virus Production, and shRNA/Stable RNA Interference.
MCF-10A and HMECtert cells were cultured as described (15, 30). MCF-10−2A, MCF-12A, and PWR-1E cells were obtained from American Type Culture Collection (Manassas, VA) and cultured according to their guidelines. MCF-10A cells expressing Bcl-2, HPV16 E7, RASV12, NeuN, MEK2DD, and PI3KE545K have been described previously (17, 19, 20, 31). The pLNCX-based retroviral vector encoding myr-Akt1 was generated from a plasmid obtained from P. Tsichlis (New England Medical Center, Tufts University, Boston, MA). Retroviral (pMKO.1puro) or lentiviral (pLKO.1puro) (32) vectors encoding shRNA sequences were used to down-regulate specific BH3-only factors. shRNA sequences for Bim, Bmf, Bad, and Bid vectors are listed in SI Materials and Methods. VSV-G-pseudotyped retro- and lentiviruses were generated, and MCF-10A lines were infected and selected as described (15, 33).
Anoikis/Cell Death Assays.
For anoikis assays, cells were plated on tissue culture plates pretreated with polyHEMA and analyzed for DNA fragmentation by using the Cell Death Detection ELISA kit (Roche Diagnostics, Mannheim, Germany) as described (30). MCF-10A monolayer cultures were treated with 0.5 μM STS (Sigma–Aldrich, St. Louis, MO), 1 μg/ml FasAb (Upstate Biotechnology, Lake Placid, NY), and 45-sec exposure of UV light [UV-C (254 nm) 30-W bulb], and DNA fragmentation was assayed by flow-cytometric determination of subG1 content as described (30). All cell death assays were performed in at least three independent experiments.
3D Morphogenesis Assays.
Cells were cultured in growth factor-reduced and reconstituted basement membrane (Matrigel; BD Biosciences, San Jose, CA) and processed for EtBr staining or indirect immunofluorescence/confocal microscopy as described (15).
RT-PCR/Real-Time RT-PCR.
Total RNA was isolated in two steps by using RNA STAT-60 (Tel-Test, Friendswood, TX) and the RNeasy MinElute Cleanup Kit (QIAGEN, Valencia, CA). RT-PCR was performed by using 50–100 ng of RNA with the SuperScript One-Step RT-PCR/Platinum Taq system (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Amplification products were visualized by agarose gel electrophoresis. Real-time RT-PCR was performed as described (34) by using 60 ng of RNA with SYBR Green PCR Master Mix, MultiScribe Reverse Transcriptase, and the ABI PRISM 7700 sequence-detection system (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol. Amplification of specific targets was verified by dissociation curve analysis. Sequences of primer pairs for RT-PCR analysis are listed in SI Materials and Methods. All real-time RT-PCR assays were performed in duplicate in at least three independent experiments.
Soft-Agar Assays.
Cells were grown in soft agar and fed as described (30). Colonies >50 μm in diameter were scored as positive for growth after 5 weeks. All assays were conducted in duplicate in at least three independent experiments.
In Situ Hybridization.
All experiments with mice were performed according to the guidelines of the Institutional Animal Care and Use Committee of Harvard Medical School. Paraffin-embedded samples of mammary gland specimens derived from C57BL/6 wild-type mice were prepared and processed as described (5). In situ hybridization to tissue sections was performed as described (35).
For additional details, see SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the TRACE group, Barb Bryant, David Rose, and Joseph Bolen for microarray support; Eva Lin and Rebeca Vazquez for technical assistance; Sara Bulmer, William Hahn, and The RNAi Consortium for shRNA vectors; Myles Brown and members of his laboratory for real-time RT-PCR expertise; the Nikon Imaging Center at Harvard Medical School for confocal microscopy support; and members of the J.S.B. laboratory for reagents and helpful discussion. This work was supported by National Cancer Institute/National Institutes of Health Grants CA080111 and CA105134 (to J.S.B.), Swiss National Science Foundation Grant PA00A-105094 (to T.S.), Fondation Recherche Medicale and Susan G. Komen Breast Cancer Foundation Grant PDF29406 (to A.A.M.), National Cancer Institute/National Institutes of Health Institutional Training Grant T32CA09361 (to M.O.), U.S. Department of Defense Breast Cancer Research Program Awards (to J.S.C.), and a National Science Foundation predoctoral fellowship (to N.L.S.).
Abbreviations
- ECM
extracellular matrix
- PI3K
phosphatidylinositol 3-kinase
- STS
staurosporine.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0700115104/DC1.
