Abstract
In adult rat testes, the blood–testis barrier (BTB) in the seminiferous epithelium must “open” (or “disassemble”) to accommodate the migration of preleptotene spermatocytes from the basal to the adluminal compartment that occurs at stage VIII of the epithelial cycle. However, the molecule(s) and/or mechanism(s) that regulate this event are unknown. In this report, C-type natriuretic peptide (CNP) was shown to be a regulator of BTB dynamics. Although Sertoli and germ cells contributed to the pool of CNP in the seminiferous epithelium, its receptor, natriuretic peptide receptor B, resided almost exclusively in Sertoli cells. CNP also expressed stage-specifically and localized predominantly at the BTB in the seminiferous epithelium at stage VIII of the epithelial cycle. A synthetic CNP-22 peptide, when added to Sertoli cell cultures, was shown to perturb Sertoli cell tight junction in vitro, causing disappearance of BTB-associated proteins (JAM-A, occludin, N-cadherin, and β-catenin) from the cell–cell interface. This inhibitory effect of CNP on the tight junction was confirmed by transient overexpression of CNP in these cells, which was mediated, at least in part, by accelerating the internalization of BTB integral membrane proteins. To validate these in vitro findings, CNP-22 was administered to testes at a dose of 0.35 or 3.5 μg per testis, which was shown to perturb the BTB integrity In vivo when the barrier function was assessed by monitoring the diffusion of a small molecular probe across the BTB. In summary, CNP secreted by Sertoli and germ cells into the BTB microenvironment regulates BTB dynamics during spermatogenesis.
Keywords: anchoring junction, spermatogenesis, tight junction, ectoplasmic specialization, endocytosis
Rat atrial extracts were shown to contain a diuretic and natriuretic factor in a report published in 1981 (1). Since then, three structurally related peptide hormones with vasodilatory properties, known as atrial natriuretic peptide (ANP), brain natriuretic peptide, and C-type natriuretic peptide (CNP), were isolated, molecularly cloned, and characterized (2). Recent studies have shown that these natriuretic peptides regulate a variety of physiological functions in mammals including blood pressure, blood volume, fat metabolism, bone growth (3), and steroidogenesis in testes (4). For instance, ANP was shown to stimulate testosterone and cGMP production in mouse Leydig cells in vitro (5–7). ANP mRNA transcripts were detected in adult rat testes (8, 9), and studies by immunohistochemistry also localized immunoreactive ANP to elongating spermatids and spermatozoa in the seminiferous epithelium of adult mouse and rat testes (10). These studies thus illustrate the significance of these gonadal peptides in testis function (4). These peptide hormones exert their biological effects on coupling onto the cell surface guanylyl cyclase (GC) receptors known as natriuretic peptide receptor (NPR)-A and NPR-B, which, in turn, catalyze the synthesis of the second messenger, cGMP, and determine the cGMP intracellular level (3, 11, 12). Thus, NPR-A and NPR-B are also referred to as GC-A and GC-B, respectively. NPR-A is activated by ANP and brain natriuretic peptide, but not CNP, whereas CNP, but not ANP or brain natriuretic peptide, activates NPR-B (13, 14). In addition, all three peptide hormones bind the natriuretic peptide clearance receptor (NPR-C) with similar affinities, but NPR-C lacks the intracellular kinase domain of NPR-A and NPR-B (15). Recent studies have also shown that ANP acts as an antipermeability factor by blocking vascular permeability factor (also known as VEGF) signaling function, perturbing tight junction (TJ) protein phosphorylation and distribution in bovine aortic endothelial cells in vitro, such as occludin and zonula occludens 1 (ZO-1) (16), illustrating that these peptides may have other biological functions besides modulating vascular dilation/constriction and steroidogenesis. Furthermore, cGMP, the downstream signaling molecule of natriuretic peptides, was shown to regulate Sertoli cell TJ permeability barrier function in vitro (17). We thus sought to examine whether these peptide hormones would regulate junction restructuring events in adult rat testes, in particular the blood–testis barrier (BTB) during spermatogenesis. But because ANP was shown to be localized almost exclusively to the elongating/elongate spermatids in adult rat testes (10), we sought to examine the distribution of CNP and its receptor NPR-B in the testis. We also conducted a series of coherent experiments to examine the role of CNP in BTB dynamics.
Results
Expression of CNP in the Seminiferous Epithelium of Adult Rat Testes.
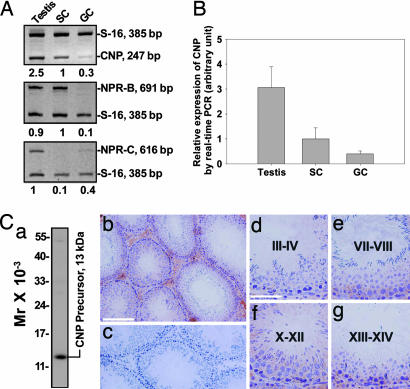
Sertoli and germ cells were shown to express CNP (Fig. 1 A and B) by RT-PCR using RNA isolated from lysates of primary Sertoli or germ cell cultures. These cell preparations had negligible contamination of other cell types when their purity was assessed by RT-PCR and/or immunoblottings using primer pairs and/or antibodies specific to germ cells (e.g., c-kit receptor), Sertoli cells (e.g., testin), Leydig cells (e.g., 3β-hydroxysteroid dehydrogenase), and peritubular myoid cells (e.g., fibronectin) as earlier reported (18), and electron microscopy (17, 19). Sertoli cells were shown to express more CNP than germ cells, but less than testes (Fig. 1 A and B), suggesting that other cell types, such as Leydig cells, macrophages, endothelial cells of the microvessels in the interstitium, or peritubular myoid cells and/or endothelial cells of the lymphatic vessels in the tunica propria, also contributed to the steady-state CNP mRNA level in the testis. Indeed, results shown in Fig. 1C are consistent with this conclusion because positive CNP staining was found in the interstitium and immunoreactive CNP was detected in Leydig cells, macrophages, and/or endothelial cells (Fig. 1 Cb and Cf), similar to the findings of two earlier reports (20, 21). The steady-state mRNA level of the CNP receptor NPR-B, however, was very low in germ cells vs. testes or Sertoli cells (Fig. 1A). The expression level of the clearance receptor NPR-C was also very low in Sertoli cells vs. germ cells (Fig. 1A). Collectively, these data illustrate that the Sertoli cell is the major cell type involved in the CNP–NPR-B–cGMP signaling in the seminiferous epithelium. By using an antibody specific to the CNP precursor (Fig. 1Cc) for immunohistochemistry, CNP was found to be localized to Leydig cells, macrophages, or endothelial cells in the interstitium, and its localization in the epithelium is consistent with its presence at the BTB (Fig. 1Cb). These immunohistochemical data are consistent with the findings that Sertoli and Leydig cells (and perhaps endothelial cells and macrophages in the interstitium) contribute most of the CNP in the testis (Fig. 1 A and B). The CNP level in the seminiferous epithelium, such as the BTB, appeared to be stage-specific, being reduced at stages III–IV (Fig. 1Cd), but remained relatively stable in other stages (see Fig. 1 Ce–Cg).
Fig. 1.
Expression and localization of CNP in adult rat testes. (A) Semiquantitative RT-PCR analyses of CNP, NPR-B, and NPR-C expression in testes, Sertoli cells (SC), and germ cells (GC) coamplified with S-16. The numbers below each lane denote the relative expression levels in each sample normalized to the level in SC or testes arbitrarily set at 1. (B) Results of quantitative real-time PCR analyses on the steady-state CNP mRNA levels in testes, Sertoli cells (SC), and germ cells (GC). (C) Immunohistochemical localization of a CNP precursor in adult testes. (Ca) Immunoblot analysis of rat testis lysate using an antibody specific to the CNP precursor. (Cb) Immunostaining of CNP in control testes. [Scale bar: 200 μm (also applies to Cc, which is a negative control stained with normal rabbit IgG).] (Cd–Cg) Stage-specific staining of CNP illustrating its localization at the BTB. [Scale bar: 80 μm in Cd (applies to Ce–Cg).]
CNP Disrupts Tight and Anchoring Junctions in Sertoli Cells.
Effects of using CNP synthetic peptide on Sertoli cell junction dynamics.
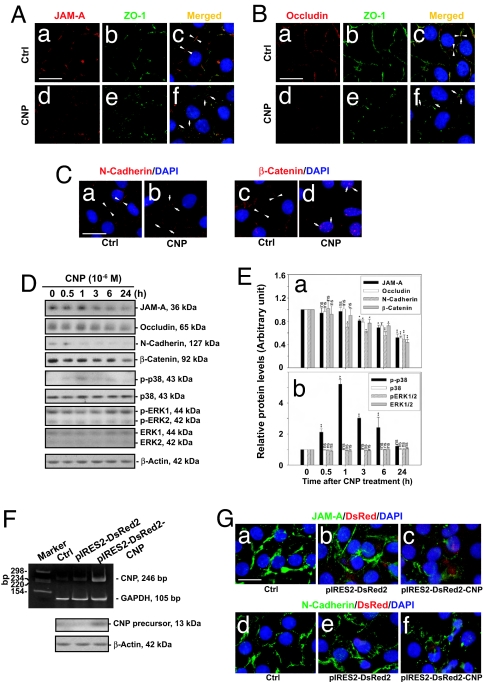
We first examined the effects of CNP on Sertoli cell junctions by adding synthetic peptides to primary Sertoli cell cultures on day 4 when functional TJ, basal ectoplasmic specialization, and desmosome-like junctions were established as assessed by electron microscopy (19) (data not shown). Immunofluorescent staining of the junction proteins JAM-A, occludin, and N-cadherin, which are BTB-associated proteins, showed organized junction fibrils at the cell–cell interface in these cultures on day 4 (Fig. 2Aa, Ba, and Ca), consistent with earlier findings that intact TJ and anchoring junctions were present in these cultures. Furthermore, the TJ adaptor protein ZO-1 was also found to be at the cell–cell contact colocalizing with JAM-A or occludin, forming an almost continuous fluorescent belt at the cell–cell interface (Fig. 2 Ab, Ac, Bb, and Bc; see white arrowheads in Fig. 2 Ac, Bc, and Ca). β-Catenin was also restricted to the cell–cell interface (see white arrowheads in Fig. 2Cc). Treatment of Sertoli cells with CNP at 1 × 10−6 M for 4 h, however, led to a disintegration of the almost continuous fluorescence belts at the cell–cell interface, illustrating a disruption of the TJ and anchoring junction protein fibrils (Fig. 2 Ad–Af, Bd–Bf, Cb, and Cd vs. Aa–Ac, Ba–Bc, Ca, and Cc). CNP treatment also caused an increase in nuclear localization of β-catenin (white arrows in Fig. 2Cd). These results illustrate that the junctions were no longer intact after CNP treatment. Indeed, immunoblotting analysis of Sertoli cells treated with CNP for different durations showed that the levels of JAM-A, occludin, and N-cadherin began to decrease significantly after ≈3 h (Fig. 2 D and E). The p38 MAPK was also activated after CNP treatment, by ≈0.5 h, reaching a maximal activation by 1 h (Fig. 2 D and E). CNP apparently did not affect the level of ERK MAPK and its activation (Fig. 2 D and E), suggesting that the activation of p38 MAPK may be one of the mechanisms that caused the disruption of TJ and anchoring junctions in cultured Sertoli cells.
Fig. 2.