References
- 1.Danial NN, Korsmeyer SJ. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 2.Frisch SM, Ruoslahti E. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- 3.Gilmore AP. Cell Death Differ. 2005;2(Suppl 12):1473–1477. doi: 10.1038/sj.cdd.4401723. [DOI] [PubMed] [Google Scholar]
- 4.Green KA, Streuli CH. Cell Mol Life Sci. 2004;61:1867–1883. doi: 10.1007/s00018-004-3366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mailleux AA, Overholtzer M, Schmelzle T, Bouillet P, Strasser A, Brugge JS. Dev Cell. 2007;12:221–234. doi: 10.1016/j.devcel.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coucouvanis E, Martin GR. Cell. 1995;83:279–287. doi: 10.1016/0092-8674(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 7.Hogan BL, Kolodziej PA. Nat Rev Genet. 2002;3:513–523. doi: 10.1038/nrg840. [DOI] [PubMed] [Google Scholar]
- 8.Reddig PJ, Juliano RL. Cancer Metastasis Rev. 2005;24:425–439. doi: 10.1007/s10555-005-5134-3. [DOI] [PubMed] [Google Scholar]
- 9.Willis SN, Adams JM. Curr Opin Cell Biol. 2005;17:617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Letai A. Cancer Cell. 2006;10:343–345. doi: 10.1016/j.ccr.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Martin SS, Vuori K. Biochim Biophys Acta. 2004;1692:145–157. doi: 10.1016/j.bbamcr.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Puthalakath H, Villunger A, O'Reilly LA, Beaumont JG, Coultas L, Cheney RE, Huang DC, Strasser A. Science. 2001;293:1829–1832. doi: 10.1126/science.1062257. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Adachi M, Kawamura R, Imai K. Cell Death Differ. 2006;13:129–140. doi: 10.1038/sj.cdd.4401686. [DOI] [PubMed] [Google Scholar]
- 14.Ramjaun AR, Tomlinson S, Eddaoudi A, Downward J. Oncogene. 2007;26:970–981. doi: 10.1038/sj.onc.1209852. [DOI] [PubMed] [Google Scholar]
- 15.Debnath J, Muthuswamy SK, Brugge JS. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- 16.Reginato MJ, Mills KR, Becker EB, Lynch DK, Bonni A, Muthuswamy SK, Brugge JS. Mol Cell Biol. 2005;25:4591–4601. doi: 10.1128/MCB.25.11.4591-4601.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J, Muthuswamy SK, Brugge JS. Nat Cell Biol. 2003;5:733–740. doi: 10.1038/ncb1026. [DOI] [PubMed] [Google Scholar]
- 18.Debnath J, Brugge JS. Nat Rev Cancer. 2005;5:675–688. doi: 10.1038/nrc1695. [DOI] [PubMed] [Google Scholar]
- 19.Isakoff SJ, Engelman JA, Irie HY, Luo J, Brachmann SM, Pearline RV, Cantley LC, Brugge JS. Cancer Res. 2005;65:10992–11000. doi: 10.1158/0008-5472.CAN-05-2612. [DOI] [PubMed] [Google Scholar]
- 20.Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. Cell. 2002;111:29–40. doi: 10.1016/s0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 21.Green DR, Evan GI. Cancer Cell. 2002;1:19–30. doi: 10.1016/s1535-6108(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 22.Zinkel S, Gross A, Yang E. Cell Death Differ. 2006;13:1351–1359. doi: 10.1038/sj.cdd.4401987. [DOI] [PubMed] [Google Scholar]
- 23.Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, Datta SR, Greenberg ME, Licklider LJ, Lowell BB, et al. Nature. 2003;424:952–956. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- 24.Ohgushi M, Kuroki S, Fukamachi H, O'Reilly LA, Kuida K, Strasser A, Yonehara S. Mol Cell Biol. 2005;25:10017–10028. doi: 10.1128/MCB.25.22.10017-10028.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Idogawa M, Adachi M, Minami T, Yasui H, Imai K. Int J Cancer. 2003;107:215–223. doi: 10.1002/ijc.11399. [DOI] [PubMed] [Google Scholar]
- 26.Valentijn AJ, Gilmore AP. J Biol Chem. 2004;279:32848–32857. doi: 10.1074/jbc.M313375200. [DOI] [PubMed] [Google Scholar]
- 27.Ness JM, Harvey CA, Strasser A, Bouillet P, Klocke BJ, Roth KA. Brain Res. 2006;1099:150–159. doi: 10.1016/j.brainres.2006.04.132. [DOI] [PubMed] [Google Scholar]
- 28.Tan TT, Degenhardt K, Nelson DA, Beaudoin B, Nieves-Neira W, Bouillet P, Villunger A, Adams JM, White E. Cancer Cell. 2005;7:227–238. doi: 10.1016/j.ccr.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Wick W, Petersen I, Schmutzler RK, Wolfarth B, Lenartz D, Bierhoff E, Hummerich J, Muller DJ, Stangl AP, Schramm J, et al. Oncogene. 1996;12:973–978. [PubMed] [Google Scholar]
- 30.Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, Deng CX, Brugge JS, Haber DA. Proc Natl Acad Sci USA. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunawardane RN, Sgroi DC, Wrobel CN, Koh E, Daley GQ, Brugge JS. Cancer Res. 2005;65:11572–11580. doi: 10.1158/0008-5472.CAN-05-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Root DE, Hacohen N, Hahn WC, Lander ES, Sabatini DM. Nat Methods. 2006;3:715–719. doi: 10.1038/nmeth924. [DOI] [PubMed] [Google Scholar]
- 33.Irie HY, Pearline RV, Grueneberg D, Hsia M, Ravichandran P, Kothari N, Natesan S, Brugge JS. J Cell Biol. 2005;171:1023–1034. doi: 10.1083/jcb.200505087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keeton EK, Brown M. Mol Endocrinol. 2005;19:1543–1554. doi: 10.1210/me.2004-0395. [DOI] [PubMed] [Google Scholar]
- 35.Bellusci S, Henderson R, Winnier G, Oikawa T, Hogan BL. Development (Cambridge, UK) 1996;122:1693–1702. doi: 10.1242/dev.122.6.1693. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.