A study to assess the effects of CNP on BTB-associated proteins at the Sertoli–Sertoli cell interface in vitro. (A) Sertoli cells were cultured in vitro for 4 days; thereafter, cells were treated with CNP at 10−6 M for 4 h. Fluorescent staining of JAM-A and ZO-1 in control (Aa–Ac) and CNP-treated (Ad–Af) cells is shown. Apposing white arrowheads in Ac indicate the TJ fibrils at the cell–cell contact site, and white arrows in Af indicate a disintegration of such TJ fibrils after CNP treatment. [Scale bar: 20 μm in Aa (also applies to Ab–Af).] (B) Fluorescent staining of occludin and ZO-1 in these cultures similar to A is also shown. Cells are shown at the same scale as in A. (C) Fluorescent staining of N-cadherin (Ca and Cb) and β-catenin (Cc and Cd) in control and CNP-treated Sertoli cells. Apposing white arrowheads depict the AJ fibrils at the cell–cell interface (Ca and Cc), and white arrows indicate the fragmented AJ fibrils (Cb and Cd). Note that β-catenin displayed nuclear localization after CNP treatment (see arrows in Cd). [Scale bar: 25 μm in Ca (also applies to Cb–Cd).] (D) Immunoblot analysis of different target proteins in Sertoli cells at different time points after CNP treatment. (E) A histogram summarizing data shown in D (n = 3). (F and G) Effects of CNP on BTB-associated proteins at the Sertoli–Sertoli cell interface after its overexpression. (F) RT-PCR and immunoblot analysis of Sertoli cells 24 h after transfection with pIRES2-DsRed2 or pIRES2-DsRed2-CNP, illustrating an increase in endogenous CNP in Sertoli cells. (G) Fluorescent staining of JAM-A (Ga–Gc) and N-cadherin (Gd–Gf) in Sertoli cells transfected with pIRES2-DsRed2 or pIRES2-DsRed2-CNP. [Scale bar: 20 μm in Ga (also applies to Gb–Gf).]
Effects of transient overexpression of CNP on Sertoli cell junction dynamics.
To verify the effects of CNP on Sertoli cell junction integrity, we next overexpressed CNP in primary Sertoli cells using a pIRES2-DsRed2 plasmid. This vector, when transfected into Sertoli cells, expressed DsRed2 (a variant of Discosoma sp. red fluorescent protein), in addition to the expression of CNP. Thus, DsRed2 signals were used to track the overexpression of CNP. The CNP steady-state mRNA and protein levels were significantly increased compared with cells transfected with the empty vector or control (Fig. 2F). Fluorescent staining of JAM-A and N-cadherin in these cultures indicated that a transient overexpression of CNP also resulted in the disintegration of junction fibrils at the cell–cell interface (Fig. 2 Gc and Gf vs. Ga, Gb, Gd, and Ge). Collectively, the results shown in Fig. 2 demonstrate that CNP affects Sertoli cell junction dynamics in vitro.
CNP Accelerates Integral Membrane Protein Internalization in Sertoli Cells.
Biochemical studies.
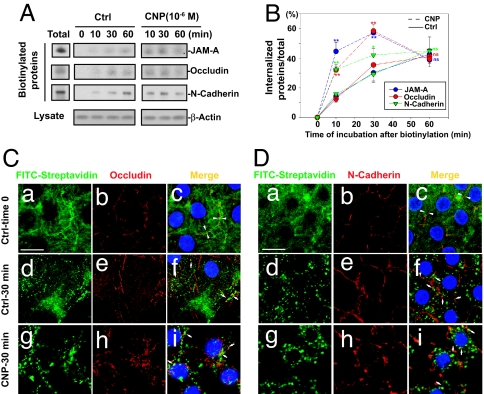
Because Sertoli cells cultured in vitro are known to establish functional TJ and anchoring junctions that mimic the BTB in vivo (19, 22–24), we sought to examine whether CNP regulates BTB dynamics by inducing changes in integral membrane protein internalization at the BTB using this in vitro model. We used a cell surface protein biotinylation technique to track protein endocytosis (Fig. 3A). All three junction proteins showed time-dependent internalization under normal conditions when Sertoli cells were incubated at 35°C for various durations (Fig. 3 A and B). The maximal levels of internalization of these proteins were detected after 1 h (Fig. 3 A and B). Preliminary experiments showed that longer incubation at this temperature (normal physiological temperature of testes) did not result in a higher level of internalized proteins (data not shown), because endocytosed proteins underwent proteolysis and/or being recycled back to the cell surface (25). Incubation at 18°C prevented the internalized proteins from further progressing into the endocytic or recycling pathway (25), but the rate of internalization between these two temperatures did not differ significantly up to 1 h (data not shown). Moreover, this lower nonphysiological temperature seemed to insensitize the cellular responses to CNP treatment (data not shown). Therefore, these experiments were performed at 35°C for up to 1 h. Treatment of Sertoli cells with CNP at 1 × 10−6 M led to an acceleration of protein internalization (Fig. 3 A and B). The maximal level of internalization after CNP treatment was reached by 30 min (Fig. 3 A and B). These results demonstrated that integral membrane proteins at the BTB could be internalized, and the rate of internalization was up-regulated by the CNP peptide, which could be the mechanism by which CNP perturbed Sertoli cell junction dynamics as reported in Fig. 2.
Fig. 3.
A study to investigate the mechanism by which CNP regulates Sertoli cell TJ barrier dynamics in vitro. (A) Immunoblot analysis of endocytosed N-cadherin, JAM-A, and occludin in Sertoli cells after cell surface biotinylation. Biotinylated proteins were allowed to undergo endocytosis at 35°C with or without CNP (1 μM) treatment. Noninternalized biotinylated cell surface proteins were stripped, and the endocytosed proteins were pulled down by NeutrAvidin-agarose and analyzed by immunoblottings. Sertoli cell cultures that were terminated at time 0 but without the biotin-stripping step represent the amount of total biotinylated proteins. (B) Kinetics of internalization of N-cadherin, JAM-A, and occludin with (dashed lines) and without [control (Ctrl), solid lines] CNP treatment. The percentage of internalized protein vs. total biotinylated protein from three independent experiments is shown. ∗, P < 0.05 by ANOVA; ∗∗, P < 0.01; ns, not significantly different. (C and D) Fluorescent staining of occludin (C) and N-cadherin (D) with biotinylated endocytosed proteins that were visualized by FITC-streptavidin using confocal microscopy. Representative images from z-stack slices are shown. At time 0 (Ca–Cc and Da–Dc) proteins appeared to be labeled heavily at the cell–cell interface, and some at the cell surface. Apposing white arrowheads depict the TJ (Cc) and AJ (Dc) fibrils at the cell–cell interface. After 30 min of incubation to allow endocytosis with (Cg–Ci and Dg–Di) or without (Cd–Cf and Dd–Df) CNP treatment, remaining biotin on the cell surface was removed, and the FITC signal represents internalized biotinylated proteins, which appeared in the punctate vesicles (Cd, Cg, Dd, and Dg) (see arrows in Cf, Ci, Df, and Di). Treatment of cells with CNP resulted in more rapid internalization of occludin and N-cadherin (Cg–Ci and Dg–Di vs. Cd–Cf and Dd–Df). [Scale bar: 15 μm in Ca and Da (applies to Cb–Ci and Db–Di).]
Confocal microscopy studies.
Confocal microscopy was used to corroborate the biochemical data that CNP promoted protein internalization at the BTB. Immediately after biotinylation of surface proteins (control: time 0), Sertoli cells were fixed and stained with FITC-streptavidin and an anti-occludin or anti-N-cadherin antibody (Fig. 3 Ca–Cc and Da–Dc) in selected cultures. Biotin was detected at the cell–cell interface where junction proteins are localized (apposing white arrowheads in Fig. 3 Cc and Dc). Biotin was also found on the cell surface, showing even distribution of green fluorescence near the cell–cell interface (Fig. 3 Ca, Cc, Da, and Dc). In some Sertoli cell cultures, after cell surface biotinylation, cells were incubated in F12/DMEM for 30 min at 35°C to allow protein internalization, and the biotin remaining on the cell surface was removed. Biotinylated proteins appeared to be localized in punctate vesicles (Fig. 3 Cd and Dd), which were shown to colocalize with dynamin (data not shown). Some of these endocytic vesicles were colocalized with occludin or N-cadherin (white arrows in Fig. 3 Cf and Df), indicating previously biotinylated occludin and N-cadherin on the cell surface were indeed endocytosed. Treatment of cells with CNP at 10−6 M (Fig. 3 Cg–Ci and Dg–Di) yielded more endocytic vesicles that were away from the cell–cell interface (white arrow in Fig. 3 Ci and Di), suggesting an acceleration of internalization of integral membrane proteins, possibly progressing into endosomes and/or lysosomes. These observations are consistent with data in Fig. 2 that CNP down-regulates BTB-associated proteins.
CNP Disrupts the BTB Integrity in Vivo.
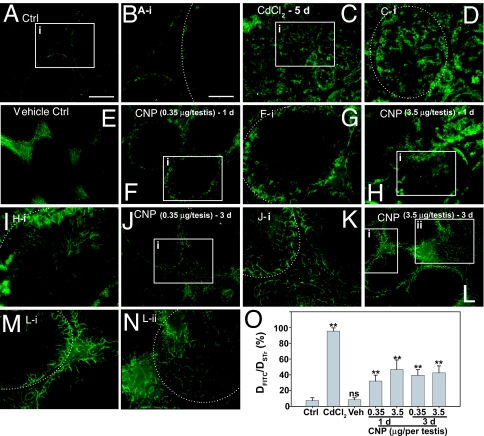
To further confirm that results of the in vitro studies regarding the effects of CNP on Sertoli cell junction dynamics were of physiologically significant, we sought to examine whether CNP peptide indeed regulated the BTB integrity in vivo. We thus examined whether treatment of testes with CNP synthetic peptide would alter the BTB integrity using a functional assay to monitor the diffusion of a small fluorescent dye, FITC (Mr 389), across the BTB after its administration via the jugular vein (Fig. 4). Because the BTB is a selective barrier that limits diffusion of small molecules from the systemic circulation into the seminiferous epithelium (26, 27), it is not surprising that FITC was excluded from entering the seminiferous epithelium in normal rat testes with fluorescence confined to the basal region of the tubules, consistent with its localization at the BTB (Fig. 4 A and B; the white broken line in Fig. 4B illustrates the relative BTB location in the seminiferous epithelium). However, when rats were treated with cadmium, which was known to perturb the BTB integrity (28, 29), FITC was readily detected in the entire seminiferous epithelium beyond the BTB, and some fluorescence was seen detected at or near the tubule lumen by day 5 (Fig. 4 C and D). In rats treated with vehicle only, fluorescence was restricted near the basement membrane and no FITC was detected beyond the BTB, similar to normal rats (Fig. 4 E vs. A and B). However, in rats treated with CNP at doses of 0.35 or 3.5 μg per testis, and the BTB integrity was assessed by 1 or 3 days, it was shown to be compromised (Fig. 4 F–N). Although the damage to the BTB integrity was not as severe as rats treated with cadmium, green fluorescence was clearly detected inside the seminiferous epithelium beyond the BTB (Fig. 4 F–N vs. A, B, and E). When these results were analyzed semiquantitatively by measuring the distance traveled by FITC beyond the BTB near the basement membrane (DFITC) vs. the radius of the seminiferous tubule (DSTr), the diffusion of FITC in testes from rats treated with CNP was significantly greater than normal testes or vehicle control (Fig. 4O). These results thus confirm the in vitro findings that an elevation of testicular CNP level affects BTB dynamics.
Fig. 4.
A functional study to assess the BTB integrity after treatment of rats with CNP vs. controls. (A and B) Normal testes (Ctrl). (C and D) Rats treated with CdCl2 for 5 days (positive control). (E) Vehicle control. (F–N) Different CNP treatment groups: 0.35 μg per testis, 1 day (F and G); 3.5 μg per testis, 1 day (H and I); 0.35 μg per testis, 3 days (J and K); and 3.5 μg per testis, 3 days (L–N). Boxed areas annotated with either i or ii in selected micrographs were magnified and are shown in adjacent micrographs. The dotted line depicts the approximate location of the basement membrane in the seminiferous tubules near the BTB. (O) A bar chart summarizing the results by calculating the distance of FITC diffused into the epithelium (DFITC) vs. the radius of a seminiferous tubule (DSTr) (average of longest and shortest axes for oval-shaped tubules) (n = 200 tubules from testes of two rats per treatment group). ∗∗, P < 0.01 by one-way ANOVA; ns, not significantly different. [Scale bars: 150 μm in A (also applies to C, E, F, H, J, and L) and 80 μm in B (also applies to D, G, I, K, M, and N).] Veh, vehicle control.
Discussion
In the seminiferous epithelium of adult rat testes, the BTB created by adjacent Sertoli cells near the basement membrane physically divides the epithelium into the basal and adluminal compartment so that postmeiotic germ cell development is segregated from the systemic circulation (26, 27). It also confers cell polarity and restricts the diffusion of molecules from the interstitium into the seminiferous epithelium. Although BTB is one of the tightest blood–tissue barriers, preleptotene spermatocytes residing in the basal compartment must traverse the BTB at stage VIII of the epithelial cycle (30), entering the adluminal compartment for further development. Thus, BTB undergoes extensive restructuring to facilitate preleptotene spermatocyte migration. However, the biochemical mechanisms that regulate this event have been unknown until recently. For instance, TNFα and TGF-β3 produced by Sertoli and germ cells have recently been shown to modulate BTB dynamics (31–34). Both cytokines induced transient but reversible BTB disruption in adult rats (32, 33), suggesting that their local production by Sertoli and germ cells plays a crucial role in “opening” the BTB to facilitate preleptotene spermatocyte migration.
Testosterone is also a regulator of Sertoli cell TJ permeability barrier function because its presence in Sertoli cells cultured in vitro was shown to facilitate the TJ barrier assembly (35, 36). However, in vivo studies using an androgen suppression model by lowering the intratesticular androgen level in adult rats, thereby inducing elongating/elongate spermatid (step 8 and beyond) depletion from the seminiferous epithelium by perturbing the apical ectoplasmic specialization function, have shown that the BTB integrity was not compromised (37). Recently, three mouse models were generated in which androgen receptor (AR) was selectively knocked out in Sertoli cells (SCARKO) (38–40). These mice were produced by Cre/loxP technology where transgenic mice carrying an AR with a floxed exon 2 were crossed with mice expressing Cre-recombinase selectively in Sertoli cells regulated by the AMH (anti-Müllerian hormone) promoter (38). Testes from these mice were shown to be markedly smaller, displaying a block in meiosis with fewer round spermatids and virtually no elongated spermatids, but the number of Sertoli cells and spermatogonia were similar to wild types. Interestingly, serum testosterone level in SCARKO mice was shown to be similar (38, 41), 50% less (40), or 40-fold more (39) in different studies when compared with wild types. By using these AR knockout mice (39) as an in vivo model to examine the role of testosterone on BTB function, it was reported that androgen is a crucial regulator of BTB, which mediated its effects via claudin-3, which was the transcriptional target of ARs in Sertoli cells (42). However, it must be noted that in this in vivo study (42) the SCARKO mice were made with a floxed exon 1, but the floxed animals had already displayed a marked hypomorphic phenotype and the ultimate AR knockout was neither complete nor Sertoli cell-selective (39). Furthermore, the steady-state protein level of claudin-3 in adult rat testes was virtually nondetectable in contrast to the high levels seen in immature rats before 30 days of age (unpublished observations) (42), illustrating that claudin-3 is highly unlikely to be an important androgen target protein at the BTB, at least in adult rats. Needless to say, although androgen was shown to regulate the levels of several BTB integral membrane proteins, such as occludin, claudin-3, and cadherins (18, 35, 36, 42), work is needed to define its molecular targets at the BTB.
In this article we have demonstrated that CNP is a regulator of BTB dynamics in adult rat testes based on results of in vitro and in vivo studies. CNP and its receptor, NPR-B (or GC-B), are known regulators of renal and cardiovascular physiology; additionally, CNP−/− mice displayed dwarfism, and NPR-B−/− mice displayed phenotypes of dwarfism, female sterility, and decreased adiposity (for a review, see ref. 3). However, the effects of CNP on male testicular function, in particular BTB and anchoring junction dynamics and/or integrity, are not known. It is likely that CNP affects BTB integrity via its effects on the intracellular cGMP level in Sertoli cells because 8-bromo-cGMP (a cGMP analog which cannot be cleaved and inactivated by cGMP phosphodiesterase) was shown to disrupt the Sertoli cell TJ barrier function dose-dependently (17). Recent studies have also shown that the effects of ANP on the endothelial TJ barrier function are also mediated via changes in the phosphorylation status of occludin and ZO-1 at the TJ site (16). More important, NPR-A (or GC-B, the receptor of ANP) was recently shown to be predominantly localized in the seminiferous tubule instead of cells in the interstitium, such as Leydig cells in rat testes (43). Different natriuretic peptides can exert differential effects on endothelial barrier function. For instance, ANP, but not CNP, is capable of blocking the thrombin-induced disruption of the rat lung microvascular endothelial barrier via a cGMP-independent, possibly Rho A-dependent, mechanism (44). Thus, ANP may work in concert with CNP to regulate junction restructuring events during spermatogenesis. In this context, it is of interest to note that recent studies have shown that the secretion of CNP is up-regulated by TNFα (45) and TGF-β (46) in cultured aortic endothelial cells. It is likely that CNP may be working synergistically with cytokines to “open” the BTB at stage VIII of the epithelial cycle to facilitate preleptotene spermatocyte migration. Functional experiments shall be designed in future studies to examine how these molecules are interacting cooperatively to regulate BTB dynamics. On this note, it is of interest that the receptors of these peptide and cytokines are restricted almost exclusively to Sertoli cells in the seminiferous epithelium (for a review, see ref. 26). Thus, as schematically shown in Fig. 5, CNP produced by Sertoli and germ cells into the BTB microenvironment, such as at stage VIII of the epithelial cycle, may trigger intracellular signaling events via NPR-B. This, in turn, accelerates endocytosis of N-cadherin, occludin and JAM-A, the three BTB-associated integral-membrane proteins, lowering their steady-state levels at the BTB between adjacent Sertoli cells, opening the BTB transiently to facilitate preleptotene spermatocyte migration. In summary, CNP, a peptide hormone produced by cardiocytes known to regulate vascular dilation/constriction and permeability, is also produced locally in the testis by Sertoli cells, and serves as an autocrine factor to regulate BTB dynamics.
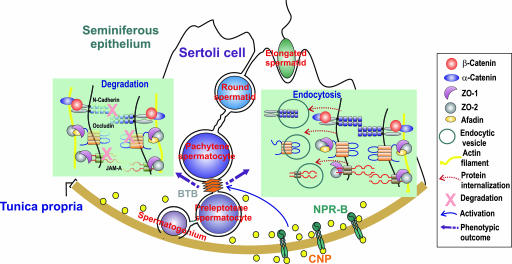
Fig. 5.
A schematic model illustrating that CNP regulates BTB dynamics by promoting endocytosis of integral membrane proteins. This effect, coupled with degradation of proteins at the BTB possibly via the ubiquitination/lysosomal pathway under the influence of other molecules, in turn reduces the steady-state levels of integral membrane proteins at the BTB. The net result opens the BTB to facilitate preleptotene spermatocyte migration that occurs at stage VIII of the epithelial cycle. It is likely that CNP is working in concert with other molecules in the microenvironment of the seminiferous epithelium, such as TGF-β3 and TNFα, to regulate BTB dynamics.
Materials and Methods
Animals and Cell Cultures.
The use of rats was approved by The Rockefeller University Animal Care and Use Committee. Sertoli cells were isolated from 20-day-old rats and plated on Matrigel-coated (diluted 1:7 with F12/DMEM; BD Biosciences) 6- or 12-well plates at 0.4–0.5 × 106 cells per square centimeter or the Lab-Tek Chamber Slide system (four chambers; Nunc/Thermo Fisher Scientific, Waltham, MA) at ≈0.15 × 106 cells per square centimeter and used on day 4 as described (32). Germ cells were isolated from 90-day-old rats (18).
Treatment of Sertoli Cells with CNP Peptide and CNP Overexpression.
To assess the effects of CNP in Sertoli cell cultures, CNP-22 [C93H157N27O28S3, Mr 2197.63 (human, porcine, and rat), amino acid residues 32–53, catalog no. H-1296.0500] was purchased from Bachem Bioscience (King of Prussia, PA) and dissolved in sterile PBS. Cultures were treated with CNP (10−7 M and 10−6 M) for various durations up to 24 h before termination. For transient overexpression of CNP in Sertoli cells, the full-length rat CNP was cloned by PCR using a primer pair specific to CNP [supporting information (SI) Table 1] and cDNAs reverse-transcribed from rat testis RNA to serve as templates. The identity of the full-length clone was confirmed by direct nucleotide sequencing at Genewiz (North Brunswick, NJ). This cDNA was ligated to pGEM-T vector (Promega, Madison, WI), which was then excised from the SpeI/SacII restriction endonuclease recognition sites within the pGEM-T vector and subcloned into pIRES2-DsRed2 (Clontech, Mountain View, CA) at the NheI/SacII sites, generating the pIRES2-DsRed2-CNP plasmid. The identity of the vector and the insert was verified by direct nucleotide sequencing at both directions before use. Primary Sertoli cells were transfected with pIRES2-DsRed2 or pIRES2-DsRed2-CNP by using Effectene reagent (Qiagen, Valencia, CA) essentially as earlier described with ≈15% efficiency (32). Cells were used by 24 h after transient transfection.
PCR, Immunohistochemistry, Microscopy, and Immunoblotting.
Semiquantitative RT-PCR and real-time PCR were performed as described in SI Table 1. Immunohistochemistry was performed as described (37). Fluorescent microscopy was performed by using an Olympus BX-40 microscope equipped with fluorescence optics, and images were acquired with an Olympus DP70 12.5 MPa Digital Camera. Immunoblot analysis was performed as described (32). Confocal microscopy was performed at The Rockefeller University Bio-Imaging Resource Center with an inverted LSM 510 Laser Scanning confocal microscope (Zeiss, Thornwood, NY). Images were processed by using LSM 510 (version 3.2) software (Zeiss). Antibodies used for pertinent morphology and immunoblotting studies are listed in SI Table 2.
Endocytosis Assay.
Endocytosis assay was performed by cell surface biotinylation as described (25, 47). Sertoli cells cultured for 4 days were washed with ice-cold PBS twice and incubated with 0.5 mg/ml sulfo-NHS-SS-Biotin (Pierce, Rockford, IL) in PBS/CM buffer [10 mM Na2HPO4 and 2 mM KH2PO4 (pH 7.4) at 22°C, containing 137 mM NaCl, 2.7 mM KCl, 1 mM CaCl2, and 0.7 mM MgCl2] at 4°C for 30 min to permit biotinylation of all cell surface proteins. This was followed by a quenching step using 50 mM NH4Cl in PBS/CM buffer at 4°C for 15 min. Cells were washed twice with PBS and incubated in F12/DMEM with (test) or without (control) CNP-22 peptide (10−6 M) at 35°C for various durations to allow endocytosis of cell surface proteins. Thereafter, cells were washed in cold PBS, treated with a biotin-stripping buffer [50 mM MESNA in 100 mM Tris·HCl (pH 8.6) at 22°C, containing 100 mM NaCl and 2.5 mM CaCl2] at 4°C for 30 min to remove remaining biotin on cell surface and quenched with 5 mg/ml iodoacetamide in PBS/CM buffer at 4°C for 15 min. After washing with PBS, cells were lysed in an IP lysis buffer [10 mM Tris (pH 7.4) at 22°C, containing 0.15 M NaCl, 2 mM PMSF, 1 mM EGTA, 1% Nonidet P-40 (vol/vol), 1 mM sodium orthovanadate, 1 μg/ml leupeptin, 1 μg/ml aprotinin, and 10% glycerol (vol/vol)]. Equal amounts of cell lysates were incubated with NeutrAvidin beads to pull down biotinylated proteins, and, after washing with PBS, biotinylated proteins were eluted in SDS sample buffer for immunoblotting using corresponding specific antibodies to assess the kinetics of protein internalization.
Functional BTB Integrity Assay.
The integrity of the BTB was assessed in a functional assay by monitoring the ability of the BTB to restrict the diffusion of a small fluorescent probe across the barrier. In brief, adult rats (≈270–300 g of body weight at ≈90–120 days of age) were treated with a low (0.35 μg of peptide per testis; n = 3 rats) or a high (3.5 μg of peptide per testis; n = 3 rats) dose of CNP-22 (CNP was prepared in saline, and each testis was at 1.6 ± 0.13 g) via an intratesticular injection using a 27-gauge needle in a final volume of ≈150 μl with ≈75 μl per site (with a total of two sites per testis) (32, 48) at time 0, and rats were used by day 1 and day 3 thereafter for FITC infusion (see below). At these doses, if the peptide had dispersed throughout the testes uniformly, and assuming a testicular volume of 1.6 ml, a concentration of 10−7 M or 10−6 M would have been achieved; however, this might not be the case given the Mr of the peptide (see SI Discussion). Controls included rats treated with saline alone (vehicle control, n = 3), no treatment (n = 3), or with CdCl2 (3 mg/kg of body weight) via i.p. [positive control, n = 2; terminated on day 5, which is known to induce BTB damage (28, 29)]. At specified time points, rats were anesthetized. FITC (Mr 389; AnaSpec, San Jose, CA) was reconstituted in DMSO and diluted in sterile saline. A total of 0.6 mg of FITC in a final volume of 200 μl was administered to each rat via the jugular vein by using a 28-gauge needle. Rats were allowed to recover. Approximately 80 min after the infusion, rats were killed and testes were removed and frozen in liquid nitrogen. Frozen testes sections were obtained and examined by fluorescence microscopy. Tubules from each rat (a total of ≈200 tubules from two testes of different rats per treatment group were randomly selected) were photographed for each time point. To yield semiquantitative data to assess BTB integrity after CNP peptide treatment, the distance of FITC that was diffused into the seminiferous epithelium beyond the BTB in tubules from treatment groups vs. controls was measured and compared with the tubule radii (diffusion/radius). We did not quantify the concentration of CNP-22 in the testes, but, because BTB damage was detected almost across the entire testes as we randomly selected the tubules for scoring, we speculate that the CNP-22 peptide was distributed evenly in treated testes.
Supplementary Material
Acknowledgments
We thank Mr. Wenxiang Zhang at The Rockefeller University Genomics Resource Center for studies using real-time PCR and Dr. Alison North at The Rockefeller University Bio-Imaging Resource Center for confocal microscopy experiments. This work was supported in part by grants from the National Institutes of Health (National Institute of Child Health and Human Development Grants U01 HD045908 and U54 HD029990, Project 3) and the CONRAD Program (Consortium for Industrial Collaboration in Contraceptive Research Grant C1G 01-72).
Abbreviations
- CNP
C-type natriuretic peptide
- ANP
atrial natriuretic peptide
- TJ
tight junction
- NPR
natriuretic peptide receptor
- GC
guanylyl cyclase
- BTB
blood–testis barrier
- ZO-1
zonula occludens 1
- AR
androgen receptor.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0610100104/DC1.
References
- 1.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 2.Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA. Pharmacol Rev. 2000;52:375–414. [PubMed] [Google Scholar]
- 3.Potter LR, Abbey-Hosch S, Dickey DM. Endocr Rev. 2006;27:47–72. doi: 10.1210/er.2005-0014. [DOI] [PubMed] [Google Scholar]
- 4.Gnessi L, Fabbri A, Spera G. Endocr Rev. 1997;18:541–609. doi: 10.1210/edrv.18.4.0310. [DOI] [PubMed] [Google Scholar]
- 5.Bex F, Corbin A. Eur J Pharmacol. 1985;115:125–126. doi: 10.1016/0014-2999(85)90595-3. [DOI] [PubMed] [Google Scholar]
- 6.Mukhopadhyah AK, Schumacher M, Leidenberger FA. Biochem J. 1986;239:463–467. doi: 10.1042/bj2390463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandey KN, Pavlou SN, Kovacs WJ, Inagami T. Biochem Biophys Res Commun. 1986;38:399–404. doi: 10.1016/0006-291x(86)90295-0. [DOI] [PubMed] [Google Scholar]
- 8.Vollmar AM, Friedrich A, Schulz R. J Androl. 1990;11:471–475. [PubMed] [Google Scholar]
- 9.Dagnino L, Drouin J, Nemer M. Mol Endocrinol. 1991;5:1292–1300. doi: 10.1210/mend-5-9-1292. [DOI] [PubMed] [Google Scholar]
- 10.Pandey K, Orgebin-Crist M. Biochem Biophys Res Commun. 1991;180:437–444. doi: 10.1016/s0006-291x(05)81312-9. [DOI] [PubMed] [Google Scholar]
- 11.Potter LR, Hunter T. J Biol Chem. 2001;276:6057–6060. doi: 10.1074/jbc.R000033200. [DOI] [PubMed] [Google Scholar]
- 12.Reubi J. Endocr Rev. 2003;24:389–427. doi: 10.1210/er.2002-0007. [DOI] [PubMed] [Google Scholar]
- 13.Koller KJ, Lowe DG, Bennett GL, Minamino N, Kangawa K, Matsuo H, Goeddel DV. Science. 1991;252:120–123. doi: 10.1126/science.1672777. [DOI] [PubMed] [Google Scholar]
- 14.Suga S, Nakao K, Hosoda K, Mukoyama M, Ogawa Y, Shirakami G, Arai H, Saito Y, Kambayashi Y, Inouye K, et al. Endocrinology. 1992;130:229–239. doi: 10.1210/endo.130.1.1309330. [DOI] [PubMed] [Google Scholar]
- 15.Maack T. Annu Rev Physiol. 1992;54:11–27. doi: 10.1146/annurev.ph.54.030192.000303. [DOI] [PubMed] [Google Scholar]
- 16.Pedram A, Razandi M, Levin ER. J Biol Chem. 2002;277:44385–44398. doi: 10.1074/jbc.M202391200. [DOI] [PubMed] [Google Scholar]
- 17.Lee NPY, Cheng CY. Endocrinology. 2003;144:3114–3129. doi: 10.1210/en.2002-0167. [DOI] [PubMed] [Google Scholar]
- 18.Lee NPY, Mruk DD, Lee WM, Cheng CY. Biol Reprod. 2003;68:489–508. doi: 10.1095/biolreprod.102.005793. [DOI] [PubMed] [Google Scholar]
- 19.Siu MKY, Wong CH, Lee WM, Cheng CY. J Biol Chem. 2005;280:25029–25047. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- 20.Collin O, Lissbrant E, Bergh A. Int J Androl. 1997;20:55–60. doi: 10.1046/j.1365-2605.1997.00108.x. [DOI] [PubMed] [Google Scholar]
- 21.Middendorff R, Muller D, Paust H, Davidoff MMA. J Clin Endocrinol Metab. 1997;81:4324–4328. doi: 10.1210/jcem.81.12.8954035. [DOI] [PubMed] [Google Scholar]
- 22.Mruk DD, Cheng CY. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- 23.Byers S, Hadley MA, Djakiew D, Dym M. J Androl. 1986;7:59–68. doi: 10.1002/j.1939-4640.1986.tb00871.x. [DOI] [PubMed] [Google Scholar]
- 24.Janecki A, Steinberger A. J Androl. 1986;7:69–71. doi: 10.1002/j.1939-4640.1986.tb00873.x. [DOI] [PubMed] [Google Scholar]
- 25.Le TL, Yap AS, Stow JL. J Cell Biol. 1999;146:219–232. [PMC free article] [PubMed] [Google Scholar]
- 26.Xia W, Mruk DD, Lee WM, Cheng CY. Cytokine Growth Factor Rev. 2005;16:469–493. doi: 10.1016/j.cytogfr.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Setchell BP. In: Molecular Mechanisms in Spermatogenesis. Cheng CY, editor. Austin, TX: Landes Bioscience; 2007. in press. [Google Scholar]
- 28.Wong CH, Mruk DD, Lui WY, Cheng CY. J Cell Sci. 2004;117:783–798. doi: 10.1242/jcs.00900. [DOI] [PubMed] [Google Scholar]
- 29.Hew KW, Heath GL, Jiwa AH, Welsh MJ. Biol Reprod. 1993;49:840–849. doi: 10.1095/biolreprod49.4.840. [DOI] [PubMed] [Google Scholar]
- 30.Russell LD. Am J Anat. 1977;148:313–328. doi: 10.1002/aja.1001480303. [DOI] [PubMed] [Google Scholar]
- 31.Lui WY, Wong CH, Mruk DD, Cheng CY. Endocrinology. 2003;144:1139–1142. doi: 10.1210/en.2002-0211. [DOI] [PubMed] [Google Scholar]
- 32.Xia W, Mruk DD, Lee WM, Cheng CY. J Biol Chem. 2006;281:16799–16813. doi: 10.1074/jbc.M601618200. [DOI] [PubMed] [Google Scholar]
- 33.Li MWM, Xia W, Mruk DD, Wang CQF, Yan HHN, Siu MKY, Lui WY, Lee WM, Cheng CY. J Endocrinol. 2006;190:313–329. doi: 10.1677/joe.1.06781. [DOI] [PubMed] [Google Scholar]
- 34.Siu MKY, Lee WM, Cheng CY. Endocrinology. 2003;144:371–387. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- 35.Janecki A, Jakubowiak A, Steinberger A. Toxicol Appl Pharmacol. 1992;112:51–57. doi: 10.1016/0041-008x(92)90278-z. [DOI] [PubMed] [Google Scholar]
- 36.Chung NPY, Cheng CY. Endocrinology. 2001;142:1878–1888. doi: 10.1210/endo.142.5.8145. [DOI] [PubMed] [Google Scholar]
- 37.Xia W, Wong CH, Lee NPY, Lee WM, Cheng CY. J Cell Physiol. 2005;205:141–157. doi: 10.1002/jcp.20377. [DOI] [PubMed] [Google Scholar]
- 38.De Gendt K, Swinnen JV, Saunders PTK, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lecureuil C, et al. Proc Natl Acad Sci USA. 2004;101:1327–1332. doi: 10.1073/pnas.0308114100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holdcraft RW, Braun RE. Development (Cambridge, UK) 2004;131:459–467. doi: 10.1242/dev.00957. [DOI] [PubMed] [Google Scholar]
- 40.Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, Lardy H, Yeh S. Proc Natl Acad Sci USA. 2004;101:6876–6881. doi: 10.1073/pnas.0307306101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan KAL, De Gendt K, Atanassova N, Walker M, Sharpe RM, Saunders PTK, Denolet E, Verhoeven G. Endocrinology. 2005;146:2674–2683. doi: 10.1210/en.2004-1630. [DOI] [PubMed] [Google Scholar]
- 42.Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Proc Natl Acad Sci USA. 2005;102:16696–16700. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muller D, Mukhopadhyay AK, Speth RC, Guidone G, Potthast R, Potter LR, Middendorff R. Endocrinology. 2004;145:1392–1401. doi: 10.1210/en.2003-0706. [DOI] [PubMed] [Google Scholar]
- 44.Klinger JR, Warburton R, Carino GP, Murray J, Murphy CN, Harrington EO. Exp Cell Res. 2006;312:401–410. doi: 10.1016/j.yexcr.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Suga S, Itoh H, Komatsu Y, Ogawa Y, Hama N, Yoshimasa T, Nakao K. Endocrinology. 1993;133:3038–3041. doi: 10.1210/endo.133.6.8243333. [DOI] [PubMed] [Google Scholar]
- 46.Suga S, Nakao K, Itoh H, Komatsu Y, Ogawa YHN, Imura H. J Clin Invest. 1992;90:1145–1149. doi: 10.1172/JCI115933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morimoto S, Nishimura N, Terai T, Manabe S, Yamamoto Y, Shinahara W, Miyake H, Tashiro S, Shimada M, Sasaki T. J Biol Chem. 2005;280:2220–2228. doi: 10.1074/jbc.M406906200. [DOI] [PubMed] [Google Scholar]
- 48.Russell LD, Saxena NK, Weber JE. Gamete Res. 1987;17:43–56. doi: 10.1002/mrd.1120170106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.